Abstract
Alcohol is one of the commonest illicit psychoactive substances consumed globally and is the world's third largest risk factor for disease and disability. It has been reported to have multiple effects on the hypothalamo-pituitary-thyroid axis and the functioning of the thyroid gland. It has been reported to cause direct suppression of thyroid function by cellular toxicity, and indirect suppression by blunting thyrotropin-releasing hormone response. It causes a decrease of peripheral thyroid hormones during chronic use and in withdrawal. Alcohol use may also confer some protective effect against thyroid nodularity, goiter, and thyroid cancer. This article presents a review of the clinically relevant effects of alcohol on the functioning of the thyroid gland and also discusses the effect of medication used in treatment of alcohol dependence on thyroid function.
Keywords: Alcohol, hypothalamus-pituitary-thyroid axis, thyroid, tobacco
INTRODUCTION
Alcohol is the world's third largest risk factor for disease and disability; causing at least 60 types of diseases and contributing to 200 others.[1] Globally, high-income countries of the developed world have the highest alcohol consumption,[1] with 50.1% of the US population (adults over 18 years of age) being current regular drinkers.[2] In contrast, findings from the Indian National Survey suggest that around 21% of adult males in the country are current drinkers. Yet, the apparent advantage in proportion is lost in the sheer number of population, as even a conservative estimate puts the number of alcohol users in the country at around 61 million, of whom around 10 million have alcohol dependence.[3] In view of the magnitude of the problem, it becomes necessary for us to understand the effect of alcohol on various body systems.
Indeed, alcohol affects almost all organs and systems of the human body.[4] The hypothalamo-pituitary axis, through the chemicals of dopamine and serotonin, is intricately linked with the development and continuation of dependence of all chemical substances,[5] and therefore it is not surprising to find that most substances of abuse in return have multiple effects on the hypothalamo-pituitary-thyroid (HPT) axis and thyroid function. Of the multitude of drug – endocrine interactions reported, this review focuses on the effect of alcohol on the HPT axis. The paper is presented as a critical clinical overview for readers, and is by no means an exhaustive systematic review because of wide scope of the subject.
LITERATURE REVIEW
Databases of Medline (Pubmed), PsycINFO, and Scopus were searched from inception till July 2012. The initial decision of the authors was to limit all searches to English language original articles on human subjects, excluding all reviews, systematic reviews, and meta-analyses. The MeSH terms “alcohol-related disorders,” “alcohol-induced disorders,” “thyroid diseases,” “thyroid gland”, and “thyroid function tests” were used to build primary search queries. For the completeness of understanding, we inform the readers that searching with the term “alcohol” or the MeSH “ethanol” results in a large number of articles, primarily dealing with the use of alcohol for thyroid nodule ablation and in thyroid surgeries, and were therefore avoided in all our searches.
To supplement the data gathered from the first round of searches, we searched with specific terms in all the above databases and Google Scholar. Such secondary search terms for thyroid functions were: thyroid, neuroendocrine, endocrine, thyroid stimulating hormone (TSH), thyrotropin-releasing hormone (TRH), T3, T4, thyroid stimulating hormone, thyrotropin-releasing hormone, thyrotropin, thyroxine, triiodothyronine, hypothalamic-pituitary axis, hypothalamo-pituitary axis, and hormone. Each of these secondary terms was combined with terms for alcohol to generate the search results.
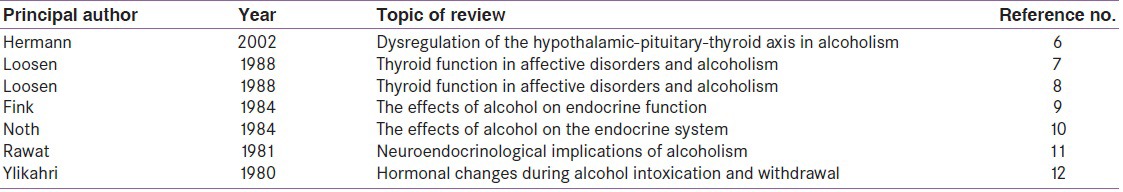
We also identified previous reviews and systematic reviews on the topic, which were rare, and manually searched all cross-references for further studies [Table 1]. Last but not least, while writing we were surprised to find paucity of human studies on fetal and neonatal thyroid functioning in relation to maternal alcohol use. There was also lack of consensus in studies as regards the effect of alcohol on the levels of peripheral thyroid hormone. For the purpose of completeness, we went back to our original database and hand searched articles related to these particular topics for inclusion.
Table 1.
Reviews and systematic reviews on the effect of alcohol on thyroid function
All abstracts were individually considered by both the reviewers for inclusion into the study. For the included abstracts, full texts were subsequently procured. Figure 1 depicts the search strategy and the studies included.
Figure 1.
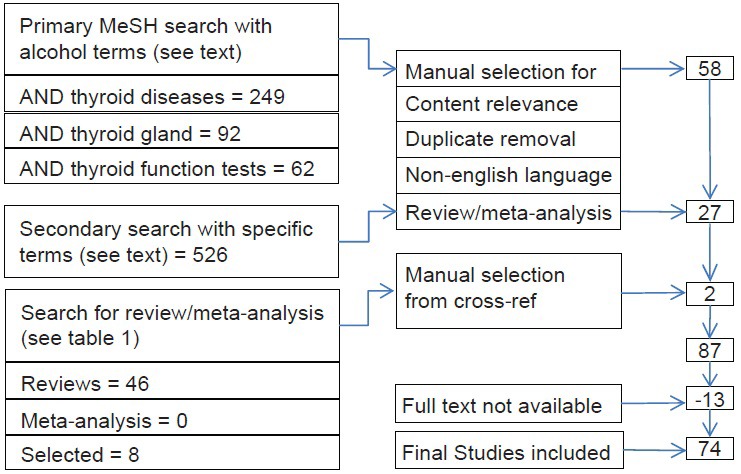
Search methodology
A majority of the selected studies were descriptive and often contradictory, suggesting a complex interrelation of alcohol with the thyroid function. Randomized control trials (RCTs) were rare though comparison groups were often used. Lack of consensus in clinical findings also hampered the development of theoretical models of causation. Theoretical constructs for how these changes in thyroid functioning are brought about by alcohol were rare. When present, they were often untested and offered as suppositions by authors. Despite these limitations, clinically important patterns of interaction between alcohol use and thyroid axis emerged from these studies, which this article tries to summarize.
