Abstract
Aging-associated declines in cognitive, emotional, and cardiovascular function are well known. Environmental stress triggers critical changes in the brain, further compromising cardiovascular and behavioral health during aging. Excessive dietary salt intake is one such stressor. Here, we tested the effect of high salt (HS) on anxiety, learning-memory function, and blood pressure (BP) in male Fischer brown Norway (FBN) rats. Adult (A; 2 mo) and old (O; 20 mo) male rats were fed normal-salt (NS; 0.4% NaCl) or HS (8% NaCl) diets for 4 wk after being implanted with telemeter probes for conscious BP measurement. Thereafter, tests to assess anxiety-like behavior and learning-memory were conducted. The rats were then killed, and samples of plasma, urine, and brain tissue were collected. We found that systolic BP was higher in O-NS (117 ± 1.2 mm Hg) than in A-NS (105 ± 0.8 mm Hg) rats (P < 0.05). Furthermore, BP was higher in O-HS (124 ± 1.4 mm Hg) than in O-NS (117 ± 1.2 mm Hg) rats (P < 0.05). Moreover, anxiety-like behavior (light-dark and open-field tests) was not different between A-NS and O-NS rats but was greater in O-HS rats than in A-NS, O-NS, or A-HS rats (P < 0.05). Short-term memory (radial arm water maze test) was similar in A-NS and O-NS rats but was significantly impaired in O-HS rats compared with A-NS, O-NS, or A-HS rats (P < 0.05). Furthermore, oxidative stress variables (in plasma, urine, and brain) as well as corticosterone (plasma) were greater in O-HS rats when compared with A-NS, O-NS, or A-HS rats (P < 0.05). The antioxidant enzyme glyoxalase-1 expression was selectively reduced in the hippocampus and amygdala of O-HS rats compared with A-NS, O-NS, or A-HS rats (P < 0.05), whereas other antioxidant enzymes, glutathione reductase 1, manganese superoxide dismutase (SOD), and Cu/Zn SOD remained unchanged. We suggest that salt-sensitive hypertension and behavioral derangement are associated with a redox imbalance in the brain of aged FBN rats.
Introduction
Many rodent strains have been used in the past for aging research (1). Two of the most commonly used rodent strains are the Fischer 344 (F344)6 and the F344/brown Norway (F344/BN) F1 hybrid rats. The F344/BN strain exhibits less biological variability (2) and fewer systemic pathologies (3) than the F344 strain. Moreover, F344/BN rats exhibit age-related similarities to humans and a substantially greater life span relative to F344 rats (4, 5), making F344/BN (FBN) the rodent model recommended by the National Institute on Aging for conducting preclinical aging research (6–9). Using this model, we set out to evaluate the impact of high-salt (HS) diet on changes in cognitive, emotional, and cardiovascular functions typically observed with aging.
It is well documented that aging is accompanied by declines in cognitive, emotional, and cardiovascular function (10–13). Many external stimuli can adversely affect the brain, contributing to cardiovascular and behavioral abnormalities during aging. In this context, excessive salt intake is considered a potent stressor. Although salt is required to maintain normal fluid-mineral balance, excessive intake of salt may have pathophysiologic consequences. One consequence of excessive salt intake is hypertension (14–18). Interestingly, sensitivity to the effects of excessive salt intake increases with aging, making the association between salt and hypertension during aging more precarious (19). In addition to hypertension and other adverse cardiovascular consequences, many animal studies indicate that excessive dietary salt intake has adverse behavioral consequences (20). Relevant to this, many rodent studies have shown an age-related increase in anxiety/hyper-emotionality (21–27). This is consistent with gerontologic surveys that have reported increases in anxiety and depression in the elderly (10, 11). Thus, considering the association between salt, aging, hypertension, cognition, and emotional behavior (19, 28, 29), FBN rats are a good model to examine the effect of high salt (HS) on the co-occurrence of aging, hypertension, learning-memory function, and anxiety-like behaviors. However, surprisingly few behavioral studies have been conducted in this model (30–35), and none have examined the influence of HS on behavior. Furthermore, the effect of HS on the co-occurrence of aging, hypertension, learning-memory impairment, and anxiety-like behaviors was never examined in this model. This is surprising considering the high comorbid prevalence of hypertension and affective disorders (19, 28, 29). Furthermore, the influence of HS intake on this comorbidity also is not known. Particularly vague is the sequence in which the hypertension vs. behavioral disorders develops—i.e., does one precede the other? Evidence from some human studies have revealed that salt-sensitive normotensive individuals show greater anxiety than salt-resistant individuals (36), and some animal data have shown that salt-sensitive rats are cognitively impaired compared with a salt-resistant strain (37). These observations would be compatible with the occurrence of the behavior alterations before the development of hypertension, because they suggest that anxiety or cognitive impairment is associated with greater salt sensitivity, which could contribute to the development of hypertension. All of this information provides a strong rationale for examining the influence of HS intake on aging-associated hypertension, anxiety, and cognitive impairment.
The current study, using the FBN model of aging, is designed to test the hypothesis that feeding an HS diet to old FBN rats increases blood pressure, leads to greater anxiety-like behavior, and causes greater learning and memory impairment than in adult FBN rats. The role of oxidative stress in these processes also was examined. Although the involvement of oxidative stress in aging (38), anxiety (39–51), hypertension (52–55), and cognitive impairment (56–58) is known, the influence of an HS diet on the role of oxidative stress in anxiety, high blood pressure (BP), and cognitive deterioration during aging is not known. Specific oxidative stress targets potentially critical to the etiology of the comorbidity of hypertension, anxiety, and cognitive impairment, including glyoxalase 1 (GLO-1), glutathione reductase 1 (GSR-1), Cu/Zn superoxide dismutase (SOD), and Mn SOD, were examined. Areas of the brain in which these variables will be monitored include the amygdala, hippocampus, and cerebral cortex, all areas considered important for anxiety, learning-memory, and hypertension comorbidity (59).
Materials and Methods
Animal model.
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, following protocols approved by the University of Houston Institutional Animal Care and Use Committee. Adult (2 mo old) and old (20 mo old) FBN rats were purchased from the National Institute on Aging colony maintained by Harlan Laboratories and acclimatized for 1 wk at the University of Houston animal facility before commencement of treatment. The adult (A) FBN rats were randomly assigned to 2 groups: 1 group was fed a normal-salt (NS) diet, hereafter referred to as A-NS, and the other group was fed an HS diet, hereafter referred to as A-HS. Similarly, the old (O) FBN rats were randomly assigned to 2 groups that were fed either an NS or an HS diet; the groups are hereafter referred to as O-NS and O-HS, respectively.
