Abstract
Iron biofortification of pearl millet (Pennisetum glaucum) is a promising approach to combat iron deficiency (ID) in the millet-consuming communities of developing countries. To evaluate the potential of iron-biofortified millet to provide additional bioavailable iron compared with regular millet and post-harvest iron-fortified millet, an iron absorption study was conducted in 20 Beninese women with marginal iron status. Composite test meals consisting of millet paste based on regular-iron, iron-biofortified, or post-harvest iron-fortified pearl millet flour accompanied by a leafy vegetable sauce or an okra sauce were fed as multiple meals for 5 d. Iron absorption was measured as erythrocyte incorporation of stable iron isotopes. Fractional iron absorption from test meals based on regular-iron millet (7.5%) did not differ from iron-biofortified millet meals (7.5%; P = 1.0), resulting in a higher quantity of total iron absorbed from the meals based on iron-biofortified millet (1125 vs. 527 μg; P < 0.0001). Fractional iron absorption from post-harvest iron-fortified millet meals (10.4%) was higher than from regular-iron and iron-biofortified millet meals (P < 0.05 and P < 0.01, respectively), resulting in a higher quantity of total iron absorbed from the post-harvest iron-fortified millet meals (1500 μg; P < 0.0001 and P < 0.05, respectively). Results indicate that consumption of iron-biofortified millet would double the amount of iron absorbed and, although fractional absorption of iron from biofortification is less than that from fortification, iron-biofortified millet should be highly effective in combatting ID in millet-consuming populations.
Introduction
Biofortification, which refers to the development of micronutrient-enhanced staple crop varieties by traditional breeding practices or by modern biotechnology, has gained increased attention in preventing micronutrient deficiencies over the last decade (1). It is potentially more sustainable and cost-effective than conventional fortification and it implicitly targets the low-income households in remote areas with large daily consumption of a few food staples and limited access to commercially marketed fortified foods (2–4). Dissemination of seeds that efficiently accumulate soil iron could increase the delivery of iron to the diets of poverty-stricken people, including women and children who are most at risk for iron deficiency (ID)7 (4). However, iron biofortification only improves iron status if the additional iron provided by the biofortified crop is bioavailable and consequently fills the gap between current iron intake and iron requirement. Furthermore, acceptance of biofortified crop varieties by farmers and consumers is crucial. This implies that the biofortified crop has a sufficiently high yield that is stable in different environments and climatic zones and that the cooking and sensory properties are comparable with nonbiofortified varieties (4, 5).
Present iron biofortification research programs are focused on enhancing iron concentrations in pearl millet, maize, wheat, rice, and beans (1, 6, 7). Pearl millet (Pennisetum glaucum) is among the most important staple crops in the semi-arid tropics of India (8) and sub-Saharan Africa, especially western Africa (9), where 26% of the average per capita cereal grain consumption has been reported to be pearl millet (10). The monotonous pearl millet-based diets with low iron concentration and low iron bioavailability contribute to ID with or without anemia in these regions (11).
Depending on genotype and environmental conditions, the iron concentration of pearl millet has been reported to vary between 1.6 and 9.6 mg/100 g (12, 13). Several studies have reported higher concentrations up to 20 mg iron/100 g pearl millet (14–16); however, such concentrations most likely include contaminant iron from post-harvest treatments and should not be used for reporting native iron concentration of pearl millet (17). The iron concentration of iron-biofortified pearl millet has been reported to be ∼7–8 mg/100 g (18, 19), which is about double the iron content of other major cereal staples. Compared with regular pearl millet, the iron-biofortified varieties usually also have higher phytic acid (PA) concentrations (18). PA is a well-known inhibitor of human iron absorption (20, 21), impairing the bioavailability of additional iron in biofortified varieties (22) as well as iron fortification compounds used for conventional fortification (23).
Several previous studies have reported improved iron status through conventional iron fortification of cereals flours (24). However, pearl millet has not been considered as a vehicle for iron fortification so far, most likely due to the limited industrial processing of pearl millet grains in sub-Saharan Africa and India (10). Therefore, iron-biofortified millet providing additional bioavailable iron could be a promising approach to combat ID in pearl millet-consuming communities with limited access to commercially fortified foods. The aim of the present study was to investigate the extent to which the additional iron in iron-biofortified pearl millet is bioavailable. Fractional iron absorption and total iron absorbed from composite meals based on iron-biofortified pearl millet were compared with the same composite meals consisting of regular-iron pearl millet or post-harvest iron-fortified pearl millet using a 5-d multiple meal design and stable isotope technique.
Methods
Participants.
The study was carried out in young Beninese women recruited in Natitingou, Atacora department, Northern Benin. Twenty-two apparently healthy, nonpregnant, nonlactating women with marginal iron stores [plasma ferritin (PF) <25 μg/L], aged between 17 and 35 y, and having a body weight <65 kg were selected from an initial screening of 133 women. Intake of vitamin and/or mineral supplements was not allowed during and 2 wk before the study. Women with symptomatic malaria (asexual P. falciparum parasitemia in blood smears + fever); known metabolic, chronic, and gastro-intestinal disease; as well as women on long-term medication were excluded from the study. No women were recruited who had donated blood or experienced substantial blood loss within 6 mo of the beginning of the study. All study participants provided informed written consent. Ethical approval for the study was given by the ethical review committee at the Ministry of Health in Benin and at the ETH Zurich, Switzerland.