ALCOHOL AND PERIPHERAL THYROID HORMONE LEVELS
The effect of chronic alcohol consumption on peripheral thyroid hormone (T3 and T4) levels is not settled.[6] Even in animal models, both a suppression of peripheral thyroid hormones[13,14,15] and an absence of alteration in their levels[14] have been reported.[13]
Human studies on current alcohol users are less, as most studies are conducted in detoxification programs and researchers face the practical difficulty in recruiting active drinkers. Some consensus seems to be developing for alcohol causing a moderate suppression of peripheral serum thyroxine (T4) levels with more significant suppression of triiodothyronine (T3) levels in chronic alcohol users [Table 2].[17,25] In addition, alcohol-dependent individuals may present with a “euthyroid sick syndrome,” evidenced by low levels of T3, high levels of reverse T3 (rT3), and normal levels of T4.[26,27,28] Extrapolation of baseline data from longitudinal studies on patients undergoing detoxification similarly suggests a decrease in peripheral T3 and T4 levels (or free T3, free T4) during chronic alcohol use.[29,30,31] Finally, in patients who relapsed and returned to their regular amount of alcohol consumption, re-lowering of plasma T4 concentrations supports this observation.[31]
Table 2.
Effect of alcohol on thyroid hormone levels from cross-sectional studies reporting positive results

In contrast, a wealth of data exists on the thyroid profile of alcoholics during withdrawal and abstinence. Peripheral thyroid hormones are suppressed during withdrawal and the degree of suppression of their levels has been associated with the severity of withdrawal.[18,32] This phenomenon is clinically interesting as thyroid suppression may accentuate the withdrawal dysphoria,[25,33] thereby increasing the relapse risk in alcoholism.[34]
Making clear sense of thyroid hormonal changes during abstinence is difficult as studies report conflicting results. For all phases of abstinence (early as well as late), there are always more number of studies that show normal values of peripheral thyroid hormones, as compared to studies that report abnormality. Hermann et al. reviewed 19 studies in 2002[6] and showed that 63–66% of studies reported normal findings of T3 and T4 for early abstinence (less than 3 weeks), whereas 75–83% studies reported normal findings for late (more than 3 weeks) abstinence. When considering the free fraction of the hormones (fT3/fT4), the proportion of studies reporting no abnormalities becomes as high as 88–100%. Therefore, the effect of alcohol withdrawal on the levels of peripheral thyroid hormone must be small and dependent on multiple other variables like comorbid mood state, hepatic status, preexisting thyroid profile, etc.
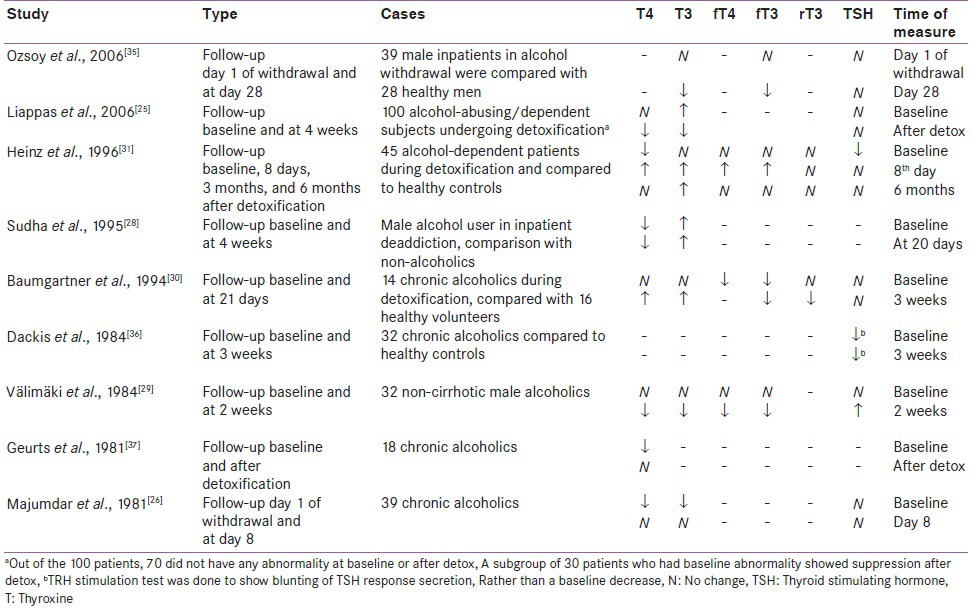
Yet, in longitudinal studies that do show abnormality [Table 3], patterns seem to emerge. One pattern is the suppression of T4 in acute withdrawal[25,28,29,30,31,37] which persisted for 2-4 weeks into abstinence.[36,38] Other studies show an increase (return to normal) of T4 levels within the first week,[31] second week,[29,37] third week,[30] or fourth week[25] after initial suppression during acute withdrawal. The levels of f T4 have been found to be similarly suppressed in acute withdrawal.[16] Total (T3) and free triiodothyronine (fT3) levels also showed a decrease in patients who had abstained from alcohol for less than 3 weeks, but mostly returned to normal in the long term.[22,23,24,30] Only one study by Ozsoy et al. in 2006 reported a reverse pattern with no change of f T3 and f T4 in early (1 day after cessation) withdrawal, but with a significant suppression in late withdrawal (28th day of abstinence), which is in direct contradiction to the reports by others.[35]
Table 3.