Survival surgery for radio telemetery pressure probe implantation and conscious BP recording.
Rats were anesthetized (3% isoflurane and oxygen mixture), and the abdominal aorta was exposed. The catheter of the rat telemetric probe device (TA11PA-C40; Data Sciences International) was inserted into the abdominal aorta, guided upstream, and secured in place using tissue adhesive (Vetbond; 3M Animal Care Products). Subsequently, rats were housed in individual cages and allowed 1 wk to recover from surgery before recording of BP and NS/HS treatments commenced. Each cage was placed on a receiver connected to a computer capable of analyzing the transmitted signals from the implanted telemeter pressure probes (Data Sciences International). During BP measurements, 2–3 rats in each group exhibited erroneous telemeter recordings and were omitted from analysis. Therefore, final BP analysis included data from 7–8 rats per group.
NS and HS diets.
For 4 wk, rats were fed chow containing a mixture of 20% crude protein, 4.5% crude fat, 6% fiber, and 7% minerals, supplemented with either 0.4% NaCl (NS; catalog no. 5053; LabDiet) or 8% NaCl (HS; catalog no. Test Diet 5AY2-Modified 5053 with 8% total added salt; LabDiet). Food and water were provided ad libitum and consumption recorded twice weekly. After 4 wk of experimental diets, rats were subjected to behavior tests.
Anxiety behavior tests.
The open-field and light-dark anxiety-like behavior tests were conducted as published previously (48, 51), using 10 rats per group. For the open-field test, the percentage of time spent in the center of the open-field arena, rearings, total activity, ambulatory activity, distance covered, and fecal boli were examined. For the light-dark box test, total time spent in the lit area of the light-dark box was recorded (48, 51).
Learning and memory function test.
The learning and memory function test was conducted by using the radial arm water maze procedure (60–62) using 10 rats in each group. The apparatus consisted of a black circular pool filled with water configured with 6 radially oriented swim paths in a dimly lit room. Each rat was randomly assigned a goal arm, released at an arm different from the goal arm, and allowed to locate the goal arm. The rats were allowed 1 min for each learning trial or memory test. The rats were subjected to a set of 6 learning trials (trials 1–6), a 5-min rest, and then another set of 6 learning trials (trials 7–12). Thirty minutes after the end of 12th trial, they were tested for short-term memory.
Brain dissections and preparation of homogenates.
Rats were anesthetized (isoflurane, no. 57319-479-06; Phoenix Pharmaceuticals) after the behavior tests. Quick decapitation was performed and the brains were quickly removed, rapidly frozen, and stored at −80°C until analysis. Blood and bladder urine also were collected at this time. We were not able to withdraw sufficient quantities of blood and urine from each rat. In 1 group we collected blood and urine from 4 rats, in the other group from 5 rats, and in the other 2 groups from 6 rats. The hippocampus, amygdala, and cerebral cortex were identified according to Paxinos and Watson (63) and removed by gross dissection and homogenized. Samples were prepared as published previously (51). Brain samples of hippocampus, amygdala, and cerebral cortex were obtained from either 3 or 4 rats per group.
Briefly, each frozen brain was put on a dry ice–cooled petri dish, and a coronal cut at 0.75 mm from the rostral end of the brain (ventral side down) was made to separate the cortex. A sagittal cut was made at 2.9 mm from midline followed by a second cut an additional 1.5 mm from midline. The two 1.4-mm slices from both hemispheres were separated. Amygdala and hippocampus nuclei were isolated from these slices by using a brain slicer (Zivic Instruments).
Western blot analysis.
Equal amounts of brain tissue homogenates were prepared, subjected to SDS-PAGE, and then Western blotting as previously published (51). Primary antibody dilutions used were as follows; GLO-1 (1:200 dilution, no. ab81461; Abcam), GSR-1 (1:200 dilution), Cu/Zn SOD (1:1000 dilution, no. 07-403; Millipore), Mn SOD (1:1000 dilution, no. 06-984; Millipore), and β-actin (1:1000 dilution). The membranes were incubated with the appropriate primary antibody for 1 h, followed by incubation with an anti-rabbit HRP-conjugated secondary antibody (1:1000) or anti-mouse HRP secondary antibody (1:1000) at room temperature for 1 h. Images of the immunoblots were captured by a Fluorchem imaging system (Alpha Innotech Corp.), and the intensity of each immunoreactive band was determined by using Alpha Ease FC 4.0 (Alpha Innotech Corp.), which was normalized to the β-actin protein control.
Indices of oxidative stress and corticosterone levels.
Concentrations of 8-hydroxy-2-deoxyguanosine (8-OH-dG) were measured in urine by using an ELISA-based kit (8-OH-dG ELISA kit, no. 589320; Cayman Chemicals). Serum 8-isoprostane concentrations (48) were measured by using an EIA kit (Cayman Chemicals). The OxyBlot Protein Oxidation Detection Kit (no. S7150; EMD Millipore Corp.) was used for immunoblot detection of carbonyl groups in different brain tissue homogenates (51). Serum corticosterone concentrations (64) were measured by using an EIA-based kit (no. 500655; Cayman Chemicals).
Statistical methods and data analysis.
Data are expressed as means ± SEMs. Significance was determined by 2-factor ANOVA followed by Tukey’s post hoc test (GraphPad Software, Inc.). P < 0.05 was considered significant. GraphPad Prism 5 software was used to determine variances among means using the in-built “Bartlett’s test for equal variance.” Pearson’s correlation analysis was conducted by using the Microsoft Excel program, wherever appropriate, to examine the correlation between groups.
Results
General variables.
Final body weights did not differ between the NS and HS adult (364 ± 7 g) or old (513 ± 14 g) rats, nor did food intake, which was 19 ± 1 g/d and 21 ± 1 g/d in adult and old rats, respectively. However, water intake was greater due to the HS diet in both adult (A-NS vs. A-HS: 19 ±1 vs. 62 ± 2 mL/d) and old (O-NS vs. O-HS: 43 ± 4 vs. 133 ±18 mL/d) rats.
Conscious BP measurement.
