Abstract
Venous thromboembolism (VTE) is a frequent complication in critically ill patients and is associated with increased rates of morbidity and mortality. The use of thromboprophylaxis to reduce the risk of VTE in this patient population is the standard of care. This review will summarize the recommendations set forth in consensus guidelines for the prevention and treatment of VTE across subgroups within the critically ill patient population. In addition, the drug properties of the recommended pharmacologic agents for thromboprophylaxis will be highlighted including their pharmacokinetics, dosing and complications. The critical care practitioner may also encounter novel oral anticoagulants with increasing frequency. These agents will be briefly reviewed in terms of their approved and investigational indications and the clinical concerns related to their use will also be discussed.
Keywords: Anticoagulant, critical care, embolism, prophylaxis, thrombosis, venous thromboembolism
INTRODUCTION
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), are frequent complications encountered in critically ill patients admitted to an intensive care unit (ICU) leading to increased rates of morbidity and mortality within this patient population.[1] Critically ill patients are at high-risk for developing a VTE for reasons such as extended periods of immobilization, mechanical ventilation, and vascular injury or surgery. The risk of developing a DVT can be as high as 81% in critically ill patients not managed with thromboprophylaxis and can occur in approximately 44% of critically ill patients despite some form of thromboprophylaxis.[1] However, VTE incidence can vary markedly depending on mode of detection. Progression to a PE has been reported in approximately 12% of patients with a documented DVT in the ICU despite thromboprophylaxis.[2] The use of thromboprophylaxis to reduce rates of VTE in the ICU is the standard of care.[1,3]
The 9th edition of the American College of Chest Physicians (ACCP) clinical practice guidelines for prevention and treatment of VTE provide updated recommendations for various subgroups of patients that emphasizes the evaluation of patient risk for VTE and move clinical practice away from universal thromboprophylaxis for all patients.[3] Prophylaxis with an anticoagulant is recommended in patients who have an elevated DVT and PE risk, which includes the critically ill provided they are not at a high bleeding risk. The use of anticoagulants in patients with an active gastroduodenal ulcer, bleeding history in the three months prior to hospital admission or platelet count less than 50,000 K/uL may increase the risk of hemorrhage. Clinicians may also reference specific recommendations set forth in their respective specialty areas to guide their practice.
The focus of this review is to outline the specific pharmacologic profiles of the recommended anticoagulants for VTE prophylaxis and treatment and provide a summary of current recommendations for thromboprophylaxis across the distinct critically ill patient populations. Also included is a discussion of novel oral anticoagulants that have or are currently undergoing investigation for VTE prophylaxis and treatment in patients that clinicians may encounter in intensive care units. Specific discussion of the underlying pathophysiology and diagnosis of VTE is beyond the scope of this review.
Mechanical prophylaxis
Mechanical devices for thromboprophylaxis can be characterized as either static or dynamic.[3,4] Static devices include graduated compression stockings (GCS) and placement of an inferior vena cava (IVC) filter, which is an invasive procedure. Dynamic methods include intermittent pneumatic compression devices (IPC) and arteriovenous foot pumps. Since up to 80% of ICU patient experience one or more episodes of minor or major bleeding, mechanical thromboprophylaxis is often an attractive option to limit potential adverse effects.[3] However, data evaluating their use alone or as an adjunct to pharmacologic prophylaxis in critically ill patients is limited. At this time, there are no randomized studies comparing mechanical prophylaxis to no prophylaxis in critically ill patients. Current guidelines recommend mechanical prophylaxis for patients in whom pharmacologic prophylaxis is contraindicated or in combination with pharmacologic thromboprophylaxis in certain sub-populations at higher VTE risk.[3,5] IPCs are the preferred device although arteriovenous foot pumps can be considered when IPCs are contraindicated due to external fixation or casts.[3] As a general rule, guidelines recommend against the use of IVC filters as thromboprophylaxis and should only be considered in specific circumstances.[3]