Effect of alcohol on thyroid hormone levels from longitudinal studies reporting positive results
ALCOHOL AND TRH ABNORMALITIES
In contrast to the conflicting fluctuations in peripheral thyroid hormone levels, a blunting of the plasma TSH response after stimulation with TRH has been reported more consistently in patients of alcohol dependence when compared to healthy control subjects [Tables 2 and 3]. The TSH blunting was more frequent in early abstinence for less than 3 weeks,[29] but was also observed after longer period of abstinence in some studies. TSH blunting also correlated positively with the severity of withdrawal symptoms in early abstinence, but not after 5-8 weeks.[18]
Although a statistically significant abnormality of the TRH response was shown in most of these studies, only 20–35% of all assessed patients showed blunting of the TRH response.[18,19,21,29,36] It is not currently known as to why only some alcoholics have TRH response abnormality. Loosen et al. in 1993[39] classified patients with TRH abnormality according to the classification of Cloninger (1987), but failed to show association with Type II alcoholism, a subtype of alcoholism with a high hereditary component.[40] Other studies too explored the possible genetic contribution for altered TRH tests by focusing on patients with a positive family history of alcoholism or on high-risk children of alcoholic fathers.[41,42] However, none of these studies found a possible genetic contribution.
Co-occurring liver cirrhosis in alcoholism was also considered as a possible contributor for the altered TRH response by its effect on the HPT axis. But a review by Loosen et al. in 1992, looking into this issue, found that while 26% patients without liver disease showed a reduced TRH response, only one of the six studies on alcohol-dependent patient with liver disease reported abnormality in TSH response. The authors, therefore, suggested that TRH response dysfunction in alcoholism cannot be attributed simply to liver cirrhosis.[6,43]
ALCOHOL AND THYROID VOLUME
Alcohol is known to have a direct toxic effect on thyroid cells, which is used therapeutically in ethanol ablation therapy of thyroid nodules.[35,44] Alcohol-induced direct toxicity may account for the reduction in the thyroid volume in alcohol-dependent patients (as compared to healthy controls) as seen in multiple ultrasound studies.[22,24,45] The severity of thyroid fibrosis in alcoholics correlates with the duration and total dose of alcohol use rather than peak blood levels, as studies show chronic alcohol users to have more fibrosis than acute binge drinkers. The reduced thyroid size is accompanied by a reduction in T3 and f T3 concentrations with normal T4, f T4, and TSH values in most studies. Although alcohol causes similar hepatic fibrosis by direct toxicity, liver damage does not seem to be a contributor to the thyroid fibrosis. A significant reduction in thyroid volume has been reported in ultrasound studies in alcohol-dependent patients without liver cirrhosis when compared to healthy controls. Additionally, patients with non-alcoholic liver cirrhosis show no differences in thyroid volume when compared to controls. Thus, the toxic effect of alcohol on thyroid appears to happen independent of liver damage.[22]
The toxic effect of alcohol on the thyroid may also confer some benefit and it has been suggested that alcohol consumption may be protective for the development of goiter. Knudsen et al. in 2001, studying 4649 healthy subjects, found association of smaller thyroid volume and lower prevalence of solitary nodules with increasing levels of alcohol consumption.[45] In contrast, a recent study found an increase in thyroid volume in chronic alcohol users.[16] The thyroid volume increase was dependent on the dose of alcohol consumed and independent of the associated smoking habits. The authors, however, did not postulate any biological basis for their divergent report.[16]
HYPOTHESIS ON THE MECHANISM OF THE EFFECT OF ALCOHOL ON THYROID
The fact that alcohol causes direct cellular toxicity on thyroid cells thereby producing thyroid suppression and reducing thyroid volume is well established. However, the mechanism of alcohol-induced reduction in TSH secretion to TRH stimulation is open to speculation. One hypothesis for this phenomenon is a possible down-regulation of the TRH receptors in the pituitary due to chronically high TRH concentrations. Physiologically, TRH levels increase when there is a decrease in peripheral concentration of thyroid hormones or where there is more demand for T3 and T4. In chronic alcoholism and in early alcohol abstinence, T3 and T4 levels are reported to be low. This pattern of peripheral low thyroid hormones in various stages of alcoholism can chronically induce a slightly elevated TRH release.[6] The increased TRH can subsequently cause feedback suppression of the TRH receptors, thereby blunting the downstream TSH secretion.
There seems to be fair amount of evidence supporting the feedback TRH receptor suppression by TRH. Chronic ethanol treatment in rats has resulted in an increase in TRH mRNA in neurons in the hypothalamus.[46,47] Additionally, experimentally increased TRH level has resulted in a down-regulation of pituitary TRH receptors in rats with both TSH secreting tumors[48,49] as well as in healthy pituitary tissue.[50] And finally, alcohol-dependent patients in abstinence, who showed a strong blunting of the TRH response, also showed high endogenous TRH levels in the CSF and vice versa,[17,51] suggesting that increased CSF TRH was the cause of the TRH response blunting.