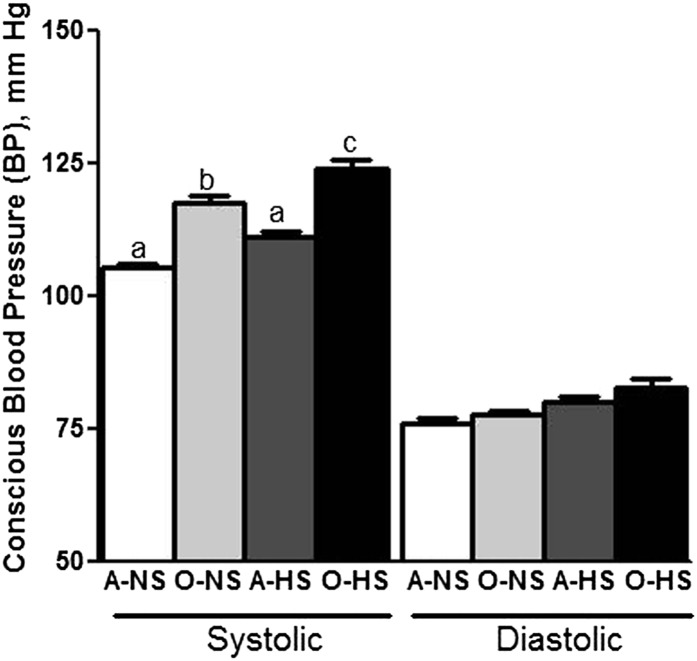
As shown in Fig. 1, systolic BP was higher in O-NS rats than in A-NS rats. HS feeding further increased systolic BP in O-HS rats but not in A-HS rats. Diastolic BP in adult and old rats fed either the NS or HS diet was not significantly different between diets.
FIGURE 1.
Conscious blood pressure measurements in adult and old rats fed normal- or high-salt diets for 4 wk. Bars are means ± SEMs, n = 7–8. Means without a common letter differ, P < 0.05. A-HS, adult, high-salt diet; A-NS, adult, normal-salt diet; O-HS, old, high-salt diet; O-NS, old, normal-salt diet.
Anxiety-like behavior tests.
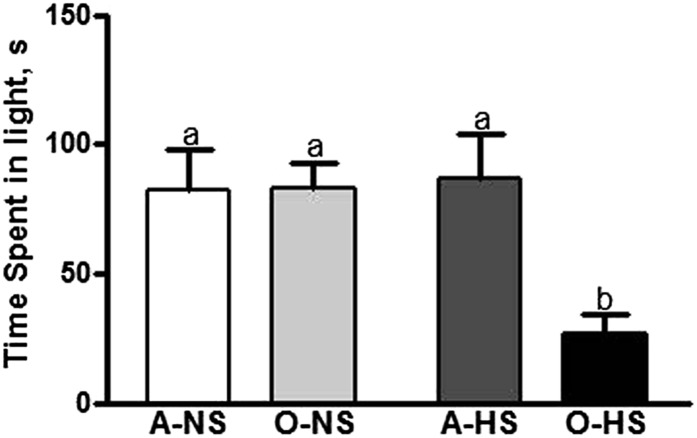
In the light-dark test, a rat is exposed to a novel environment with protected (dark) and unprotected (light) areas. Unwillingness to explore the lit area and willingness to spend more time in the dark during a 5-min test session is indicative of high anxiety-like behaviors. Adult rats fed either an NS or an HS diet a spent similar amount of time in the light compartment (Fig. 2). Time spent in the light compartment by O-NS rats also was similar to that of A-NS rats but was significantly less than that of O-HS rats compared with A-NS or O-NS rats.
FIGURE 2.
Anxiety-like behavior shown by using a light-dark test in adult and old rats fed normal- or high-salt diets for 4 wk. Bars are means ± SEMs, n = 10. Means without a common letter differ, P < 0.05. A-HS, adult, high-salt diet; A-NS, adult, normal-salt diet; O-HS, old, high-salt diet; O-NS, old, normal-salt diet.
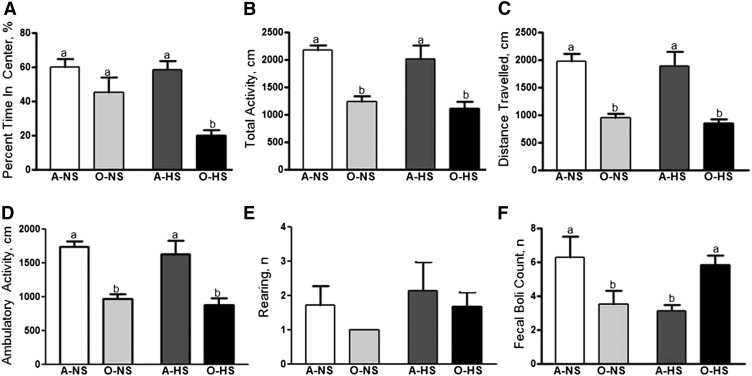
In the open-field test, that percentage of time spent in the center of the arena was similar in A-NS or A-HS rats. However, O-HS rats spent much less time in the center compared with O-NS rats (Fig. 3A). Furthermore, total activity (Fig. 3B), distance traveled (Fig. 3C), ambulatory activity (Fig. 3D), and rearing (Fig. 3E) were not significantly altered with HS treatment in adult rats. Old rats, on the other hand, had lower total (Fig. 3B) and ambulatory (Fig. 3D) activity and covered less distance (Fig. 3C) than did A-NS and A-HS rats. The number of rearings recorded by the Optomax software (Opto-varimax) in the open-field test also were slightly reduced in O-NS and O-HS rats when compared with adult rats (Fig. 3E). Standing on the hind legs and sniffing the novel environment is a typical rodent tendency demonstrative of their curious behavior. Reduced rearing is indicative of increased anxiety. Fecal boli significantly increased with HS intake in old rats but decreased with HS intake in adult rats (Fig. 3F). Total weight or size of the fecal boli did not differ between groups. Increased defecation in the novel environment is indicative of increased anxiety-like behavior of rodents.
FIGURE 3.
Anxiety-like behavior shown by using an open-field test in adult and old rats fed normal- or high-salt diets for 4 wk. The open-field test determined percentage of time spent in the center of the open-field arena (A), total activity (B), distance traveled (C), ambulatory activity (D), rearing (E), and fecal boli (F) for 15 min using Opto-varimax software. Bars are means ± SEMs, n = 10. Means without a common letter differ, P < 0.05. A-HS, adult, high-salt diet; A-NS, adult, normal-salt diet; O-HS, old, high-salt diet; O-NS, old, normal-salt diet.