Study design.
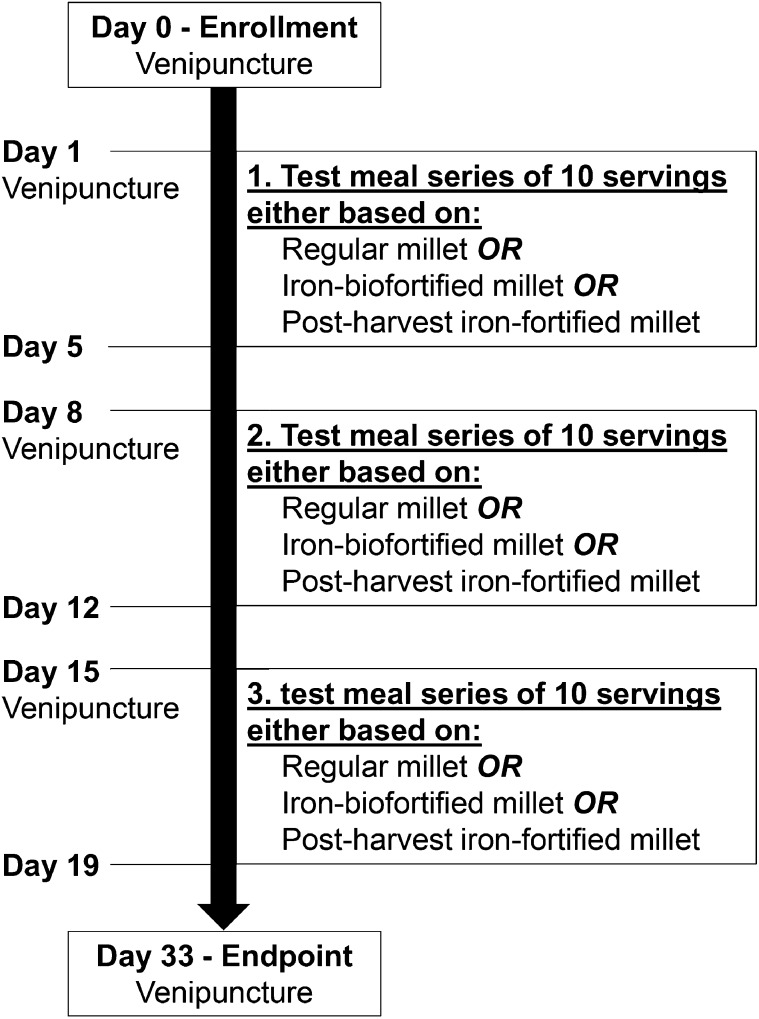
A randomized cross-over design with multiple meals was used with each woman serving as her own control. Every woman received 3 different types of test meals in series of 10 servings for 5 d each. The order of the 3 different series was randomized. The 3 different types of test meals were either based on regular-iron, iron-biofortified, or post-harvest iron-fortified pearl millet (Fig. 1). Servings of one test meal type were always labeled with the same isotope: 54Fe was used for the post-harvest iron-fortified meals, 57Fe for the regular-iron meals, and 58Fe for the iron-biofortified meals. The labeled test meals were administered twice per day in the morning and at noon from Wednesday to Sunday for 3 consecutive weeks (days 1–5, days 8–12, and days 15–19). The serving in the morning was administered between 0630 and 0930 h after an overnight fast and the second serving was administered at least 3 h later. The participants consumed the test meals completely in the presence of the investigators and were not allowed to eat or drink between the test meals and for 3 h after the second meal.
FIGURE 1.
Schematic diagram of the study design.
During screening (baseline measurements), 2 wk before the first meal feeding series, body weight and height of the participants were measured and a first blood sample was drawn for iron status determination [hemoglobin (Hb), PF, C-reactive protein (CRP)] and malaria parasitemia diagnosis. Stool and urine samples were taken for the detection of soil-transmitted helminths and a pregnancy test. A second (d 1), third (d 8), and fourth (d 15) blood sample was drawn for iron status (Hb, PF, CRP) and malaria parasitemia determination immediately before starting a meal feeding series. Fourteen days after the last test meal (d 33; endpoint measurements), body weight and height measures were repeated and a fifth blood sample was drawn for iron isotopic and malaria parasitemia analysis. Iron absorption was determined by using a stable isotope technique in which the incorporation into erythrocytes of isotopic iron labels was measured 14 d after the administration of the last test meal (13). Soil-transmitted helminth analyses and pregnancy tests from the screening were confirmed by stool and urine samples taken at endpoint. Ear temperature was measured at screening and endpoint and always before the meal feedings.
Test meals.
Test meals were composite meals of traditional Beninese millet paste served either with a leafy vegetable sauce in the morning or an okra sauce at noon. Bottled water (300 g) was administered in 2 servings of 80 and 220 g with each test meal. Each test meal serving consisted of 60 g pearl millet flour prepared into millet paste (325 ± 2 g/serving) accompanied by either 110 ± 2 g leafy vegetable sauce or 80 ± 2 g okra sauce. The 2 sauces were prepared freshly for each study day according to a standardized procedure adapted from local recipes. Recipes of the 2 sauces are shown in Supplemental Table 1.