Another hypothesis regarding thyroid dysfunction in alcoholism suggests that alcoholism results in Euthyroid Sick Syndrome (ESS), a condition characterized by reduced T3 concentrations and elevated concentrations of the thyroid hormone metabolite rT3.[25] ESS is observed in a variety of other non-thyroidal illnesses like chronic renal failure, liver dysfunction, after stress of surgery and with various medications.[52,53] However, current evidence does not favor ESS as a cause of thyroid dysfunction in alcoholism. In contrast to elevated rT3 concentrations in the ESS, rT3 concentrations are normal in alcoholics[18,29,30,31,37] and reduced T4 concentrations in alcoholism are sometimes accompanied by normal or elevated concentrations of T3, a pattern not seen in ESS.[28,36,37] Therefore, thyroid abnormality in alcoholism is not fully explained by ESS.
ALCOHOL AND THYROID CANCER
Several studies report a decrease in thyroid cancer risk with alcohol use.[54,55,56,57] A large prospective study following up 1,280,296 women with 421 new cases of thyroid cancer showed a clear reduction in risk of thyroid cancer with greater alcohol consumption,[58] and two smaller prospective studies[59,60] similarly reported a small reduction in risk, albeit not reaching statistical significance. Comorbid tobacco use, which also causes a reduction of thyroid cancer, has been a consistent confounding factor in these studies, and in a pooled analysis of 2725 thyroid cancer cases, from 14 case–control studies,[54] the statistical association of decreased cancer in alcohol consumption disappeared after adjusting for smoking.
The most conclusive evidence to date of the effect of alcohol on thyroid cancer comes from the finding of the large NIH-AARP Diet and Health Study, which prospectively followed up 490,000 participants including 292,000 men over a period of 7½ years during which 170 men and 200 women developed thyroid cancer. The study clearly showed a significant reduction of cancer risk when consuming two or more drinks per day (RR = 0.57) as compared to no drinking. Even consuming one or more drinks of beer per day was associated with a decreased risk when compared with no beer drinking, particularly in men (RR = 0.47), whereas no clear dose – response relationship was found with wine consumption. The inverse association was stronger in males, whereas in females, though trends of decrease were found, it did not reach statistical significance. The strength of the inverse association persisted even when adjusted for smoking and non-smokers had a further reduction in risk (RR = 0.33) when compared to smokers (RR = 0.78). The decrease in risk was seen in all types of thyroid cancer, although the authors observed a clearer inverse association for papillary (RR = 0.58) as compared with follicular thyroid cancer (RR = 0.86).[61]
A theoretical hypothesis for this protective phenomenon stems from the fact that TSH is known to increase the proliferation of follicular thyroid cells in laboratory studies.[62,63] Alcohol may prevent the proliferative effect of TSH on the thyroid follicle, thereby reducing the occurrence of cancer.[61]
ALCOHOL AND AUTOIMMUNE THYROID DISEASES
Alcohol is known to decrease the frequency of autoimmune disorders like rheumatoidarthritis[64] and systemic lupus erythematosus.[65] One recent prospective Danish study reported significant protective role of alcohol in preventing autoimmune hypothyroidism. The study diagnosed 140 cases of autoimmune hypothyroid from 2,027,208 person-years of observation and reported a negative association between alcohol consumption and the incidence of overt autoimmune hypothyroidism. One interesting finding of the study was that though modest to high alcohol consumption of 1-20 units/week protects from developing autoimmune overt hypothyroidism, a higher consumption of ≥21 units/week did not show the protective effect.[66]
EFFECT OF MATERNAL ALCOHOL CONSUMPTION ON FETAL THYROID HORMONAL STATUS
Though maternal consumption of alcohol results in fetal alcohol syndrome with multiple congenital defects, thyroid largely seems to remain unharmed by the in utero alcohol exposure. Most animal studies report no effect of maternal alcohol administration on neonatal and adult offspring animals,[67,68] although Hannigan et al. in 1990 reported significantly lower serum total thyroxine (T4) concentrations in young rats exposed to alcohol in utero than normal and pair-fed control rats.[69] Human studies have similarly reported that though alcohol consumption during pregnancy is related to multiple alcohol-related birth defects (ARBDs), the level of the neonatal T4 is mostly within normal limits.[70] Thus, alcohol does not seem to have significant effect on the thyroid axis in the neonate.
TREATMENT OF ALCOHOLISM AND THE THYROID AXIS
Apart from the changes that happen to the thyroid hormones during the time of alcohol withdrawal as well as early and late abstinence, treatment of alcoholism uses medications that may themselves interfere with the thyroid functioning. Benzodiazepines are most commonly used to tide over the acute withdrawal symptoms of alcohol. Chlordiazepoxide, a long-acting benzodiazepine, is the medication of choice though diazepam and clonazepam are often used, as well as lorazepam in cases of hepatic damage. Benzodiazepines as a group appear to be safe with no prominent action on the thyroid axis,[71] and animal studies report that diazepam did not affect the availability of thyroid hormone in blood circulation.[72] Conversely, the plasma concentration of diazepam seems to increase in hypothyroid rats, but remains normal in hyperthyroidism.[73]
For continuation of abstinence, disulfiram, acamprosate, and naltrexone are most commonly used. Disulfiram, an irreversible inhibitor of aldehyde dehydrogenase, is used as a deterrent agent to prevent drinking. It results in accumulation of acetaldehyde in the body subsequent to alcohol consumption, which results in unpleasant effects after drinking. Disulfiram is known to have prominent anti-thyroid action, and as early as in 1954 it was shown to react chemically with iodine forming a complex that creates a state of relative iodine deficiency and hampers the iodine trapping by the thyroid.[74] With deterrent agents becoming less popular in treatment, the research interest in disulfiram has decreased as evidenced by most papers on the drug being from 1980s.[75,76]
Of the various anti-craving agents, acamprosate, an N-Methyl-D-aspartate (NMDA) receptor antagonist, does not seem to have any effect on thyroid, whereas naltrexone, an opioid receptor antagonist, might have slight thyroid stimulating effect without any clinical significance.[77,78] Baclofen, an agonist for the GABA-B receptors and long used for treatment for spasticity, is now being used as an anti-craving agent. Baclofen does not seem to have any effect on the peripheral thyroid hormones, but can produce a blunting of the TRH-stimulated TSH release in normal persons[79] though its effect on the already deranged thyroid axis needs to be studied.
