Abstract
Background:
Mechanical ventilation is an important tool in the management of respiratory failure in the critically ill patient. Although mechanical ventilation can be a life-saving intervention, it is also known to carry several side-effects and risks. Adequate oxygenation is one of the primary goals of mechanical ventilation. However, while on mechanical ventilation, patients frequently experience hypoxic events resulting from various causes, which need to be properly evaluated and treated.
Materials and Methods:
Data were obtained by prospectively reviewing all intensive care admissions during the period from March 2009 to March 2010 at a 651-bed urban medical center. Patients who developed hypoxemia (oxygen saturation ≤88% and a PaO2≤60 torrs) while on mechanical ventilation were investigated for the cause of hypoxic event.
Results:
During the study period, 955 patients required mechanical ventilation from which 79 developed acute hypoxia. The causes of acute hypoxia in decreasing order of occurrences were pulmonary edema, atelectasis, pneumothorax, pneumonia, ARDS, endotracheal tube malfunction, airway bleeding, and pulmonary embolism.
Conclusions:
Appropriate evaluation of all hypoxic events must begin at the bedside. A step-by-step approach must include a thorough physical examination. Evaluation of the endotracheal tube can immediately reveal dislodgement, bleeding, and secretions. Correlation of physical examination findings with those on chest radiograph is essential. Each hypoxic event requires a different intervention depending on its etiology. Instead of simply increasing the fraction of oxygen in the inspired air to overcome hypoxia, a concerted effort in appropriate problem solving can reduce the likelihood of an incorrect diagnosis and management response.
Keywords: Hypoxemia, hypoxia, mechanical ventilation
INTRODUCTION
Mechanical ventilation is an important tool in the management of respiratory failure in the critically ill patient. Mechanical ventilation is required for the management of respiratory failure resulting from various clinical conditions including acute respiratory distress syndrome (ARDS), pneumonia, sepsis, chronic obstructive pulmonary disease (COPD), and asthma. Although mechanical ventilation can be a lifesaving intervention, it is also known to carry several side-effects and risks. Various complications of mechanical ventilation include hypotension, respiratory distress (“fighting the ventilator”), with consequent increase in airway pressure, ARDS, ventilator-associated pneumonia (VAP), and complications related to endotracheal tubes.[1] Providing adequate oxygenation (PaO2 60-70 torrs) is one of the primary goals of mechanical ventilation. However, while on mechanical ventilation, patients frequently experience hypoxic events resulting from various causes, which need to be properly evaluated and treated.[1] There is limited published data focusing on causes of hypoxic events in mechanically ventilated patients. This study examines the causes and frequency of various hypoxic events while on mechanical ventilation.
MATERIALS AND METHODS
Data were obtained by prospectively reviewing all intensive care admissions during the period from March 2009 to March 2010 at a 651-bed urban medical center with 36 adult critical care beds. All mechanically ventilated patients ≥18 years of age were studied. Patients who developed hypoxemia (oxygen saturation ≤88% and a PaO2≤60 torrs) while on mechanical ventilation were investigated for the cause of hypoxic event. In this study, all patients admitted to the medical intensive care unit and mechanically ventilated for any cause of respiratory failure were considered for inclusion. Patients who could not be stabilized with adequate oxygenation regardless of the mode of ventilation and inspired fraction of oxygen delivered by the ventilator were not considered for inclusion in this study. Only stable patients on mechanical ventilation that developed acute hypoxia, not as a result of the underlying cause of respiratory failure, were further studied and included. If on evaluation of the patient the cause of the hypoxia was considered to be secondary to the underlying cause of respiratory failure than the patient would be excluded.
