Abstract
The actions of vitamin D are not confined to bone. Vitamin D receptors are present in nearly all the nuclei and its actions are manifold. Populations deficient in vitamin D are at higher risk of developing autoimmune diseases, diabetes, cancer, infections, allergies and other chronic illnesses.
Keywords: Allergies, asthma, autoimmune disease, cancer, cardiovascular, diabetes, extraskeletal, falls, infections, Vitamin D
INTRODUCTION
Vitamin D, one of the fat-soluble vitamins, is unique in that it is a steroid hormone with endocrine, paracrine and autocrine effects. It is also unique among the vitamins having both endogenous production and exogenous sources. Vitamin D has long been known for its classic role for bone health and calcium homeostasis. With milk and food products being fortified with vitamin D, it was believed that rickets has been conquered in the developed world. It is emerging that rickets is only the tip of the iceberg of vitamin D deficiency. With increasing data of low levels of vitamin D in the general population and in other disease states, vitamin D insufficiency is emerging as an unrecognized pandemic. The discovery that most tissues in the body have vitamin D receptors and several have the one hydroxylase enzyme to convert 25(OH)D (25 hydroxy cholecalciferol) to the active form unlike previously thought has provided new insights into the pleiotropic role of this vitamin. During the past decade, association between Vitamin D insufficiency and increased risk of various non–skeletal morbidities have been recognized including its role in decreasing the risk of many chronic illnesses like allergies, asthma, autoimmune diseases, diabetes, cancers, infections and cardiovascular disease. This review attempts at revisiting the role of this important vitamin in the light of recent developments and provides a comprehensive account of the extraskeletal effects.
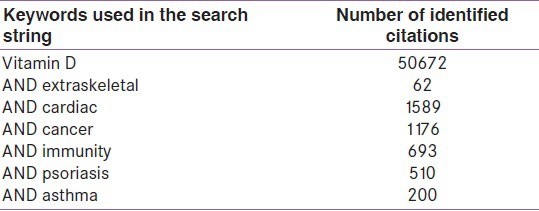
A search of the literature was done using PubMed, Ovid, and Cochrane database using the following keywords in the search string – Vitamin D AND extraskeletal, falls, mortality, diabetes, cardiovascular, allergy, asthma, autoimmune diseases, cancer, infections, multiple sclerosis, psychiatric and pain. The search was limited to articles written in the English language and all articles relevant to the present review were searched. Meta-analysis of RCTs (randomized control trials) and RCTs were given a higher place in hierarchy.
A search using PubMed database yielded 50672 citations for vitamin D, it emerging from a nutrient to one of the most talked about and researched drugs. The results of the literature search are as in Table 1.
Table 1.
Literature search using PubMed database
Vitamin D has a long and interesting history. For more than 500 million years during evolution, phytoplanktons and zooplanktons have been producing vitamin D[1] and it is one of the oldest hormones.[2] These do not have a skeleton and so the original function of vitamin D may have been unrelated to the classic role of bone homeostasis. The turn of the twentieth century saw the epidemic of rickets in Northern Europe and United States[3] along with increased upper respiratory infections in the affected children. Folk tradition used cod liver oil as a protective agent even in the mid 19th century. After its commercial production, an advertisement for cod liver oil in the April 18, 1890, issue of Science proclaimed “prevention or cure of cough and colds in both the young and old.” These effects were rediscovered only in 1941[4] and again in 2006.[5] Vitamin D was identified in the early 1920s by Adolf Windaus after the discovery of the antirachitic effects of cod liver oil. It is now recognized to play an essential role not only for bone health but for overall health and well being.[6,7]
Finsen received the Nobel Prize in 1903 for his observation that exposure to sunlight is effective in treating several skin disorders, including lupus vulgaris. The influence of latitude and altitude on the epidemiology of cancer was initially reported by Hoffman[8] in 1915 and later by other authors.[9,10] The 25 hydroxylation of vitamin D in the liver and one hydroxylation in the kidney to form active 1,25(OH)2D was appreciated in the 1970s.[10] The role of Vitamin D receptor (VDR) for functioning of Vitamin D and its presence in kidneys, small intestine and osteoblasts to up-regulate genes for calcium homeostasis was subsequently recognized.[10,11,12] Later, investigations demonstrated the presence of VDR in every tissue and cell in the body including skin, colon, brain, pancreas, breast, activated T and B lymphocytes, monocytes and macrophages.[12,13] A breakthrough into the extraskeletal effects of Vitamin D was reported in the early 1980s with the demonstration of anti-proliferative activity of 1,25(OH)2D on human myeloid leukemia cell line (HL- 60).[14] This was followed by several observations in cell lines from melanoma, colon and prostate cancers.[15,16,17] Non-renal activation of 25(OH)D was suggested in studies of patients with sarcoidosis and tuberculosis in the 1980s,[10,18] followed by observation of 25(OH)D in macrophages.[19] One hydroxylase activity was identified in cultured cells from skin, colon, prostate, breast, lung and brain.[10,12,15,16.20,21,22] VDR is found in nearly every tissue and cell and the ability to activate 25(OH)D to 1,25(OH)2D is also widely distributed. The functions with which Vitamin D is now associated and the diseases in which its role is implicated is also as diverse as the tissues in which VDR is located.
