Abstract
Recent evidence suggests that self/non-self discrimination exists among roots; its mechanisms, however, are still unclear. We compared the growth of Buchloe dactyloides cuttings that were grown in the presence of neighbors that belonged to the same physiological individual, were separated from each other for variable periods, or originated from adjacent or remote tillers on the same clone. The results demonstrate that B. dactyloides plants are able to differentiate between self and non-self neighbors and develop fewer and shorter roots in the presence of other roots of the same individual. Furthermore, once cuttings that originate from the very same node are separated, they become progressively alienated from each other and eventually relate to each other as genetically alien plants. The results suggest that the observed self/non-self discrimination is mediated by physiological coordination among roots that developed on the same plant rather than allogenetic recognition. The observed physiological coordination is based on an as yet unknown mechanism and has important ecological implications, because it allows the avoidance of competition with self and the allocation of greater resources to alternative functions.
Keywords: Buchloe dactyloides, competition, development, phenotypic plasticity, physiological coordination
Virtually all multicellular organisms possess recognition systems that allow them to distinguish self from non-self with precision (1-4). For example, self/non-self recognition systems enable the prevention of inbreeding by self-pollination in plants (4-5) as well as the cooperation between kin in various clonal marine invertebrates (6-8). Because competition entails allocation of limiting resources to nonreproductive functions (9-11), natural selection is expected to favor mechanisms that minimize wasteful competition among parts of the same individual (12), clonemates, and kin (13).
Recent evidence suggests that roots are able to alter their growth according to the presence or absence of specific neighbors (14) and to segregate spatially in “territories” (15). The evidence also suggests the existence of two different types of self/non-self discrimination among roots. Mahall and Callaway (16-18) found that the desert shrub Ambrosia dumosa differentially avoids root elongation in the presence of roots of other Ambrosia individuals. A somewhat contrasting response was demonstrated in soy (Glycine max, ref. 19), strawberry (Fragaria chiloensis, ref. 20) and garden pea (Pisum sativum, ref. 21), whereby roots avoid competition with other roots on the same plant. These findings raise the following questions regarding self/non-self discrimination in roots: (i) How common is the ability of plants to avoid competition with self? (ii) What mechanism enables plants to discriminate self from non-self neighbors? Is it based on allogenetic recognition and extraordinarily high genetic specificity of individuals within the population, such as in plant reproductive self-incompatibility systems (e.g., ref. 5) or mammal immune systems (e.g., ref. 22), or on physiological coordination among roots that are part of the same plant (18, 23-25)? Although coordination among organs of the same plant is well documented for correlative inhibition among shoots (e.g., refs. 23 and 26-28), roots (29-30), as well as reciprocal growth induction of shoots and roots (24, 31), it is usually not studied in the context of self/non-self interactions. However, the results of previous studies suggest the involvement of physiological coordination in self/non-self root discrimination (18, 20, 21). The prevention of contact inhibition between roots of the same A. dumosa plant (18) as well as the avoidance of self competition between roots of F. chiloensis (20) was found to be at least partially based on physiological coordination between roots that develop on the same plant. In an earlier study, P. sativum plants were grown so that they had two roots and two shoots that could be either longitudinally separated into two genetically identical but physiologically distinct individuals or left intact. Root growth was significantly greater in the presence and the direction of roots that belonged to different plants, regardless of their genetic identity. However, Pisum cuttings originated from different individuals had the greatest root growth (21). These findings demonstrated that the avoidance of self competition in roots was at least partially based on physiological coordination between roots that develop on the same plant but that the involvement of allogenetic recognition mechanisms could not be ruled out (21).
The purpose of the present study is to investigate the presence of self/non-self discrimination in the clonal perennial grass Buchloe dactyloides and test the hypothesis that self/non-self discrimination in roots is based solely on physiological coordination between roots that develop on the same plant, rather than allogenetic recognition. This physiological mechanism is especially appealing in the case of root interactions because it is highly unlikely that roots are able to both exude and perceive complex organic “identifying molecules,” such as glycoproteins, which are known from self-incompatibility systems (5), because they are expected to be susceptible to rapid decomposition by soil bacteria and fungi (32).