The three most common causes of respiratory failure among the patients developing acute hypoxic events were septic shock (22%), cardiopulmonary arrest (18%), and pneumonia (13%). The remaining causes included COPD exacerbation (8%), postoperative respiratory failure (6%), stroke (5%), myocardial infarction (5%), status epilepticus (4%), drug overdose (4%), ARDS (4%), congestive heart failure exacerbation (3%), gastrointestinal bleeding (3%), pulmonary edema (3%), aspiration pneumonia (1%), ischemic bowel (1%), pulmonary embolism (PE) (1%), and trauma (1%) in decreasing order of occurrence [Table 1]. Patients presenting with signs of systemic inflammatory response syndrome, high suspicion or evidence of infection, end organ dysfunction, and refractory hypotension were considered to have septic shock. Patients that presented with clinical signs and symptoms suggestive of pneumonia (i.e., cough, fever, and sputum production), leukocytosis, evidence of infiltrate on chest X-ray, and not otherwise meeting the criteria of septic shock were considered to have pneumonia. Patients that met criteria of the American-European Consensus Conference on ARDS with acute onset disease with bilateral diffuse infilatrates of chest X-ray without evidence of cardiovascular disease and PaO2 to FiO2 ratio of less than 200 were considered to have ARDS as the cause of respiratory failure.
Table 1.
Causes of respiratory failure
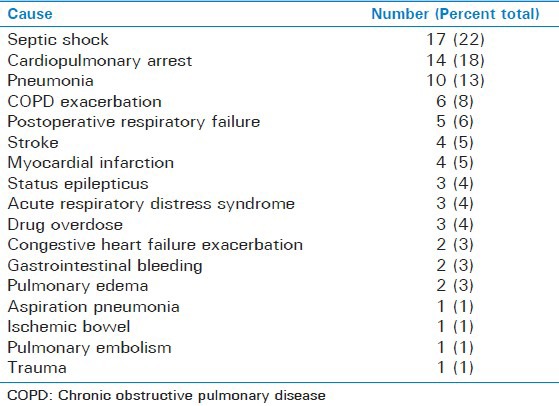
All hypoxic events recorded were not due to the initial cause of respiratory failure. Prior to the occurrence of each hypoxic event, all the patients had been stable with adequate oxygenation. The patients developing hypoxic events were clinically evaluated and a chest X-ray and other relevant clinical test were conducted to determine the cause of hypoxic event while on mechanical ventilation.
RESULTS
The study included 955 patients receiving mechanical ventilation. There was a total of 79 patients (8%) who developed acute hypoxia while on mechanical ventilation. There were 39 males and 40 females. The average age was 58 years (ranging between 22 and 91 years). The most common causes of acute hypoxia was pulmonary edema (29%). In the pulmonary edema group, the average ejection fraction was 37% (ranging between 20% and 55%) and the average serum albumin in these patients was 2.49 g/dL (ranging between 1.3 and 2.9 g/dL). The scond most common cause of acute hypoxia was atelectasis (28%). Common causes of atelectasis among the study patients were mucous plugging (20%), poor suctioning (6%), and right main-stem intubation (2%) leading to right upper lobe collapse or left lung collapse. The third most common cause was pneumothorax (13%) leading to hypoxemia. In the pneumothorax group, five patients (6%) had COPD, three (5%) had ARDS, and two (3%) had bronchial asthma.