Nongenetically modified iron-biofortified (ICTP8203) and regular-iron (DG-9444) pearl millet (P. glaucum) was planted and harvested by HarvestPlus India and then shipped to Benin as whole grains. A portable household mill (HAWOS Billy 200; Hawos Kornmühlen) was used to obtain the flour for the millet paste preparation. Millet paste was prepared freshly on each study day by weighing and mixing millet flour and water at a ratio of 1.2:1. This blend was then added to boiling water (blend:boiling water ∼1:2.3) and cooked for 26 ± 3 min with intermittent stirring.
Regular-iron, iron-biofortified, and post-harvest iron-fortified millet meals were labeled with 0.4 mg 57Fe, 0.4 mg 58Fe, and 0.4 mg 54Fe, respectively. The stable iron isotopes were in the form of a solution and were diluted in the first serving of water (80 g) and administered after one-half of the millet paste and sauce was consumed. To ensure complete intake of isotopic labels, the second serving of water (220 g) was served in the same plastic tumbler. Ferrous sulfate solution (4 g/L) was used for the post-harvest fortification of regular-iron millet. The iron concentration per serving of regular-iron millet meals was adjusted to approximately the same concentration as in the iron-biofortified millet meals. To prevent potential organoleptic changes in the meals, the necessary amount of iron, 0.9 mL ferrous sulfate solution (4 g Fe/L), was diluted in the same 80 g of water as the isotopic labels and administered after one-half of the millet paste and sauce was consumed.
Test meal analysis.
Iron concentrations in the millet flours, millet pastes, and sauces were analyzed by graphite-furnace atomic absorption spectrophotometry (AA240Z; Varian) after mineralization by microwave digestion (MLS ETHOSplus; MLS) using a mixture of HNO3 and H2O2. The PA concentration was measured by using a modification of the Makower method (25) in which iron was replaced by cerium in the precipitation step. After the mineralization of the precipitates, inorganic phosphate was determined according to Van Veldhoven and Mannaerts (26) and converted into PA concentrations. The total polyphenol (PP) concentration was determined by using a modification of the Folin-Ciocalteau method (27) and was expressed as gallic acid equivalents. As sauces were prepared daily with freshly bought vegetables, iron, PA, and PP concentrations of each preparation (15/sauce) were measured and expressed as means ± SDs.
Preparation of isotopically labeled iron.
Isotopically labeled 54FeSO4, 57FeSO4, and 58FeSO4 were prepared from isotopically enriched elemental iron (54Fe-metal: 99.9% enriched; 57Fe-metal: 97.9% enriched; 58Fe-metal: 99.9% enriched; all Chemgas) by dissolution in 0.1 mol/L sulfuric acid. The solutions were flushed with argon to keep the iron in the +II oxidation state. Prepared iron tracer solutions were analyzed for iron isotopic composition and tracer iron concentration by reversed isotope dilution MS.
Blood analysis.
Hb was measured in whole blood on the day of collection by using HemoCue hemoglobin 201+ anemia was defined as Hb <12 g/dL (28). PF and CRP were measured using an IMMULITE automatic system (Siemens). ID was defined as PF <15 μg/L and ID anemia as Hb <12 g/dL plus PF <15 μg/L (28). The expected high-sensitivity CRP concentrations for healthy individuals were <5 mg/L (29). Thick and thin blood smears were stained in duplicate by using the Giemsa coloration technique and were independently examined by 2 experienced microscopists (30).
Each isotopically enriched blood sample was analyzed in duplicate for its isotopic composition. Whole blood was mineralized by microwave digestion and iron was separated by anion-exchange chromatography and a subsequent precipitation step with ammonium hydroxide (31). Iron isotope ratios were determined by a multicollector inductively coupled plasma MS instrument (NEPTUNE, Thermo Finnigan).
Stool and urine analysis.
Each stool sample was analyzed in duplicate for the detection of soil-transmitted helminths using the KatoKatz method (30). Urine samples were collected for a pregnancy test (HcG distinct rapid test device; Ziva Impex) and the detection of Schistosoma hematobium eggs using the syringe filtration technique (30).
Calculation of Fe absorption.
The amounts of 54Fe, 57Fe, and 58Fe labels in the blood were calculated based on the shift in iron isotope ratios and the estimated amount of iron circulating in the body. Circulating iron was calculated based on the blood volume estimated from height and weight and measured Hb concentration (32). The calculations were based on the principles of isotope dilution, taking into account that iron isotopic labels were not monoisotopic, using the methods described by Turnlund et al. (33). Calculation of iron absorption is shown in detail in . For calculation of fractional absorption, 80% incorporation of the absorbed iron into RBCs was assumed (34).
Statistical analysis.
Analyses were conducted with SPSS statistical software (SPSS 16.0) and Excel (Windows 7; Microsoft). Results of food analysis, age, anthropometric features, Hb, PF, and CRP were presented as means ± SDs if normally distributed. If not normally distributed, the results were presented as geometric mean values with the 95% CI in parentheses. Results of iron absorption were presented as geometric mean values with the 95% CI in parentheses. Iron absorption from different test meals within the same participant was compared by repeated-measures ANOVA followed by a Bonferroni corrected pairwise comparison. Comparison of millet flour composition (iron, PP, PA) was done by Mann-Whitney U tests. Differences were considered significant at P < 0.05. All data were converted to their logarithms for statistical analysis and reconverted for reporting. The study was powered to detect an intra-subject difference of 30% in fractional iron absorption with an α level of 0.05.