We predicted that root growth should decrease in the presence of other roots of the same plant and increase in the presence of roots that belong to physiologically alien plants. We tested our hypotheses by comparing root growth in genetically identical plant pairs that were separated from each other for variable periods or originated from adjacent or remote tillers on the same clone. Accordingly, the experimental plant pairs belonged to either the same physiological individual, were separated from each other for variable periods, or originated from adjacent or remote tillers on the same clone. We predicted that root growth and allocation would not only be greater in separated than in intact roots of the same plant (21), but that they would be positively correlated to the separation time of the roots from each other. Similarly, although shoots that originate from tightly adjacent nodes were expected to be highly coordinated with each other, more remote shoots were expected to show decreased coordination. We therefore predicted that root growth would be relatively greater in plant pairs that originated from distant compared to adjacent tillers on the same clone.
The results indicate that B. dactyloides is able to discriminate between “self” and “non-self” neighbors and that the physiological identity is more important than the genetic identity of neighboring roots for the determination of root growth.
Materials and Methods
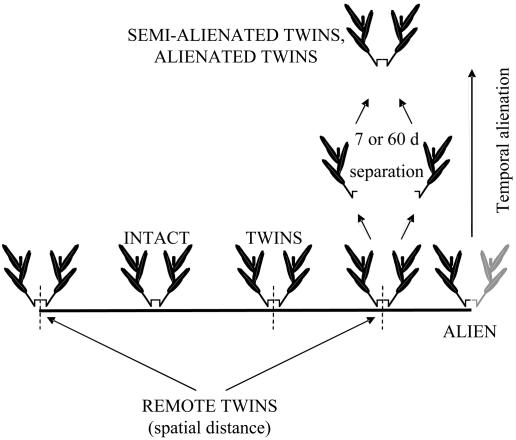
We used cuttings of buffalo grass B. dactyloides (Poaceae) collected from the lawns of the Sede Boker campus of Ben-Gurion University of the Negev, Israel. The plant is a stoloniferous dominant turfgrass native to the shortgrass prairies of the North American Great Plains (33). In this species, the nodes are spaced along the stolon in tight pairs that often develop highly symmetrical lateral branches on opposite sides of the stolon (Fig. 1). We used such symmetrical two-branched cuttings that were either longitudinally severed into two genetically identical but physiologically separate individuals or left intact (Fig. 1). The cuttings readily developed adventitious roots from the nodes immediately upon planting.
Fig. 1.
B. dactyloides cuttings with two equal halves were planted INTACT, or as TWINS, ALIEN, SEMI-ALIENATED, and ALIENATED TWINS (temporal alienation experiment), or REMOTE TWINS (spatial distance experiment). Root growth could be compared between treatments in which the plants had different physiological and genetic identities.
Temporal Alienation. Root growth was observed after the plants were grown in the presence of neighbors of variable physiological and genetic identities. The plants were assigned to one of the following treatments (Fig. 1): INTACT, in which the two plant halves remained physiologically integrated; TWINS, in which the two plant halves originated from the same node but were physiologically separate immediately before the onset of the experiment; SEMI-ALIENATED and ALIENATED, in which the two plant halves were grown under the same growth conditions but in separate pots for 7 and 60 days, respectively, before growing together; and ALIEN, in which the two plant halves originated from two different (physiologically and genetically alien) clones.