CONCLUSION
The effect of alcohol on the HPT axis is significant and alcohol consumption affects almost all aspects of the functioning of the thyroid gland. Given the comorbidity of mood disorders in alcoholism and the relation of mood disorders with hypothyroidism, these findings open up interesting theoretical possibilities to explain the increased occurrence of mood disorders in alcoholism. Although current studies have mostly looked into the effect of alcohol on the neuro-endocrine axis, such associations are rarely unidirectional. The altered thyroid levels in withdrawal may adversely affect alcohol abstinence by changing the hormonal milieu in the brain, increasing withdrawal dysphoria, and increasing craving. At present, such studies are few and upcoming, but it creates the possibility of understanding and treating alcohol use disorders from a whole new perspective.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.World Health Organisation. Global status report on alcohol and health 2011 [Internet] WHO. 2011. [Last accessed on 2012 Sep 24]. Available from: http://www.who.int/topics/alcohol_drinking/en .
- 2.Centers for disease control and prevention. Summary health statistics for U.S. adults: National health interview survey, 2010 [Internet] FASTSTATS-Alcohol Use. 2010. [Last accessed on 2012 Sep 24]. Available from: http://www.cdc.gov/nchs/fastats/alcohol.htm .
- 3.Ray R. The extent, patterns and trends of drug abuse in India-National Survey [Internet] Ministry of Social Justice and empowerment and United Nations office on drugs and crime. 2004. [Last accessed on 2012 Aug 2]. Available from: http://www.unodc.org/india/national_Survey.html .
- 4.Balhara Y, Mathur S. Alcohol abuse: A major public health problem-South Asian perspective. Addict Disord Treat. 2011 In Press. [Google Scholar]
- 5.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 6.Hermann D, Heinz A, Mann K. Dysregulation of the hypothalamic-pituitary-thyroid axis in alcoholism. Addiction. 2002;97:1369–81. doi: 10.1046/j.1360-0443.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 7.Loosen PT. Thyroid function in affective disorders and alcoholism. Neurol Clin. 1988;6:55–82. [PubMed] [Google Scholar]
- 8.Loosen PT. Thyroid function in affective disorders and alcoholism. Endocrinol Metab Clin North Am. 1988;17:55–82. [PubMed] [Google Scholar]
- 9.Fink R. The effects of alcohol on endocrine function. Contemp Issues Clin Biochem. 1984;1:271–88. [PubMed] [Google Scholar]
- 10.Noth RH, Walter RM., Jr The effects of alcohol on the endocrine system. Med Clin North Am. 1984;68:133–46. doi: 10.1016/s0025-7125(16)31246-9. [DOI] [PubMed] [Google Scholar]
- 11.Rawat AK. Neuroendocrinological implications of alcoholism. Prog Biochem Pharmacol. 1981;18:35–57. [PubMed] [Google Scholar]
- 12.Ylikahri RH, Huttunen MO, Härkönen M. Hormonal changes during alcohol intoxication and withdrawal. Pharmacol Biochem Behav. 1980;13(Suppl 1):131–7. doi: 10.1016/s0091-3057(80)80021-9. [DOI] [PubMed] [Google Scholar]
- 13.Mason GA, Noonan LR, Garbutt JC, Caldwell JD, Shimoda K, Walker CH, et al. Effects of ethanol and control liquid diets on the hypothalamic-pituitary-thyroid axis of male Fischer-344 rats. Alcohol Clin Exp Res. 1992;16:1130–7. doi: 10.1111/j.1530-0277.1992.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner A, Heyne A, Campos-Barros A, Köhler R, Müller F, Meinhold H, et al. Hypothalamic-pituitary-thyroid axis in chronic alcoholism. II. Deiodinase activities and thyroid hormone concentrations in brain and peripheral tissues of rats chronically exposed to ethanol. Alcohol Clin Exp Res. 1994;18:295–304. doi: 10.1111/j.1530-0277.1994.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen DD. Chronic daily ethanol and withdrawal: 5.Diurnal effects on plasma thyroid hormone levels. Endocrine. 2003;22:329–34. doi: 10.1385/ENDO:22:3:329. [DOI] [PubMed] [Google Scholar]
- 16.Valeix P, Faure P, Bertrais S, Vergnaud AC, Dauchet L, Hercberg S. Effects of light to moderate alcohol consumption on thyroid volume and thyroid function. Clin Endocrinol (Oxf) 2008;68:988–95. doi: 10.1111/j.1365-2265.2007.03123.x. [DOI] [PubMed] [Google Scholar]
- 17.Garbutt JC, Mayo JP, Little KY, Gillette GM, Mason GA, Dew B, et al. Dose-response studies with thyrotropin-releasing hormone: evidence for differential pituitary responses in men with major depression, alcoholism, or no psychopathology. Alcohol Clin Exp Res. 1996;20:717–22. doi: 10.1111/j.1530-0277.1996.tb01677.x. [DOI] [PubMed] [Google Scholar]
- 18.Pienaar WP, Roberts MC, Emsley RA, Aalbers C, Taljaard FJ. The thyrotropin releasing hormone stimulation test in alcoholism. Alcohol Alcohol. 1995;30:661–7. [PubMed] [Google Scholar]
