Abstract
Background:
To evaluate seizure characteristic among multiple sclerosis patients with coexistent seizure activity compared to control group.
Materials and Methods:
This study is a cross-sectional study which was conducted by reviewing the clinical records of patients with definite diagnosis of MS according to McDonald's criteria from March 2007 to June 2011, who referred to the MS clinic of the university.
Results:
A total of 920 patients with a diagnosis of MS were identified, among whom 29 patients (3.15%) with seizure activity (case) due to MS with the mean age of 32.6 ± 6.23 years were analyzed. Also, fifty MS patients without any seizure occurrence with the mean age of 33.7 ± 7.4 years were used as our control group. In case group, seizure was general tonic clonic in 23 patients (79.3%), complex partial in four (13.8%), and simple partial in two (5.9%). The 26 available interictal EEGs in MS patients showed abnormal EEG pattern in 22 (84.6%) of them, including focal epileptic form discharge or focal slowing in 10 (38.5%), generalized discharge (spike-wave, polyspike, or general paroxysmal fast activity) in 10 (38.5%), and general slowing activity in 10 record (38.5%). MRI reviews of the 26 available brain MRIs showed subcortical white mater lesions in 22 (84.6%) of patients with seizure. All MRIs were performed within one month after the first seizure episode. Amongst 48 available MRIs in our control group, 91.7% (44 cases) showed periventricular lesions and in 8.3% (4 cases) subcortical white matter lesions were reported.
Conclusion:
The result of this study demonstrated the higher rate of subcortical whit matter lesion in MS patients with seizure occurrence compared to control group.
Keywords: Electroencephalography, magnetic resonance imaging, multiple sclerosis, seizure
INTRODUCTION
Multiple sclerosis (MS) is considered to be a chronic autoimmune degenerative disease of the central nervous system which predominantly affects young females.[1] Broad spectrum of sign and symptoms of MS are related to various brain regions which are affected with multifocal lesions that cause episodes of demyelination and axonal injury at unpredictable intervals.[2] Although the etiology of MS still remains unclear, both genetic and environmental risk factors are thought to play an important role in susceptibility and outcome of the disease.[3]
Although seizure is a rare manifestation of MS, a higher rate of seizures in patients with MS has been reported,[4] and evidence from magnetic resonance imaging (MRI) studies have linked the occurrence of seizures to cortical and subcortical lesions.[5,6,7,8]
Here, we aimed to study the rate of seizure occurrence and its characteristic in patients with MS in a cross-sectional hospital registry-based study in Isfahan, Iran.
MATERIALS AND METHODS
This study is a cross-sectional study which was conducted by reviewing the clinical records of patients with definite diagnosis of MS according to McDonald's criteria[9] from March 2007 to June 2011, who referred to the MS clinic of the Kashani hospital, Isfahan University of Medical Sciences, Isfahan Iran.
Clinical and demographic information were gathered from Kashani hospital registry database, by specially designed forms. The populations of this study consist of 29 MS patients with definite seizure activity and as our control group, fifty MS patients who had never experienced seizure were chosen and EEG and MRI were performed on recruited patients.
Patients were categorized as relapsing/remitting MS (RRMS), secondary progressive MS (SPMS), and primary progressive MS (PPMS). To determine the degree of disability, Kurtzke's Expanded Disability Status Scale (EDSS) was used.[10] Data on electroencephalography (EEG), magnetic resonance imaging (MRI) findings, and prescribed antiepileptic drugs (AED) were retrieved. MRIs were performed within first month after the first seizure and reported by a neuroradiologist. Reports of the International League against Epilepsy (ILAE) Commission on Classification and Terminology were used for the diagnosis and classification of the seizures.[11] Data were analyzed by SPSS software and the results are reported as mean ± SD.
This study was approved by the local ethics committee and all patients in this study signed an informed consent form to access and review their medical records.