Results
Participant characteristics.
The data of 20 women were included in the study. The data of one woman were excluded due to impaired health conditions during the whole study, and data of another woman were excluded due to high CRP concentration (49.3 mg/L) measured before the second meal-feeding series. At baseline, all women had marginal iron status (PF <25 μg/L) and normal CRP concentrations (<5 mg/L) (Table 1). Five of the women were iron deficient without anemia, 10 were iron-deficient anemic, and 1 woman was anemic at baseline.
TABLE 1.
Age, anthropometric features, and Hb, PF, and CRP concentrations of Beninese women at baseline1
| Variable | Summary value |
| Age, y | 20.6 ± 2.9 |
| Weight, kg | 54.2 ± 6.2 |
| Height, cm | 161 ± 7 |
| BMI, kg/m2 | 20.9 ± 2.6 |
| Hb, g/L | 119 ± 13 |
| PF, μg/L | 11.9 ± 5.1 |
| Plasma CRP, mg/L | 0.51 (0.33, 0.88) |
Values are means ± SDs or geometric means (95% CIs), n = 20. CRP, C-reactive protein; Hb, hemoglobin; PF, plasma ferritin.
During the study, PF concentrations between 25 and 32 μg/L were measured in 2 women before 1 of the 3 meal-feeding series. Additionally, 2 women had PF concentrations between 25 and 32 μg/L before 2 of the 3 meal feeding series. One additional woman had PF concentrations between 25 and 30 μg/L prior to all the 3 meal-feeding series. Three women had slightly elevated CRP concentrations (5.5–7.5 mg/L) before 1 of the 3 meal-feeding series. None of the women had a CRP concentration >5 mg/L before more than one meal-feeding series. All women were negative for soil-transmitted helminths at baseline, but at endpoint, 3 women were positive for S. mansoni. None of the women had a positive malaria blood smear at baseline or endpoint.
Test meal composition.
The iron concentration of the iron-biofortified millet was ∼3.5 times that of regular-iron millet (Table 2). Depending on the sauce, the final iron concentrations of the composite test meals were ∼50–60% higher in meals based on iron-biofortified and post-harvest iron-fortified millet than in meals based on regular-iron millet (Table 3). The iron concentrations of the leafy vegetable sauce and okra sauce were 2.0 ± 0.5 and 1.2 ± 0.2 mg/100 g fresh matter (FM), respectively. The PA concentration in the iron-biofortified millet flour was ∼200 mg/100 g higher than that in the regular-iron millet flour (Table 2), resulting in a difference of ∼120 mg PA/serving of millet paste (Table 3). The PA concentrations of the leafy vegetable sauce (2 ± 1 mg/100 g FM) and okra sauce (10 ± 4 mg/100 g FM) were low and had no relevant influence on the PA:iron (PA:iron molar ratio) in the test meals. PA:iron was highest in the regular-iron millet meals followed by the iron-biofortified millet meals and lowest in the post-harvest iron-fortified millet meals (Table 3). The PP concentrations in the 2 millet types were similar (Table 2). The leafy vegetable sauce and okra sauce had PP concentrations of 145 ± 24 and 93 ± 9 mg gallic acid equivalents/100 g FM, respectively.
TABLE 2.
Total PPs, PA, and iron in regular-iron and iron-biofortified pearl millet flour1
| Pearl millet | PP | PA | Iron | PA:iron |
| mg/100 g flour | ||||
| Regular-iron pearl millet (DG-9444) | 106 ± 4a | 653 ± 17 | 2.5 ± 0.1b | 22.1:1 |
| Iron-biofortified pearl millet (ICPT8203) | 87 ± 1b | 852 ± 35 | 8.8 ± 0.3a | 8.2:1 |
Values are means ± SDs or molar ratios, n = 3. Labeled means in a column without a common letter differ, P < 0.05. PA, phytic acid; PA:iron, phytic acid:iron molar ratio; PP, polyphenol.
TABLE 3.
Total PPs, PA, and iron in millet pastes and composite millet meals consumed by Beninese women1
| Pearl millet meal | PP | PA | Iron2 | PA:iron3 |
| mg/serving | ||||
| Regular-iron millet paste | 72 ± 2 | 392 ± 10 | 1.5 ± 0.2 | |
| Composite millet meal4 | ||||
| + Leafy vegetable sauce | 231 ± 27 | 394 ± 10 | 4.1 ± 0.5 | 8.1:1 |
| + Okra sauce | 146 ± 7 | 400 ± 10 | 2.9 ± 0.3 | 11.7:1 |
| Iron-biofortified millet paste | 65 ± 1 | 511 ± 21 | 5.5 ± 0.6 | |
| Composite millet meal4 | ||||
| + Leafy vegetable sauce | 224 ± 27 | 513 ± 21 | 8.1 ± 0.8 | 5.4:1 |
| + Okra sauce | 139 ± 7 | 519 ± 21 | 6.9 ± 0.6 | 6.4:1 |
| Post-harvest iron-fortified millet paste | 72 ± 2 | 392 ± 10 | 1.5 ± 0.25 | |
| Composite millet meal4 | ||||
| + Leafy vegetable sauce | 231 ± 27 | 394 ± 10 | 7.8 ± 0.5 | 4.3:1 |
| + Okra sauce | 146 ± 7 | 400 ± 10 | 6.6 ± 0.3 | 5.1:1 |
Values are means ± SDs, n = 3. PA, phytic acid; PA:iron, phytic acid:iron molar ratio; PP, polyphenol.