- 19.Anderson DL, Nelson JC, Haviland MG, MacMurray JP, Cummings MA, McGhee WH, et al. Thyroid stimulating hormone and prolactin responses to thyrotropin releasing hormone in nondepressed alcoholic inpatients. Psychiatry Res. 1992;43:121–8. doi: 10.1016/0165-1781(92)90126-n. [DOI] [PubMed] [Google Scholar]
- 20.Sellman JD, Joyce PR. The clinical significance of the thyrotropin-releasing hormone test in alcoholic men. Aust N Z J Psychiatry. 1992;26:577–85. doi: 10.3109/00048679209072092. [DOI] [PubMed] [Google Scholar]
- 21.Müller N, Hoehe M, Klein HE, Nieberle G, Kapfhammer HP, May F, et al. Endocrinological studies in alcoholics during withdrawal and after abstinence. Psychoneuroendocrinol. 1989;14:113–23. doi: 10.1016/0306-4530(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 22.Hegedüs L, Rasmussen N, Ravn V, Kastrup J, Krogsgaard K, Aldershvile J. Independent effects of liver disease and chronic alcoholism on thyroid function and size: The possibility of a toxic effect of alcohol on the thyroid gland. Metab Clin Exp. 1988;37:229–33. doi: 10.1016/0026-0495(88)90100-x. [DOI] [PubMed] [Google Scholar]
- 23.Casacchia M, Rossi A, Stratta P. Thyrotropin-releasing hormone test in recently abstinent alcoholics. Psychiatry Res. 1985;16:249–51. doi: 10.1016/0165-1781(85)90113-1. [DOI] [PubMed] [Google Scholar]
- 24.Hegedüs L. Decreased thyroid gland volume in alcoholic cirrhosis of the liver. J Clin Endocrinol Metab. 1984;58:930–3. doi: 10.1210/jcem-58-5-930. [DOI] [PubMed] [Google Scholar]
- 25.Liappas I, Piperi C, Malitas PN, Tzavellas EO, Zisaki A, Liappas AI, et al. Interrelationship of hepatic function, thyroid activity and mood status in alcohol-dependent individuals. In Vivo. 2006;20:293–300. [PubMed] [Google Scholar]
- 26.Majumdar SK, Shaw GK, Thomson AD. Thyroid status in chronic alcoholics. Drug Alcohol Depend. 1981;7:81–4. doi: 10.1016/0376-8716(81)90119-8. [DOI] [PubMed] [Google Scholar]
- 27.Loosen PT, Dew BW, Prange AJ. Long-term predictors of outcome in abstinent alcoholic men. Am J Psychiatry. 1990;147:1662–6. doi: 10.1176/ajp.147.12.1662. [DOI] [PubMed] [Google Scholar]
- 28.Sudha S, Balasubramanian K, Arunakaran J, Govindarajulu P. Preliminary study of androgen, thyroid and adrenal status in alcoholic men during deaddiction. Indian J Med Res. 1995;101:268–72. [PubMed] [Google Scholar]
- 29.Välimäki M, Pelkonen R, Härkönen M, Ylikahri R. Hormonal changes in noncirrhotic male alcoholics during ethanol withdrawal. Alcohol Alcohol. 1984;19:235–42. [PubMed] [Google Scholar]
- 30.Baumgartner A, Rommelspacher H, Otto M, Schmidt LG, Kürten I, Gräf KJ, et al. Hypothalamic-pituitary-thyroid axis in chronic alcoholism. I. HPT axis in chronic alcoholics during withdrawal and after weeks of abstinence. Alcohol Clin Exp Res. 1994;18:284–94. doi: 10.1111/j.1530-0277.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 31.Heinz A, Bauer M, Kuhn S, Krüger F, Gräf KJ, Rommelspacher H, et al. Long-term observation of the hypothalamic-pituitary-thyroid axis in alcohol-dependent patients. Acta Psychiatr Scand. 1996;93:470–6. doi: 10.1111/j.1600-0447.1996.tb10679.x. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg M, Hehir R, Hurowitz M. Intravenous triiodothyronine in acute alcoholic intoxication. Preliminary report. N Engl J Med. 1960;263:1336–9. doi: 10.1056/NEJM196012292632603. [DOI] [PubMed] [Google Scholar]
- 33.Haggerty JJ, Jr, Prange AJ., Jr Borderline hypothyroidism and depression. Annu Rev Med. 1995;46:37–46. doi: 10.1146/annurev.med.46.1.37. [DOI] [PubMed] [Google Scholar]
- 34.Hartka E, Johnstone B, Leino EV, Motoyoshi M, Temple MT, Fillmore KM. A meta-analysis of depressive symptomatology and alcohol consumption over time. Br J Addict. 1991;86:1283–98. doi: 10.1111/j.1360-0443.1991.tb01704.x. [DOI] [PubMed] [Google Scholar]
- 35.Ozsoy S, Esel E, Izgi HB, Sofuoglu S. Thyroid function in early and late alcohol withdrawal: Relationship with aggression, family history, and onset age of alcoholism. Alcohol Alcohol. 2006;41:515–21. doi: 10.1093/alcalc/agl056. [DOI] [PubMed] [Google Scholar]
- 36.Dackis CA, Bailey J, Pottash AL, Stuckey RF, Extein IL, Gold MS. Specificity of the DST and the TRH test for major depression in alcoholics. Am J Psychiatry. 1984;141:680–3. doi: 10.1176/ajp.141.5.680. [DOI] [PubMed] [Google Scholar]
- 37.Geurts J, Demeester-Mirkine N, Glinoer D, Prigogine T, Fernandez-Deville M, Corvilain J. Alterations in circulating thyroid hormones and thyroxine binding globulin in chronic alcoholism. Clin Endocrinol (Oxf) 1981;14:113–8. doi: 10.1111/j.1365-2265.1981.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 38.Emsley RA, Roberts MC, Aalbers C, Taljaard FJ, Kotze TJ. Endocrine function in alcoholic Korsakoff's syndrome. Alcohol Alcohol. 1994;29:187–91. [PubMed] [Google Scholar]