Learning and memory function.
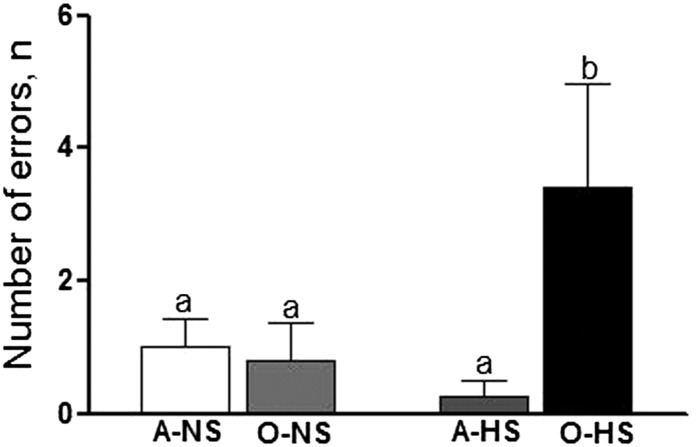
Both adult and old rats fed the NS diet (A-NS, O-NS) made comparable errors on the STM test. On the other hand, whereas the HS diet did not affect the ability of adult rats to make errors on the STM test, O-HS rats made significantly more errors than did the A-HS rats (Fig. 4).
FIGURE 4.
Short-term memory shown by using a radial arm water maze memory test in adult and old rats fed normal- or high-salt diets for 4 wk. Bars are means ± SEMs, n = 10. Means without a common letter differ, P < 0.05. A-HS, adult, high-salt diet; A-NS, adult, normal-salt diet; O-HS, old, high-salt diet; O-NS, old, normal-salt diet.
Analysis of indices of oxidative stress and concentrations of corticosterone and enzymes of antioxidant defense.
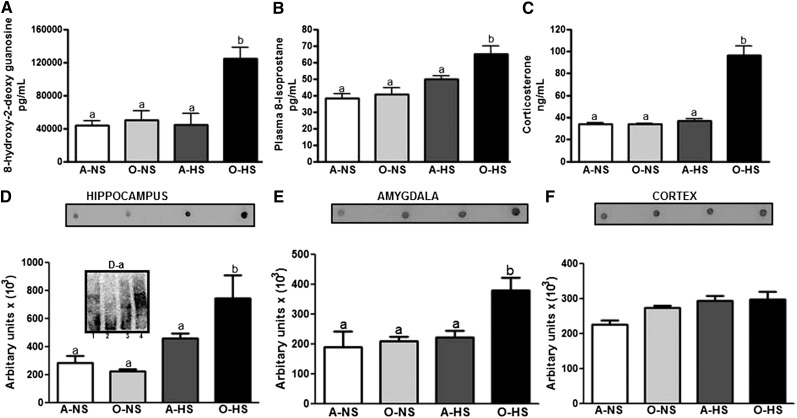
Oxidative stress variables, including 8-OH-dG (in urine) (Fig. 5A), 8-isoprostane (in plasma) (Fig. 5B), and protein carbonylation (in the brain) ( ), were measured. Urinary 8-OH-dG (Fig. 5A) and plasma 8-isoprostane (Fig. 5B) were significantly greater in O-HS rats compared with O-NS rats. No significant changes were observed between A-NS and A-HS rats. Protein carbonylation was measured in oxidative stress–susceptible brain areas previously reported to be important for BP, anxiety, and learning-memory function (46, 47, 51). Protein carbonylation was significantly greater in the hippocampus (Fig. 5D) and amygdala (Fig. 5E) of O-HS rats when compared with O-NS rats, whereas the concentrations were not altered in the cortex (Fig. 5F). No changes were observed in protein carbonylation between A-NS and A-HS rats. Thus, 3 different markers of oxidative stress all indicate that O-HS rats have higher oxidative stress than A-NS or A-HS rats. Plasma corticosterone concentrations in O-HS rats were significantly greater than in A-NS, O-NS, or A-HS rats (Fig. 5C). Corticosterone concentrations were similar among A-NS, O-NS, and A-HS rats (Fig. 5C).
FIGURE 5.
Markers of oxidative stress 8-OH-dG and concentrations of 8-isoprostane and corticosterone in adult and old rats fed normal- or high-salt diets for 4 wk. Concentrations of urinary 8-OH-dG (A), serum 8-isoprostane (B), protein carbonylation (C–E), and serum corticosterone (F) were measured as described in Materials and Methods. Dot blots in panels C–E represent 10 μL homogenate-oxyblot reaction mixtures (D-a). Bars are means ± SEMs, n = 4–6. Means without a common letter differ, P < 0.05. A-HS, adult, high-salt diet; A-NS, adult, normal-salt diet; O-HS, old, high-salt diet; O-NS, old, normal-salt diet; 8-OH-dG, 8-hydroxy-2-deoxyguanosine.
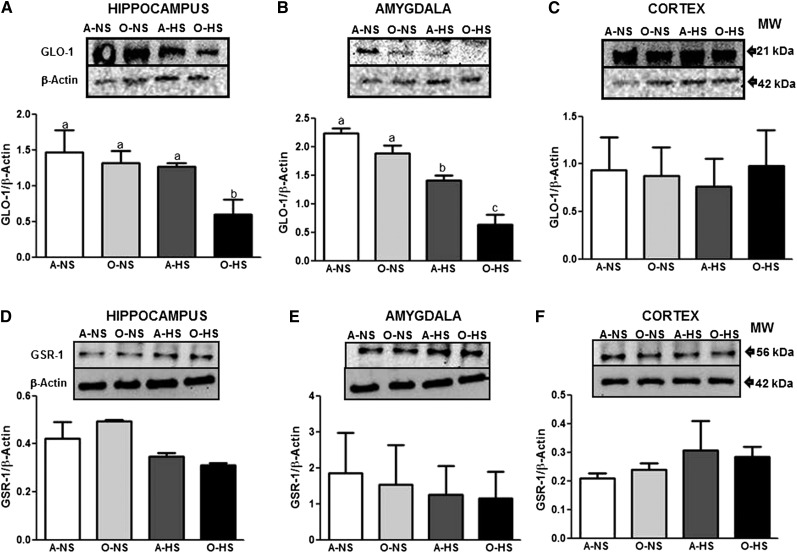
Protein expression levels of GLO-1 (), GSR-1 ( ), Cu/Zn SOD (Supplemental Fig. 1A–C), and Mn SOD (Supplemental Fig. 1D–F) were examined in the hippocampus, amygdala, and cortex. Whereas Mn SOD, Cu/Zn SOD, and GSR-1 protein expression remained unchanged in all groups, GLO-1 protein expression levels were less in the hippocampus (Fig. 6A) and amygdala (Fig. 6B) but not in the cortex (Fig. 6C) of O-HS rats when compared with A-NS, A-HS, and O-NS rats. Finally, Pearson’s correlation analysis was conducted, and a negative correlation was observed between increase in oxidative stress (protein carbonylation) and GLO-1 antioxidant enzyme levels in the hippocampus (r = −0.89, P < 0.05) and amygdala (r = −0.79, P < 0.05) of old HS-treated rats.