To ensure that plants of different treatments would start the experiment at the same age and developmental status, they were prepared by harvesting fresh cuttings from their mother plants at the end of the alienation period. The plants were planted after growing the fresh cuttings in tap water for 7 days before the last segment of the experiment, in which they were grown together (Fig. 1). The experiment started in January 23, 2002, and was conducted in the ecological growth facility at the Sede Boker campus of Ben Gurion University of the Negev. This structure has sides that are automatically controlled to open during daylight, when it is not raining, and protects against extreme temperatures, rain, and morning dew. Measurements were made 52 days after plants (INTACT) or plant halves (all other treatments) started growing together, when roots started to grow through the drainage holes of the pots.
Spatial Distance. Root growth was observed after the plants were grown in the presence of adjacent or remote shoots on the same clone. The plants were assigned to one of the following treatments (Fig. 1): INTACT, TWINS, and ALIEN, as described above, and REMOTE TWINS, in which the two plant halves originated from two different nodes located three internodes apart on the same stolon (Fig. 1).
Because this experiment was conducted in a different season and under slightly different environmental conditions than the temporal alienation experiment, it had to be handled separately and a complete set of the treatments had to be repeated. The experiment started in June 16, 2002, and was conducted in a shade house (70% black knitted shadecloth, Polysack, Nir Yitchak, Israel) at the Sede Boker campus of Ben Gurion University of the Negev, Israel. Measurements were made 20 days after the experiment started. The difference in the length of the two experiments reflected the seasonal differences in growth rates of the plants.
Growth Conditions, Measurements, and Statistical Analyses. The cuttings were prepared so that they had two equal, 30- to 50-mm-long, 1- to 1.5-mm-thick shoots, bearing five unfurled leaves each. The initial length variation allowed between the two shoots of the same plant (or plant halves) was <5 mm. Each plant (or set of two plant halves) was planted in the middle of a 360-ml well drained pot filled with fine vermiculite. Pots were arranged in blocks based on the initial length of their shoots (plants belonging to the same block had equal initial stem and longest leaf lengths; ±5 mm). Each block consisted a full replication of all of the treatments (number of blocks equaled the number of replications), and the position of each pot within each block and the position of each replication block on the bench were assigned randomly. The plants were alternately watered with deionized water and 0.1 strength Hoagland solution (34) every other day. During planting, special care was given to ensure that the alignment of the two halves of severed plants would be similar to that of INTACT plants.
Seminal and lateral roots were counted if they were longer than 1 and 0.5 mm, respectively. Root length was recorded by scanning the harvested roots using delta-t scan software (Delta-T, Cambridge, U.K.). Because a large proportion of the fine roots was translucent, the roots were stained with Brilliant Cresyl blue before scanning. Dry biomass was estimated after drying the plants at 70°C in a ventilated oven for 3 days.
Statistical analyses were conducted by using systat 10.0 (35). All dependent variables were tested for normality and log-transformed when they did not meet the assumptions of parametric statistics. We used one-way ANOVAs to test differences among treatment averages. The effect of the initial cutting size was included as a covariate, but because it was not significant in any analysis, it is not reported. Differences between individual treatment averages were further estimated by using Fisher's least significant difference comparisons.
Results
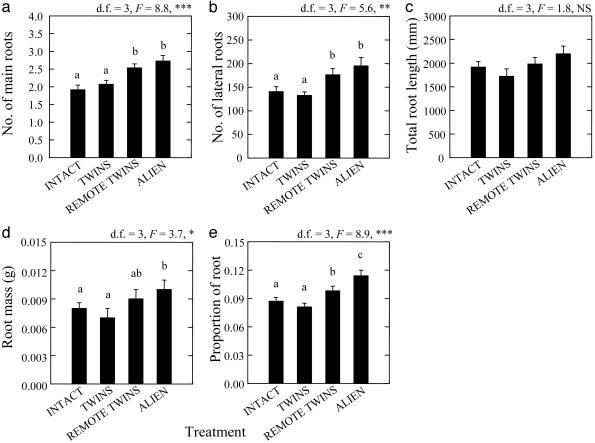
Temporal Alienation. The results indicate that the identity of the neighboring roots could be important for the determination of root growth. Although no significant differences were found between INTACT and TWINS, root growth was 39-142% greater for all developmental variables in ALIEN and ALIENATED TWINS than for INTACT and TWINS plants, with intermediate scores for SEMI-ALIENATED TWINS (Fig. 2). Because both plant halves in all but the ALIEN treatment originated from the same genetic individual, the increased root growth in ALIENATED and SEMI-ALIENATED TWINS and the similar root growth in ALIENATED TWINS and ALIEN (Fig. 2) indicated that the observed self/non-self discrimination was based on physiological coordination among roots that developed on the same plant, rather than allogenetic recognition.
