Abstract
Background:
Migraine is common worldwide. In recent years, vitamin D deficiency has been determined as a global health problem. A few studies have shown inverse relationship between serum vitamin D levels and headache. Thus, in this study, we assessed relationship between serum vitamin D levels with migraine.
Materials and Methods:
The present study was a cross-sectional. Seventy-six migraine patients aged 10-61 years were included. The multiple linear regression was used to show association between serum 25-0H-D3 and migraine. Adjustments were performed for age, sex, waist circumference, body mass index (BMI), number of chronic diseases, and education level.
Results:
The positive weak relationship was observed between serum vitamin D and headache diary result (P = 0.042, r = 0.19). But, no significant relationship was observed between serum vitamin D and migraine severity (P = 0.741).
Conclusion:
High levels of serum 25-OH-D3 was related to higher headache diary result. After adjustment for confounding variables, this significant association remained. No significant relationship was shown between serum vitamin D and migraine severity.
Keywords: Migraine, relationship, vitamin D
INTRODUCTION
Headache is common during childhood and adolescence.[1] Migraine and tension-type headache are most common primary headache disorders, that affects 80% of people worldwide.[2] Migraine is a neurological disorder[3] that is debilitating, progressive and chronic.[4] Mechanism of migraine pain in the brain is due to release of pain-producing inflammatory substances around the nerves and blood vessels in the head.[5] Main characteristic of the migraine attacks is headache[6] that may take several hours to 2-3 days[5] and is often severe,[6] pulsating, and one-sided.[5] Other related symptoms include nausea, sometimes vomiting, intolerance to light and sound,[5] neck pain, and muscle tension.[7] Migraine is related to almost 2-fold greater risk of ischemic attacks.[8] In addition, migraine in adults are associated with seasonal allergies, asthma, epilepsy, continuous nightmares, atopic disorders, stroke, cardiovascular disorders, sleep problems, motion sickness, epistaxis and among women of reproductive age are related to preeclampsia and uterine bleeding.[1] The most common causes of migraine is hunger or not eating enough, which is important especially in young people.[6] Migraine is the 19th cause of disability in the world[9] and involves in 10-20% of the population during their lifetime.[10] Recent data indicate that one in every four American adults, suffer from frequent or severe headache including migraine.[1] Women are approximately three times more likely to get migraine disease as compared with men.[6] Migraine prevalence in Turkey is 16.4% (8.5% in men and 24.6% in women);[11] in European adults it is 14.7%,[12] England (7.6% in men and 18.3% in women),[13] Germany 13.4%;[2] in Africa it is 3-7%;[5] and in Asia (3% in men and 10% in women).[5]
Moreover, migraine is one of the most common headaches in Iran.[14] Headache prevalence is 63.4% among Ilam students, that is, 8.1% students have migraine,[15] 7.3% among Ardabil students,[16] 8.85% in Rasht among high school students,[17] and 1.7% in Shiraz among students aged 6-13 years.[18]
In recent years, vitamin D deficiency has been known as a global public health problem.[19] A total of 30-80% children and adults have vitamin D deficiency around the world.[20] In Iran, the prevalence of vitamin D deficiency is 75.1% among women and 72.1% among men.[21] In Isfahan, prevalence of mild, moderate, and severe vitamin D deficiency are 19.6%, 23.9%, and 26.9% respectively, which is more common among women and children.[22] The prevalence of vitamin D deficiency is higher in winter and autumn than in summer and spring.[22]
Vitamin D deficiency is associated with different types of disorders such as musculoskeletal disorders (rickets, osteomalacia, osteoporosis, myopathy), cancer (at least 17 cancers such as cancers of the breast, prostate, colon, ovary, pancreas, etc.), autoimmune disorders (diabetes mellitus, multiple sclerosis, osteoarthritis, rheumatoid arthritis, Crohn's disease, etc.), cardiovascular disorders (hypertension, congestive heart failure, myocardial infarction), kidney disorders, mental disorders (depression, schizopherenia), skin disorders (psoriasis), etc.[23] Evidence have shown that lower levels of vitamin D are related to higher headache,[23,24,25,26,27] but a few studies have proved this. Hence, purpose of the present study is to examine association between serum levels of vitamin D and migraine.