PHYSIOLOGY OF EXTRASKELETAL EFFECTS OF VITAMIN D
The small intestine, kidneys and bone are the primary organs responsive to the endocrine effects of vitamin D and are involved in the classic function of mineral and bone homeostasis. But the effects of vitamin D are beyond the maintenance of skeletal health. The new pluripotent nature of vitamin D and induction of non-classical responses prompted a re-evaluation of the cellular and molecular mechanisms associated with this vitamin. The presence of VDR in other tissues[13] and the identification of 1 hydroxylase enzyme in extra-renal tissues lead to search for and increasing evidence that extra-renal synthesis of 1,25(OH)2 D[20,21] may be important for regulating cell growth and differentiation[1,13,23,24] via paracrine, intracrine and autocrine mechanism. Skin has the enzymatic machinery to convert Vitamin D3 to 1,25(OH)2D as it has both 25 hydroxylase and 1 hydroxylase activity. It is recognized that in addition to the calcium-regulating tissues like intestine, bone and kidneys, most other tissues mentioned earlier have nuclear receptors for 1,25(OH)2 D.[17,24,25] Although the exact physiological function of 1,25(OH)2D in these tissues is not fully understood, it is recognized that 1,25(OH)2D is a potent inhibitor of cellular proliferation and inducer of cell maturation[17,24,25,26] and probably has a role in preventing carcinogenesis in these tissues. There has been an interesting association with increased risk of dying of colon, breast, prostate and ovarian cancer in individuals living at high latitudes.[22,27] Garland[28] reported that concentration of 25(OH)D more than 20 ng/mL decreases the risk of dying from colon cancer by more than 200%.
Vitamin D, which is photosynthesized in the skin or obtained from diet, enters the circulation bound to Vitamin D-binding protein. It is hydroxylated in the liver to 25(OH)D and then in the kidney at the 1 position to form the active form of 1,25(OH)2 D. This circulates bound to vitamin D-binding protein. At the target cell, it enters and binds to VDR in the cytoplasm, which then enters the nucleus and heterodimerizes with the retinoic acid X receptor to increase transcription of Vitamin D-dependent genes that control both classical function of bone and calcium metabolism and other non-classical functions. Circulating 1,25(OH)2D is catabolized by 24 hydroxylase to 1,24,25(OH)2D to inactive form.[29] The 1,25(OH)2D present in extra-renal tissue is also degraded locally after its effect to palcalcitoic acid and does not enter the circulation. Nagpal and colleagues reported that 1,25(OH)2D through its transcriptional activity was capable of regulating directly or indirectly at least 200 genes,[30] which control proliferation, differentiation, apoptosis and angiogenesis. 1,25(OH)2D is also produced locally in the tissues. This regulates many cellular functions like:
Promotion of innate immunity. When a monocyte/macrophage is stimulated by an infective agent like Mycobacterium tuberculosis or its liposaccharides through its toll-like receptor (TLR), expression of VDR and 1 hydroxylase is up-regulated. 1,25(OH)2D enters the nucleus and increases expression of cathelicidin (CD), which is a peptide capable of promoting innate immunity and induces destruction of infective agents like TB.
1,25(OH)2D also acts locally on activated T and B lymphocytes, which regulate cytokine and immunoglobulin synthesis. 1,25(OH)2D inhibits T cell proliferation, in particular T helper (Th1) cells capable of producing interferon (IFN-۲) and interleukin (IL-2), which prevent further antigen presentation and recruitment of T lymphocytes.[31,32] It also enhances the production of IL4, IL5 and IL10 shifting balance from Th1 to Th2 phenotype.[31] It also inhibits the formation of Th17 cells, which play an important role in autoimmunity.[31]
Local 1,25(OH)2D in various cells regulate a variety of genes that control proliferation and keep cell cycle in G1 /S phase like p21 and p27 and also genes that inhibit angiogenesis and induce apoptosis. Locally produced 1,25(OH)2D does not enter the circulation as it is locally metabolized by 24 hydroxylase to calcitroic acid, which is inert.