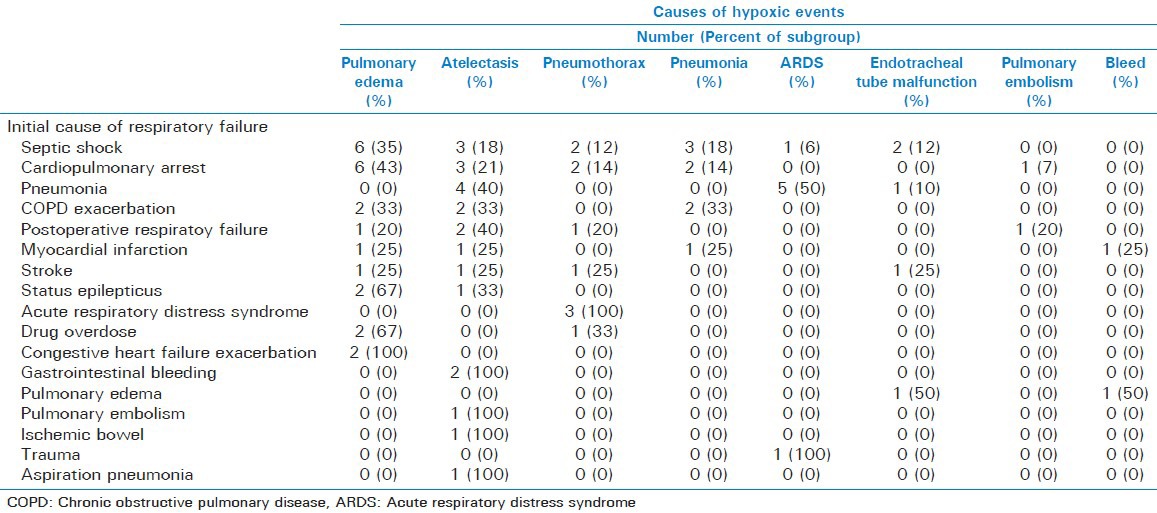
The remaining causes of acute hypoxia included pneumonia (10%), ARDS (9%), endotracheal tube malfunction (6%), airway bleeding (3%), and PE (3%) in decreasing order of occurrence [Table 2]. Subgroup analyses show that none of the causes of acute hypoxia were secondary to the underlying cause of respiratory failure [Table 3].
Table 2.
Causes of hypoxic events
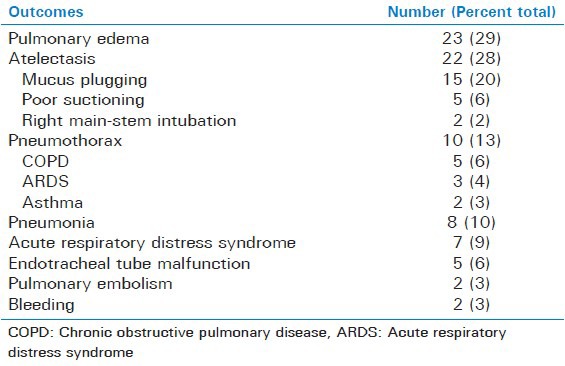
Table 3.
Subgroup analyses showing the causes of respiratory failure and eventual cause of acute hypoxia
DISCUSSION
Various pathophysiological mechanisms of hypoxia generally include alveolar hypoventilation, impaired diffusion, venous admixture, ventilation-perfusion mismatch, and low mixed venous oxygen. Understanding the physiological basis of hypoxia can help elucidate its causes. While a patient is mechanically ventilated, the cause of a hypoxic event can be due to the ventilator and equipment problems, progression of the pre-existing disease that resulted in respiratory failure, and development of a new disease process or a side-effect from an intervention. We will explore in detail the most common causes of acute hypoxia in patients on mechanical ventilation.