FIGURE 6.
GLO-1 and GSR-1 protein levels in the hippocampus, amygdala, and cortex in adult and old rats fed normal- or high-salt diets for 4 wk. Protein levels of GLO-1 (A–C) and GSR-1 (D–F) were determined by Western blotting. The upper panels in A–F are representative blots for GLO-1 (A–C) and GSR-1 (D–F) as well as the protein loading control β-actin (A–F). Bar graphs in panels A–C and D–F are ratios of GLO-1:β-actin and GSR-1:β-actin, respectively. Bars are means ± SEMs, n = 3–4. Means without a common letter differ, P < 0.05. A-HS, adult, high-salt diet; A-NS, adult, normal-salt diet; GLO-1, glyoxalase 1; GSR-1, glutathione reductase 1; O-HS, old, high-salt diet; O-NS, old, normal-salt diet.
Discussion
Excessive dietary salt intake is considered a risk factor for hypertension (14–18). However, the relationship between salt intake, hypertension, and aging remains unclear. Previously, we reported that F344 rats neither showed age-related increase in blood pressure nor age-related salt-sensitivity (65, 66), whereas FBN rats demonstrated age-related hypertension measured under anesthesia (67) as well as when conscious as seen in the present study (Fig. 1). Perhaps, sodium (HS intake) accumulation in the brain triggers a higher BP set point in aged FBN rats. This is plausible given the role of the central nervous system in BP regulation. A BP set point is regulated via central nervous system–dependent sympathetic/parasympathetic mechanisms (68). This seems likely because hypertonic saline (sodium) delivery directly into cerebral ventricle increases BP and peripheral sympathetic tone (69, 70). The occurrence of a similar mechanism responsible for salt sensitivity in the FBN model is an attractive possibility.
Next, we tested whether HS intake causes behavioral (anxiety) and cognitive (learning-memory function) disturbances in the FBN aging model. In the light-dark test, HS treatment increased anxiety-like behavior in old but not in adult rats. This indicates that HS is anxiogenic with advancing age. We did not observe any changes in the time that the A-NS and O-NS rats spent in the lit and dark areas, suggesting that their baseline anxiety-like behaviors are comparable. Earlier, Hasenöhrl et al. (31) reported that old FBN rats spend less time in the lit area than adult rats. However, we defined old as 21 mo of age, whereas Hasenöhrl et al. defined old as 32 mo of age. The total and ambulatory activity, distance traveled, and rearing were not significantly altered by feeding an HS diet to adult rats, whereas HS-fed old rats were less active, and hence covered lesser distance than adult rats. The number of rearings also was slightly reduced in old rats. The overall decline in locomotor activity observed in our study is a commonly observed aging deficit (26, 71–73). It is well known that aging is accompanied by decreases in both quantity and quality of motor activity in humans and in animals (74, 75). Defecation on the other hand, indicative of increased anxiety-like behavior of rodents, was significantly increased with HS intake in old rats, despite the fact that food intake during 4 wk of NS/HS treatment was not different. Thus, whereas activity-based measurement did not show any marked differences in old rats upon HS treatment, fecal boli assessment clearly showed that HS-diet-fed old rats exhibited increased anxiety-like behavior. It is interesting to note that fecal boli were increased upon HS diet intake in old rats but were decreased by HS diet in adult rats. We do not know the exact reason for this observation. However, our data seem to be reasonable considering that maintenance of sodium balance is regarded as critical for proper cellular function and that perturbation of this balance during aging makes high sodium a risk factor for pathophysiologic outcomes during aging. Hence, whereas adult rats do not seem to be susceptible to HS, aged rats show anxiety-like behavior upon HS treatment.
Having established that an HS diet increased anxiety-like behavior in O-HS rats as indicated by the reduced time spent in the center zone, the question of the cause of the increased anxiety remains. Increased concentrations of serum corticosterone, often (but not always) are associated with increased anxiety (64), and serum corticosterone was increased by HS feeding in old but not in adult FBN rats. Therefore, HS appears to be associated with increased activity of the hypothalamic-pituitary-adrenocortical axis stress response in this aging model. This seems reasonable, because aging is believed to engender enhanced adrenal corticosteroid production in this strain (31). Collectively, these data suggest that the FBN rat strain develops age-related hypertension but not behavioral changes unless challenged with HS.
The STM test indicated that an HS diet impairs learning and memory in old rats without affecting memory in adult rats. These are novel observations because the effect of salt on learning and memory function in this model has, to our knowledge, never been examined. Our data are particularly intriguing in consideration of human studies that indicate hypertension to be a powerful risk factor for Alzheimer disease, the most common cause of dementia in the elderly (76). Thus, cognitive deterioration induced by hypertension is known, although a pathophysiologic mechanistic link is still missing.
