Abstract
Little is known about the impact of n3 (ω3) PUFAs on polarization of CD4+ T cells into effector subsets other than Th1 and Th2. We assessed the effects of dietary fat [corn oil (CO) vs. fish oil (FO)] and fermentable fiber [cellulose (C) vs. pectin (P)] (2 × 2 design) in male C57BL/6 mice fed CO-C, CO-P, FO-C, or FO-P diets for 3 wk on the ex vivo polarization of purified splenic CD4+ T cells (using magnetic microbeads) into regulatory T cells [Tregs; forkhead box P3 (Foxp3+) cells] or Th17 cells [interleukin (IL)-17A+ and retinoic acid receptor-related orphan receptor (ROR) γτ+ cells] by flow cytometry. Treg polarization was unaffected by diet; however, FO independently reduced the percentage of both CD4+ IL-17A+ (P < 0.05) and CD4+ RORγτ+ cells (P < 0.05). Moreover, expression of another critical Th17-cell–related transcription factor, signal transducer and activator of transcription 3, was reduced by FO. Dietary FO reduced the surface expression of both IL-6R and IL-23R on polarized Th17 cells (P ≤ 0.05), thus interfering with the promotive effects of these critical cytokines on Th17 polarization. Additionally, C57BL/6 mice fed diets enriched in eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), or DHA + EPA similarly reduced Th17-cell polarization in comparison to CO by reducing expression of the Th17-cell signature cytokine (IL-17A; P = 0.0015) and transcription factor (RORγτ P = 0.02), whereas Treg polarization was unaffected. Collectively, these data show that n3 PUFAs exert a direct effect on the development of Th17 cells in healthy mice, implicating a novel n3 PUFA–dependent, anti-inflammatory mechanism of action via the suppression of the initial development of this inflammatory T-cell subset.
Introduction
Dietary fish oil (FO)8 enriched in n3 PUFAs, namely DHA and EPA, exerts anti-inflammatory effects through multiple mechanisms in preclinical and clinical settings (1–4). Dietary n3 PUFAs have been shown to alter T-cell plasma membrane microorganization (lipid rafts) at the immunologic synapse, ultimately suppressing T-cell activation, signal transduction, and nuclear translocation/activation of transcription factors (5–9). Moreover, we have shown that dietary FO suppresses the polarization of splenic CD4+ T cells into the inflammatory Th1 subset, with no effect on the polarization of T cells into Th2 cells (10, 11). However, little is known about the effect of n3 PUFAs on CD4+ T-cell polarization into other effector subsets, specifically inflammatory Th17 cells and immunoregulatory T cells (Tregs).
Th17 cells induce tissue inflammation associated with the pathogenesis of autoimmune diseases and aid in the clearance of mucosal infections by pathogens that are not adequately handled by Th1 and Th2 cells (12–14). Previous research has shown that during colitis, n3 PUFAs reduce the percentage of colonic and splenic Th17 cells [CD4+ interleukin (IL)-17A+] and suppress the colonic Th17-associated inflammatory microenvironment (15, 16). Moreover, in obese colitic mice, dietary n3 PUFAs reduced splenic T-cell ex vivo Th17-cell polarization (15), suggesting that n3 PUFAs may suppress one or more intrinsic aspects of Th17-cell differentiation/polarization.
In mice, the process of CD4+ T-cell differentiation/polarization into Th17 cells involves initial cytokine signaling from the combination of IL-6 and transforming growth factor (TGF) β (17, 18). IL-21, induced in a signal transducer and activator of transcription 3 (STAT3)–dependent manner by IL-6, acts in an autocrine manner with TGF-β to drive Th17 cell generation (19–21). Subsequently, IL-23 signaling is required to maintain or expand differentiated Th17 cells (22). All of the cytokine pathways involved in Th17-cell differentiation result in the upregulation of the expression of 2 critical transcription factors: STAT3, which binds directly to both the IL-17 and IL-21 promoters (23, 24), and retinoic acid receptor-related orphan receptor (ROR) γτ, whose expression is necessary for Th17-cell differentiation (22, 25). Ultimately, Th17 cells secrete IL-17A, IL-17F, IL-21, and IL-22, and these cytokines likely cooperate to induce tissue inflammation (13).
Functionally, Tregs play an active role in establishing and maintaining immunologic unresponsiveness to self-constituents (i.e., immunologic self-tolerance) and negative control of various immune responses to nonself-antigens (26). Therefore, Treg-cell–mediated suppression serves as a vital mechanism of negative regulation of immune-mediated inflammation and features prominently in autoimmune and autoinflammatory disorders, cancer, and metabolic inflammation (27). Tregs are uniquely identified by the expression of the transcription factor forkhead box P3 (Foxp3), which specifically controls their development and function (28–30). A defect in the Foxp3 gene produces a phenotype exhibiting hyperactivation of CD4+ T cells and overproduction of proinflammatory cytokines (31). The level of Foxp3 protein expression in Tregs is critical for suppressor function (32), and sustained Foxp3 expression in mature Tregs is necessary for the maintenance of both the Treg phenotype and suppressor function (27, 33). In addition to T-cell receptor (TCR) signaling, the induction of Foxp3 expression in peripheral CD4+ T cells is facilitated by high amounts of TGF-β signaling through the TGF-β receptor (TGF-βR) (34–36). Therefore, the Treg and Th17 polarization programs both require TGF-β however, inflammatory Th17 cells are produced when additional inflammatory cytokine signaling is present within the polarization microenvironment.
Fermentable fiber-derived SCFAs (e.g., butyrate) exert immunomodulatory effects including downregulation of T-cell responses, induction of T-cell anergy, and modulation of antigen presentation capacity (37–39). In the context of inflammatory bowel disease, similar to FO, SCFAs have been shown to exert beneficial anti-inflammatory effects (40–45). Moreover, butyrate alone or in combination with other SCFAs has been shown to shift T-cell responses toward an anti-inflammatory phenotype, in part by reducing Th1-cell cytokine production (46), similar to the effects of dietary FO (10, 11). Therefore, in this study, we assessed the combined ability of dietary n3 PUFAs and soluble fiber to coordinately affect the general inflammatory response elicited by immune cell populations in healthy mice. Additionally, we sought to determine the coordinated impact of these dietary components on Th17 cell and Treg ex vivo polarization capacity. Last, we independently assessed the ability of each of the 2 main bioactive fatty acids present in FO, namely DHA and EPA, to influence Th17 cell and Treg ex vivo polarization.
Materials and Methods
Animals and diets.