Iron values of the millet pastes include only native iron. Iron values of the composite millet meals include native iron and 0.4 mg iron as 54Fe, 57Fe, or 58Fe. Post-harvest iron-fortified composite millet meals contained 3.7 mg iron added as 56FeSO4.
Values are molar ratios of the composite millet meals.
Values are means ± SDs based on the means from the analysis of single components (pastes, n = 3; sauces, n = 15). SDs were adapted by calculating the square root of the sum from the square of the SDs from the single analysis of pastes and sauces. Iron, PA, and PP concentrations of the sauces alone are in text.
Value does not include 3.7 mg fortification iron, which was added later on to the composite millet meal.
Iron absorption measurements.
Mean fractional iron absorption from the iron-biofortified millet meals did not differ (P = 1.0) compared with the regular-iron millet meals (Table 4), resulting in a doubling of total iron absorbed from the iron-biofortified meals (P < 0.0001). The mean fractional absorption from the post-harvest iron-fortified millet meals was ∼40% higher than from the iron-biofortified millet meals (P < 0.01) and the regular-iron millet meals (P < 0.05). Total iron absorbed from the test meals based on post-harvest iron-fortified millet was therefore higher than from the regular-iron and iron-biofortified millet meals (P < 0.0001 and P < 0.05, respectively).
TABLE 4.
Fractional and total iron absorption per composite millet meal consumed by Beninese women1
| Composite millet meal | Fractional iron absorption | Total iron absorption2 | Ratio of fractional absorption (meal A:meal B, C) |
| % of dose | mg/d | ||
| Regular-iron millet meal | 7.5 (5.7, 10.0)b | 0.53 (0.40, 0.70)c | —3 |
| Iron-biofortified millet meal | 7.5 (5.6, 10.1)b | 1.13 (0.83, 1.52)b | 1.0 |
| Post-harvest iron-fortified millet meal | 10.4 (8.2, 13.2)a | 1.50 (1.18, 1.91)a | 0.7 |
Values are geometric means (95% CIs), n = 20. Labeled means in a column without a common letter differ, P < 0.05.
Total iron absorption is based on the fractional absorption and iron concentrations of one portion of millet paste with leafy vegetable sauce and one portion of millet paste with okra sauce. Iron concentrations of the test meals are shown in Table 3.
—, No value; the fractional iron absorption from the regular-iron millet meal (A) is compared with that from the iron-biofortified millet meal (B) and the post-harvest iron-fortified millet meal (C).
Discussion
The current study has 2 major findings. The first is that fractional absorption did not differ between the regular-iron and the iron-biofortified millet meals, leading to a significantly increased quantity of total iron absorbed from the iron-biofortified millet meals compared with regular-iron millet meals. This indicates that iron-biofortified pearl millet would provide higher amounts of bioavailable iron than regular-iron pearl millet when consumed in a composite meal. Our findings with iron-biofortified millet differ from a previous study comparing iron bioavailability from regular and iron-biofortified beans. The iron-biofortified beans did not provide a greater amount of absorbed iron when administered in multiple composite meals for 5 d (22). The authors argued that the higher PA concentrations in the iron-biofortified beans compared with the regular-iron beans led to a molar excess of PA and therefore more strongly inhibited the bioavailability of additional iron irrespective of the PA:iron, which was around 9:1 in both types of bean meals. The simultaneous increase of iron and PA in iron-biofortified crops has been reported in previous studies (18, 22) and makes it difficult for plant breeders to develop iron-biofortified crops providing bioavailable iron. Our results suggest that, in contrast to common beans, the additional iron in iron-biofortified pearl millet is not strongly inhibited by the additional PA in iron-biofortified millet. This is most probably because the PA concentrations in the 2 millet types used in our study were generally lower than in the bean varieties, and because the difference in total PA between the regular-iron and iron-biofortified millet was only ∼200 mg/100 g compared with ∼500 mg/100 g between the regular-iron and iron-biofortified beans. Furthermore, the difference in iron concentrations between the regular-iron and iron-biofortified millet was greater than in the beans.
Although the PA:iron in the iron-biofortified millet meals was lower than in the regular-iron millet meals, fractional iron absorption was the same from both meal types. This can be explained by a relative decrease in fractional iron absorption with increased quantity of ingested iron. Cook et al. (35) added 1, 3, and 5 mg labeled FeSO4 to a bread roll meal and reported that fractional iron absorption decreases as the amount of iron ingested increases but that more iron was absorbed from the meals with higher iron concentrations.