Pharmacologic prophylaxis
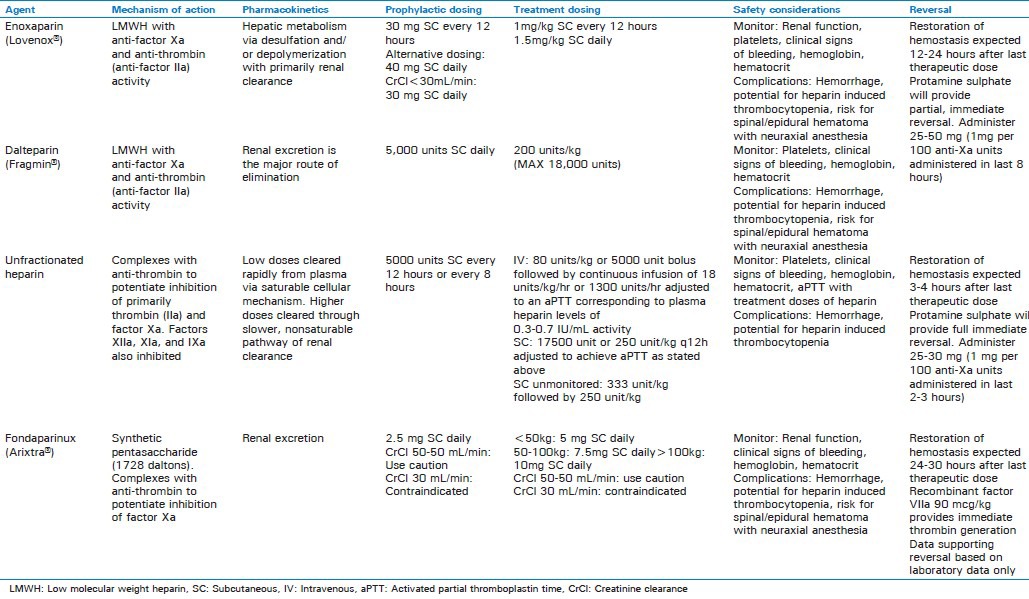
Options for pharmacologic thromboprophylaxis include low dose unfractionated heparin (LDUH), low molecular weight heparins (LMWH) (i.e., enoxaparin and dalteparin), and pentasaccharides (i.e., fondaparinux). Specific information for each agent including dosing and monitoring recommendations is outlined in Table 1. Although there are few studies evaluating pharmacologic prophylaxis for the critically ill patient, current guidelines recommend the use of either LMWH or LDUH over no prophylaxis.[3] Selection of a specific agent for prophylaxis can be challenging in these patients, as the clinician must balance the risk of thrombosis and hemorrhage. Furthermore, one may need to take into consideration additional published literature to support the selection of prophylaxis for certain subgroups of critically ill patients.
Table 1.
Trauma
Major trauma patients who receive no thromboprophylaxis are reported to have increased rates of asymptomatic VTE with an incidence as high as 58% detected by a single venogram during hospital admission.[9] However, the use of a diagnostic test as a surrogate marker for clinically relevant VTE events is criticized.[10] The risk of symptomatic VTE is estimated to be 3% to 5% among major trauma patients. Thromboprophylaxis should include mechanical methods, preferably IPC, or administration of LDUH or LMWH in major trauma patients.[3] Traumatic injury to the spinal cord and brain and surgical intervention to the spine place the trauma patient at higher risk for VTE, estimated to be 8% to 10%, and thromboprophylaxis recommendations include both pharmacologic prevention with LDUH or LMWH and mechanical prophylaxis with IPC if no lower extremity injury exists.[3] The Eastern Association for the Surgery of Trauma (EAST) guidelines for trauma patients published in 2002 suggest LMWH over LDUH for VTE prevention in major trauma patients.[5] The use of a pharmacologic agent to prevent VTE may be limited by relative contraindications for chemical prophylaxis including severe head injury, non-operatively managed liver or spleen injures, renal failure, spinal column fracture with epidural hematoma, severe thrombocytopenia, and coagulopathy.[11]
Burn injury
The incidence of VTE in burn patients is fairly low in comparison to most ICU populations ranging from 1% to 3% with no routine prophylaxis and approximately 2% with pharmacologic prophylaxis.[12,13,14,15] As there are limited studies evaluating pharmacologic prophylaxis in burn patients; consensus guidelines extrapolate data from other populations to recommend routine prophylaxis with LDUH or LMWH in burn patients who have additional risk factors for VTE.[11] Additional VTE risk factors in burn patients include age, morbid obesity, extensive or lower-extremity burns, lower-extremity trauma, insertion of a femoral venous catheter, and prolonged immobility.[16] A single retrospective analysis compared the incidence of symptomatic DVT in burn patients receiving enoxaparin or LDUH.[17] Symptomatic DVT was documented in 5 (0.45%) patients receiving LDUH and none in the enoxaparin group (P = 0.07). However, patients receiving LDUH had more VTE risk factors and primarily received heparin 5000 units subcutaneously every 12 hours, potentially biasing the results. Two recent studies evaluated the impact of burn injury on enoxaparin dosing for thromboprophylaxis. The results suggest that patients with large burns and associated hypermetabolism may require doses above 30 mg every 12 hours of enoxaparin to achieve a targeted anti-factor Xa range for prophylaxis.[16,18] However, specific dosing for either LDUH or LMWH is not defined in this population, and clinical correlation between anti-factor Xa levels and thrombosis risk has not been established.