Specific pathogen–free male C57BL/6 mice were maintained under barrier conditions and housed as described (47). Over a 4-wk period, mice were fed 1 of 4 isocaloric semipurified diets that were adequate in all nutrients but differed in their compositions of dietary lipid [15% total lipids: corn oil (CO) vs. FO] and fiber (cellulose vs. pectin), yielding 4 dietary groups: CO + cellulose (CO-C), CO + pectin (CO-P), FO + cellulose (FO-C), and FO + pectin (FO-P).
In a second study, male C57BL/6 mice aged 12–15 wk were fed for 3 wk 1 of 4 isocaloric semipurified diets that met the National Research Council nutrition requirements (48, 49) and that were adequate in all nutrients but differed only in their lipid compositions: 3% CO control diet, combined DHA + EPA enriched (0.5% DHA + 0.5% EPA + 2% CO), DHA enriched (1% DHA + 2% CO), or EPA enriched (1% EPA + 2% CO) (50). Highly purified lipid sources were used, i.e., DHA ethyl ester (>70% pure, Incromega DHA700E SR; Bioriginal Food & Science Corp.) and EPA free fatty acid (>95% pure; SLA Pharma). Diet compositions are provided in Supplemental Table 1, and diets were replaced daily. Details regarding the diet fatty acid composition and percentage of energy from lipids and percentage of energy from n3 PUFAs are listed in Supplemental Table 2. All procedures adhered to U.S. Public Health Service policy and were approved by the Institutional Animal Care and Use Committee at Texas A&M University.
Isolation of splenic mononuclear cells.
Splenic mononuclear cells were isolated from a subset of mice via Lympholyte-M (Cedarlane), and staining conditions were conducted as described previously (51). Antigen-presenting cells (APCs) were identified by surface expression of phycoerythrin (PE)-anti-major histocompatibility complex (MHC) class II [i.e., I-A(b), AF6-1201; BD Biosciences]. T cells were identified by expression of APC-anti-CD3α (145-2C11; eBioscience), PE-anti-CD4 (GK1.5; eBioscience), or PE-anti-CD8a (53-6.7; eBioscience).
In vitro T-cell stimulation and cytokine production.
Spleens were removed aseptically, and CD4+ T cells were isolated by positive selection using magnetic CD4 (L3T4) microbeads (Miltenyi Biotec). Cell purity exceeded 90% and did not differ between dietary groups, as described previously (6). Purified CD4+ T-cell cultures containing 2.5 × 105 viable cells/well (assessed by trypan blue exclusion) were either unstimulated [complete Roswell Park Memorial Institute (RPMI) alone] or stimulated with either 5 μg/mL of plate-bound anti-CD3 (clone 145-2C11; BD Biosciences) plus 20 μg/mL of soluble anti-CD28 (clone 37.51; eBioscience) or with 10 μg/mL LPS (E. coli 055:B5; Sigma Aldrich) and were incubated at 37°C for 24 h; culture supernatants were stored at −80°C. Secreted IL-1β, IL-4, IL-6, IL-10, IL-12p70, IL-17A, interferon (IFN) γ, and tumor necrosis factor (TNF) α were simultaneously measured by using the Bio-Plex Pro Mouse Cytokine Group I multiplex kit (Bio-Rad) and the Bio-Plex 200 System and accompanying software package, Bio-Plex Manager 6.0 (Bio-Rad).
Splenic T-cell ex vivo polarization conditions.
A total of 2 × 105 viable (assessed via trypan blue exclusion) CD4+ T cells were added to a round-bottom 96-well plate (BD Biosciences), and all cultures were stimulated with 5 μg/mL of plate-bound anti-CD3 (145-2C11; BD Biosciences) plus 5 μg/mL of soluble anti-CD28 (37.51; eBioscience) (nonpolarized condition). Ex vivo polarization was performed as described previously (15). For Treg polarizing conditions, cultures were supplemented with 2 μg/L TGF-β (BioLegend). For Th17-cell polarizing conditions, cultures were stimulated with 2 μg/L TGF-β, 10 μg/L IL-6, 20 μg/L IL-23 (BioLegend), 10 μg/mL anti-IFN-γ (AN-18; eBioscience), and 10 μg/mL anti-IL-4 (11B11; eBioscience). All cultures were incubated at 37°C for 72 h and subsequently stimulated with 1× brefeldin A (diluted from a 10× stock; eBioscience), 0.001 mmol/L ionomycin (EMD Chemicals) and 50 μg/L phorbol myristate acetate (Sigma Aldrich) for an additional 5 h before antibody staining for flow cytometry analysis.
Surface and intracellular antibody staining conditions.
Intracellular staining was performed as described previously (15, 16). Tregs were identified by PE-anti-Foxp3 (FJK-16s; eBioscience), and Th17 cells were identified by APC-anti-IL-17A (eBio17B7; eBioscience) or APC-anti-RORγτ (AFKJS-9; eBioscience) expression. Cytokine receptor surface expression was detected by PE-anti-IL-23R (O78-1208; BD Biosciences), PE-anti-IL-21R (4A9; BD Biosciences), and PE-anti-CD126 (IL-6R α chain, D7715A7; BD Biosciences) expression. All flow cytometric analyses were conducted by using an Accuri C6 flow cytometer (BD Accuri).
Assay of total and phospho-STAT3 expression in Th17 polarized T-cell lysates.
Protein lysates were isolated from Th17 polarized splenic CD4+ T-cell cultures as previously described (52), and the resultant protein concentration was determined by using Coomassie Plus Protein assay (Pierce). Equal amounts of protein were used from each sample to independently measure both total and phospho-STAT3 (Tyr 705) by ELISA (eBioscience). Subsequently, the ratio of total:phosphorylated (i.e., activated) STAT3 was determined as described elsewhere (47).
Statistical analysis.
Data from the combined dietary fat + fiber study (2 × 2 design) were analyzed by 2-factor ANOVA with the main effects fat and fiber, with the exception of the data presented in Table 1 (splenic mononuclear immune cell populations), which were analyzed by 1-factor ANOVA. T-cell polarization data were initially analyzed by 2-factor ANOVA with the main effects of diet and treatment (i.e., nonpolarized vs. polarized culture conditions). Data from the second study were analyzed by 1-factor ANOVA (main effect: dietary group). Least-squares means were used for all post hoc comparisons. Differences were considered significant at P ≤ 0.05. All data were tested for normality by Shapiro-Wilk, Kolmogorov-Smirnov, Cramer-von Mises, and Anderson-Darling tests. All data are presented as means ± SEMs, and all analyses were conducted by using the SAS system (SAS Institute) for Windows (version 9.0).
TABLE 1.