Oxidative stress is known to be independently involved in hypertension (52–55), anxiety (39–51), and aging-associated learning-memory impairment (57, 58). Whether an HS diet affects oxidative stress, contributing to the comorbidity of hypertension, anxiety, and cognitive deficits during aging, is an interesting question. On the basis of our observations of increased oxidative stress in response to an HS diet in old but not in adult rats, it seems reasonable to suggest that oxidative stress pathways are susceptible to HS, triggering a pathway(s) in the brain that leads to erroneous physiologic and behavioral outcomes. Selective alteration in the concentrations of GLO-1, an enzyme of antioxidant defense, previously reported to be involved in oxidative stress–mediated anxiety (47, 48, 51), also is quite intriguing, because concentrations of Cu/Zn SOD and Mn SOD were unchanged. GLO-1 is part of the glyoxalase system present in the cytosol of cells (77). Cu/Zn SOD also is considered a cytosolic SOD, although it is localized to the intermembrane space of the mitochondria. By contrast, Mn SOD is largely localized to the mitochondrial matrix (78, 79). Perhaps, the cytosolic antioxidant response to high dietary salt feeding supersedes mitochondrial antioxidant defense mechanisms. The selective nature of the changes in GLO-1 after HS feeding in only old FBN rats also is suggested by the observed increase in oxidative stress in the hippocampus and amygdala but not in the cortex. Actually, GLO-1 participates in the detoxification of methylglyoxal, a cytotoxic byproduct of glycolysis that induces protein modification [advanced glycation end-products (AGEs)], oxidative stress, and apoptosis. Oxidative stress, in turn, results in the formation of AGEs (47, 50, 80, 81) and contributes to cytotoxicity and inflammation. GLO-1 detoxifies dicarbonylation and methylglyoxal-mediated cytotoxicity (82). In vitro, overexpression of GLO-1 prevents methylglyoxal accumulation (83), whereas GLO-1 inhibition increases methylglyoxal concentration and decreases cellular viability (84). Given its role in AGE formation, oxidative stress, and cytotoxicity, GLO-1 has also been implicated in aging (77, 85–89). Therefore, we speculate that HS increases oxidative stress, which decreases GLO-1, thereby increasing glycation-promoting cytotoxicity and inflammation, all of which then contribute to the comorbidity of hypertension, anxiety, and learning-memory impairment during aging.
Supplementary Material
Acknowledgments
The authors thank M. F. Lokhandwala for many helpful discussions during the course of this study. They also thank Douglas C. Eikenburg for editing and critically reading the manuscript. G.C., M.A., and S.S. conceived and designed the experiments; F.A., A.T.D., G.P., F.J., R.B., and C.M. conducted research and analyzed the data; S.S., M.A., K.A., G.P., and G.C. wrote the manuscript; and S.S. had the primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGE, advanced glycation end-product; A-HS, adult, high-salt diet; A-NS, adult, normal-salt diet; BP, blood pressure; F344, Fischer 344; F344/BN, Fischer 344 and brown Norway; FBN, Fischer brown Norway; GLO-1, glyoxalase 1; GSR-1, glutathione reductase 1; HS, high salt; NS, normal salt; O-HS, old, high-salt diet; O-NS, old, normal-salt diet; SOD, superoxide dismutase; STM, short-term memory ; 8-OH-dG, 8-hydroxy-2-deoxyguanosine.
Literature Cited
- 1.Masoro EJ. Rats as models for the study of obesity. Exp Aging Res. 1980;6:261–70. [DOI] [PubMed] [Google Scholar]
- 2.Phelan JP, Austad SN. Selecting animal models of human aging: inbred strains often exhibit less biological uniformity than F1 hybrids. J Gerontol. 1994;49:B1–11. [DOI] [PubMed] [Google Scholar]
- 3.Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15:319–28. [DOI] [PubMed] [Google Scholar]
- 4.Sarrieau A, Chaouloff F, Lemaire V, Mormede P. Comparison of the neuroendocrine responses to stress in outbred, inbred and F1 hybrid rats. Life Sci. 1998;63:87–96. [DOI] [PubMed] [Google Scholar]
- 5.Sarrieau A, Mormede P. Hypothalamic-pituitary-adrenal axis activity in the inbred Brown Norway and Fischer 344 rat strains. Life Sci. 1998;62:1417–25. [DOI] [PubMed] [Google Scholar]
- 6.Rice KM, Linderman JK, Kinnard RS, Blough ER. The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology. 2005;6:335–43. [DOI] [PubMed] [Google Scholar]
- 7.Rogers J. Summary and recommendations of the Venice Conference on Animal Models for Aging Research. Neurobiol Aging. 1991;12:699–701. [DOI] [PubMed] [Google Scholar]
- 8.van der Staay FJ, Blokland A. Behavioral differences between outbred Wistar, inbred Fischer 344, brown Norway, and hybrid Fischer 344 × brown Norway rats. Physiol Behav. 1996;60:97–109. [DOI] [PubMed] [Google Scholar]
- 9.Walker EM, Jr, Nillas MS, Mangiarua EI, Cansino S, Morrison RG, Perdue RR, Triest WE, Wright GL, Studeny M, Wehner P, et al. Age-associated changes in hearts of male Fischer 344/Brown Norway F1 rats. Ann Clin Lab Sci. 2006;36:427–38. [PubMed] [Google Scholar]
- 10.Griffiths RA, Good WR, Watson NP, O'Donnell HF, Fell PJ, Shakespeare JM. Depression, dementia and disability in the elderly. Br J Psychiatr. 1987;150:482–93. [DOI] [PubMed]
- 11.van't Veer-Tazelaar PJ, van Marwijk HW, Jansen AP, Rijmen F, Kostense PJ, van Oppen P, van Hout HP, Stalman WA, Beekman AT. Depression in old age (75+), the PIKO study. J Affect Disord. 2008;106:295–9. [DOI] [PubMed] [Google Scholar]
- 12.Basile J. Hypertension in the elderly: a review of the importance of systolic blood pressure elevation. J Clin Hypertens. 2002;4:108–12, 19. [DOI] [PMC free article] [PubMed]
- 13.Andrawes WF, Bussy C, Belmin J. Prevention of cardiovascular events in elderly people. Drugs Aging. 2005;22:859–76. [DOI] [PubMed] [Google Scholar]
- 14.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23:363–84. [DOI] [PubMed] [Google Scholar]
- 15.Henry JP. Stress, salt and hypertension. Soc Sci Med. 1988;26:293–302. [DOI] [PubMed] [Google Scholar]
- 16.Henry JP, Stephens PM. Psychosocial stress induces high blood pressure in a population of mammals on a low-salt diet. J Hypertens. 1988;6:139–44. [PubMed] [Google Scholar]
- 17.Miller SB, Friese M, Dolgoy L, Sita A, Lavoie K, Campbell T. Hostility, sodium consumption, and cardiovascular response to interpersonal stress. Psychosom Med. 1998;60:71–7. [DOI] [PubMed] [Google Scholar]