MATERIALS AND METHODS
Study population
This cross-sectional study was conducted among migraine patients in Isfahan city, Iran, in autumn 2012. Totally, 89 migraine patients (72.4% women and 27.6% men) aged 10-61 years were selected. All participants completed an informed consent form.
Baseline data and anthropometric assessment
First, for each participant, data regarding age, sex, weight, height, waist circumference, body mass index (BMI), education, medical history, consumption of vitamin and mineral supplements were collected. Weight was taken by analogue scale with light clothing and without shoes with accuracy of 0.5 kg and height was taken by tape measure without shoes accurately. BMI was determined using body weight (in kilogram) divided by height (in meter square). Waist circumference was measured by inelastic tape at narrowest part of body, below the ribs.
Biochemical assessment
To assess serum levels of vitamin D, 25(OH) D3 was measured by enzyme-linked immunosorbent assay (ELISA). Results of serum vitamin D were classified. According to the ELISA method in laboratory: Deficiency (serum vitamin D less than 12 ng/ml); insufficiency (serum vitamin D between 12 and 30 ng/ml); and sufficiency (serum vitamin D more than 30 ng/ml). Calcium, phosphor, and albumin were measured for the diagnosis of primary hyperparathyroidism.
Migraine assessment
Migraine disease was confirmed by neurologist. Severity, average duration of migraine attacks, and frequency of attacks per month was completed by neurologist. Migraine severity was measured by Visual Analogue Scale (VAS).[28] The headache diary result (HDR) was determined as: Duration of headache × frequency of headache.[28]
Statistical methods
Statistical analysis was conducted using the SPSS 18.0 software (SPSS, Inc. Chicago, IL, USA). Multiple linear regression analysis was performed to determine relationship between serum vitamin D levels and migraine headaches. P values less than 0.05 were considered as significance levels.
RESULTS
In this study, from 89 migraine patients at baseline, 13 patients refused to participate. Finally, 76 migraine patients with mean age of 33.1 ± 11.1 years were included for the analysis. There were 55 women with mean age of 33.4 ± 10.5 years and 21 men with mean age of 32.4 ± 12.8 years. Demographical characteristics of patients are shown in Table 1. Mean migraine severity was 6.6 ± 1.1, which was higher among males. But, mean HDR was higher among females. Deficiency, insufficiency, and sufficiency of vitamin D were observed 13.2%, 68.4%, and 18.4% among migraine patients, respectively.
Table 1.
Demographical characteristics of patients with migraine
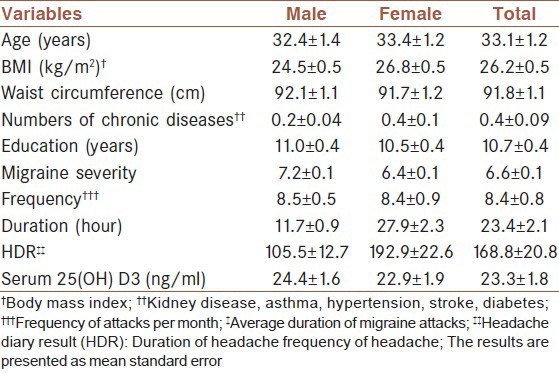
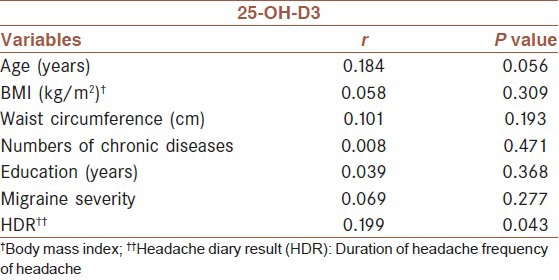
Table 2 shows unadjusted Pearson's correlation coefficients of serum 25-OH-D3 with another variable. No significant correlation was observed between serum vitamin D levels with migraine severity. However, a significant positive association was found between serum vitamin D levels and HDR.