Fig. 2.
Self/non-self discrimination in B. dactyloides plants as expressed by means (n = 16-18 per treatment) ± SE of number of main (a) and lateral (b) roots, total root length (c), root mass (d), and proportion of total plant biomass allocated to roots (e). Shown are roots developed in the presence of another root on the same plant (INTACT), roots that originated from the same node but were physiologically separate immediately before the onset of the experiment (TWINS), roots that originated from the same node but were physiologically separate for 7 (SEMI-ALIENATED) or 60 days (ALIENATED) before the onset of the experiment, or roots of a different clone (ALIEN). Results of one-way ANOVAs appear above each figure. Bars that share the same superscript were not significantly different (P > 0.05) in least significant difference comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Spatial Distance. The results indicate that the developmental background of the plants could be an important determinant of root growth. Root growth was 16-46% greater for all developmental variables in ALIEN than in INTACT and TWINS plants (Fig. 3). When the two plant halves originated just three nodes apart on the same stolon (REMOTE TWINS), their root growth was 15-29% greater for different root growth variables compared to TWIN plants (Fig. 3), suggesting that the physiological coordination among roots that develop on the same plant depends on their proximity.
Fig. 3.
Self/non-self discrimination in B. dactyloides plants as expressed by means (n = 35-37 per treatment) ± SE of number of main (a) and lateral (b) roots, total root length (c), root mass (d), and proportion of total plant biomass allocated to roots (e). Shown are roots developed in the presence of another root on the same plant (INTACT), roots that originated from the same node but were physiologically separate immediately before the onset of the experiment (TWINS), roots that originated from two different nodes located three internodes apart on the same stolon (REMOTE TWINS), or roots of a different clone (ALIEN). Results of one-way ANOVAs appear above each figure. Bars that share the same superscript were not significantly different (P > 0.05) in least significant difference comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS = P > 0.05.
Discussion
The results demonstrate that B. dactyloides plants are able to respond differently to self and non-self neighbors and develop fewer and shorter roots in the presence of other roots of the same plant. The increased root growth in ALIENATED TWINS and REMOTE TWINS compared with INTACT and TWINS and the similar root growth in ALIENATED TWINS and ALIEN (Figs. 2 and 3) suggest that the observed self/non-self discrimination was mediated by physiological coordination among roots that developed on the same plant rather than allogenetic recognition. Furthermore, the consistent similarity in root growth in INTACT and TWINS (also found in P. sativum, ref. 21) and the similar root growth in ALIENATED TWINS and ALIEN plants (Figs. 2 and 3) imply that the coordination among roots that develop on the same plant is mediated by signals that are coordinated in organs of the same plant even some time after their separation. However, once cuttings that originate from the very same node are separated, they become progressively alienated to each other and eventually relate to each other as true ALIEN plants. Additionally, the physiological coordination among organs of the same individual plant is limited to relatively short distances (Fig. 3). This finding is consistent with the ecological rationale of physiological coordination among organs of the same plant: the avoidance of competition is expected to be the most meaningful among closely spaced roots of the same plant or ramets of the same clone that are potentially in direct competitive interactions with each other (21).