RESULTS
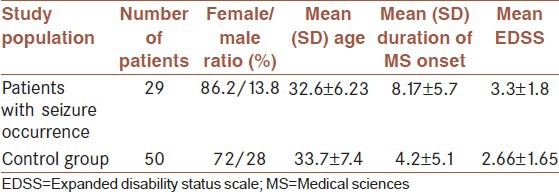
A total of 920 patients with a diagnosis of MS were identified, among whom 34 patients (3.69%) had seizures. Five patients were excluded due to underlying etiologies of seizureother than MS; one with the history of brain tumor, one with CNS infection, and three with febrile seizure. Twenty nine patients (3.15%) remained for analysis with the mean age of 32.6 ± 6.23 years (range, 22-44) and most were females (25 case; 86.2%). Seven patients (24.1%) had the history of seizure before MS onset and 22 patients (75.9%) had experienced seizures after the onset of the disease. The mean duration of MS onset was 8.17 ± 5.7 years (range, 1-20) and the mean of th e interval between MS onset and the first seizure occurrence-when occurring after MS onset-was 3.54 ± 3.6 years (range, 0-11). Also, fifty MS patients with no seizure occurrence were selected as our control group. The mean age of 33.7 ± 7.4 years with female predominance (F = 72%, M = 28%) was reported in our control group, and the mean duration of MS onset was 4.2 ± 5.1 [Table 1].
Table 1.
Demographic data of enrolled cases
There was no report of status epilepticus seizure. Seizure was general tonic clonic in 23 patients (79.3%), complex partial in four (13.8%), and simple partial in two (5.9%). We could not establish whether those with generalized seizure were primary or secondary. The 26 available interictal EEGs showed abnormal EEG pattern in 22 (84.6%) of patients, including focal epileptic form discharge or focal slowing in 10 (38.5%), generalized discharge (spike-wave, polyspike, or general paroxysmal fast activity) in 10 (38.5%), and general slowing activity in 10 record (38.5%).
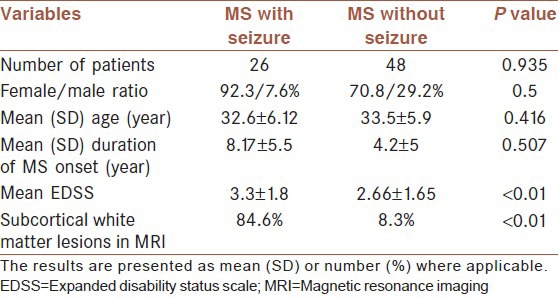
MRI reviews of the 26 available brain MRIs showed subcortical white mater lesions in 22 (84.6%) of patients with seizure. All MRIs were performed within one month after the first seizure episode. However, amongst 48 available MRIs in our control group, 91.7% (44 cases) showed periventricular lesions and in 8.3% (4 cases) subcortical white matter lesions were reported [Table 2].
Table 2.
MRI finding in multiple sclerosis patients with and without seizure activity
The mean duration of MS onset was 8.17 years (range, 1-20) and the mean EDSS was 3.3 (range, 0-8.5). At the time of the first seizure occurrence and at the time of study, MS type was predominantly relapsing remitting type (61.7% and 58.8%, respectively). In our control group, the mean duration of MS onset was 4.2 ± 5.1 and the mean EDSS was 2.66 ± 1.65. Also, like in population of study, MS type was predominantly relapsing remitting type (28 cases, 59.6%).
Among these 29 patients with seizure activity; one case (3.4%) was self-limited and got seizure-free without any treatment, two cases (6.9%) reported an intractable seizure disorder (despite adequate treatment), twenty five cases (86.2%) had an excellent response (controlled with monotherapy), and one case (3.4%) had a good response to AEDs (controlled with poly therapy). Mostly used drugs were sodium valproate (400-1000 mg) and carbamazepine (200-600 mg) (41.4% and 34.5%, respectively).
DISCUSSION
Amongst our study population, 3.15% had seizure; however, since there are no studies about the prevalence of seizure in general population of Iran, we could not assess if the occurrence of seizure is higher in MS patients or not. Several lines of hospital-and population-based MS-patient cohorts have studied the occurrence of seizure in which the prevalence of seizures assessed to be ranging from 0.5% to 8.3%.[4] Overall, three studies documented the incidence of seizure in MS. Amongst these, two demonstrated about three-fold incidence of seizure in MS patients compared to age-matched control group; however, these studies were conducted on small populations of MS patients.[12,13]
Another study choose the population of the study amongst the MS patients who had seizure after the first demyelinating episode and demonstrated no difference between incidence of seizure in MS patients compared to general population.[14] However, since seizure may be the presentation of MS,[15] patients selection of this study remains questionable. Since there are no studies about the prevalence of seizure in general population of Iran, we could not assess if the occurrence of seizure is higher in MS patients or not.