General surgery
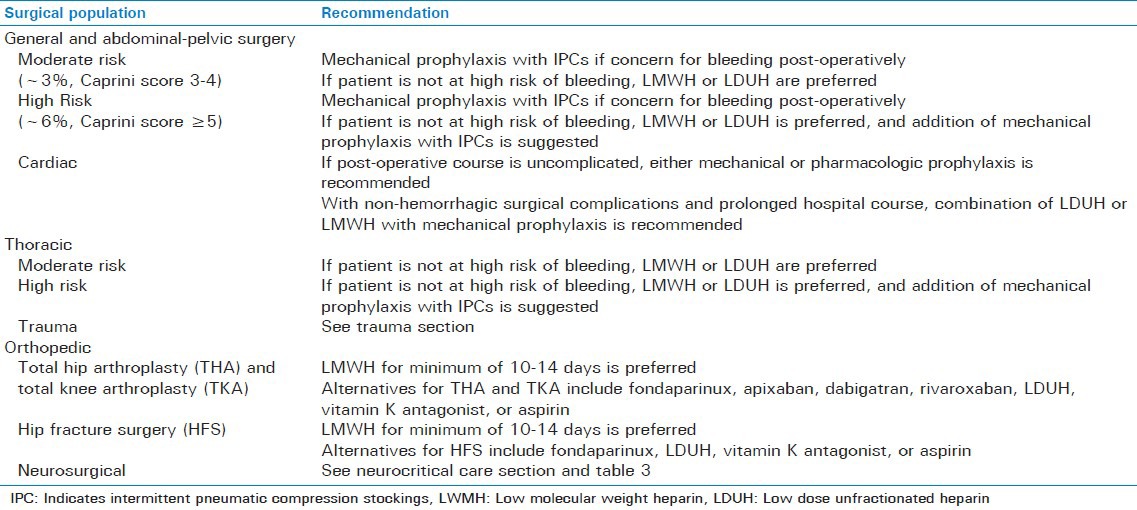
Approximately 1/3 of the 150,000 to 200,000 VTE-attributed deaths in the United States reported annually occur after surgery.[19] The probability of thrombosis depends on the surgical procedure and associated post-operative course, as well as underlying patient characteristics.[11] In addition, surgical complications including post-operative infections (pneumonia, urinary tract infection, and sepsis), acute renal failure and need for transfusion have also been linked with an increased risk of VTE after surgery.[11] Due to the complicated nature of determining risk of VTE in surgical patients, several models have been developed to estimate total risk related to patient and procedural factors. For example, the Caprini model scores risk of VTE based on a variety of risk factors and classifies patients as very low (0-1 point), low (2 points), moderate (3-4 points) and high (≥5 points).[20,21] This model has been validated in general surgery, vascular, urologic, plastic and reconstructive surgery patients and is used in the current surgical VTE prevention guidelines.[11] Specific recommendations based on VTE risk and surgical populations are outlined in Table 2.
Table 2.
Recommendations for pharmacologic venous thromboembolism prophylaxis based on surgical population[11]
Neurocritical care
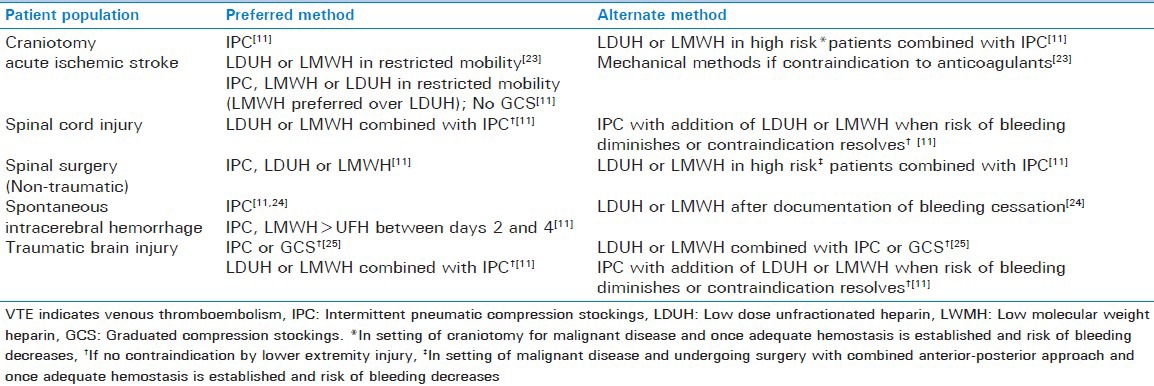
The neurocritical care patient population encompasses a diverse group of underlying pathologies, including neurosurgery, stroke (hemorrhagic and ischemic), traumatic brain injury (TBI) and spinal cord injury (SCI). Thromboprophylaxis in this patient population remains controversial with sparse high quality data in certain subgroups and considerable differences in clinical practice, particularly with the timing of therapy initiation.[22] Timing of initiation will vary based on underlying pathology and risk of hemorrhagic complications, particularly with intracerebral hemorrhage. There is no uniform anticoagulant for thromboprophylaxis agent recommended across the subsets of neurocritical care patients and intermittent compression devices in general are the method of choice [Table 3]. No consensus guidelines are available for patients with subarachnoid hemorrhage or brain tumors.
Table 3.