Pulmonary edema
Pulmonary edema was the leading cause of acute hypoxic events in our patients. Twenty-three (29%) patients in this study developed hypoxia due to pulmonary edema. The average ejection fraction of the patients developing pulmonary edema in our patients was 37% (ranging between 20% and 55%). The average serum albumin in these patients was 2.49 g/dL (ranging between 1.3 and 2.9 g/dL). Pulmonary edema is a common complication of excessive fluid resuscitation in mechanically ventilated patients leading to an increase in intravascular volume and increased left ventricular end-diastolic pressure. Pulmonary edema develops as a result of excess fluid filtration through the capillaries into the lungs. The accumulation of this fluid is dependent on various factors including vascular permeability, hydrostatic, and oncotic pressures.[2] The vast majority of patients developing pulmonary edema in this study had low cardiac ejection fraction. Most of the study patients had multiple comorbidities including advanced age, poor nutritional status resulting in hypoalbuminemia, and a history of structural and functional heart disease. Typically, these cases of pulmonary edema responded to fluid restriction and forced diuresis with loop diuretics.[3] The most commonly used drug is intravenous furosemide in doses of 20-40 mg, which leads to an increase in the sodium and water excretion resulting in decreased left ventricular end-diastolic pressure. In hypoalbuminemic patients, the use of intravenous albumin aids in preventing third spacing of fluids.[4] In patients with kidney disease or those refractory to fluid restriction or drug therapy, hemodialysis is the only option in decreasing intravascular volume.[5]
Atelectasis
Common causes of atelectasis among the study patients were mucous plugging (20%), poor suctioning (6%), and right main-stem intubation (2%), leading to right upper lobe collapse or left lung collapse. Immobility in critically ill patient along with impaired mucociliary clearance, ineffective cough leading to mucous accumulation are risk factors for atelectasis. Atelectasis is a common cause of hypoxic events and can further lead to complications including pneumonia.[6,7,8] The role of bronchoscopy for relieving atelectasis and hypoxemia by removing retained secretions is both well studied and effective.[6,7,8,9,10,11]
Published case studies have described atelectasis of the right upper lobe resulting from inadvertent intubation of the right main-stem bronchus.[12] Presence of bilateral breath sounds after intubation does not reliably exclude right main-stem intubation. Prompt confirmation with chest roentgenogram is recommended.[13,14] Right main-stem intubation can obstruct the orifice of the right upper lobe brochus due to its close proximity to the carina. Also commonly observed is left lower lobe collapse or entire left lung collapse with right main-stem intubations.
Five patients (6%) developed atelectasis from poor suctioning. In addition, four patients were found to have left lower lobe atelectasis that was likely the result of poor suctioning of the left main stem bronchus due to its natural angulation. Proper suctioning technique requires a catheter that is half the diameter of the endotracheal tube inserted at the level of the carina with minimal pressure suctioning started only after retraction 1-2 cm away from the carina for a maximum duration of 10 s.[15]
Pneumothorax
Ten patients (13%) in the study developed pneumothorax leading to hypoxemia. Five patients (6%) had COPD, three (5%) had ARDS, and two (3%) had bronchial asthma. Pneumothorax results from pulmonary barotrauma leading to alveolar rupture and subsequent air leak into the pleural space due to positive pressure ventilation.[11] Risk factors include COPD, ARDS, asthma, and use of positive end expiratory pressure (PEEP).[16,17,18] Pneumothorax can be prevented by maintaining the peak inspiratory pressure (PIP) below 35 cm of water. In this study, nearly half of the patients who developed pneumothorax had underlying lung disease including COPD and asthma. Average peak pressures in patients with underlying lung disease were between 30 and 35 cm H2O and in patients with ARDS were 40 cm H2O. As mentioned, controlling PIP is one of the most important steps in preventing iatrogenic barotrauma. For ARDS patients, we recommend ARDS Network Trial Guidelines for ventilator management.[19] In general, appropriate management includes maintaining low PIP and use of small tidal volumes of 5-7 ml/kg ideal bodyweight.[19,20] Several procedures such as bronchoscopy, central line placement, and thoracentesis carry the risk of pneumothorax as a complication.[21] One should remain vigilant in identifying post-procedural pneumothorax for at least 24 h after completion of the procedure.