Percentage of splenic immune cell subsets from mice fed diets containing fish oil or corn oil plus cellulose or pectin for 3 wk1
| Splenic mononuclear cells | CO-C | CO-P | FO-C | FO-P | P value |
| % | % | % | % | ||
| MHC II+ cells | 70.9 ± 2.60 | 65.1 ± 2.50 | 61.8 ± 5.10 | 60.7 ± 1.90 | 0.22 |
| CD3+ cells | 19.3 ± 1.80a | 25.3 ± 2.50ab | 33.6 ± 4.90b | 31.8 ± 2.20b | 0.02 |
| CD3+ CD4+ cells | 11.9 ± 1.10a | 14.1 ± 1.40a | 20.7 ± 1.40b | 20.2 ± 2.60b | 0.004 |
| CD3+ CD8+ cells | 6.30 ± 2.20a | 7.70 ± 1.20ab | 11.7 ± 2.20a,b | 12.9 ± 3.10b | 0.05 |
Values are means ± SEMs, n = 5/diet. Labeled means in a row without a common letter differ, P ≤ 0.05. CO-C, corn oil + cellulose; CO-P, corn oil + pectin; FO-C, fish oil + cellulose; FO-P, fish oil + pectin; MHC, major histocompatibility complex.
Results
Dietary combination of fat and fiber has no effect on splenic T-cell populations and in vitro cytokine production.
The influence of diet on the percentage of splenic immune cell subsets was determined by flow cytometry and the results are presented in Table 1. Within the APC compartment, there was no difference between dietary groups in MHC II surface expression. Conversely, the T-cell compartment was affected by diet, because spleens from mice fed both FO-containing diets (FO-C and FO-P) harbored an increased percentage of total T cells (CD3+ cells; P = 0.02) in comparison to the CO-C group but did not differ from those fed the CO-P diet. Similarly, mice fed both FO-containing diets exhibited increased percentages of splenic CD3+ CD4+ T cells compared with either of the CO-containing diets (P = 0.004). Compared with the CO-C diet, the percentage of splenic CD3+ CD8+ cells was increased only by the FO-P diet (P = 0.05).
The cytokine response to stimulation was subsequently assessed in purified splenic CD4+ T cells. After stimulation through the TCR via anti-CD3/CD28, there was no combined effect of dietary fat and fiber on the secretion of any of the cytokines assessed (Table 2). Dietary fiber exerted no independent effect on in vitro cytokine secretion after anti-CD3/CD28 stimulation. However, the FO-containing diets reduced the secretion of IL-6 (P = 0.03), IFN-γ (P = 0.05), and IL-17A (P = 0.04) compared with CO. After inflammatory challenge via LPS (Table 2), the cells from mice fed the FO-P diet exhibited a reduction in TNF-α secretion compared with all other diets (P = 0.03). Moreover, dietary lipid source had an independent effect wherein cells from mice fed FO secreted less TNF-α in response to LPS compared with cells from the CO-fed mice (P = 0.05). For all other cytokines produced in response to LPS stimulation, no other differences were apparent between dietary groups. Under both stimulation conditions, IL-12p70 was undetectable.
TABLE 2.
Splenic CD4+ T cell in vitro cytokine production after 24-h stimulation with anti-CD3/CD28 or LPS from mice fed diets containing FO or CO plus cellulose or pectin for 3 wk1
| Cytokine | CO-C | CO-P | FO-C | FO-P | P-interaction | CO | FO | P-fat | Cellulose | Pectin | P-fiber |
| CD3/CD28, pg/mL | |||||||||||
| IL-4 | 344 ± 48.0 | 399 ± 129 | 571 ± 43.9 | 471 ± 274 | 0.69 | 372 ± 241 | 526 ± 62.7 | 0.23 | 479 ± 167.9 | 439 ± 59.6 | 0.99 |
| IL-6 | 113 ± 19.3 | 184 ± 43.1 | 88.2 ± 25.2 | 94.8 ± 20.9 | 0.27 | 144 ± 22.4a | 91.5 ± 14.2b | 0.03 | 139.2 ± 15.6 | 102 ± 22.6 | 0.19 |
| IL-10 | 20.7 ± 2.40 | 27.4 ± 6.60 | 30.4 ± 14.3 | 23.1 ± 4.10 | 0.71 | 24.1 ± 3.20 | 26.7 ± 6.50 | 0.94 | 25.5 ± 5.90 | 25.3 ± 3.8 | 0.71 |
| IL-17A | 173 ± 45.2 | 304.4 ± 78.2 | 134 ± 40.7 | 111 ± 47.1 | 0.23 | 239 ± 30.3a | 124 ± 45.0b | 0.04 | 156 ± 38.2 | 232 ± 30.6 | 0.39 |
| IFN-γ | 74.9 ± 22.5 | 241 ± 101 | 51.1 ± 20.5 | 77.8 ± 43.6 | 0.22 | 149 ± 48.9a | 64.5 ± 3.90b | 0.05 | 64.3 ± 15.1 | 160 ± 59.5 | 0.10 |
| TNF-α | 56.5 ± 12.7 | 84.4 ± 49.1 | 35.4 ± 12.2 | 29.8 ± 10.6 | 0.26 | 70.5 ± 21.7 | 32.6 ± 8.10 | 0.32 | 57.1 ± 8.80 | 46.0 ± 21.1 | 0.87 |
| IL-1β | 3.90 ± 0.70 | 4.30 ± 1.10 | 3.80 ± 1.90 | 2.50 ± 0.30 | 0.49 | 4.10 ± 0.60 | 32.2 ± 0.80 | 0.44 | 3.90 ± 0.80 | 3.40 ± 0.50 | 0.71 |
| LPS, pg/mL | |||||||||||
| IL-4 | N/A | N/A | N/A | N/A | — | N/A | N/A | — | N/A | N/A | — |
| IL-6 | 99.9 ± 24.9 | 150 ± 24.3 | 125 ± 17.8 | 120 ± 15.4 | 0.33 | 122 ± 18.0 | 117 ± 13.0 | 0.71 | 107 ± 16.4 | 133 ± 14.7 | 0.23 |
| IL-10 | 22.7 ± 4.40 | 42.7 ± 17.8 | 25.2 ± 5.20 | 21.7 ± 2.40 | 0.28 | 32.7 ± 7.80 | 23.4 ± 2.60 | 0.70 | 23.9 ± 3.30 | 32.2 ± 7.10 | 0.51 |
| IL-17A | 7.00 ± 1.80 | 8.60 ± 2.20 | 6.80 ± 1.90 | 7.30 ± 1.40 | 0.77 | 7.80 ± 1.40 | 7.00 ± 1.20 | 0.68 | 6.90 ± 1.30 | 7.90 ± 1.30 | 0.60 |
| IFN-γ | N/A | N/A | N/A | N/A | — | N/A | N/A | — | N/A | N/A | — |
| TNF-α | 15.9 ± 5.20a | 35.4 ± 17.9a | 23.5 ± 5.30a | 9.10 ± 4.50b | 0.03 | 31.6 ± 8.80a | 16.3 ± 3.50b | 0.05 | 31.9 ± 3.40 | 24.3 ± 8.60 | 0.35 |
| IL-1β | 12.6 ± 2.70 | 16.9 ± 2.50 | 12.0 ± 2.20 | 7.00 ± 3.10 | 0.30 | 14.5 ± 1.80 | 11.3 ± 1.90 | 0.21 | 12.3 ± 1.70 | 13.4 ± 2.00 | 0.59 |
Values are means ± SEMs, n = 5/diet. Labeled means in a row without a common letter differ, P ≤ 0.05. CO, corn oil; CO-C, corn oil + cellulose; CO-P, corn oil + pectin; FO, fish oil; FO-C, fish oil + cellulose; FO-P, fish oil + pectin; IFN-γ, interferon γ IL, interleukin; N/A, not available; TNF-α, tumor necrosis factor α.