Recommendations for venous thromboembolism prophylaxis in neurocritical care
Medical
Compared to other critically ill patient populations there are fewer studies involving patients in the medical ICU regarding prevention of VTE. The incidence of asymptomatic DVT in patients not receiving thromboprophylaxis among medical and medical-surgical ICU patients ranges from 9% to 32% depending on screening method and 7% to 13% in those receiving heparin products.[26,27,28] Only one symptomatic PE was reported in a single study among 100 patients.[26] In general, the ACCP guideline recommendations for critically ill patients may be referenced in this patient population.[3] These recommendations include pharmacologic prevention with a LMWH or LDUH. Mechanical prophylaxis with GCS or IPC should be used if bleeding risk is present and pharmacologic prophylaxis should be re-evaluated when the risk of bleeding subsides.
Pregnancy
Pregnancy is associated with a significantly increased thrombotic risk, with a 3 to 4-fold higher risk throughout pregnancy.[29] Postpartum, the risk increases further to 20-fold, and returns towards baseline 6-8 weeks after delivery.[29,30,31] The primary reason for increased thrombotic risk is hormonally induced hypercoagulability as a result of increased venous capacitance, decreased outflow, and increased production of factors VII, VIII, X and von Willebrand factor.[30,31,32] In addition, mechanical obstruction due to the gravid uterus, decreased mobility, and vascular injury during delivery may also increase risk of VTE.[32,33] In deciding which patients require anticoagulation for prevention of VTE, risk and benefit to both mother and fetus must be weighed. Although there is little data to guide therapy in this population, current guidelines recommend LMWH as the preferred agent for both prophylaxis and treatment of VTE during pregnancy.[34] However, neither heparin nor LMWH cross the placenta and are both considered safe.[30,34] Based on limited data supporting fetal safety, pentasaccharides (i.e., fondaparinux) and direct thrombin inhibitors (DTIs) are not recommended during pregnancy.[34] Postpartum after cesarean section, patients with at least one major or two minor risk factors should receive either LMWH or mechanical prophylaxis.[34]
Malignancy
Patients with cancer who are critically ill or have undergone surgery are at significantly higher risk of VTE compared to those without cancer.[35] The increased risk associated with cancer is based on alterations in all three components of Virchow's triad as a result of the underlying malignancy and associated management (chemotherapy and surgery) as well as the presence of additional risk factors for thrombosis (i.e., prior VTE, obesity, immobility).[35,36] VTE in cancer patients is associated with significant morbidity and mortality, and is the 2nd most common cause of death in hospitalized patients with cancer.[36] The majority of studies evaluating thromboprophylaxis in cancer patients focus on perioperative management in patients undergoing surgical interventions. Based on these studies, current guidelines recommend an extended duration (i.e., 4 weeks) of thromboprophylaxis with LMWH for high VTE risk patients admitted for abdominal or pelvic surgery for cancer.[11] If a patient is at increased risk for bleeding, mechanical devices should be used until pharmacologic thromboprophylaxis can be initiated.
Methods for VTE treatment in critically Ill
Management of VTE in critically ill patients can be especially difficult as the selection and dosing of therapeutic anticoagulation may be affected by several aspects of critical illness such as liver or kidney dysfunction [Table 1]. Furthermore, an ICU patient is subject to invasive monitoring and surgical intervention that carry inherent bleeding risks, which can complicate anticoagulant use. Nonetheless, therapeutic anticoagulation is appropriate for the majority of critically ill patients experiencing a VTE and recommended for patients with a high clinical suspicion while diagnostic tests are being carried out.[37] There are no specific recommendations with respect to one anticoagulant over another in the critically ill patient. Initial anticoagulation may include a LMWH, intravenous unfractionated heparin (UFH) or fondaparinux. A critical care clinician may suggest UFH for VTE management in the critically ill given that it is minimally affected by organ dysfunction, has a relatively shorter half-life and is reversible with protamine. The administration of agents such as enoxaparin or fondaparinux may not be appropriate for critically ill patients secondary to potential interference with subcutaneous absorption.[38] Non-heparin anticoagulants may be used, particularly in the setting of HIT such as bivalirudin, danaparoid, desirudin or argatroban.
Complications of VTE prevention and treatment
The complications associated with the prevention and treatment of VTE that are of utmost concern in the ICU setting include hemorrhage and heparin induced thrombocytopenia (HIT) [Table 1].[39] Bleeding complications with UFH and LMWH are due to the underlying mechanism of action with inhibition of anti-thrombin III, whereas HIT is related to formation of IgG antibodies that recognize complexes of platelet factor 4 (PF4) and heparin on the surface of platelets.[39,40] Risk of hemorrhage depends on drug dosing and route of administration, as well as patient specific characteristics (i.e., renal/liver dysfunction, age, and underlying hematologic abnormalities) and treatment related variables (i.e., surgical interventions and concomitant medications).[39] While major bleeds are the most concerning adverse effect associated with VTE prevention and treatment, the risk of hemorrhage can be minimized thorough risk/benefit assessment prior to initiation, and appropriate dosing and monitoring. In addition, for the majority of traditional anticoagulants used for prevention and treatment of VTE in the acute setting, agents for reversal exist [Table 1].