- 18.Weber CS, Thayer JF, Rudat M, Sharma AM, Perschel FH, Buchholz K, Deter HC. Salt-sensitive men show reduced heart rate variability, lower norepinephrine and enhanced cortisol during mental stress. J Hum Hypertens. 2008;22:423–31. [DOI] [PubMed] [Google Scholar]
- 19.Zicha J, Dobesova Z, Vokurkova M, Rauchova H, Hojna S, Kadlecova M, Behuliak M, Vaneckova I, Kunes J. Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiol Res. 2012;61 Suppl 1:S35–87. [DOI] [PubMed] [Google Scholar]
- 20.Ernsberger P, Azar S, Iwai J. Open-field behavior in two models of genetic hypertension and the behavioral effects of salt excess. Behav Neural Biol. 1983;37:46–60. [DOI] [PubMed] [Google Scholar]
- 21.Blokland A, Raaijmakers W. Age-related changes in correlation between behavioral and biochemical parameters in Lewis rats. Behav Neural Biol. 1993;60:52–61. [DOI] [PubMed] [Google Scholar]
- 22.Boguszewski P, Zagrodzka J. Emotional changes related to age in rats–a behavioral analysis. Behav Brain Res. 2002;133:323–32. [DOI] [PubMed] [Google Scholar]
- 23.Frussa-Filho R, Otoboni JR, Uema FT, Palermo-Neto J. Effects of age and isolation on the evolution of catalepsy during chronic haloperidol treatment. Braz J Med Biol Res. 1992;25:925–8. [PubMed] [Google Scholar]
- 24.Lamberty Y, Gower AJ. Age-related changes in spontaneous behavior and learning in NMRI mice from middle to old age. Physiol Behav. 1992;51:81–8. [DOI] [PubMed] [Google Scholar]
- 25.Li JW, Watanabe M, Fujisawa Y, Shibuya T. Relation between age-related changes in hyper-emotionality and serotonergic neuronal activities in the rat limbic system. Yakubutsu Seishin Kodo. 1995;15:231–8. [PubMed] [Google Scholar]
- 26.Miyagawa H, Hasegawa M, Fukuta T, Amano M, Yamada K, Nabeshima T. Dissociation of impairment between spatial memory, and motor function and emotional behavior in aged rats. Behav Brain Res. 1998;91:73–81. [DOI] [PubMed] [Google Scholar]
- 27.Nagahara AH, Handa RJ. Age-related changes in c-fos mRNA induction after open-field exposure in the rat brain. Neurobiol Aging. 1997;18:45–55. [DOI] [PubMed] [Google Scholar]
- 28.Prohaska TR, Leventhal EA, Leventhal H, Keller ML. Health practices and illness cognition in young, middle aged, and elderly adults. J Gerontol. 1985;40:569–78. [DOI] [PubMed] [Google Scholar]
- 29.Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich). 2008;10:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bethel-Brown CS, Zhang H, Fowler SC, Chertoff ME, Watson GS, Stanford JA. Within-session analysis of amphetamine-elicited rotation behavior reveals differences between young adult and middle-aged F344/BN rats with partial unilateral striatal dopamine depletion. Pharmacol Biochem Behav. 2010;96:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasenöhrl RU, Weth K, Huston JP. Intraventricular infusion of the histamine H(1) receptor antagonist chlorpheniramine improves maze performance and has anxiolytic-like effects in aged hybrid Fischer 344 × Brown Norway rats. Expl Brain Res. 1999;128:435–40. [DOI] [PubMed] [Google Scholar]
- 32.Segar TM, Kasckow JW, Welge JA, Herman JP. Heterogeneity of neuroendocrine stress responses in aging rat strains. Physiol Behav. 2009;96:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanford JA, Osterhaus GL, Vorontsova E, Fowler SC. Measuring forelimb force control and movement in Fischer 344/Brown Norway rats: effects of age and lorazepam. Behav Pharmacol. 2006;17:725–30. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Bethel CS, Smittkamp SE, Stanford JA. Age-related changes in orolingual motor function in F344 vs F344/BN rats. Physiol Behav. 2008;93:461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Stanford JA. Acute and rebound effects of lorazepam on orolingual motor function in young versus aged Fischer 344/Brown Norway rats. Behav Pharmacol. 2008;19:161–5. [DOI] [PubMed] [Google Scholar]
- 36.Deter HC, Buchholz K, Schorr U, Schachinger H, Turan S, Sharma AM. Psychophysiological reactivity of salt-sensitive normotensive subjects. J Hypertens. 1997;15:839–44. [DOI] [PubMed] [Google Scholar]
- 37.Desmond ME, Duzy MJ, Federici BD. Second messenger regulation of occlusion of the spinal neurocoel in the chick embryo. Dev Dynam. 1993;197:291–306. [DOI] [PubMed] [Google Scholar]
- 38.Butterfield A, Swomley AM, Sultana R. Amyloid beta-peptide 1–42-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal. Epub 2013 Feb 14. [DOI] [PMC free article] [PubMed]
- 39.Bouayed J, Rammal H, Younos C, Soulimani R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur J Pharmacol. 2007;564:146–9. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira MR, Silvestrin RB, Mello EST, Moreira JC. Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotor and exploratory activity of adult rats: effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology. 2007;28:1191–9. [DOI] [PubMed] [Google Scholar]
- 41.Ditzen C, Jastorff AM, Kessler MS, Bunck M, Teplytska L, Erhardt A, Kromer SA, Varadarajulu J, Targosz BS, Sayan-Ayata EF, et al. Protein biomarkers in a mouse model of extremes in trait anxiety. Mol Cell Proteomics. 2006;5:1914–20. [DOI] [PubMed] [Google Scholar]
- 42.Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–6. [DOI] [PubMed] [Google Scholar]
- 43.Kromer SA, Kessler MS, Milfay D, Birg IN, Bunck M, Czibere L, Panhuysen M, Putz B, Deussing JM, Holsboer F, et al. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J Neurosci. 2005;25:4375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M, Nussbaumer M, Czibere L, Turck CW, Singewald N, et al. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev. 2007;31:89–102. [DOI] [PubMed] [Google Scholar]
- 45.Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326:369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res. 2010;1359:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salim S, Asghar M, Taneja M, Hovatta I, Chugh G, Vollert C, Vu A. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res. 2011;1404:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010;208:545–52. [DOI] [PubMed] [Google Scholar]
- 49.Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, Souza TM, Portela LV, Perry ML. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007;81:198–203. [DOI] [PubMed] [Google Scholar]
- 50.Thornalley PJ. Unease on the role of glyoxalase 1 in high-anxiety-related behaviour. Trends Mol Med. 2006;12:195–9. [DOI] [PubMed] [Google Scholar]
- 51.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–40. [DOI] [PubMed] [Google Scholar]