In population of our study, the prevalence of generalized tonic colonic seizure was much higher compared to any other form of seizure, although complex partial seizure had about twice the prevalence of simple partial seizure which is the same as normal population; however, in another study, it was demonstrated that MS patients’ simple partial seizure were more frequent.[16]
In our study at the time of first seizure occurrence, MS type was predominantly relapsing-remitting and also in our control group, MS type was mostly relapsing-remitting, which can be due to the higher prevalence of relapsing-remitting type in general MS population. Several lines of studies have demonstrated the occurrence of seizures in relapsing-remitting and also primary or secondary progressive MS. Like our findings, a few studies have suggested that occurrence of seizure are more likely during the relapses;[8,17] however, this observation was not confirmed by other studies.[18,19]
MRI findings in our patients demonstrated subcortical lesions compared to the frequency of periventricular lesions in control group. However, the interval between the first seizure occurrence and MRI performance in few patients were long. In line with these findings, several studies have shown the relation between ictal symptoms and cortical and subcortical lesions.[5,6,7,17,19,20] Although a recent cohort study demonstrated no convincing correlation between MRI findings and ictal behavior, the fact that MRI in this study was not performed at the time of seizure activity should be taken into account as a weakness of the study.[21]
Results of intracranial EEG in our study demonstrated abnormal pattern in most of the patients which included focal epileptic form discharge or focal slowing, generalized discharge, and general slowing activity; Abnormal EEG patterns in line with our findings were also described by other studies.[22] However, in this study, interpretation of EEG abnormalities was not straight forward, as the time of EEG recording in relation to the first seizure onset and anticonvulsant treatment was incongruent between patients.
All antiepileptic drugs have been used in management of seizure in patients with MS without favoring any particular treatment. However, it has been showed that these treatments in MS patients can mimic disease activity. The fact that seizures which accrues during the relapses have much more benign course that does not require treatment is in contrast with seizures which occurs unrelated to replaces that needs more aggressive treatment.[23] Among our 29 MS patients with seizure activity, most of them were controlled with monotherapy and had an excellent response. Mostly used drugs were valproate sodium and carbamazepine. However, in contrast with some of other studies, none of our cases demonstrated disease activity after antiepileptic therapy.
CONCLUSION
The result of our study demonstrated a link between seizure occurrence and subcortical whit matter lesion in MS patients which is in line with what has been previously discussed by other studies.
Include the research project number: 290041
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Harris MK, Maghzi AH, Etemadifar M, Kelley RE, Gonzalez-Toledo E, Minagar A. Acute demyelinating disorders of the central nervous system. Curr Treat Options Neurol. 2009;11:55–63. doi: 10.1007/s11940-009-0008-6. [DOI] [PubMed] [Google Scholar]
- 2.Benedikz J, Stefánsson M, Guomundsson J, Jónasdóttir A, Fossdal R, Gulcher J, et al. The natural history of untreated multiple sclerosis in Iceland. A total population-based 50 year prospective study. Clin Neurol Neurosurg. 2002;104:208–10. doi: 10.1016/s0303-8467(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 3.Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: Risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9:727–39. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- 4.Koch M, Uyttenboogaart M, Polman S, De Keyser J. Seizures in multiple sclerosis. Epilepsia. 2008;49:948–53. doi: 10.1111/j.1528-1167.2008.01565.x. [DOI] [PubMed] [Google Scholar]