HIT is an antibody mediated adverse reaction primarily related to the use of UFH and LMWH.[41] The incidence of HIT varies from 0.1-5% based on patient population, with the highest risk reported in cardiovascular and orthopedic surgery and the lowest in non-critically ill medical and obstetric patients.[40,42] In addition, choice of anticoagulant significantly impacts risk as UFH has a 10-fold higher risk of HIT compared to LWMH.[40] The hallmark characteristic of HIT is thrombocytopenia (<150 × 109/L or 30-50% reduction from baseline) which is traditionally observed about 5 days after initiation of UFH or LMWH. Occasionally thrombocytopenia can occur within 24 hours in patients with prior exposure or up to 3 weeks after discontinuation of UFH or LMWH in delayed onset HIT.[40]
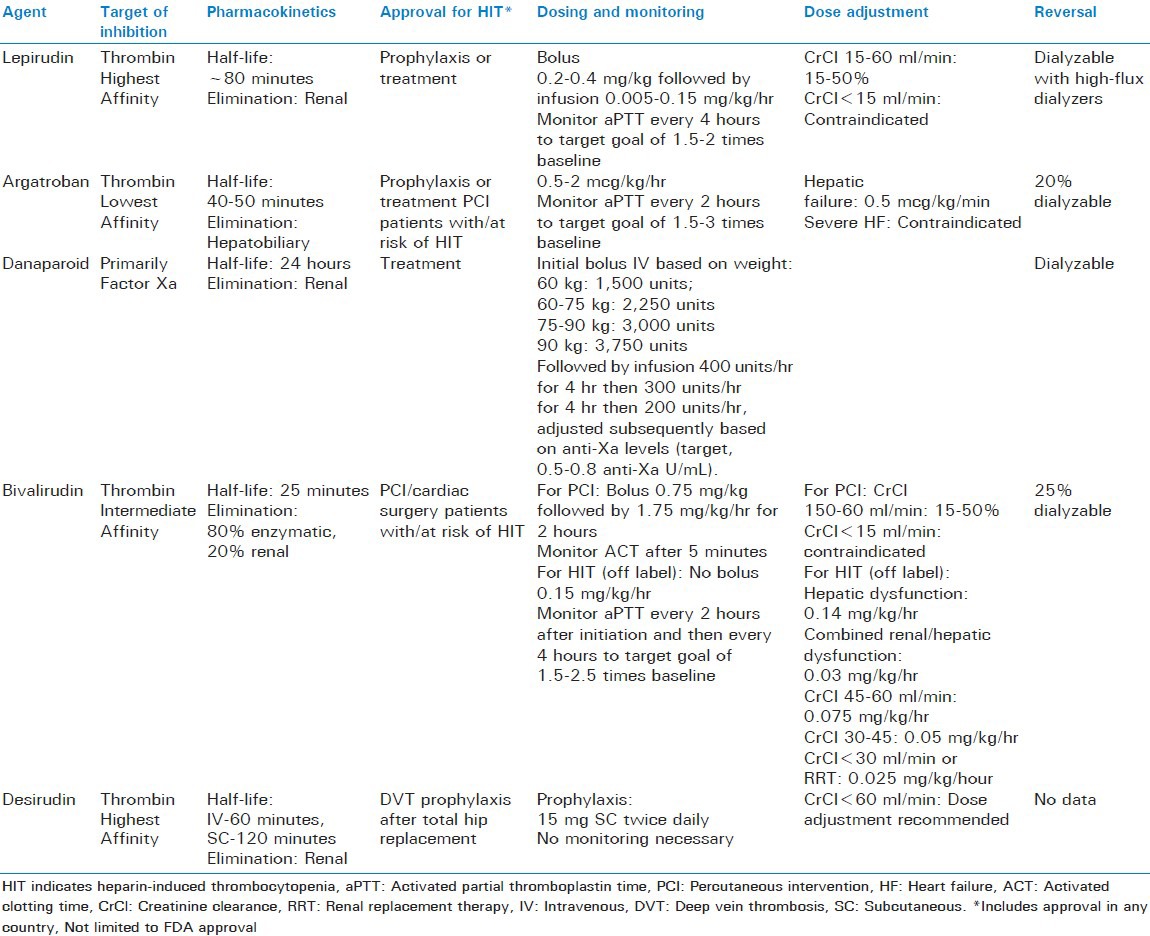
Treatment of HIT includes discontinuation of all forms of UFH or LMWH and initiation of a DTI. Currently available DTIs include lepirudin, argatroban, bivalirudin and desirudin, and guidelines recommend initiation of one of these agents rather than immediate initiation of vitamin K antagonist therapy.[40] Lepirudin, danaparoid or argatroban are recommended for patients with normal renal function, while argatroban is preferred for patients with renal insufficiency. In patients requiring renal replacement therapy, both argatroban and danaparoid are options. Although many institutions have implemented protocols for bivalirudin for management of HIT, published data is limited primarily to patients undergoing percutaneous intervention.[40,43,44] Data for desirudin is also currently lacking. Baxter Healthcare Corporation also recently announced the decision to discontinue production of lepirudin in 2012 and Organon, Inc. also discontinued manufacturing danaparoid in the United States in 2002, leaving argatroban as the only guideline supported DTI for management of HIT in some countries. Further studies are needed to evaluate the efficacy and safety of bivalirudin and desirudin for management of HIT in critically ill patients. Specific information regarding each DTI is summarized in Table 4.