Pneumonia
Eight patients (10%) in our study developed hypoxemia due to new-onset pneumonia. Pneumonia occurring 48 h after intubation is defined as ventilator-associated pneumonia. There are two varieties of VAP based on the time of onset: early onset and late onset with the former diagnosed during the first 3-4 days of intubation and the latter occurring after 4 days of intubation.[22,23] Early onset VAP is usually the result of oropharyngeal infectious organisms, Hemophilus influenza, Streptococcus pneumonia, and Staphylococcus aureus. Late onset pneumonia results from gram negative bacteria including Pseudomonas aeruginosa and Acinetobacter.[22,24] Several risk factors are associated with VAP which include duration of mechanical ventilation, supine postion, poor oral care, aspiration due to enteric feeding, use of antacids, COPD, ARDS, and sepsis.[22,24,25] The implementation of a modified ventilator bundle driven by respiratory therapy which includes head elevation to 45°, sedation vacations, hand washing, and spontaneous breathing trials to prevent prolonged intubation have been shown to dramatically reduce the incidence of VAP.[26]
Bleeding
In this study, two patients (3%) became hypoxic from bleeding. Presence of blood in the endotracheal tube is an alarming finding. Bleeding can arise from the airway or alveoli. Suction trauma is the leading cause of airway bleeding. Repetitive suctioning of the airway to remove secretions leads to inflammation and eventually vascular erosion and bleeding. Bronchoscopy in our patients localized the bleeding site in the airways. Stress-induced ultrastructural changes that disrupt the capillary–alveolar endothelium can also result in pulmonary edema and alveolar hemorrhage.[27] Various other pathologies responsible for hemorrhage include coagulopathy, thrombocytopenia, crack cocaine, cytotoxic drugs, radiation therapy, autoimmune disorders, vasculitis, and idiopathic causes.[28,29] A systematic approach to identify the cause of bleeding is essential. Bronchoscopic examination is crucial in the workup of patients with endobronchial bleeding.
Endotracheal tube malfunction
Five patients (6%) in our study developed hypoxemia due to endotracheal tube malfunction. Endotracheal tube malfunction occurred in patients who were not adequately sedated. Cough reflex of these patients in respiratory distress led to the displacement of the endotracheal tube which led to hypoxemia requiring re-intubation. One patient had cuff deflation of the endotracheal tube requiring re-intubation. Several methods of stabilizing the endotracheal tube exist which include commercial tube holders, adhesive tape, cotton, and twill. No particular method of stabilization has been proven to be superior.[30] Routine evaluation of the endotracheal tube position, reliable anchoring of the endotracheal tube, and adequate sedation are recommended to minimize tube dislodgement.
Pulmonary embolism
Only two patients (3%) in our study developed PE. Deep venous thrombosis (DVT) and PE are often unrecognized in the critically ill patients. Patients who experience sudden hypoxemia with the presence of tachycardia or hypotension may have undetected PE.[31] Frequency of PE in hospitalized patients is nearly 15% in autopsy studies.[32] Prevalence of thromboembolic disease in patients hospitalized for pulmonary disease is estimated between 8% and 25%.[33] In a study of intubated COPD patients, the presence of DVT was between 15% and 28%, but no proven PE was observed.[33] In a large study of ICU patients, the incidence of PE was 1.9% with nearly a third of cases diagnosed in patients with acute respiratory distress.[34] The mortality rate in ICU patients who develop PE is 52.9%.[34] Currently, there are no prediction models for PE in the ICU setting.[31] The use of Wells’ score and the Geneva revised score poorly correlate with the presence of PE.[34] Risk factors for DVT and PE in the ICU include history of DVT, end-stage renal failure, platelet transfusions, vasopressor use, length of stay, age, and mechanical ventilation.[31,35] Diagnosis of PE can be confirmed with the use of CT angiogram, but the use of the test is limited to those patients who are stable and at minimal risk of contrast-related complications. Chest radiograph images can be normal in a third of cases.[34] The low incidence in our study can be attributed to the standard use of thromboembolism prophylaxis in all mechanically ventilated patients.
Acute respiratory distress syndrome
In this study, seven patients (9%) developed hypoxia due to worsening to ARDS. Several risk factors exist for the development of ARDS. Five cases in our study were due to pneumonia. All of our patients had normal cardiac ejection fraction and central venous pressures ≤ 12. Despite improvement in mortality rates, ARDS still carries a mortality rate of 43%.[36] Mortality in ARDS occurs primarily as a result of sepsis and multiorgan failure, as was the case in the five deaths in our patients. Use of low tidal volume, ≤6 cc/kg, with plateau pressure < 30 cm H2O and avoidance of fluid overload remain the standard of care in ARDS management.[37]
CONCLUSION
In this prospective study of 955 mechanically ventilated patients, 79 (8%) developed significant hypoxia. Various causes of acute hypoxic events included pulmonary edema (29%), atelectasis (28%), pneumothorax (13%), pneumonia (10%), ARDS (9%), endotracheal tube malfunction (6%), PE (3%), and bleeding (3%) in decreasing order of frequency.