Ex vivo Th17-cell polarization is reduced by n3 PUFAs.
Under the nonpolarized TCR-stimulated condition (stimulation with anti-CD3/CD28), there was no difference between dietary groups and basal expression was low for Foxp3 (8.3 ± 0.7%), IL-17A (25.0 ± 1.3%), and RORγτ (13.3 ± 0.6%). From each dietary group, data from nonpolarized and polarized cultures were initially analyzed by 2-factor ANOVA (main effects: diet and treatment), and for each T-cell subset marker (Foxp3, IL-17A, and RORγτ) there was a significant effect of treatment (i.e., polarization status; P < 0.05) but no significant interaction. Therefore, within each dietary group, we subtracted the nonpolarized expression levels from the outcome in Th17 and Treg polarized cultures to yield a corrected value of polarized expression of Th17 and Treg signature markers. These data were subsequently analyzed by 2-factor ANOVA (main effects: fat and fiber) to determine the individual and/or combined effects of the dietary fat and fiber combinations used in the experimental diets. Representative dot plots for polarized Tregs and Th17 cells are shown in Supplemental Figure 1.
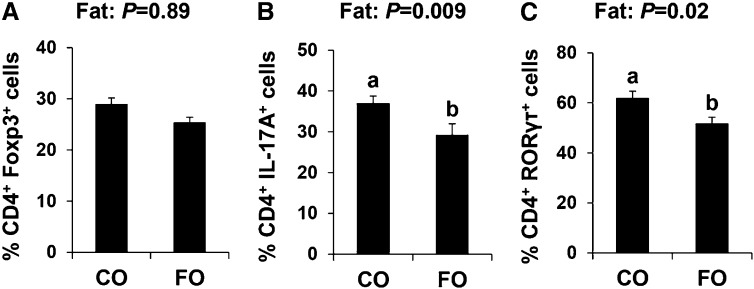
There was no dietary influence on the ability of splenic CD4+ T cells to polarize to Tregs (Fig. 1A). There was no interactive effect of dietary fat and fiber on IL-17A or RORγτ expression. Additionally, the dietary fiber source had no significant effect on the expression of the Th17 signature markers (IL-17A and RORγτ). However, there was an independent effect of dietary fat on Th17 polarization status (Fig. 1B, C). The proportion of CD4+ cells expressing both IL-17A and the transcription factor that drives its expression, RORγτ, were reduced significantly in FO-fed mice compared with CO-fed mice (P ≤ 0.05). Collectively, these results indicate that n3 PUFAs reduce Th17-cell polarization and therefore render CD4+ T cells refractive to ex vivo Th17 polarization signals.
FIGURE 1.
Splenic CD4+ T-cell Treg (A) and Th17-cell (B, C) polarization from male mice fed diets containing FO or CO plus cellulose or pectin for 3 wk. Data were analyzed by 2-factor ANOVA (main effects: dietary fat and fiber), and only pooled data from a significant main effect (dietary fat) is shown. Results are the outcome of the percentage of cells in polarized cultures minus the percentage of cells in nonpolarized TCR-stimulated cultures. Values are means ± SEMs, n = 12/diet. Labeled means without a common letter differ, P ≤ 0.05. CO, corn oil; FO, fish oil; Foxp3, forkhead box P3; IL-17A, interleukin 17A; RORγτ, retinoic acid receptor–related orphan receptor γτ TCR, T-cell receptor; Treg, regulatory T cell.
n3 PUFAs reduce STAT3 activation under Th17 polarizing conditions.
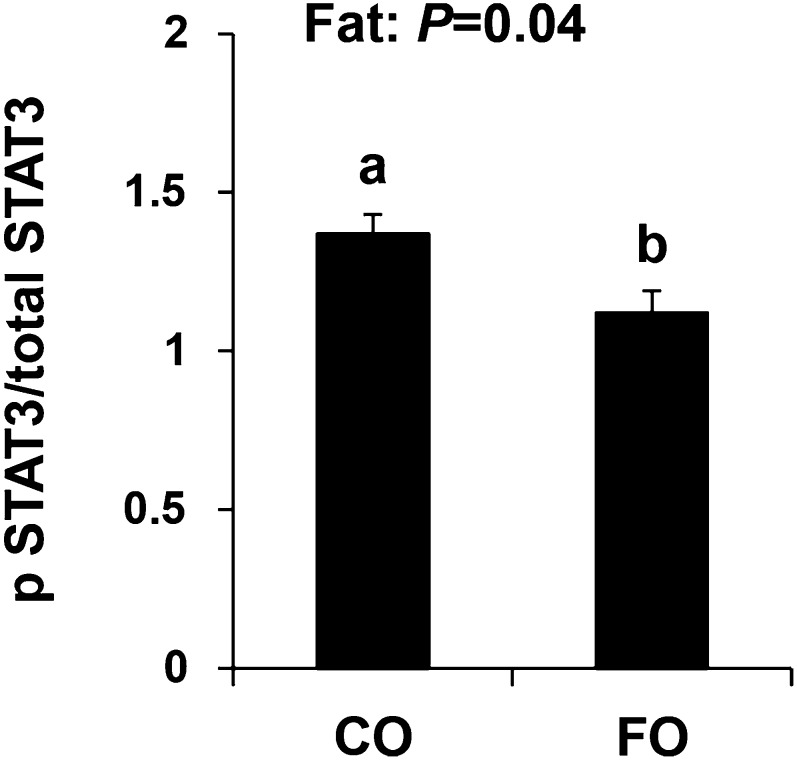
STAT3 is a transcription factor that regulates the expression of RORγτ, and signaling through STAT3 is a critical requirement of Th17-cell differentiation (53, 54). Therefore, we assessed the influence of diet on STAT3 expression by examining the ratio of activated (i.e., phosphorylated) relative to total STAT3 expression in Th17 polarized CD4+ T-cell cultures. Dietary FO reduced the ratio of activated:total STAT3 compared with the CO diet (P ≤ 0.05; Fig. 2). There was no independent effect of dietary fiber nor was there a combined effect of fat and fiber on STAT3 phosphorylation.