1,25(OH)2D produced in kidneys enters the circulation. It down-regulates renin production in the kidney and stimulates insulin secretion in the β islet cells of pancreas acting in an endocrine manner.
Prevalence of Vitamin D deficiency
Nature provided our forefathers who were basically hunter-gatherers with an efficient vitamin D production system by adequate exposure to sun. However, the changes of evolution and modernization, clothing, increasing hours spent indoors with limited sun exposure, obesity, environment pollution blocking the ultraviolet (UV) rays and use of sunscreens resulted in reduced vitamin D synthesis. Today, poor vitamin D status is increasing in an alarming manner to the level of a pandemic. The Third National Health and Nutrition Examination Survey (NHANES III) has revealed that a large segment of American population (61–92%) have low Vitamin D levels. This has been confirmed in many other studies in the US and other parts of the world.[33,34,35,36,37] Even in a sundrenched country like India, Vitamin D insufficiency has been reported to be prevalent. Until the late 20th century, there were no studies assessing vitamin D status of Indian population. Initial reports of vitamin D deficiency among the people in Punjab appeared in 1973.[38] Later, more reports were published showing the prevalence of Vitamin D deficiency even in healthy people, including doctors and nurses.[39,40,41] Vitamin D status of children was low in both urban and rural population.[42]
VITAMIN D STATUS
In the past, Vitamin D deficiency was defined by the serum level of Vitamin D, which was associated with the development of rickets. But recent studies assessing the relationship between serum 25(OH)D and parathyroid hormone level revealed that the optimum level is much higher.[2] This introduced the terminology of sufficiency, deficiency and insufficiency, but the debate about what constitutes these levels continues. The circulating level of 25(OH)D is the most suitable indicator of Vitamin D status because it is easily measured, stable and has the longest half life of 3 weeks.[43,44] A clear consensus exists that the best indicator to evaluate vitamin D status is 25(OH)D but no consensus exists as to the level of 25(OH)D to define deficiency. By combining the results of studies correlating the 25(OH)D level with parathyroid hormone stimulation, rise of 1,25(OH)2D level and calcium absorption, Heaney concluded that optimal calcium absorption occurred at 25(OH)D levels of 32 ng/mL or more.[44,45,46,47] A simplistic classification of vitamin D status is suggested in the Mayo Clinic proceedings 2011 [Table 2].[48]
Table 2.
Classification of Vitamin D status by 25(OH)D concentration[48]

So the criterion for optimal Vitamin D status has moved away from a concentration needed to achieve skeletal health to that which demonstrates optimal benefits on non-skeletal health outcomes.
In this review, the role of Vitamin D in healthy and deficiency states in relation to extraskeletal tissues and organs is discussed under the following sections.
Vitamin D and cardiovascular diseases
Vitamin D and diabetes
Vitamin D and cancer
Vitamin D and autoimmune diseases – multiple sclerosis
Vitamin D and innate immunity – TB/HIV
Vitamin D and psoriasis
VITAMIN D AND CARDIOVASCULAR DISEASE
To the potpourri of risk factors for cardiovascular disease (CVD), Vitamin D deficiency has been added as a novel risk factor. Several observational studies point to a strong association between Vitamin D deficiency and cardiovascular mortality. The mechanism of how Vitamin D improved CVD outcomes remains obscure. Potential hypothesis include role of calcitriol in the down-regulation of many genes including those involved in renin production, proliferation of cardiac and vascular muscle cells, down-regulation of C reactive protein (CRP) and other pro-inflammatory markers. Renin-angiotensin-aldosterone system, insulin resistance and secondary hyperparathyroidism are thought to mediate some of the cardiovascular effects of Vitamin D deficiency. Data analysis of NHANES III by Fiscella and colleagues revealed 40% higher risk of death due to CVD and stroke in black population with calcitriol level in the lowest quartile.[49] Vitamin D deficiency has been reported to be associated with higher risk of metabolic syndrome, hypertension (HTN) and adverse cardiovascular events.[49,50] Early 1980s and 1990s saw an emergence of several observational data suggesting an environmental factor like Vitamin D related to latitude, season and sun exposure as an etiology for the mortality difference noted in ischemic heart disease (IHD).[51,52]
Several cohort and case control studies like the men in the health professionals follow-up study and Framingham offspring study revealed high risk for developing CVD in subjects with lower levels of 25(OH)D.[50,53] Severe Vitamin D deficiency not only increases the risk of developing CVD but also increases risk of sudden cardiac death[54] by 3–5 times in patients with established CVD and 50% increase in fatal stroke.[55] Similar findings were seen in hemodialysis patients.[56] In contrast, a study from South India reported that very high levels of 25(OH)D were associated with increased risk of IHD.[57] Studies have also shown an increased risk of incident hypertension and metabolic syndrome with suboptimal Vitamin D status.[58,59]