Pulmonary edema is the most common cause of hypoxia as a result of fluid overload either due to over-hydration, renal disease, or low ejection fraction. Physical examination findings, chest radiographs, and echocardiograms are useful in confirming the diagnosis. Treatment with diuretics or hemodialysis, if needed, can reverse hypoxia.
Atelectasis commonly results from mucous plugging as a result of poor suctioning or right main-stem intubation. A prompt chest radiograph can confirm right main-stem intubation requiring tube readjustment to reverse atelectasis. Proper suctioning of the mucous plugs causing atelectasis can similarly relieve the endobronchial obstruction and relieve hypoxia. Proper suctioning technique with removal of secretions can prevent eventual plugging and atelectasis. Bronchoscopy plays a significant role in the removal of retained secretions to alleviate hypoxia due to atelectasis.
Pneumothorax causing hypoxia may occur in patients with COPD, asthma, ARDS, and high PEEP and after certain invasive pulmonary procedures or placement of a central line. Maintaining low PIP (<35 cm H2O) and using low tidal volumes (5-7 ml/kg ideal body weight) can help prevent barotrauma resulting in pneumothorax. Early recognition of a pneumothorax, typically on a chest radiograph, can help avoid tension pneumothorax and eventual cardiopulmonary arrest.
Ventilator-associated pneumonias both either early or late onset can lead to hypoxia. The diagnosis is usually confirmed by a chest radiograph. Prevention of VAP may be successfully achieved with the use of strategic ventilator bundles which include various infection control techniques.
Worsening hypoxia may result from the development or progression to ARDS. In our study, most cases of ARDS resulted from pneumonia.
Endotracheal tube malfunction commonly occurs as a result of tube displacement due to uncontrolled cough or struggle by the patients under inadequate sedation. The use of proper tube stabilizers and anchoring techniques along with adequate sedation can help minimize tube dislodgements. Ruptured cuff or over-distended cuff with tracheal ballooning due to tracheomalacia requires replacement of the tube.
PE is an infrequent cause of a hypoxic event in our study. The use of thromboembolism prophylaxis in our patients may explain the low occurrence of PE and supports its use in all ventilated patients. Lack of predictive models in the ICU can cause many cases to go undiagnosed with serious consequences. Clinical suspicion for PE must be considered in patients who suddenly develop hypoxia with otherwise unexplained tachycardia or hypotension.
Bleeding in the airway, although an alarming sign, is usually the result of suction trauma. Bronchoscopic examination is crucial in the workup of endobroncial bleeding. A systematic approach to identify the cause of bleeding is essential.
Appropriate evaluation of all hypoxic events must begin at the bedside. A step-by-step approach must include a thorough physical examination, in general, and chest examination, in particular, with special attention to auscultatory findings. Evaluation of the endotracheal tube can immediately reveal dislodgement, bleeding, and secretions. Correlation of physical examination findings with those on chest radiograph is essential. Each hypoxic event may require a different intervention depending on its etiology. When presented with a hypoxic event, the physician must consider all the above outlined causes. Instead of simply increasing the fraction of oxygen in the inspired air to overcome hypoxia, a concerted effort in appropriate problem solving can reduce the likelihood of an incorrect diagnosis and inappropriate management response.
Footnotes
Source of Support: Pulmonary division fund, St. Joseph's regional medical center
Conflict of Interest: None declared.