FIGURE 2.
Th17-cell expression of phosphorylated/total STAT3 from mice fed diets containing FO or CO plus cellulose or pectin for 3 wk. Data were analyzed by 2-factor ANOVA (main effects: dietary fat and fiber). Only pooled data from a significant main effect (dietary fat) are shown. Values are means ± SEMs, n = 8/diet. Labeled means without a common letter differ, P ≤ 0.05. CO, corn oil; FO, fish oil. IL, interleukin; pSTAT, phosphorylated signal transducer and activator of transcription; STAT, signal transducer and activator of transcription.
n3 PUFAs reduce T-cell responsiveness to Th17 polarizing cytokines.
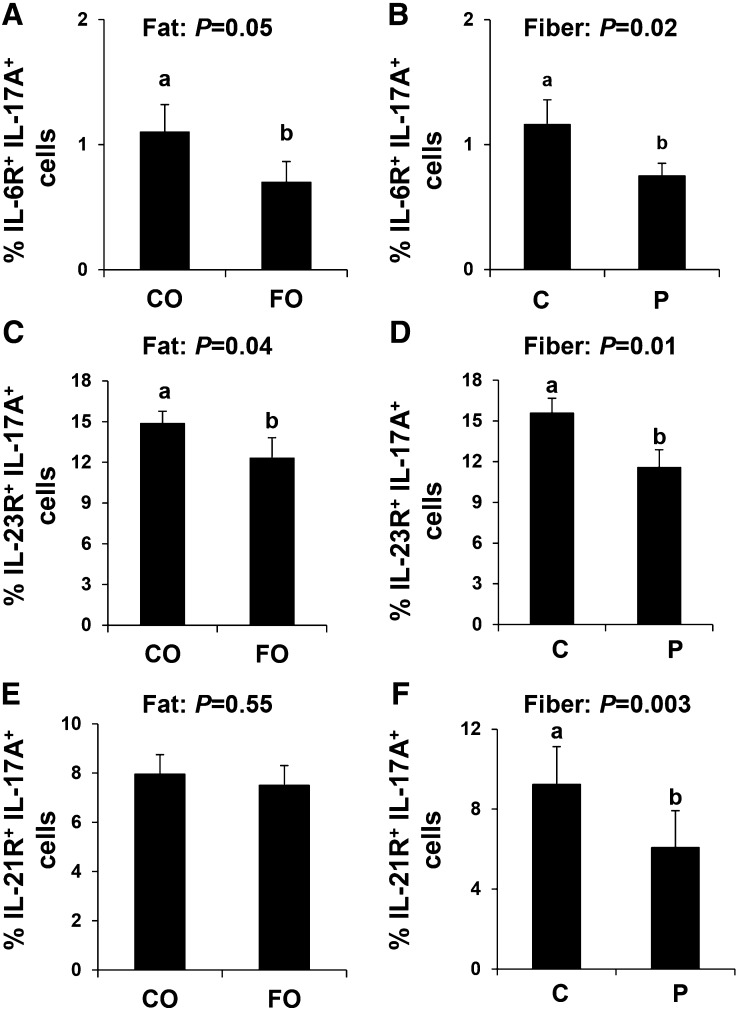
We assessed the ability of IL-17A+ T cells to respond to key polarizing cytokines by measuring surface expression of IL-6R, IL-23R, and IL-21R; representative dot plots are shown in Supplemental Figure 2. In mice, the combination of TGF-β1 and IL-6 is sufficient to drive Th17-cell polarization (18, 55); however, because TGF-β1 signaling is common for both Th17 and Treg polarization (34), we initially assessed expression of IL-6R on the surface of Th17 polarized cells (IL-17A+ IL-6R+ cells). There was no combined effect of dietary fat and fiber on IL-6R expression (P = 0.07); however, each of these dietary components exerted a significant independent effect on the proportion of IL-17A+ IL-6R+ cells (fat: P = 0.05; fiber: P = 0.02). Therefore, independent of fiber, FO reduced the percentage of IL-17A+ IL-6R+ cells compared with CO (Fig. 3A). Moreover, the proportion of IL-17A+ IL-6R+ cells from mice consuming dietary pectin was reduced compared with those consuming cellulose (Fig. 3B). Interestingly, the proportion of IL-17A+ IL-23R+ cells and IL-17A+ IL-21R+ cells was reduced in mice consuming pectin as the dietary fiber source compared with those consuming cellulose (Fig. 3D, F).
FIGURE 3.
Th17-cell surface cytokine receptor expression from mice fed diets containing FO or CO plus cellulose or pectin for 3 wk. Th17 cells (CD4+ IL-17A+) coexpressing IL-6R (A, B), IL-23R (C, D), and IL-21R (E, F). Data were analyzed by 2-factor ANOVA. Only pooled data from a significant main effect (dietary fat: A, C, and E) and dietary fiber (B, D, and F) are shown. Values are means ± SEMs, n = 10/diet. Labeled means without a common letter differ, P ≤ 0.05. C, cellulose; CO, corn oil; FO, fish oil; IL, interleukin; P, pectin.
IL-23 signaling does not induce Th17-cell differentiation but instead is important for supporting Th17-cell expansion and for the ongoing maintenance of an established Th17-cell phenotype (18, 55). Consistent with FO reducing Th17 polarization, the percentage of IL-17A+ IL-23R+ cells was reduced in mice consuming FO compared with those consuming CO (P = 0.04; Fig. 3C). IL-21R expression, which establishes an autocrine signaling loop that drives the expression of STAT3, did not differ between CO- and FO-fed mice (Fig. 3E).
Purified dietary EPA and DHA equally reduce Th17-cell ex vivo polarization.