The second finding of the present study is that the post-harvest iron-fortified millet meals provided a greater amount of absorbed iron than the iron-biofortified millet meals. The fortification iron added to the regular-iron millet meals raised the iron concentration to that of the iron-biofortified millet meals. The lower PA concentration in regular-iron millet compared with iron-biofortified millet led to the lower PA:iron in the post-harvest iron-fortified millet meals. The lower PA:iron (below <6:1) could explain why the fractional iron absorption from post-harvest iron-fortified millet meals is higher than from the other 2 meal types. It has been reported that iron absorption from composite meals improves with PA:iron <6:1 (36).
The composite meals used in our study were very close to the traditional Beninese preparations of pearl millet paste accompanied by a leafy vegetable sauce or okra sauce. However, if available and affordable, local people like to add meat, fish, or traditional Beninese cheese to the sauces. We did not use these ingredients, because it would have introduced heme iron or proteins that might have interfered with iron absorption. We did not measure ascorbic acid concentrations in the 2 sauces but assume negligible amounts of ascorbic acid after cooking (37). PP concentrations in the test meals were relatively high. Some PPs are potent inhibitors of iron absorption (38); however, the relatively high fractional iron absorptions in our study indicate little interference of PPs with iron absorption. Unlike sorghum, pearl millet does not contain condensed tannins with catechol and galloyl groups (39), which are suspected to inhibit iron absorption (40). We also assume that the sauces did not contain many PPs with iron-binding structures. Afitin, an indispensable fermented Beninese condiment (41), can contain considerable amounts of PA (42); however, we used reduced amounts of a long-fermented afitin (24 h) to prepare our sauces. Moreover, the standardized sauces in our study did not contain ingredients that would have added a considerable amount of PA, such as peanut or pumpkin seed paste. The use of ingredients or sauces high in PA would affect the amount of bioavailable iron from iron-biofortified millet as it would that of regular-iron millet. However, we cannot completely rule out if ingredients high in PA would not add PA in molar excess and bind the additional iron from the iron-biofortified millet and thus reduce its benefit compared with regular-iron millet.
The daily per capita consumption of millet in women 18–45 y of age from rural Northern Benin is 159 g (43). Hundred fifty-nine g of iron-biofortified millet prepared into millet paste with 7.5% iron bioavailability, as measured in our study, would provide ∼72% of the median daily iron requirements for menstruating women older than 18 y (44). The same amount of regular-iron millet would only provide ∼20% of the requirements (calculations do not include iron from sauces). Millet consumption data for young Beninese children are not available, but data from Burkina Faso, which borders to Northern Benin, can be extrapolated. Assuming equal fractional iron absorption in young children, Beninese children 12–36 mo of age, who consume an average of 32 g millet/d (45), could satisfy ∼46% of the 0.5 mg absorbed iron required per day (44) with iron-biofortified millet consumption but only ∼13% if the regular-iron millet is consumed.
An early radioisotope study measuring iron bioavailability from composite pearl millet meals reported iron absorption of 4.8 and 1.2%, respectively, from a pearl millet couscous meal with fish and a pearl millet gruel meal with peanut paste. The study calculated the iron absorption by iron-replete men with an average serum ferritin of 83 μg/L in relation to exchangeable iron, because the meals were contaminated by iron from soil residues and/or by dust (46). In our study, fractional iron absorption values from meals based on regular-iron millet were higher than those reported in the previous study and relatively high for meals with such high PA:iron. The reason for this finding is probably the upregulated iron absorption in our mainly iron-deficient female participants (47).
In conclusion, our study shows that the total amount of iron absorbed from iron-biofortified and post-harvest iron-fortified pearl millet is about 2- and 3-times higher than from regular-iron pearl millet. The PA:iron appeared to be the major determinant of the total amount of iron absorbed. Our results suggest that, despite delivering higher PA concentrations, biofortification of pearl millet could be a valuable approach to increase the bioavailable iron supply in remote millet-consuming communities with limited access to conventionally post-harvest fortified foods. Efficacy studies are now needed to investigate if iron-biofortified millet provides sufficient additional iron to improve iron status and combat ID in such populations. Although our study does not exactly represent conventional iron fortification, we think that the results can be extrapolated to the absorption of fortification iron, because we assume that the iron in the aqueous solution consumed with the meal enters the common exchangeable iron pool where its bioavailability is influenced by enhancers and inhibitors in the same way as the bioavailability of an iron premix added to the millet flour before preparation. FeSO4 would be a suitable iron compound in terms of bioavailability, but further research is needed in relation to sensory changes. Although fractional iron absorption is somewhat better from iron-fortified than iron-biofortified millet, post-harvest fortification of pearl millet could be more challenging because of the lack of central milling facilities or because of the difficulties in fortifying flour at the community level. We therefore conclude that iron biofortification of pearl millet is a promising approach to easily and rapidly increase bioavailable iron in the diets of millet-consuming communities in western Africa and probably also in India, depending on the type of foods it is consumed with.
Supplementary Material
Acknowledgments
The authors thank HarvestPlus, especially Erick Boy, for providing the regular-iron and iron-biofortified pearl millet. C.I.C., I.M.E., C.Z., E.M., J.D.H., and R.F.H. designed research; C.I.C., E.M., and F.T. conducted research; C.I.C. and C.Z. analyzed data; C.I.C., I.M.E., and R.F.H. wrote the paper; and C.I.C., I.M.E., and R.F.H. had primary responsibility for final content. All authors read and approved the final version of the paper.