All these observational and cohort studies lead to RCTs focusing on Vitamin D as a potential antihypertensive agent. An RCT conducted on German women shows reduction of systolic BP after Vitamin D supplementation.[60] Reduction of BP was also demonstrated following exposure to UV A in comparison with UV B in another RCT.[61] But other studies showed no similar effect.[62,63] Therefore, studies on vitamin D supplementation have not consistently demonstrated a positive effect on reducing BP.[64]
Two studies that prospectively examined vitamin D supplementation on cardiovascular mortality did not show better survival compared with controls.[63,65] A meta-analysis found an 8% reduction in all-cause mortality with modest amounts of Vitamin D supplementation.[66] A recent meta-analysis of 51 RCTs showed that vitamin D was associated with non-significant effect on death (RR 0.96), MI (RR 1.02), and stroke (RR 1.05) with no change in BP, resulting in the conclusion that data available to date are unable to show a significant decrease in mortality and cardiovascular risk associated with vitamin D.[67]
Several epidemiological studies have shown a strong association between Vitamin D insufficiency and risk of CVD, diabetes and metabolic syndrome. Several prospective studies suggested that Vitamin D deficiency predisposes to incident HTN, IHD and sudden cardiac death or cardiac failure. Initial RCTs of Vitamin D in the treatment of HTN yielded mixed results, and no large RCTs on cardiovascular end points have been published.[64,66] The results of future trials should provide guidance on how to manage Vitamin D status in clinical practice.[66] The current evidence does not strongly support screening for Vitamin D deficiency in all patients with established CVD or who are at risk for CVD.[64] Well-designed prospective RCTs are necessary to further investigate the role of Vitamin D supplementation for cardiovascular risk reduction.[66]
VITAMIN D AND DIABETES
Epidemiologic and observational studies have kindled interest in the potential role of Vitamin D and inflammatory process in the pathogenesis, prevention and control of, both type I and type II diabetes.[68] Animal and human studies have suggested that vitamin D is a potential modifier of diabetes.[69,70,71] Possible mechanisms in modulating diabetes risk are detailed below.
Type I diabetes mellitus (DM) is characterized by an autoimmune destruction of pancreatic islet cells. Animal studies suggest that the immunomodulatory and anti-inflammatory actions of vitamin D may reduce the autoimmune insulinitis of type I DM.[69,70] Vitamin D can suppress the antigen presenting capacity of macrophages, inhibit dentritic cell maturation, modulate the development of CD4 lymphocytes and inhibit the production of interferon۲ (IFN۲) and interleukin2 (IL2) among other cytokines, which are known to activate macrophages and cytotoxic T cells leading to islet cell destruction in type I DM.
Type II DM was found to be associated with an increase in levels of tumor necrosis factor a and b, CRP, plasminogen activator inhibitor I (PAI – I) and interleukin-6(IL-6). By modulation of immune and inflammatory process, Vitamin D may decrease insulin resistance and increase insulin secretion in type II DM.
Pancreatic islets have both VDR and Vitamin D-dependent calcium-binding protein (CaBP). The effects of vitamin D on b cells may be by its regulation of extracellular calcium and calcium flux through the β cell or through calcium independent pathways. Vitamin D deficiency may also impair insulin secretion through its associated increase in parathormone levels. It may reduce insulin resistance by its immunomodulatory and anti-inflammatory effects. Epidemiologically the type I DM is 10–15 times more common in far northern or southern latitudes compared with tropical latitudes. In a long-term follow-up study from Finland, it was shown that children who took 2000 IU per day Vitamin D during first year of life reduced their risk of developing type I DM by 88%. Moreover, the children who had evidence of Vitamin D deficiency had a 2.4-fold increased risk of developing type I DM.[71] A 33% reduction in risk of type I DM was found in children supplemented with Vitamin D in the European community sponsored concerted action on the epidemiology and prevention of diabetes study (EURODIAB).[72] Similar data was seen in a birth cohort study with Vitamin D supplementation in the first year of life.[73] Maternal intake of vitamin D may also have a protective effect on the baby. A meta-analysis concluded that Vitamin D supplementation in infancy might be protective against the development of type I DM.[71] A temporary reduction in insulin dose was also seen following supplementation with calcitriol in adult patients with type I diabetes.[74] Animal studies showed benefit of vitamin D supplementation on inhibiting onset of autoimmune diabetes[69] and arresting the disease process.