- 5.Thompson AJ, Kermode AG, Moseley IF, MacManus DG, McDonald WI. Seizures due to multiple sclerosis: Seven patients with MRI correlations. J Neurol Neurosurg Psychiatry. 1993;56:1317–20. doi: 10.1136/jnnp.56.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreau T, Sochurkova D, Lemesle M, Madinier G, Billiar T, Giroud M, et al. Epilepsy in patients with multiple sclerosis: Radiological-clinical correlations. Epilepsia. 1998;39:893–6. doi: 10.1111/j.1528-1157.1998.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 7.Truyen L, Barkhof F, Frequin ST, Polman CH, Tobi H, Hommes OR, et al. Magnetic resonance imaging of epilepsy in multiple sclerosis: A case control study. Implications for treatment trials with 4-aminopyridine. Mult Scler. 1996;1:213–7. [PubMed] [Google Scholar]
- 8.Sokic DV, Stojsavljevic N, Drulovic J, Dujmovic I, Mesaros S, Ercegovac M, et al. Seizures in multiple sclerosis. Epilepsia. 2001;42:72–9. doi: 10.1046/j.1528-1157.2001.48699.x. [DOI] [PubMed] [Google Scholar]
- 9.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 11.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 12.Olafsson E, Hauser WA. Prevalence of epilepsy in rural Iceland: A population-based study. Epilepsia. 1999;40:1529–34. doi: 10.1111/j.1528-1157.1999.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 13.Nicoletti A, Sofia V, Biondi R, Lo Fermo S, Reggio E, Patti F, et al. Epilepsy and multiple sclerosis in Sicily: A population-based study. Epilepsia. 2003;44:1445–8. doi: 10.1046/j.1528-1157.2003.09203.x. [DOI] [PubMed] [Google Scholar]
- 14.Nyquist PA, Cascino GD, McClelland RL, Annegers JF, Rodriguez M. Incidence of seizures in patients with multiple sclerosis: A population-based study. Mayo Clin Proc. 2002;77:910–2. doi: 10.4065/77.9.910. [DOI] [PubMed] [Google Scholar]
- 15.Trouillas P, Courjon J. Epilepsy with multiple sclerosis. Epilepsia. 1972;13:325–33. doi: 10.1111/j.1528-1157.1972.tb05267.x. [DOI] [PubMed] [Google Scholar]
- 16.Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: Newly diagnosed epileptic seizures in a general population. Lancet. 1990;336:1267–71. doi: 10.1016/0140-6736(90)92959-l. [DOI] [PubMed] [Google Scholar]
- 17.Büttner T, Hornig CR, Dorndorf W. Multiple sclerosis and epilepsy. An analysis of 14 case histories. Nervenarzt. 1989;60:262–7. [PubMed] [Google Scholar]
- 18.Kinnunen E, Wikstrom J. Prevalence and prognosis of epilepsy in patients with multiple sclerosis. Epilepsia. 1986;27:729–33. doi: 10.1111/j.1528-1157.1986.tb03602.x. [DOI] [PubMed] [Google Scholar]
- 19.Ghezzi A, Montanini R, Basso PF, Zaffaroni M, Massimo E, Cazzullo CL. Epilepsy in multiple sclerosis. Eur Neurol. 1990;30:218–23. doi: 10.1159/000117350. [DOI] [PubMed] [Google Scholar]
- 20.Gambardella A, Valentino P, Labate A, Sibilia G, Ruscica F, Colosimo E, et al. Temporal lobe epilepsy as a unique manifestation of multiple sclerosis. Can J Neurol Sci. 2003;30:228–32. doi: 10.1017/s031716710000264x. [DOI] [PubMed] [Google Scholar]
- 21.Nyquist PA, Cascino GD, Rodriguez M. Seizures in patients with multiple sclerosis seen at Mayo Clinic, Rochester, Minn, 1990-1998. Mayo Clin Proc. 2001;76:983–6. doi: 10.4065/76.10.983. [DOI] [PubMed] [Google Scholar]
- 22.Kelley BJ, Rodriguez M. Seizures in patients with multiple sclerosis: Epidemiology, pathophysiology and management. CNS Drugs. 2009;23:805–15. doi: 10.2165/11310900-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spatt J, Chaix R, Mamoli B. Epileptic and non-epileptic seizures in multiple sclerosis. J Neurol. 2001;248:2–9. doi: 10.1007/s004150170262. [DOI] [PubMed] [Google Scholar]