Footnotes
Abbreviations used: CRP, C-reactive protein; FM, fresh matter; Hb, hemoglobin; ID, iron deficiency; PA, phytic acid; PA:iron, phytic acid:iron molar ratio; PF, plasma ferritin; PP, polyphenol.
Literature Cited
- 1.Nestel P, Bouis HE, Meenakshi JV, Pfeiffer W. Biofortification of staple food crops. J Nutr. 2006;136:1064–7. [DOI] [PubMed] [Google Scholar]
- 2.Meenakshi JV, Johnson NL, Manyong VM, Degroote H, Javelosa J, Yanggen DR, Naher F, Gonzalez C, Garcia J, Meng E. How cost-effective is biofortification in combating micronutrient malnutrition? An ex ante assessment. World Dev. 2010;38:64–75. [Google Scholar]
- 3.Qaim M, Stein AJ, Meenakshi JV. Economics of biofortification. Agr Econ. 2007;37:119–33. [Google Scholar]
- 4.Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer WH. Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr Bull. 2011;32:S31–40. [DOI] [PubMed] [Google Scholar]
- 5.Bouis HE, Welch RM. Biofortification: a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010;50:S20–32. [Google Scholar]
- 6.Gregorio GB. Progress in breeding for trace minerals in staple crops. J Nutr. 2002;132:S500–2. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz-Monasterio JI, Palacios-Rojas N, Meng E, Pixley K, Trethowan R, Pena RJ. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. J Cereal Sci. 2007;46:293–307. [Google Scholar]
- 8.Parthasarathy Rao P, Birthal PS, Reddy BVS, Rai KN, Ramesh S. Diagnostics of sorghum and pearl millet grains-based nutrition in India. J SAT Agr Res. 2006 Aug [cited 2013 Jul];2(1):[about 4 p.]. Available from: http://ejournal.icrisat.org/cropimprovement/v2i1/v2i1diagnostics.pdf.
- 9.Vietmeyer ND, Ruskin FR. Lost crops of Africa: grains. Washington, DC: National Academy Press; 1996.
- 10.Ndjeunga J, Nelson CH. Prospects for a pearl millet and sorghum food processing industry in west Africa semi-arid tropics. In: Towards sustainable sorghum production, utilization, and commercialization in west and central Africa. Proceedings of a Technical Workshop of the West and Central Africa Sorghum Research Network; 1999 [cited 2013 Jan]. Available from: http://oar.icrisat.org/4891/.
- 11.Zimmermann MB, Chaouki N, Hurrell RF. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: a longitudinal cohort study in Moroccan children. Am J Clin Nutr. 2005;81:115–21. [DOI] [PubMed] [Google Scholar]
- 12.Barikmo I, Ouattara F, Oshaug A. Differences in micronutrients content found in cereals from various parts of Mali. J Food Compost Anal. 2007;20:681–7. [Google Scholar]
- 13.Lestienne I, Buisson M, Lullien-Pellerin V, Picq C, Treche S. Losses of nutrients and anti-nutritional factors during abrasive decortication of two pearl millet cultivars (Pennisetum glaucum). Food Chem. 2007;100:1316–23. [Google Scholar]
- 14.Abdalla AA, El Tinay AH, Mohamed BE, Abdalla AH. Effect of traditional processes on phytate and mineral content of pearl millet. Food Chem. 1998;63:79–84. [Google Scholar]
- 15.Badau MH, Nkama I, Jideani IA. Phytic acid content and hydrochloric acid extractability of minerals in pearl millet as affected by germination time and cultivar. Food Chem. 2005;92:425–35. [Google Scholar]
- 16.Ragaee S, Abdel-Aal EM, Noaman M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006;98:32–8. [Google Scholar]
- 17.Kayode APP, Linnemann AR, Nout MJR, Van Boekel MAJS. Impact of sorghum processing on phytate, pnenolic compounds and in vitro solubility of iron and zinc in thick porridges. J Sci Food Agric. 2007;87:832–8. [Google Scholar]
- 18.Hama F, Icard-Verniere C, Guyot JP, Rochette I, Diawara B, Mouquet-Rivier C. Potential of non-GMO biofortified pearl millet (Pennisetum glaucum) for increasing iron and zinc content and their estimated bioavailability during abrasive decortication. Int J Food Sci Technol. 2012;47:1660–8. [Google Scholar]
- 19.HarvestPlus. Iron pearl millet; 2009 [cited 2012 Oct]. Available from: http://www.unscn.org/layout/modules/resources/files/HarvestPlus_Pearl_Millet_Strategy.pdf.