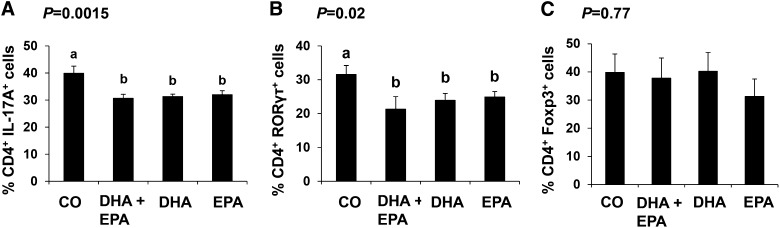
Having determined that dietary FO exerts a suppressive effect on the capability of splenic CD4+ T cells to polarize into Th17 cells, we compared diets containing EPA and DHA, alone or in combination (representative dot plots shown in Supplemental Fig. 3). There was no difference between dietary groups in the proportion of cells expressing any T-cell subset signature cytokine or transcription factor under basal, nonpolarized, TCR-stimulated conditions (IL-17A: 10.5 ± 1.9%; RORγτ: 10.0 ± 1.5%; Foxp3: 8.8 ± 1.2%). Under Th17 polarizing conditions, splenic CD4+ T cells from mice fed any of the 3 n3 PUFA–containing diets (EPA, DHA, or DHA + EPA) did not differ from each other and were refractive to Th17-cell polarization compared with the CO control diet, as evidenced by a reduced percentage of IL-17A– and RORγτ-expressing cells (P ≤ 0.05) (Fig. 4A, ). Under Treg polarizing conditions, the proportion of Foxp3-expressing cells was unaffected by diet (Fig. 4C).
FIGURE 4.
Splenic CD4+ T cell (A, B) Th17 and (C) Treg polarization from male mice fed CO, DHA + EPA, DHA or EPA enriched diets for 3 wk. Data were analyzed by 1-factor ANOVA, and P values for the effect of diet are shown. Results are the outcome of the percentage of cells in polarized cultures minus the percentage of cells in nonpolarized TCR-stimulated cultures. Values are means ± SEMs, n = 5/diet. Labeled means without a common letter differ, P ≤ 0.05. CO, corn oil; Foxp3, forkhead box P3; IL-17A, interleukin 17A; RORγτ, retinoic acid receptor–related orphan receptor γτ TCR, T-cell receptor; Treg, regulatory T cell.
Discussion
We assessed the combined effect of dietary lipid and fiber sources on CD4+ T-cell cytokine response to in vitro stimulation and the ability to respond to ex vivo Th17 and Treg polarization signals. In contrast to the suppressive effect that dietary FO and fermentable fiber–derived SCFAs independently exert on inflammatory Th1-cell polarization (10, 11, 46), there was no beneficial combined effect of dietary fat and fiber, and the FO-P diet did not significantly affect any of the endpoints assessed apart from reducing LPS-stimulated TNF-α secretion (Table 2). Moreover, dietary fiber did not exert an independent effect on the T-cell response to stimulation or polarization status; however, it did reduce the proportion of IL-17A+ cells coexpressing cytokine receptors (Fig. 3B, D, F). Further studies are required to elucidate the precise mechanism of action. Conversely, dietary FO exerted multiple independent effects on CD4+ T-cell function. The percentage of splenic CD4+ T cells was increased in FO-fed mice (Table 1). Dietary FO reduced splenic CD4+ T-cell secretion of IL-6, IFN-γ, and IL-17A in response to TCR stimulation and reduced TNF-α secretion in response to LPS (Table 2). We have reported previously that n3 PUFAs suppress Th1-cell polarization without affecting Th2 polarization (10, 11) and reduce Th17 polarization in obese colitic mice (15). Here we extend those observations by demonstrating that dietary n3 PUFAs render splenic CD4+ T cells refractive to ex vivo Th17-cell polarization, whereas the ability to polarize into Foxp3-expressing Tregs is unaffected (Fig. 1). Differentiation/polarization of Th17 cells requires 3 steps: 1) induction is initiated by IL-6 and TGF-β (although polarizing cytokine redundancy exists in place of IL-6 as IL-1 or IL-21 can also contribute to this step) (19, 20, 56), 2) amplification is primarily driven in an autocrine fashion by the production of IL-21, and 3) stabilization/maintenance of the Th17-cell phenotype is maintained by IL-23 (18, 55).
Polarized Th17 cells were identified in this study on the basis of the detection of 2 key signature markers, namely the cytokine IL-17A and the transcription factor RORγτ (55), and expression of both of these signature markers was independently reduced by dietary FO. Suppression of key transcription factors that drive Th17-cell polarization may represent an important mechanism through which n3 PUFAs render T cells refractive toward Th17 polarization. Therefore, in addition to reducing the percentage of cells expressing RORγτ, which is necessary for Th17-cell differentiation and which coordinates the activities of other essential transcription factors involved in Th17-cell polarization (22, 25), the ratio of activated, i.e., phosphorylated:total, STAT3 expression was also reduced by FO under Th17 polarizing conditions (Fig. 2). STAT3 is a critical transcription factor for the Th17 polarization program, because T-cell-conditional STAT3 deletion has been shown to prevent the development of Th17 cells in part through decreased induction of RORγτ and RORα (54, 55, 57). Moreover, the induction of RORγτ is dependent on STAT3, which is preferentially activated by IL-6 and IL-21 (13, 21, 54). Therefore, expression of 2 critical transcription factors involved in Th17-cell polarization were reduced by FO; however, other factors are involved in the transcriptional regulation of Th17-cell differentiation, including interferon regulatory factor 4 (IRF4), RORα, runt related transcription factor 1 (Runx1), basic leucine zipper transcription factor, ATF-like (BATF), and the aryl hydrocarbon receptor (AhR) (57–62). For example, both RORγτ and RORα are expressed at high levels in differentiated Th17 cells; therefore, the loss of only 1 of these transcription factors results in the partial loss of Th17 cytokine expression, and loss of both RORγτ and RORα abrogates Th17-cell differentiation (57). Hence, transcriptional redundancy, within the Th17-cell polarization process, ensures that the capacity to generate these cells is retained and explains why Th17-cell polarization was reduced by FO rather than abrogated, despite reduced expression of both RORγτ and STAT3.