Table 2.
Correlation of serum 25-OH-D3 with demographical variables and variables related to migraine
Multiple linear regression was used to determine the relationship between serum 25-OH-D3 with migraine severity and HDR.
Results of multiple linear regression for relationship between patients characteristics with migraine severity and HDR are shown in Tables 3 and 4, respectively.
Table 3.
Results of multiple linear regression for relationship between patients characteristics and migraine severity

Table 4.
Results of multiple linear regression for relationship between patients characteristics with HDR†
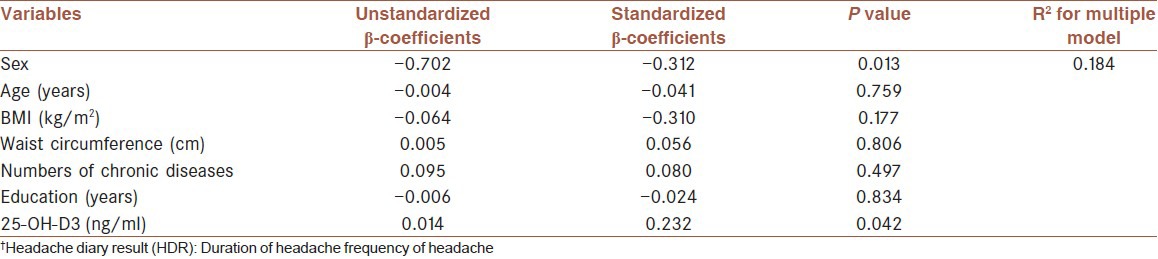
In addition, after adjustment for confounding variables such as age, sex, BMI, waist circumference, education, and number of chronic diseases, significant association was not found between serum levels of vitamin D and migraine severity (P = 0.741). But, a significant positive relationship was shown between serum vitamin D levels with HDR (P = 0.042). So that, one unit elevated serum levels of vitamin D is related to increased 0.014 log HDR (unstandardized β-coefficients = 0.014). According to values shown in Tables 3 and 4, effect of gender on migraine severity and HDR is higher than other variables.
However, when we compared the relationship between vitamin D groups (deficiency, insufficiency, and sufficiency) with migraine severity and HDR, no association was observed. These results are shown in Table 5.
Table 5.
Results of multiple linear regression for relationship between vitamin D groups with migraine severity and HDR†

DISCUSSION
In this study, we have demonstrated a significant positive association between serum levels of vitamin D and HDR, and this relationship remained significant after adjustment for confounding variables such as age, sex, BMI, waist circumference, education, and number of chronic diseases, which was inconsistent with previous studies. However, there was no significant relationship between serum vitamin D and migraine severity. In this study, also, we investigated this relationship according to different vitamin D groups, but, relationship was not shown between vitamin D groups and migraine.