- 20.Hallberg L, Brune M, Rossander L. Iron-absorption in man: ascorbic-acid and dose-dependent inhibition by phytate. Am J Clin Nutr. 1989;49:140–4. [DOI] [PubMed] [Google Scholar]
- 21.Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr. 1992;56:573–8. [DOI] [PubMed] [Google Scholar]
- 22.Petry N, Egli I, Gahutu JB, Tugirimana PL, Boy E, Hurrell R. Stable iron isotope studies in Rwandese women indicate that the common bean has limited potential as a vehicle for iron biofortification. J Nutr. 2012;142:492–7. [DOI] [PubMed] [Google Scholar]
- 23.Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res. 2004;74:445–52. [DOI] [PubMed] [Google Scholar]
- 24.Hurrell R, Ranum P, de Pee S, Biebinger R, Hulthen L, Johnson Q, Lynch S. Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food Nutr Bull. 2010;31:S7–21. [DOI] [PubMed] [Google Scholar]
- 25.Makower RU. Extraction and determination of phytic acid in beans (Phaseolus-vulgaris). Cereal Chem. 1970;47:288–96. [Google Scholar]
- 26.Van Veldhoven PP, Mannaerts GP. Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem. 1987;161:45–8. [DOI] [PubMed] [Google Scholar]
- 27.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Parker L, editor. Oxidants and antioxidants. Pt A. San Diego: Academic Press; 1999. p. 152–78. [Google Scholar]
- 28.WHO. Iron deficiency anemia: assessment, prevention and control. Geneva: WHO; 2001. [Google Scholar]
- 29.Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlstrom A, Petersen PH, et al. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP reference material (CRM 470). Eur J Clin Chem Clin Biochem. 1996;34:517–20. [PubMed] [Google Scholar]
- 30.Cercamondi CI, Egli IM, Ahouandjinou E, Dossa R, Zeder C, Salami L, Tjalsma H, Wiegerinck E, Tanno T, Hurrell RF, et al. Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization a double stable-isotope study in young Beninese women. Am J Clin Nutr. 2010;92:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotz K, Krayenbuehl PA, Walczyk T. Mobilization of storage iron is reflected in the iron isotopic composition of blood in humans. J Biol Inorg Chem. 2012;17:301–9. [DOI] [PubMed] [Google Scholar]
- 32.Brown E, Hopper J, Jr, Hodges JL, Jr, Bradley B, Wennesland R, Yamauchi H. Red cell, plasma, and blood volume in the healthy women measured by radiochromium cell-labeling and hematocrit. J Clin Invest. 1962;41:2182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnlund JR, Keyes WR, Peiffer GL. Isotope ratios of molybdenum determined by thermal ionization mass-spectrometry for stable-isotope studies of molybdenum metabolism in humans. Anal Chem. 1993;65:1717–22. [DOI] [PubMed] [Google Scholar]
- 34.Hosain F, Marsaglia G, Noyes W, Finch CA. The nature of internal iron exchange in man. Trans Assoc Am Physicians. 1962;75:59–63. [PubMed] [Google Scholar]
- 35.Cook JD, Minnich V, Moore CV. Rasmusse A, Bradley WB, Finch CA. Absorption of fortification iron in bread. Am J Clin Nutr. 1973;26:861–72. [DOI] [PubMed] [Google Scholar]
- 36.Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:S1461–7. [DOI] [PubMed] [Google Scholar]
- 37.Somsub W, Kongkachuichai R, Sungpuag P, Charoensiri R. Effects of three conventional cooking methods on vitamin C, tannin, myo-inositol phosphates contents in selected Thai vegetables. J Food Compost Anal. 2008;21:187–97. [Google Scholar]
- 38.Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr. 1999;81:289–95. [PubMed] [Google Scholar]
- 39.Dykes L, Rooney LW. Sorghum and millet phenols and antioxidants. J Cereal Sci. 2006;44:236–51. [Google Scholar]
- 40.Brune M, Hallberg L, Skanberg AB. Determination of iron-binding phenolic groups in foods. J Food Sci. 1991;56:128. [Google Scholar]
- 41.Azokpota P, Hounhouigan DJ, Nago MC. Microbiological and chemical changes during the fermentation of African locust bean (Parkia biglobosa) to produce afitin, iru and sonru, three traditional condiments produced in Benin. Int J Food Microbiol. 2006;107:304–9. [DOI] [PubMed] [Google Scholar]
- 42.Avallone S, Bohuon P, Hemery Y, Treche S. Improvement of the in vitro digestible iron and zinc content of okra (Hibiscus esculentus L.) sauce widely consumed in Sahelian Africa. J Food Sci. 2007;72:S153–8. [DOI] [PubMed] [Google Scholar]
- 43.Ategbo EAD. Food and nutrition insecurity in northern Benin: impact on growth performance of children and on year to year nutritional status of adults. Wageningen: Wageningen University; 1993.
- 44.FAO/WHO/UNU. Vitamin and mineral requirements in human nutrition. Report of a joint FAO/WHO expert consultation. 2nd ed. Bangkok: FAO/WHO; 2004. [Google Scholar]
- 45.Hama F. Rétention et biodisponibilité du fer et du zinc au cours des procédés de préparation des plats traditionnels à base de céréales locales ou biofortifiées, consommés par les jeunes enfants au Burkina Faso. Montpellier: Université Montpellier 2; 2012.
- 46.Guiro AT, Galan P, Cherouvrier F, Sall MG, Hercberg S. Iron-absorption from African pearl-millet and rice meals. Nutr Res. 1991;11:885–93. [Google Scholar]
- 47.Zimmermann MB, Biebinger R, Egli I, Zeder C, Hurrell RF. Iron deficiency up-regulates iron absorption from ferrous sulphate but not ferric pyrophosphate and consequently food fortification with ferrous sulphate has relatively greater efficacy in iron-deficient individuals. Br J Nutr. 2011;105:1245–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.