REFERENCES
- 1.Keith RL, Pierson DJ. Complications of mechanical ventilation. A bedside approach. Clin Chest Med. 1999;17:439–51. doi: 10.1016/s0272-5231(05)70326-9. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AE. Capillary fluid filtration. Starling forces and lymph flow. Circ Res. 1981;49:557–75. doi: 10.1161/01.res.49.3.557. [DOI] [PubMed] [Google Scholar]
- 3.Brater DC. Diuretic therapy. N Engl J Med. 1998;339:387–95. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 4.Dubois MJ, Orellana-Jimenez C, Melot C, De Backer D, Berre J, Leeman M, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit Care Med. 2006;34:2536–40. doi: 10.1097/01.CCM.0000239119.57544.0C. [DOI] [PubMed] [Google Scholar]
- 5.Susini G, Zucchetti M, Bortone F, Salvi L, Cipolla CM, Rimondini A, et al. Isolated ultrafiltration in cardiogenic pulmonary edema. Crit Care Med. 1990;18:14–7. doi: 10.1097/00003246-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Kreider ME, Lipson DA. Bronchoscopy for atelectasis in the ICU: A case report and review of the literature. Chest. 2003;124:344–50. doi: 10.1378/chest.124.1.344. [DOI] [PubMed] [Google Scholar]
- 7.Fowler AA, Scoggins WG, O’Donohue WJ. Positive end-expiratory pressure in the management of lobar atelectasis. Chest. 1978;74:497–500. doi: 10.1378/chest.74.5.497. [DOI] [PubMed] [Google Scholar]
- 8.Gibb KA, Carden DL. Atelectasis. Emerg Med Clin North Am. 1983;1:371–8. [PubMed] [Google Scholar]
- 9.Lindholm CE, Ollman B, Snyder J, Millen EG, Grenvik A. Cardiorespiratory effects of flexible fiberoptic bronchoscopy in critically ill patients. Chest. 1978;74:362–8. doi: 10.1378/chest.74.4.362. [DOI] [PubMed] [Google Scholar]
- 10.Barrett CR. Flexible fiberoptic bronchoscopy in the critically ill patient: Methodology and indications. Chest. 1978;73:746–9. [PubMed] [Google Scholar]
- 11.Vijay K, Mahajan M, Catron PW, Huber GL. The value of fibroptic bronchoscopy in the management of pulmonary collapse. Chest. 1978;73:817–20. doi: 10.1378/chest.73.6.817. [DOI] [PubMed] [Google Scholar]
- 12.Kozo S, Hiroshi G, Hacker DC, Arakawa K. Right upper lobe atelectasis after inadvertent right main bronchial intubation. Anesth Analg. 1983;62:851–4. [PubMed] [Google Scholar]
- 13.Brunel W, Coleman DL, Schwartz DE, Peper E, Cohen NH. Assessment of routine chest roentgenograms and the physical examination to confirm endotracheal tube position. Chest. 1989;96:1043–5. doi: 10.1378/chest.96.5.1043. [DOI] [PubMed] [Google Scholar]
- 14.McGillicuddy DC, Babineau MR, Fisher J, Ban K, Sanchez LD. Is a postintubation chest radiograph necessary in the emergency department. Int J Emerg Med. 2009;2:247–9. doi: 10.1007/s12245-009-0133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen CM, Rosendahl-Nielsen M, Hjermind J, Egerod I. Endotracheal suctioning of the adult intubated patient-what is the evidence? Intensive Crit Care Nurs. 2009;25:21–30. doi: 10.1016/j.iccn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Gammon RB, Shin MS, Buchalter SE. Pulmonary barotrauma in mechanical ventilation. Patterns and risk factors. Chest. 1992;102:568–72. doi: 10.1378/chest.102.2.568. [DOI] [PubMed] [Google Scholar]
- 17.Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28:406–13. doi: 10.1007/s00134-001-1178-1. [DOI] [PubMed] [Google Scholar]
- 18.Eisner MD, Thompson BT, Schoenfeld D, Anzueto A, Matthay MA. Acute Respiratory Distress Syndrome Network. Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:978–82. doi: 10.1164/ajrccm.165.7.2109059. [DOI] [PubMed] [Google Scholar]