- 39.Loosen PT, Chambliss B, Ekhator N, Burns D, Geracioti TD, Orth DN. Thyroid and adrenal dysfunction in abstinent alcoholic men: Locus of disturbance. Neuropsychopharmacol. 1993;9:255–66. doi: 10.1038/npp.1993.61. [DOI] [PubMed] [Google Scholar]
- 40.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–6. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro MG, Irwin M, Hauger RL, Schuckit MA. TSH response to TRH and family history of alcoholism. Biol Psychiatry. 1990;27:905–10. doi: 10.1016/0006-3223(90)90472-e. [DOI] [PubMed] [Google Scholar]
- 42.Garcia MR, Ryan ND, Rabinovitch H, Ambrosini P, Twomey J, Iyengar S, et al. Thyroid stimulating hormone response to thyrotropin in prepubertal depression. J Am Acad Child Adolesc Psychiatry. 1991;30:398–406. doi: 10.1097/00004583-199105000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Loosen PT, Sells S, Geracioti T, Garbutt JC. Thyroid hormones and alcoholism. In: Watson R, editor. Drug and alcohol abuse reviews. clifton, NJ: Humana Press; 1992. pp. 283–306. [Google Scholar]
- 44.Livraghi T, Paracchi A, Ferrari C, Bergonzi M, Garavaglia G, Raineri P, et al. Treatment of autonomous thyroid nodules with percutaneous ethanol injection: preliminary results. Work in progress. Radiology. 1990;175:827–9. doi: 10.1148/radiology.175.3.2188302. [DOI] [PubMed] [Google Scholar]
- 45.Knudsen N, Bülow I, Laurberg P, Perrild H, Ovesen L, Jørgensen T. Alcohol consumption is associated with reduced prevalence of goitre and solitary thyroid nodules. Clin Endocrinol (Oxf) 2001;55:41–6. doi: 10.1046/j.1365-2265.2001.01325.x. [DOI] [PubMed] [Google Scholar]
- 46.Zoeller RT, Fletcher DL, Simonyl A, Rudeen PK. Chronic ethanol treatment reduces the responsiveness of the hypothalamic-pituitary-thyroid axis to central stimulation. Alcohol Clin Exp Res. 1996;20:954–60. doi: 10.1111/j.1530-0277.1996.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 47.Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res. 1998;22(5 Suppl):243S–7. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- 48.Gershengorn MC. Bihormonal regulation of the thyrotropin-releasing hormone receptor in mouse pituitary thyrotropic tumor cells in culture. J Clin Invest. 1978;62:937–43. doi: 10.1172/JCI109222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nemeroff CB, Bissette G, Martin JB, Brazeau P, Vale W, Kizer JS, et al. Effect of chronic treatment with thyrotropin-releasing hormone or an analog of TRH (linear beta-alanine TRH) on the hypothalamic-pituitary-thyroid axis. Neuroendocrinol. 1980;30:193–9. doi: 10.1159/000123000. [DOI] [PubMed] [Google Scholar]
- 50.Sheppard MC, Shennan KI. Desensitization of rat anterior pituitary gland to thyrotrophin releasing hormone. J Endocrinol. 1984;101:101–5. doi: 10.1677/joe.0.1010101. [DOI] [PubMed] [Google Scholar]
- 51.Adinoff B, Nemeroff CB, Bissette G, Martin PR, Linnoila M. Inverse relationship between CSF TRH concentrations and the TSH response to TRH in abstinent alcohol-dependent patients. Am J Psychiatry. 1991;148:1586–8. doi: 10.1176/ajp.148.11.1586. [DOI] [PubMed] [Google Scholar]
- 52.Kaptein EM, Kaptein JS, Chang EI, Egodage PM, Nicoloff JT, Massry SG. Thyroxine transfer and distribution in critical nonthyroidal illnesses, chronic renal failure, and chronic ethanol abuse. J Clin Endocrinol Metab. 1987;65:606–16. doi: 10.1210/jcem-65-4-606. [DOI] [PubMed] [Google Scholar]
- 53.Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: Changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993;39:499–518. doi: 10.1111/j.1365-2265.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 54.Mack WJ, Preston-Martin S, Dal Maso L, Galanti R, Xiang M, Franceschi S, et al. A pooled analysis of case-control studies of thyroid cancer: Cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control. 2003;14:773–85. doi: 10.1023/a:1026349702909. [DOI] [PubMed] [Google Scholar]
- 55.Jee SH, Samet JM, Ohrr H, Kim JH, Kim IS. Smoking and cancer risk in Korean men and women. Cancer Causes Control. 2004;15:341–8. doi: 10.1023/B:CACO.0000027481.48153.97. [DOI] [PubMed] [Google Scholar]
- 56.Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guénel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: A countrywide case-control study in New Caledonia. Am J Epidemiol. 2007;166:1140–9. doi: 10.1093/aje/kwm204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagano J, Mabuchi K, Yoshimoto Y, Hayashi Y, Tsuda N, Land C, et al. A case-control study in Hiroshima and Nagasaki examining non-radiation risk factors for thyroid cancer. J Epidemiol. 2007;17:76–85. doi: 10.2188/jea.17.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 59.Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer. 2001;93:745–50. doi: 10.1002/ijc.1377. [DOI] [PubMed] [Google Scholar]