Another mechanism by which n3 PUFAs reduce Th17-cell polarization could be decreased responsiveness to polarizing cytokines. Signaling from the cytokine microenvironment dictates the direction of T-cell polarization, and the combination of TGF-β and IL-6 is required for murine Th17-cell differentiation (18, 55), whereas TGF-β alone directs Treg polarization (34). IL-23 signaling through IL-23R is important for supporting Th17-cell expansion and for the ongoing maintenance of an established Th17-cell phenotype (18, 55). Both IL-6 and IL-21 are strong inducers of T-cell IL-23R expression (21, 55). In vivo, IL-23 induces IL-17, IL-1, and IL-6 secretion from innate immune cells (55, 63, 64), thereby further promoting the release of Th17-cell polarizing signals and perpetuating the expansion of this cell type. Dietary FO reduced the percentage of Th17 cells coexpressing IL-6R and IL-23R compared with CO-fed mice (Fig. 3A, C), thereby limiting both the initial polarization and ongoing maintenance signaling of Th17 cells, respectively (18, 55). Interestingly, Th17 cell surface expression of IL-21R was unaffected by FO (Fig. 3E). IL-21 is produced by Th17 cells and has been shown to induce Th17-cell differentiation in combination with TGF-β (19–21). IL-21 is believed to function in an autocrine manner by amplifying the precursor frequency of differentiating Th17 cells (19–21, 55). Because the surface expression of IL-21R was unaffected by FO, it is possible that IL-21–mediated polarization of Th17 cells remained intact, because responsiveness to this cytokine was unaffected by diet. Moreover, IL-21 was not a component of the Th17 cytokine polarization cocktail, and therefore any IL-21–mediated signaling would be a byproduct of cellular secretion. Further studies are required to determine if n3 PUFAs affect the cytokine secretory potential of polarized Th17 cells. Collectively, our data show that several, but not all, of the major pathways involved in Th17-cell differentiation/polarization were interrupted by FO.
Future studies are required to determine the effect of FO on the kinetics of cytokine receptor expression during Th17-cell polarization. We measured the percentage of cells expressing surface IL-6R, IL-21R, and IL-23R after 3 d of ex vivo Th17-cell polarization. Presumably, maximal expression would occur at an earlier time point, particularly for IL-6R, which is required for initial responsiveness to polarization stimuli (18, 55). A functional IL-6R is composed of 2 subunits, the IL-6R α chain, whose expression is induced by TGF-β, and gp130, which is constitutively expressed. TCR stimulation plus exposure to IL-6 leads to the downregulation and shedding of the IL-6R α chain and responsiveness to IL-6 (13). Receptor shedding may explain the low levels of expression of IL-6R on Th17 cells. Moreover, dietary FO independently reduced IL-6R expression (Fig. 3A), and in vitro secretion of IL-6 was reduced after TCR stimulation (Table 2). Responsiveness to IL-6 signaling is critical for Th17-cell polarization and the induction of STAT3 expression (21, 22, 54, 55). Because the IL-6R subunit, gp130, has been previously shown to localize to lipid rafts (65, 66) and n3 PUFA incorporation into CD4+ T-cell membranes increases lipid raft size and reduces efficient signaling (5, 7), n3 PUFA–mediated reduction of Th17-cell polarization may be secondary to alterations in membrane lipid nanodomains; however, further studies are required to elucidate this putative mechanism of action.
Having determined that dietary FO reduces Th17-cell polarization and renders these cells refractive to polarizing stimuli, we conducted a second study to elucidate which specific bioactive long-chain n3 PUFAs found in FO could be implicated by feeding diets enriched in purified DHA alone, EPA alone, or a combination of the 2. Both the EPA- and DHA-enriched diets reduced Th17-cell status, as assessed by the intracellular expression of IL-17A and RORγτ (Fig. 4A, B), to the same extent as the combined diet (DHA + EPA). Despite reducing the level of n3 PUFAs in the diet from 11.5 to 1% by weight (study 1 vs. study 2), all n3 PUFA–containing diets reduced Th17-cell polarization, indicating that the outcome is not dependent on dosage. Clearly, further studies are needed to titrate the level of dietary n3 PUFAs required to suppress T-cell polarization. The amount of n3 PUFAs in the 11.5% FO diet fell within the range typically consumed by the Greenland Inuit (2.7–6.3% of energy) (67, 68). By reducing the total n3 PUFA content to 2% of energy, we showed similar Th17-cell suppressive effects at a level of n3 PUFA intake that is more readily attainable in the diet and within the range consumed in human clinical trials and the traditional Japanese diet (8, 69, 70).
In summary, the present study shows that dietary n3 PUFAs alter the intrinsic properties of splenic CD4+ T cells, such that they become refractive to polarization into inflammatory Th17 cells but not into Treg effector cells. Collectively, our data show that n3 PUFAs, irrespective of which long-chain bioactive fatty acid is present, impair Th17-cell polarization through mechanisms involving significant reductions in critical transcription factor expression (RORγτ and STAT3) and cytokine receptor expression (IL-6R and IL-23R). Further studies are required to fully characterize the effect of n3 PUFAs on cytokine receptor expression kinetics, activation of downstream signaling pathways, and polarized Th17 cell functional capacity. Complete suppression of Th17-cell polarization capability by n3 PUFAs would compromise mucosal immune defense; however, reducing the magnitude of the induction of Th17 cells has utility in improving inflammatory disease severity and the clinical outcome as seen previously with n3 PUFAs in murine inflammatory bowel disease and obesity models (15, 16, 47). The present study shows that critical inherent aspects of the T cell are altered by n3 PUFAs, leading to a reduced capacity to polarize into Th17 effector cells, independent from in vivo changes in the tissue cytokine microenvironment or niche (largely APC-dependent) wherein Th17 cells develop during inflammatory pathologies.
Supplementary Material
Acknowledgments
J.M.M., D.N.M., and R.S.C. designed the research, wrote the manuscript, and had primary responsibility for the final content; J.M.M., T.Y.H., and H.F.T. conducted the research; and J.M.M. analyzed the data. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AhR, aryl hydrocarbon receptor; APC, antigen-presenting cell; BATF, basic leucine zipper transcription factor, ATF-like; CO, corn oil; FO, fish oil; Foxp3, forkhead box P3; IFN, interferon; IL, interleukin; IRF4, interferon regulatory factor 4; MHC, major histocompatibility complex; PE, phycoerythrin; ROR, retinoic acid receptor–related orphan receptor; Runx1, runt related transcription factor 1; STAT, signal transducer and activator of transcription; TCR, T-cell receptor; TGF, transforming growth factor; TNF, tumor necrosis factor; Treg, regulatory T cell.