- 52.Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension. 2008;51:367–75. [DOI] [PubMed] [Google Scholar]
- 53.Banday AA, Lokhandwala MF. Loss of biphasic effect on Na/K-ATPase activity by angiotensin II involves defective angiotensin type 1 receptor-nitric oxide signaling. Hypertension. 2008;52:1099–105. [DOI] [PubMed] [Google Scholar]
- 54.Banday AA, Lokhandwala MF. Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am J Physiol Renal Physiol. 2008;295:F698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banday AA, Muhammad AB, Fazili FR, Lokhandwala M. Mechanisms of oxidative stress-induced increase in salt sensitivity and development of hypertension in Sprague-Dawley rats. Hypertension. 2007;49:664–71. [DOI] [PubMed] [Google Scholar]
- 56.Sultana R, Butterfield DA. Oxidative modification of brain proteins in Alzheimer's disease: perspective on future studies based on results of redox proteomics studies. J Alzheimers Dis. 2013;33 Suppl 1:S243–51. [DOI] [PubMed] [Google Scholar]
- 57.Lee WH, Kumar A, Rani A, Herrera J, Xu J, Someya S, Foster TC. Influence of viral vector-mediated delivery of superoxide dismutase and catalase to the hippocampus on spatial learning and memory during aging. Antioxid Redox Signal. 2012;16:339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanguilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front Aging Neurosci. 2011;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Charney DS, Drevets WC. The neurobiological basis of anxiety disorders. Am Coll Neuropsychopharmacol. 2002;63:901–28. [Google Scholar]
- 60.Aleisa AM, Helal G, Alhaider IA, Alzoubi KH, Srivareerat M, Tran TT, Al-Rejaie SS, Alkadhi KA. Acute nicotine treatment prevents REM sleep deprivation-induced learning and memory impairment in rat. Hippocampus. 2011;21:899–909. [DOI] [PubMed] [Google Scholar]
- 61.Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Caffeine prevents sleep loss-induced deficits in long-term potentiation and related signaling molecules in the dentate gyrus. Eur J Neurosci. 2010;31:1368–76. [DOI] [PubMed] [Google Scholar]
- 62.Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, Alkadhi KA. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep. 2010;33:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paxinos G, Watson C. The rat brain stereotaxic coordinates. 6th ed 1986. [DOI] [PubMed] [Google Scholar]
- 64.Arranz L, Guayerbas N, De la Fuente M. Impairment of several immune functions in anxious women. J Psychosom Res. 2007;62:1–8. [DOI] [PubMed] [Google Scholar]
- 65.Chugh G, Lokhandwala MF, Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension. 2012;59:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chugh G, Asghar M. Salt-sensitivity during aging: good or bad [abstract]? Hypertension. 2012;60:A499. [Google Scholar]
- 67.Chugh G, Lokhandwala MF, Asghar M. Oxidative stress alters renal D1 and AT1 receptor functions and increases blood pressure in old rats. Am J Physiol Renal Physiol. 2011;300:F133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi H, Yoshika M, Komiyama Y, Nishimura M. The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens Res. 2011;34:1147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki S, Takeda K, Okajima H, Takahashi H, Yoshimura M, Nakagawa M, Ijichi H. Pressor responses to intracisternal injection of hypertonic NaCl in rats. J Cardiovasc Pharmacol. 1984;6:349–54. [DOI] [PubMed] [Google Scholar]
- 70.Buñag RD, Miyajima E. Sympathetic hyperactivity elevates blood pressure during acute cerebroventricular infusions of hypertonic salt in rats. J Cardiovasc Pharmacol. 1984;6:844–51. [DOI] [PubMed] [Google Scholar]
- 71.Lamberty Y, Gower AJ. Spatial processing and emotionality in aged NMRI mice: a multivariate analysis. Physiol Behav. 1993;54:339–43. [DOI] [PubMed] [Google Scholar]
- 72.Gage FH, Kelly PA, Bjorklund A. Regional changes in brain glucose metabolism reflect cognitive impairments in aged rats. J Neurosci. 1984;4:2856–65. [DOI] [PMC free article] [PubMed]
- 73.Sprott RL, Eleftheriou BE. Open-field behavior in aging inbred mice. Gerontologia. 1974;20:155–62. [DOI] [PubMed] [Google Scholar]
- 74.Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of Parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–6. [DOI] [PubMed] [Google Scholar]
- 75.Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–9. [DOI] [PubMed] [Google Scholar]
- 76.Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thornalley PJ. Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;31:1343–8. [DOI] [PubMed] [Google Scholar]
- 78.Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. Eur J Neurosci. 2011;33:1264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patki G, Lau YS. Impact of exercise on mitochondrial transcription factor expression and damage in the striatum of a chronic mouse model of Parkinson's disease. Neurosci Lett. 2011;505:268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med. 1993;14:287–371. [DOI] [PubMed] [Google Scholar]
- 81.Thornalley PJ. Protecting the genome: defence against nucleotide glycation and emerging role of glyoxalase I overexpression in multidrug resistance in cancer chemotherapy. Biochem Soc Trans. 2003;31:1372–7. [DOI] [PubMed] [Google Scholar]
- 82.Di Loreto S, Zimmitti V, Sebastiani P, Cervelli C, Falone S, Amicarelli F. Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int J Biochem Cell Biol. 2008;40:245–57. [DOI] [PubMed] [Google Scholar]
- 83.Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhla B, Luth HJ, Haferburg D, Weick M, Reichenbach A, Arendt T, Munch G. Pathological effects of glyoxalase I inhibition in SH-SY5Y neuroblastoma cells. J Neurosci Res. 2006;83:1591–600. [DOI] [PubMed] [Google Scholar]
- 85.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–45. [DOI] [PubMed] [Google Scholar]
- 86.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. [DOI] [PubMed] [Google Scholar]
- 87.Fleming TH, Humpert PM, Nawroth PP, Bierhaus A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: a mini-review. Gerontology. 2011;57:435–43. [DOI] [PubMed] [Google Scholar]
- 88.Morcos M, Du X, Pfisterer F, Hutter H, Sayed AA, Thornalley P, Ahmed N, Baynes J, Thorpe S, Kukudov G, et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008;7:260–9. [DOI] [PubMed] [Google Scholar]
- 89.Thornalley PJ, Rabbani N. Glyoxalase in tumourigenesis and multidrug resistance. Semin Cell Dev Biol. 2011;22:318–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.