A few case reports have indicated the role of vitamin D in headache, including migraine.[23,25,26] Thys-Jacobs conducted two case reports in this field in 1994.[25,26] A case report study was conducted in two female patients with migraine associated with menstruation and premenstrual syndrome. These patients had low levels of vitamin D and with consumption of vitamin D and calcium supplement (1600-1200 IU per day) significant reduction in migraine attacks and premenstrual symptoms were observed during 2 months treatment.[25] Another study performed among postmenopausal patients with migraine and low levels of vitamin D showed that with supplementation of vitamin D and calcium, the frequency and duration of migraine attacks decreased.[26] Prakash and Shah found that daily intake of vitamin D calcium supplementation (1500 IU vitamin D3 and 1000 mg calcium) among eight patients with vitamin D deficiency, osteomalacia, and chronic tension-type headache confirmed an improvement in headache during 4-6 weeks. In this study, serum levels of calcium became normal after a week of treatment, but improvement in headache was after several weeks of treatment, hence, vitamin D is probably more important than calcium in alleviating headache.[23]
A few studies have shown an association between low levels of vitamin D with higher incidence of chronic pain and headache.[24,27,29] In 2008, Turner et al. reported the prevalence of vitamin D deficiency at 26% among 267 patients with chronic pain (including 25 patients with headache).[30] In another study, chronic pain was significantly associated with vitamin D status among English women, but, this association was not observed among men.[29] In Norway, a multi-ethnic study with cross-sectional descriptive designs, hypovitaminosis D (levels less than 50 nmol/l) were reported among 58% of patients with musculoskeletal pain, headache, and fatigue. There was an inverse relation between headache with vitamin D, and reduction in frequency of headache attacks was observed with increased levels of vitamin D; also this relationship remained after adjustment for age, sex, season, and geographical region (OR = 2.6, P = 0.008). Serum levels of vitamin D were lower in patients with headache than in other patients. Vitamin D levels was lower in winter and spring than in summer and autumn.[27]
Wheeler and O’Brien showed that patients with migraine have low vitamin D levels.[31,32] Wheeler, reported that 14.8% of patients with chronic migraine had serum levels of vitamin D less than or equal to 20 ng/ml and 25.9% of patients had serum vitamin D levels between 20 and 30 ng/ml.[31] In Greece, prevalence and frequency of headaches was more in northern areas and regions with average low temperature than in southern regions.[33] Serum vitamin D levels is higher among individuals who live at lower latitudes and is an important factors for low prevalence of headache.[19] Data have shown an increasing frequency of headache attacks during the fall and winter and minimum attacks in summer,[19] which is probably due to low levels of vitamin D in the fall and winter and indicates possibility role of vitamin D in causing headache.[19]
In a cross-sectional study including 11,614 participants in the sixth survey of Tromsø study, significant relationship was not observed between serum levels of vitamin D and migraine, but association between nonmigraine headache and vitamin D levels was only significant in nonsmokers group and adjustments were performed for age, BMI, sex, season, chronic diseases, education level, physical activity, and alcohol consumption.[24]
Accurate role of vitamin D deficiency in headache is unknown.[19] The main mechanisms in causing headache include possible sensitization of second and third neurons due to continuous stimulation of sensory receptors of periosteal coverage (because of bone swelling) and also, central sensitization (because of bone swelling). Other possible mechanisms of headache in patients with vitamin D deficiency are low serum levels of magnesium.[23] Abnormal metabolism of magnesium is involved in the pathogenesis of tension-type headache.[23] Magnesium deficiency in the brain, blood, erythrocyte, monocyte, and platelet have been found among patients with tension-type headaches and other types of headache. About 40-50% of patients with tension-type headache have low serum levels of magnesium.[23] In different studies, patients with tension-type headache have responded to treatment with magnesium.[23] Vitamin D deficiency may lead to tension-type headache using decreased absorption of magnesium, because, intestinal absorption of magnesium through food is dependent on vitamin D.[23] Another mechanisms include the presence of vitamin D receptors, 1-hydroxylase (the enzyme responsible for the formation of the vitamin D active form) and vitamin D binding protein in the brain, particularly hypothalamus.[19]
This study has several limitations. First, the present study is cross-sectional, hence, we cannot show a causal link, thus, more clinical trial studies are needed to be performed. Second, the sample size of present study is small and more studies are required to do with larger sample size. Third, further adjustments for confounding variables such as physical activity and alcohol consumption probably will be needed because these variables may affect the serum levels of vitamin D.
The strength of this study is that this is the first cross-sectional study on the relationship between vitamin D and migraine in Iran.