Literature Cited
- 1.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142 Suppl:592S–9S. [DOI] [PubMed] [Google Scholar]
- 2.Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN. Immunomodulatory effects of (n-3) fatty acids: putative link to inflammation and colon cancer. J Nutr. 2007;137 Suppl:200S–4S. [DOI] [PubMed] [Google Scholar]
- 3.Itariu BK, Zeyda M, Hochbrugger EE, Neuhofer A, Prager G, Schindler K, Bohdjalian A, Mascher D, Vangala S, Schranz M, et al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am J Clin Nutr. 2012;96:1137–49. [DOI] [PubMed] [Google Scholar]
- 4.Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107 Suppl 2:S171–84. [DOI] [PubMed] [Google Scholar]
- 5.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–60. [DOI] [PubMed]
- 6.Hou TY, Monk JM, Fan YY, Barhoumi R, Chen YQ, Rivera GM, McMurray DN, Chapkin RS. n-3 Polyunsaturated fatty acids suppress phosphatidylinositol 4,5-bisphosphate-dependent actin remodelling during CD4+ T-cell activation. Biochem J. 2012;443:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 Polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–43. [DOI] [PMC free article] [PubMed]
- 8.Kim W, McMurray DN, Chapkin RS. n-3 Polyunsaturated fatty acids—physiological relevance of dose. Prostaglandins Leukot Essent Fatty Acids. 2010;82:155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yog R, Barhoumi R, McMurray DN, Chapkin RS. n-3 Polyunsaturated fatty acids suppress mitochondrial translocation to the immunologic synapse and modulate calcium signaling in T cells. J Immunol. 2010;184:5865–73. [DOI] [PMC free article] [PubMed]
- 10.Zhang P, Kim W, Zhou L, Wang N, Ly LH, McMurray DN, Chapkin RS. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J Nutr. 2006;136:2391–8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J Nutr. 2005;135:1745–51. [DOI] [PubMed] [Google Scholar]
- 12.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–48. [DOI] [PubMed] [Google Scholar]
- 13.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monk JM, Hou TY, Turk HF, Weeks B, Wu C, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS ONE. 2012;7:e49739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monk JM, Jia Q, Callaway E, Weeks B, Alaniz RC, McMurray DN, Chapkin RS. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J Nutr. 2012;142:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. [DOI] [PubMed] [Google Scholar]
- 19.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. [DOI] [PubMed] [Google Scholar]
- 27.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. [DOI] [PubMed] [Google Scholar]
- 29.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. [DOI] [PubMed] [Google Scholar]
- 30.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. [DOI] [PubMed] [Google Scholar]
- 31.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. [DOI] [PubMed] [Google Scholar]
- 32.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–70. [DOI] [PubMed] [Google Scholar]
- 33.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–84. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007;178:7667–77. [DOI] [PubMed]
- 36.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–21. [DOI] [PubMed]
- 37.Böhmig GA, Krieger PM, Saemann MD, Wenhardt C, Pohanka E, Zlabinger GJ. n-Butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology. 1997;92:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert KM, Weigle WO. Th1 cell anergy and blockade in G1a phase of the cell cycle. J Immunol. 1993;151:1245–54. [PubMed]
- 39.Siavoshian S, Blottiere HM, Bentouimou N, Cherbut C, Galmiche JP. Butyrate enhances major histocompatibility complex class I, HLA-DR and ICAM-1 antigen expression on differentiated human intestinal epithelial cells. Eur J Clin Invest. 1996;26:803–10. [DOI] [PubMed] [Google Scholar]
- 40.Butzner JD, Parmar R, Bell CJ, Dalal V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut. 1996;38:568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Sabatino A, Morera R, Ciccocioppo R, Cazzola P, Gotti S, Tinozzi FP, Tinozzi S, Corazza GR. Oral butyrate for mildly to moderately active Crohn's disease. Aliment Pharmacol Ther. 2005;22:789–94. [DOI] [PubMed] [Google Scholar]
- 42.Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51–6. [DOI] [PubMed] [Google Scholar]
- 43.Segain JP, Raingeard de la Bletiere D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottiere HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinhart AH, Hiruki T, Brzezinski A, Baker JP. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther. 1996;10:729–36. [DOI] [PubMed] [Google Scholar]
- 45.Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavaglieri CR, Nishiyama A, Fernandes LC, Curi R, Miles EA, Calder PC. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–90. [DOI] [PubMed] [Google Scholar]
- 47.Monk JM, Kim W, Callaway E, Turk HF, Foreman JE, Peters JM, He W, Weeks B, Alaniz RC, McMurray DN, et al. Immunomodulatory action of dietary fish oil and targeted deletion of intestinal epithelial cell PPARdelta in inflammation-induced colon carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–8. [DOI] [PubMed] [Google Scholar]
- 49.NRC. Subcommittee on Laboratory Animal Nutrition. Nutrient requirements of laboratory animals. 4th rev. ed. Washington: National Academy of Sciences; 1995.
- 50.Turk HF, Monk JM, Fan YY, Callaway ES, Weeks B, Chapkin RS. Inhibitory effects of omega-3 fatty acids on injury induced epidermal growth factor transactivation contribute to delayed wound healing. Am J Physiol Cell Physiol. 2013;304:C905–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan YY, Monk JM, Hou TY, Callway E, Vincent L, Weeks B, Yang P, Chapkin RS. Characterization of an arachidonic acid-deficient (Fads1 knockout) mouse model. J Lipid Res. 2012;53:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turk HF, Kolar SS, Fan YY, Cozby CA, Lupton JR, Chapkin RS. Linoleic acid and butyrate synergize to increase Bcl-2 levels in colonocytes. Int J Cancer. 2011;128:63–71. [DOI] [PMC free article] [PubMed]
- 53.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–7. [DOI] [PubMed] [Google Scholar]
- 54.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. [DOI] [PubMed] [Google Scholar]
- 55.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brüstle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–66. [DOI] [PubMed] [Google Scholar]
- 59.Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–18. [DOI] [PubMed] [Google Scholar]
- 65.Buk DM, Waibel M, Braig C, Martens AS, Heinrich PC, Graeve L. Polarity and lipid raft association of the components of the ciliary neurotrophic factor receptor complex in Madin-Darby canine kidney cells. J Cell Sci. 2004;117:2063–75. [DOI] [PubMed] [Google Scholar]
- 66.Yanagisawa M, Nakamura K, Taga T. Roles of lipid rafts in integrin-dependent adhesion and gp130 signalling pathway in mouse embryonic neural precursor cells. Genes Cells. 2004;9:801–9. [DOI] [PubMed] [Google Scholar]
- 67.Damsgaard CT, Frokiaer H, Lauritzen L. The effects of fish oil and high or low linoleic acid intake on fatty acid composition of human peripheral blood mononuclear cells. Br J Nutr. 2008;99:147–54. [DOI] [PubMed] [Google Scholar]
- 68.Feskens EJ, Kromhout D. Epidemiologic studies on Eskimos and fish intake. Ann N Y Acad Sci. 1993;683:9–15. [DOI] [PubMed] [Google Scholar]
- 69.Nagata C, Takatsuka N, Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. 2002;156:824–31. [DOI] [PubMed] [Google Scholar]
- 70.Okuyama H, Kobayashi T, Watanabe S. Dietary fatty acids—the n-6/n-3 balance and chronic elderly diseases. Excess linoleic acid and relative n-3 deficiency syndrome seen in Japan. Prog Lipid Res. 1996;35:409–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.