- 19.Brower RG, Ware LB, Berthiaume Y, Matthay MA. Treatment of ARDS. Chest. 2001;120:1347–67. doi: 10.1378/chest.120.4.1347. [DOI] [PubMed] [Google Scholar]
- 20.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 21.Chen KY, Jerng JS, Liao WY, Ding LW, Kuo LC, Wang JY, et al. Pneumothorax in the ICU. Chest. 2002;122:678–83. doi: 10.1378/chest.122.2.678. [DOI] [PubMed] [Google Scholar]
- 22.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 23.Vallés J, Artigas A, Rello J, Bonsoms N, Fontanals D, Blanch L, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med. 1995;122:179–86. doi: 10.7326/0003-4819-122-3-199502010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Bregeon F, Papazian L, Visconti A, Gregoire R, Thirion X, Gouin F. Relationship of microbiologic diagnostic criteria to morbidity and mortality in patients with ventilator-associated pneumonia. JAMA. 1997;277:655–62. [PubMed] [Google Scholar]
- 25.McClure JR, Cooke RP, Lal P, Pickles D, Majjid S, Grant CA, et al. Outcome of late-onset hospital-acquired pneumonia related to causative organism. J Hosp Infect. 2009;71:348–52. doi: 10.1016/j.jhin.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Blamoun J, Alfakir M, Rella ME, Wojcik JM, Solis RA, Anees Khan M, et al. Efficacy of an expanded ventilator bundle for the reduction of ventilator-associated pneumonia in the medical intensive care unit. Am J Infect Control. 2009;37:172–5. doi: 10.1016/j.ajic.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz DR, Maroo A, Malhotra A, Kesselman H. Negative pressure pulmonary hemorrhage. Chest. 1999;115:1194–7. doi: 10.1378/chest.115.4.1194. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger NA, Albin RJ. A review of the respiratory effects of smoking cocaine. Am J Med. 1989;87:664–8. doi: 10.1016/s0002-9343(89)80401-2. [DOI] [PubMed] [Google Scholar]
- 29.Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest. 2010;137:1164–71. doi: 10.1378/chest.08-2084. [DOI] [PubMed] [Google Scholar]
- 30.Gardner A, Hughes D, Cook R, Henson R, Osborne S, Gardner G. Best practice in stabilisation of oral endotracheal tubes: A systematic review. Aust Crit Care. 2005;18(158):160–5. doi: 10.1016/s1036-7314(05)80029-3. [DOI] [PubMed] [Google Scholar]
- 31.Cook DJ, Donadini MP. Pulmonary embolism in medical-surgical critically ill patients. Hematol Oncol Clin North Am. 2010;24:677–82. doi: 10.1016/j.hoc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995;108:978–81. doi: 10.1378/chest.108.4.978. [DOI] [PubMed] [Google Scholar]
- 33.Fraisse F, Holzapfel L, Coulaud JM, Simonneau G, Bedock B, Feissel M, et al. Nadroparin in the prevention of deep vein thrombosis in acute decompensated COPD. Am J Respir Crit Care Med. 2000;161:1109–14. doi: 10.1164/ajrccm.161.4.9807025. [DOI] [PubMed] [Google Scholar]
- 34.Bahloul M, Chaari A, Kallel H, Abid L, Hamida CB, Dammak H, et al. Pulmonary embolism in intensive care unit: Predictive factors, clinical manifestations and outcome. Ann Thorac Med. 2010;5:97–103. doi: 10.4103/1817-1737.62473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geerts W, Selby R. Prevention of venous thromboembolism in the ICU. Chest. 2003;124:357–63. doi: 10.1378/chest.124.6_suppl.357s. [DOI] [PubMed] [Google Scholar]
- 36.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 37.Girard TD, Bernard GR. Mechanical ventilation in ARDS: A state-of-the-art review. Chest. 2007;131:921–9. doi: 10.1378/chest.06-1515. [DOI] [PubMed] [Google Scholar]