CONCLUSION
High levels of serum 25-OH-D3 were related to higher HDR. After adjustment for confounding variables, this significant association remained. No significant relationship was shown between serum vitamin D and migraine severity. Additional studies are needed to be performed with a larger sample size.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lateef TM, Cui L, Nelson KB, Nakamura EF, Merikangas KR. Physical Comorbidity of Migraine and Other Headaches in US Adolescents. J Pediatr. 2012;161:308–13. doi: 10.1016/j.jpeds.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon MS, Katsarava Z, Obermann M, Fritsche G, Oezyurt M, Kaesewinkel K, et al. Prevalence of primary headaches in Germany: Results of the German Headache Consortium Study. J Headache Pain. 2012;13:215–23. doi: 10.1007/s10194-012-0425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battista J, Badcock DR, McKendrick AM. Migraine increases centre-surround suppression for drifting visual stimuli. PLoS One. 2011;6:e18211. doi: 10.1371/journal.pone.0018211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inaloo S, Dehghani SM, Farzadi F, Haghighat M, Imanieh MH. A comparative study of celiac disease in children with migraine headache and a normal control group. Turk J Gastroenterol. 2011;22:32–5. doi: 10.4318/tjg.2011.0153. [DOI] [PubMed] [Google Scholar]
- 5.Headache disorders. [Last accessed on Mar 2004]. Available from: http://www.who.int/mediacentre/factsheets/fs277/en .
- 6.Steiner TJ. Lifting The Burden: The Global Campaign to Reduce the Burden of Headache Worldwide. Aids for management of common headache disorders in primary care. J Headache Pain. 2007;8:S26–9. [PubMed] [Google Scholar]
- 7.Unalp A, Dirik E, Kurul S. Prevalence and clinical findings of migraine and tension-type headache in adolescents. Pediatr Int. 2007;49:943–9. doi: 10.1111/j.1442-200X.2007.02484.x. [DOI] [PubMed] [Google Scholar]
- 8.Schürks M. Genetics of migraine in the age of genome-wide association studies. J Headache Pain. 2012;13:1–9. doi: 10.1007/s10194-011-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahdavi R, Tarighat EA, Ebrahimi M, Talebi M, Ghaemmaghami J. Effects of Oral Magnesium for Migraine Prophylaxis. J Pharm Sci. 2009;15:103–8. [Google Scholar]
- 10.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: Systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ertas M, Baykan B, Orhan EK, Zarifoglu M, Karli N, Saip S, et al. One-year prevalence and the impact of migraine and tension-type headache in Turkey: A nationwide home-based study in adults. J Headache Pain. 2012;13:147–57. doi: 10.1007/s10194-011-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stovner LJ, Andree C. Prevalence of headache in Europe: A review for the Eurolight project. J Headache Pain. 2010;11:289–99. doi: 10.1007/s10194-010-0217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner TJ, Scher AI, Stewart WF, Kolodner K, Liberman J, Lipton RB. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia. 2003;23:519–27. doi: 10.1046/j.1468-2982.2003.00568.x. [DOI] [PubMed] [Google Scholar]
- 14.Safavi M, Nazari F, Mahmoody M. The relationship of migraine headache and life style among women. Iran J Nurs. 2008;21:89–100. [Google Scholar]
- 15.Modara F, Rostamkhani M. Prevalence of tension and migraine headache s among the students of Ilam Medical University. J Ilam Univ Med Sci. 2008;15:13–9. [Google Scholar]
- 16.Hashemilar M, AminiSani N, Savadi D, Yousefian M. Prevalence ofmigraine among students of Ardabil University of Medical Sciences. J Ardabil Univ Med Sci. 2004;3:64–9. [Google Scholar]