- 60.Navarro Silvera SA, Miller AB, Rohan TE. Risk factors for thyroid cancer: A prospective cohort study. Int J Cancer. 2005;116:433–8. doi: 10.1002/ijc.21079. [DOI] [PubMed] [Google Scholar]
- 61.Meinhold CL, Park Y, Stolzenberg-Solomon RZ, Hollenbeck AR, Schatzkin A, Berrington de Gonzalez A. Alcohol intake and risk of thyroid cancer in the NIH-AARP Diet and Health Study. Br J Cancer. 2009;101:1630–4. doi: 10.1038/sj.bjc.6605337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams ED. TSH and thyroid cancer. Horm Metab Res Suppl. 1990;23:72–5. [PubMed] [Google Scholar]
- 63.Leone V, D’Angelo D, Pallante P, Croce CM, Fusco A. Thyrotropin regulates thyroid cell proliferation by up-regulating miR-23b and miR-29b that target SMAD3. The Journal of clinical endocrinology and metabolism [Internet] 2012. [Last accessed on 2012 Aug 2]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22730517 . [DOI] [PubMed]
- 64.Maxwell JR, Gowers IR, Moore DJ, Wilson AG. Alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatol (Oxford) 2010;49:2140–6. doi: 10.1093/rheumatology/keq202. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Pan H-F, Ye D-Q, Su H, Li X-P. Moderate alcohol drinking might be protective for systemic lupus erythematosus: A systematic review and meta-analysis. Clin Rheumatol. 2008;27:1557–63. doi: 10.1007/s10067-008-1004-z. [DOI] [PubMed] [Google Scholar]
- 66.Carle A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen L, et al. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism-a population-based case-control study. European journal of endocrinology/European Federation of Endocrine Societies [Internet] 2012. [Last accessed on 2012 Jul 25]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22802427 . [DOI] [PubMed]
- 67.Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287:E318–26. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- 68.Ramadoss J, Tress U, Chen WJ, Cudd TA. Maternal adrenocorticotropin, cortisol, and thyroid hormone responses to all three-trimester equivalent repeated binge alcohol exposure: Ovine Mode. Alcohol. 2008;42:199–205. doi: 10.1016/j.alcohol.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hannigan JH, Bellisario RL. Lower serum thyroxine levels in rats following prenatal exposure to ethanol. Alcohol Clin Exp Res. 1990;14:456–60. doi: 10.1111/j.1530-0277.1990.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 70.Hannigan JH, Martier SS, Naber JM. Independent associations among maternal alcohol consumption and infant thyroxine levels and pregnancy outcome. Alcohol Clin Exp Res. 1995;19:135–41. doi: 10.1111/j.1530-0277.1995.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 71.Mistraletti G, Donatelli F, Carli F. Metabolic and endocrine effects of sedative agents. Curr Opin Crit Care. 2005;11:312–7. doi: 10.1097/01.ccx.0000166397.50517.1f. [DOI] [PubMed] [Google Scholar]
- 72.Constantinou C, Bolaris S, Valcana T, Margarity M. Diazepam affects the nuclear thyroid hormone receptor density and their expression levels in adult rat brain. Neurosci Res. 2005;52:269–75. doi: 10.1016/j.neures.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 73.Xu F, Zhang Y, Lou Y. Effects of different thyroid status on the pharmacokinetics of diazepam. Yao Xue Xue Bao. 1998;33:571–5. [PubMed] [Google Scholar]
- 74.Christensen J, Wase AW, Van Dyke JH. Tetraethylthiuram disulphide (disulfiram) and thyroid activity. Acta Pharmacol Toxicol (Copenh) 1955;11:163–7. doi: 10.1111/j.1600-0773.1955.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 75.Benitz KF, Kramer AW, Jr, Dambach G. Comparative studies on the morphologic effects of calcium carbimide, propylthiouracil, and disulfiram in male rats. Toxicol Appl Pharmacol. 1965;7:128–62. doi: 10.1016/0041-008x(65)90084-0. [DOI] [PubMed] [Google Scholar]
- 76.Ramirez G, Ables MF, Butcher DE, Morris AD. Evaluation of the hypothalamic-hypophysial, thyroid, and gonadal axes before and after disulfiram administration in patients with chronic alcoholism. South Med J. 1988;81:1407–11. doi: 10.1097/00007611-198811000-00017. [DOI] [PubMed] [Google Scholar]
- 77.Ilias I, Kakoulas I, Christakopoulou I, Katsadoros K. Thyroid function of former opioid addicts on naltrexone treatment. Acta Medica (Hradec Kralove) 2001;44:33–5. [PubMed] [Google Scholar]
- 78.Khalil RB, Richa S. Thyroid adverse effects of psychotropic drugs: A review. Clin Neuropharmacol. 2011;34:248–55. doi: 10.1097/WNF.0b013e31823429a7. [DOI] [PubMed] [Google Scholar]
- 79.Elias AN, Szekeres AV, Stone S, Weathersbee P, Valenta LJ, Haw T. Gaba-ergic and dopaminergic regulation of thyroid stimulating hormone. Effects of baclofen and metoclopramide. Horm Res. 1984;19:171–5. doi: 10.1159/000179884. [DOI] [PubMed] [Google Scholar]