Table 4.
Intravenous direct thrombin inhibitors for heparin-induced thrombocytopenia with or without thrombosis[40,43,44,45]
Like standard therapies for VTE prophylaxis and treatment, DTIs are associated with a significant risk of bleeding. However, the incidence and risk factors for bleeding with these agents are not fully established. In addition, unlike traditional anticoagulant therapies there are no available reversal agents for bleeding associated with DTIs and the optimal strategy for restoring hemostasis is not clear.[7]
Novel anticoagulants
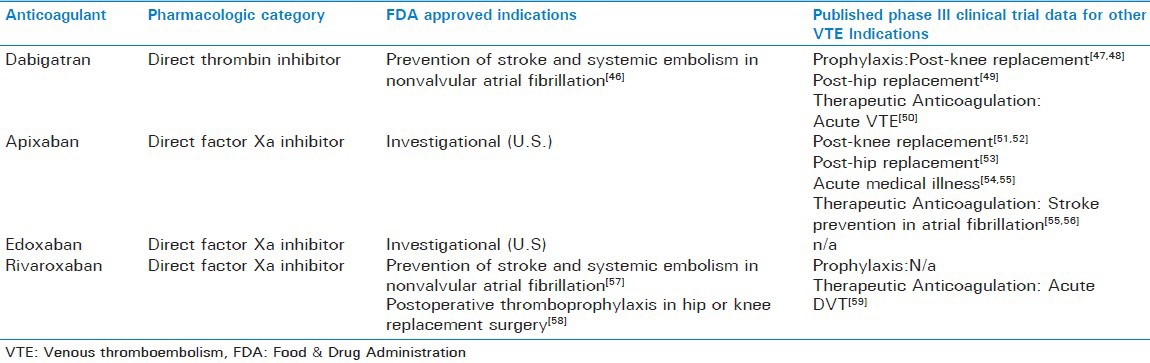
Oral DTIs and direct factor Xa inhibitors comprise two novel therapeutic classes of anticoagulants that differ pharmacologically from traditional agents used to prevent and treat VTE. At this time dabigatran and rivaroxaban have approved use in and outside of the United States, while apixaban has only entered the international market. There are numerous agents that remain under investigation (i.e., edoxaban, betrixaban), including two subcutaneous indirect factor Xa inhibitors, idrabiotaparinux and semuloparin.
Clinical indications for the novel anticoagulants vary but their primary use is for stroke prevention in the setting of atrial fibrillation and for VTE prevention after hip or knee replacement surgery [Table 5]. Unfortunately, there is lack of readily available monitoring for these agents and a specific antidote to reverse their action, of particular concern in emergent situations such as a severely injured trauma patient.[60,61,62,63] Dialysis is suggested in the product labeling to reduce serum concentrations of dabigatran over several hours but this option has its limitations in the unstable bleeding patient.[62] Procoagulant products such as activated prothrombin complex concentrates (i.e., FEIBA) may aid in the reversal of dabigatran and rivaroxaban as reversal of laboratory markers have been demonstrated but clinical correlation is still needed.[61,64]
Table 5.
Novel oral anticoagulants and venous thromboembolism related research
In the setting of acute and critical illness the irreversibility and lack of monitoring to determine the degree of anticoagulation of the novel oral anticoagulants lends great concern for this complex patient population. Furthermore, questions remain about appropriate dosing in the elderly population and in severe renal or liver impairment, and experience with these agents in critically ill patients is not available at this time.