The recently discovered physiological self/non-self discrimination phenomena in roots (18, 20, 21) imply the involvement of an as yet unexplored type of signaling system. The fact that identical genetic individuals perceive each other as “aliens” after a relatively short separation period suggests that the mechanism involves nonspecific signaling vectors that convey individually specific signals. Although at this stage the particulars of the mechanism can only be speculated, we hypothesize that the physiological specificity of plants is mediated by internal oscillations of hormones such as auxin and cytokinins (e.g., ref. 36) and/or electricity (37) that are perceived by roots through the soil. Such signals are known to be highly dynamic in time (38), and thus individually unique. Such signals can be potentially perceived and monitored both within plants and outside roots (e.g., refs. 39 and 40). Accordingly, the perception of “self” is based on resonant amplification (41) of oscillatory signals in the vicinity of other roots of the same plant. Such resonant amplification could not occur in roots that are not oscillating with the same rhythm (21). Although oscillations can be coordinated within short distances within the plant (INTACT) or its recently severed parts (TWINS), it is expected that they would deteriorate after longer separation between previously intact organs (ALIENATED TWINS) or remote organs on the same plant (REMOTE TWINS), even merely due to the progressive accumulation of chance differences in hormonal/electrical oscillations between the separated plants.
The avoidance of wasteful allocation of resources to competition with self may enable plants to allocate more to other functions, including aboveground organs (Figs. 2e and 3e), and reproduction (19, 42). Thus, it is predicted that physiological self/non-self mechanisms that enable plants to avoid competition with self are common among higher plants and other plantae organisms (e.g., algae, lichens), where the operation of genetically based recognition systems is restricted.
Acknowledgments
We thank Hagit Ram for technical assistance and Peter Alpert, Tsvi Sachs, Simon Barak, Omer Falik, and Elli Groner for stimulating discussions and helpful comments on early versions of the manuscript. This study was supported in part by a research grant from the Israel Science Foundation (to A.N.). This is publication no. 411 of the Mitrani Department of Desert Ecology.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Grosberg, R. K. (1988) Q. Rev. Biol. 63, 377-412. [Google Scholar]
- 2.Frank, U., Bak, R. P. M. & Rinkevich, B. (1996) J. Exp. Mar. Biol. Ecol. 197, 191-201. [Google Scholar]
- 3.Penn, D. J. & Potts, W. K. (1999) Am. Nat. 153, 145-164. [DOI] [PubMed] [Google Scholar]
- 4.Richman, A. (2000) Mol. Ecol. 9, 1953-1963. [DOI] [PubMed] [Google Scholar]
- 5.Dixit, R. & Nasrallah, J. B. (2001) Plant Physiol. 125, 105-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosberg, R. K. & Quinn, J. F. (1989) Evolution (Lawrence, Kans.) 43, 504-515. [DOI] [PubMed] [Google Scholar]
- 7.Sabbadin, A. (1994) Anim. Biol. 3, 157-163. [Google Scholar]
- 8.Chadwick-Furman, N. & Rinkevich, B. (1994) Coral Reefs 13, 57-63. [Google Scholar]
- 9.Novoplansky, A., Sachs, T., Cohen, D., Bar, R., Budenheimer, J. & Reisfeld, R. (1990) Solar Energy Mat. 21, 17-23. [Google Scholar]