- 17.Ghayeghran AR, Fathsami Sh. Survey on prevalence of Migraine in high school students of Rasht city. J Guilan Univ Med Sci. 2004;13:22–5. [Google Scholar]
- 18.Ayatollahi SM, Khosravi A. Prevalence of migraine and tension-type headache in primary-school children in Shiraz. East Mediterr Health J. 2006;12:809–17. [PubMed] [Google Scholar]
- 19.Prakash S, Mehta NC, Dabhi AS, Lakhani O, Khilari M, Shah ND. The prevalence of headache may be related with the latitude: A possible role of Vitamin D insufficiency? J Headache Pain. 2010;11:301–7. doi: 10.1007/s10194-010-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andşran N, Çelik N, Akça H, Doğan G. Vitamin D deficiency in children and adolescents. J Clin Res Pediatr Endocrinol. 2012;4:25–9. doi: 10.4274/jcrpe.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moradzadeh K, Larijani B, Keshtkar AA, Hossein-Nezhad A, Rajabian R, Nabipour I, et al. Normative Values of Vitamin D among Iranian Population: A Population Based Study. Int J Osteoporos Metabolic Dis. 2008;1:8–15. [Google Scholar]
- 22.Hovsepian S, Amini M, Aminorroaya A, Amini P, Iraj B. Prevalence of vitamin D deficiency among adult population of Isfahan City, Iran. J Health Popul Nutr. 2011;29:149–55. doi: 10.3329/jhpn.v29i2.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash S, Shah ND. Chronic tension-type headache with vitamin D deficiency: Casual or causal association? Headache. 2009;49:1214–22. doi: 10.1111/j.1526-4610.2009.01483.x. [DOI] [PubMed] [Google Scholar]
- 24.Kjćrgaard M, Eggen AE, Mathiesen EB, Jorde R. Association Between Headache and Serum 25-Hydroxyvitamin D; the Tromsø Study: Tromsø 6. Headache. 2012;52:1499–505. doi: 10.1111/j.1526-4610.2012.02250.x. [DOI] [PubMed] [Google Scholar]
- 25.Thys-Jacobs S. Vitamin D and calcium in menstrual migraine. Headache. 1994;34:544–6. doi: 10.1111/j.1526-4610.1994.hed3409544.x. [DOI] [PubMed] [Google Scholar]
- 26.Thys-Jacobs S. Alleviation of migraines with therapeutic vitamin D and calcium. Headache. 1994;34:590–2. doi: 10.1111/j.1526-4610.1994.hed3410590.x. [DOI] [PubMed] [Google Scholar]
- 27.Knutsen KV, Brekke M, Gjelstad S, Lagerløv P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: A cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand J Prim Health Care. 2010;28:166–71. doi: 10.3109/02813432.2010.505407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asadi B, Khorvash F, Najaran A, Khorvash F. Cyproheptadine versus propranolol in the prevention of migraine headaches in children. Pak J Med Sci. 2012;28:309–11. [Google Scholar]
- 29.Atherton K, Berry DJ, Parsons T, Macfarlane GJ, Power C, Hypponen E. Vitamin D and chronic widespread pain in a white middle-aged British population: Evidence from a cross-sectional population survey. Ann Rheum Dis. 2009;68:817–22. doi: 10.1136/ard.2008.090456. [DOI] [PubMed] [Google Scholar]
- 30.Turner MK, Hooten WM, Schmidt JE, Kerkvliet JL, Townsend CO, Bruce BK. Prevalence and clinical correlates of vitamin D inadequacy among patients with chronic pain. Pain Med. 2008;9:979–84. doi: 10.1111/j.1526-4637.2008.00415.x. 2250 1.7. [DOI] [PubMed] [Google Scholar]
- 31.Wheeler SD. Vitamin D deficiency in chronic migraine. Headache. 2008;48:S52–3. [Google Scholar]
- 32.O’Brien H, Hershey AD, Kabbouche MA, Austin SB, Frazier AL, Wright RJ. Prevalence of vitamin D deficiency among pediatric patients with recurrent headaches. Headache. 2010;50:23. [Google Scholar]
- 33.Mitsikostas DD, Tsaklakidou D, Athanasiadis N, Thomas A. The prevalence of headache in Greece: Correlations to latitude and climatological factors. Headache. 1996;36:168–73. doi: 10.1046/j.1526-4610.1996.3603168.x. [DOI] [PubMed] [Google Scholar]