CONCLUSION
The critically ill encompasses a diverse group of patients who are considered high-risk for developing a DVT and PE. Routine thromboprophylaxis is standard of care in this patient population using mechanical methods, pharmacologic prophylaxis, or both. The latest edition of the ACCP guidelines on the management of VTE recommend the use of IPC combined with a LDUH or LMWH for the thromboprophylaxis in the critically ill if no bleeding risk is present. Reference to specific literature published within certain subgroups of patients may provide additional guidance in the selection of prophylaxis. In the setting of an active VTE, therapeutic anticoagulation is generally indicated but no specific pharmacologic class is recommended. Selection of an anticoagulant for VTE prevention or treatment in a critically ill patient may be based on several factors including the pharmacology of the anticoagulant, anticipated complications of therapy, the renal or hepatic function of the patient, and the underlying needs of the patient. Being familiar with specific drug properties of traditional anticoagulants and emerging agents, as well as the evidence to support their use is important for clinicians involved in the selection of a pharmacologic agent for VTE prevention, treatment or for the management of complications such as bleeding and HIT.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med. 2001;161:1268–79. doi: 10.1001/archinte.161.10.1268. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim EH, Iregui M, Prentice D, Sherman G, Kollef MH, Shannon W. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med. 2002;30:771–4. doi: 10.1097/00003246-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed. American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S–47. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limpus A, Chaboyer W, McDonald E, Thalib L. Mechanical thromboprophylaxis in critically ill patients: A systematic review and meta-analysis. Am J Crit Care. 2006;15:402–10. quiz/discussion, 11-2. [PubMed] [Google Scholar]
- 5.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: The EAST practice management guidelines work group. J Trauma. 2002;53:142–64. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–6. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 7.Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e351S–418. doi: 10.1378/chest.11-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e227S–77. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington DT, Mozingo DW, Cancio L, Bird P, Jordan B, Goodwin CW. Thermally injured patients are at significant risk for thromboembolic complications. J Trauma. 2001;50:495–9. doi: 10.1097/00005373-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Purdue GF, Hunt JL. Pulmonary emboli in burned patients. J Trauma. 1988;28:218–20. doi: 10.1097/00005373-198802000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Rue LW, 3rd, Cioffi WG, Jr, Rush R, McManus WF, Pruitt BA., Jr Thromboembolic complications in thermally injured patients. World J Surg. 1992;16:1151–4. doi: 10.1007/BF02067085. discussion 5. [DOI] [PubMed] [Google Scholar]
- 12.Wahl WL, Brandt MM. Potential risk factors for deep venous thrombosis in burn patients. J Burn Care Rehabil. 2001;22:128–31. doi: 10.1097/00004630-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th ed) 2008;133:381S–453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 14.Bushwitz J, LeClaire A, He J, Mozingo D. Clinically significant venous thromboembolic complications in burn patients receiving unfractionated heparin or enoxaparin as prophylaxis. J Burn Care Res. 2011;32:578–82. doi: 10.1097/BCR.0b013e31822dc3c7. [DOI] [PubMed] [Google Scholar]
- 15.Lin H, Faraklas I, Cochran A, Saffle J. Enoxaparin and antifactor Xa levels in acute burn patients. J Burn Care Res. 2011;32:1–5. doi: 10.1097/BCR.0b013e318204b346. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Faraklas I, Saffle J, Cochran A. Enoxaparin dose adjustment is associated with low incidence of venous thromboembolic events in acute burn patients. J Trauma. 2011;71:1557–61. doi: 10.1097/TA.0b013e31823070f9. [DOI] [PubMed] [Google Scholar]
- 17.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: An analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–7. doi: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 18.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–8. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(Suppl 3):304–12. [PubMed] [Google Scholar]
- 20.Scales DC, Riva-Cambrin J, Le TL, Pinto R, Cook DJ, Granton JT. Prophylaxis against venous thromboembolism in neurointensive care patients: Survey of Canadian practice. J Crit Care. 2009;24:176–84. doi: 10.1016/j.jcrc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA. 1995;274:335–7. [PubMed] [Google Scholar]
- 22.Cade JF. High risk of the critically ill for venous thromboembolism. Crit Care Med. 1982;10:448–50. doi: 10.1097/00003246-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Marik PE, Andrews L, Maini B. The incidence of deep venous thrombosis in ICU patients. Chest. 1997;111:661–4. doi: 10.1378/chest.111.3.661. [DOI] [PubMed] [Google Scholar]
- 24.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ., 3rd Trends in the incidence of venous thromboembolism during pregnancy or postpartum: A 30-year population-based study. Ann Intern Med. 2005;143:697–706. doi: 10.7326/0003-4819-143-10-200511150-00006. [DOI] [PubMed] [Google Scholar]
- 25.James AH. Pregnancy and thrombotic risk. Crit Care Med. 2010;38:S57–63. doi: 10.1097/CCM.0b013e3181c9e2bb. [DOI] [PubMed] [Google Scholar]