- 10.Dudley, S. A. & Schmitt, J. (1996) Am. Nat. 147, 445-465. [Google Scholar]
- 11.Weinig, C. (2000) Evolution (Lawrence, Kans.) 54, 441-451. [DOI] [PubMed] [Google Scholar]
- 12.Kimura, M. & Simbolon, H. (2002) Ecol. Res. 17, 323-338. [Google Scholar]
- 13.Grosberg, R. K. & Hart, M. W. (2000) Science 289, 2111-2114. [DOI] [PubMed] [Google Scholar]
- 14.Krannitz, P. G. & Caldwell, M. M. (1995) Flora 190, 161-167. [Google Scholar]
- 15.Schenk, H. J., Callaway, R. M. & Mahall, B. E. (1999) Adv. Ecol. Res. 28, 145-180. [Google Scholar]
- 16.Mahall, B. E. & Callaway, R. M. (1991) Proc. Natl. Acad. Sci. USA 88, 874-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahall, B. E. & Callaway, R. M. (1992) Ecology 73, 2145-2151. [Google Scholar]
- 18.Mahall, B. E. & Callaway, R. M. (1996) Am. J. Bot. 83, 93-98. [Google Scholar]
- 19.Gersani, M., Brown, J. S., O'Brien, E. E., Maina, G. M. & Abramsky, Z. (2001) J. Ecol. 89, 660-669. [Google Scholar]
- 20.Holzapfel, C. & Alpert, P. (2003) Oecologia 134, 72-77. [DOI] [PubMed] [Google Scholar]
- 21.Falik, O., Reides, P., Gersani, M. & Novoplansky, A. (2003) J. Ecol. 91, 525-531. [Google Scholar]
- 22.Beck, S. Geraghty, D., Inoko, H. & Rowen, L. (1999) Nature 401, 921-923.10553908 [Google Scholar]
- 23.Snow, R. (1931) Proc. R. Soc. London Ser. B 108, 305-316. [Google Scholar]
- 24.Sachs, T. (1991) Pattern Formation in Plant Tissues (Cambridge Univ. Press, Cambridge, U.K.).
- 25.Sachs, T. & Novoplansky, A. (1997) in The Ecology and Evolution of Clonal Growth in Plants, eds. de Kroon, H. & van Groenendael, J. M. (Backhuys, Leiden, The Netherlands), pp. 55-78.
- 26.Novoplansky, A., Cohen, D. & Sachs, T. (1989) Physiol. Plant 77, 136-140. [Google Scholar]
- 27.Aarssen, L. W. (1995) Oikos 74, 149-156. [Google Scholar]
- 28.Novoplansky, A. (2003) New Phytol. 160, 111-118. [DOI] [PubMed] [Google Scholar]
- 29.Snow, R. (1938) New Phytol. 37, 173-185. [Google Scholar]
- 30.Gersani, M. & Sachs, T. (1992) Plant Cell Environ. 15, 463-469. [Google Scholar]
- 31.Srivastava, L. M. (2001) Plant Growth and Development (Academic, New York).
- 32.Kjoller, A. & Struwe, S. (1989) Opera-Botanica 100, 147-152. [Google Scholar]
- 33.Mintenko, A. S., Smith, S. R., Cattani, D. J. (2002) Crop Sci. 42, 2018-2024. [Google Scholar]
- 34.Hoagland, D. R. & Arnon, I. (1950) The Water Culture Method for Growing Plants Without Soil (California Agriculture Experiment Station, Berkeley), Circular no. 347.
- 35.SPSS, Inc. (2000) systat for windows (SPSS, Inc., Chicago), Version 10.0.
- 36.Ortuno, A., Sanchez, B. J., Moral, J. R., Acosta, M. & Sabater, F. (1990) Physiol. Plant 78, 211-217. [Google Scholar]
- 37.Shepherd, V. A. (1999) Curr. Sci. 77, 101-107. [Google Scholar]
- 38.Minorsky, P. V. (2001) Plant Physiol. 126, 928-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisenseel, M. H., Becker, H. F. & Ehlgoetz, J. G. (1992) Plant Physiol. 100, 16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer, A. & Weisenseel, M. H. (1997) Plant Physiol. 114, 989-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walleczek, J. (2000) in Self-Organized Biological Dynamics and Nonlinear Control: Toward Understanding Complexity, Chaos and Emergent Function in Living Systems, ed. Walleckek, J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 1-12.
- 42.Maina, G. G., Brown, J. S. & Gersani, M. (2002) Plant Ecol. 160, 235-247. [Google Scholar]