- 26.Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16:153–68. doi: 10.1016/s1521-6926(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 27.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–16. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 28.James AH, Tapson VF, Goldhaber SZ. Thrombosis during pregnancy and the postpartum period. Am J Obstet Gynecol. 2005;193:216–9. doi: 10.1016/j.ajog.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 29.Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;1412(suppl):e691S–736. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C, Lee AY. Malignancy and venous thrombosis in the critical care patient. Crit Care Med. 2010;38:S64–70. doi: 10.1097/CCM.0b013e3181c9e26f. [DOI] [PubMed] [Google Scholar]
- 31.Brown A. Preventing venous thromboembolism in hospitalized patients with cancer: Improving compliance with clinical practice guidelines. Am J Health Syst Pharm. 2012;69:469–81. doi: 10.2146/ajhp110187. [DOI] [PubMed] [Google Scholar]
- 32.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Priglinger U, Delle Karth G, Geppert A, Joukhadar C, Graf S, Berger R, et al. Prophylactic anticoagulation with enoxaparin: Is the subcutaneous route appropriate in the critically ill? Crit Care Med. 2003;31:1405–9. doi: 10.1097/01.CCM.0000059725.60509.A0. [DOI] [PubMed] [Google Scholar]
- 34.Alban S. Adverse effects of heparin. Handb Exp Pharmacol. 2012:207211–63. doi: 10.1007/978-3-642-23056-1_10. [DOI] [PubMed] [Google Scholar]
- 35.Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e495S–530. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dager WE, White RH. Treatment of heparin-induced thrombocytopenia. Ann Pharmacother. 2002;36:489–503. doi: 10.1345/aph.1A204. [DOI] [PubMed] [Google Scholar]
- 37.Selleng K, Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia in intensive care patients. Crit Care Med. 2007;35:1165–76. doi: 10.1097/01.CCM.0000259538.02375.A5. [DOI] [PubMed] [Google Scholar]
- 38.Kiser TH, Fish DN. Evaluation of bivalirudin treatment for heparin-induced thrombocytopenia in critically ill patients with hepatic and/or renal dysfunction. Pharmacotherapy. 2006;26:452–60. doi: 10.1592/phco.26.4.452. [DOI] [PubMed] [Google Scholar]
- 39.Kiser TH, Mann AM, Trujillo TC, Hassell KL. Evaluation of empiric versus nomogram-based direct thrombin inhibitor management in patients with suspected heparin-induced thrombocytopenia. Am J Hematol. 2011;86:267–72. doi: 10.1002/ajh.21955. [DOI] [PubMed] [Google Scholar]
- 40.Levi M, Eerenberg E, Kamphuisen PW. Bleeding risk and reversal strategies for old and new anticoagulants and antiplatelet agents. J Thromb Haemost. 2011;9:1705–12. doi: 10.1111/j.1538-7836.2011.04432.x. [DOI] [PubMed] [Google Scholar]
- 41.Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. N Engl J Med. 2011;365:2039–40. doi: 10.1056/NEJMc1111095. [DOI] [PubMed] [Google Scholar]
- 42.Kaatz S, Kouides PA, Garcia DA, Spyropolous AC, Crowther M, Douketis JD, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87(Suppl 1):S141–5. doi: 10.1002/ajh.23202. [DOI] [PubMed] [Google Scholar]
- 43.Pradaxa [package insert] Ridgefield, CT: Boehringer Ingelhein Pharmaceuticals, Inc; 2012. Product information for Pradaxa. Boehringer Ingelheim Pharmaceuticals IR, CT 06877. 2012. [Google Scholar]
- 44.Xarelto [package insert] 2011. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011. Product information for Xarelto. Janssen Pharmaceuticals IT, NJ 08560. [Google Scholar]
- 45.Marlu R, Hodaj E, Paris A, Albaladejo P, Crackowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217–24. doi: 10.1160/TH12-03-0179. [DOI] [PubMed] [Google Scholar]
- 46.Conner CS, Rumack BH, Watanabe AS. DRUGDEX. Englewood, Colorado: Micromedex Inc microfiches; Dabigatran Etexilate Mesylate In DRUGDEX® System Thomson Reuters (Healthcare) Inc; [Last accessed on 2012 Aug 24]. University of Colorado Medical Center. Available from: http://www.thomsonhc.com . [Google Scholar]
- 47.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 48.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–23. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 49.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, et al. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Guidelines for the management of severe traumatic brain injury. V. Deep vein thrombosis prophylaxis. J Neurotrauma. 2007;24(Suppl 1):S32–6. doi: 10.1089/neu.2007.9991. [DOI] [PubMed] [Google Scholar]
- 50.Lee CJ, Ansell JE. Direct thrombin inhibitors. Br J Clin Pharmacol. 2011;72:581–92. doi: 10.1111/j.1365-2125.2011.03916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 52.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: The RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–85. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 53.Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, et al. RE-MOBILIZE Writing Committee. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 54.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–56. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 55.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 56.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 57.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet. 2010;375:807–15. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 58.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–98. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 59.Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–77. doi: 10.1056/NEJMoa1110899. [DOI] [PubMed] [Google Scholar]
- 60.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 61.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 62.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 63.Turpie AG, Lassen MR, Eriksson BI, Gent M, Berkowitz SD, Misselwitz F, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–53. doi: 10.1160/TH10-09-0601. [DOI] [PubMed] [Google Scholar]
- 64.Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]