Observational studies have shown seasonal variation of diabetic control in type II DM.[75] Two large RCTs using combined vitamin D and calcium found that it lowers the risk of type II DM.[76,77] In a large cross-sectional study of 23,000 adults, insulin resistance was found to be inversely correlated with Vitamin D levels.[78] Similar association has been reported in other studies.[79,80] Cohort studies also found association between low vitamin D levels and type II DM[81] and showed a protective effect of high levels.[82] In contrast, other studies showed no benefit.[83,84] Pittas et al. in a meta-analysis concluded that vitamin D and calcium insufficiency may negatively influence glycemia whereas combined supplementation with both may be beneficial in optimizing glucose metabolism.[85] In animal studies of vitamin D deficient rats, reduced insulin secretion was found to improve following vitamin D replacement.
There is some evidence to suggest that Vitamin D may play a role in the prevention and treatment of type I and II DM through its action on systemic inflammation, insulin secretion and resistance. Further studies are needed to elucidate the mechanism of action and find the appropriate dose and type of Vitamin D to provide the optimal benefit.[68] The potential role of vitamin D and calcium supplementation in alleviating the increasing menace of diabetes needs to be further studied,[68] these interventions being inexpensive and easy to implement in clinical practice.
VITAMIN D AND CANCER
If 25(OH)D is available, specific VDRs found in nearly all tissues produce 1,25 (OH)2D in a paracrine manner. Vitamin D plays a physiological role in cell differentiation in normal and tumor cells. Pre-clinical and epidemiological data suggest a role for Vitamin D in the prevention and treatment of cancer. Polymorphism of VDR gene has been associated with high risk of cancer. Women with mutations of VDR gene have higher risk of breast cancer.[86] The risk of development and death due to cancer is higher is higher latitudes and is possibly related to lesser exposure to direct sunlight, particularly during childhood and teenage.[87,88] Women who had low blood level of 25(OH)D (<12 ng/ mL) at the start of study had significantly higher risk of development of colorectal cancer compared with those who had 25(OH)D level >24 ng/ mL.[89] 1,25(OH)2D is capable of regulating genes that control proliferation, differentiation, apoptosis and angiogenesis.[90] More studies will be needed to confirm and approve Vitamin D as a co-prescription with anti-cancer drugs. Most clinical trials involving Vitamin D and its metabolites have been conducted in prostate cancer patients.[91] A double-blind placebo-controlled trial to determine if 1,25(OH)2D could be used in pre-leukemia showed promising results initially but was proved to be unsuccessful in the end because of development of hypercalcemia and blast crisis.[92] In prostatic cancer, even though administration of 2000 IU Vitamin D per day resulted in fall in PSA levels, severe hypercalcemia necessitated the halting of the trial. Several non-hypercalcemic analogs of 1,25(OH)2D show antitumor activity in a subset of cancer patients with high VDR expression and is associated with good prognosis. Some transcription factor represses VDR gene expression in human colon cancer cells. In human colon cancers, elevated expression of transcription factors correlates with down-regulation of VDR.[93] The malignant cells are capable of annulling the anti-proliferative activity of Vitamin D by increasing the expression of transcriptional factors.
VITAMIN D AND AUTOIMMUNE DISEASES
The action of Vitamin D on the immune system appears to be mediated through T and B cells. When T and B cells are stimulated, VDR genes are activated and produce 1,25(OH)2 D, which suppresses proliferation and immunoglobulin synthesis. 1,25(OH)2D inhibits T cells proliferation and prevents formation of gamma interferon and interleukin-2 (IL-2) by the helper T cells (TH1). It also enhances suppressor T cell (TH2) activity, thereby enhancing production of IL-4, IL-5 and IL-10. The immunomodulating activity of 1,25(OH)2D may lead to increased risk of diseases, which may have an autoimmune background. Use of Vitamin D receptor ligands have increased the action of natural killer cells and enhanced the activation of phagocytes. 1,25(OH)2D has been shown to be useful in animal models of multiple sclerosis and Crohn's disease. In humans, there is epidemiologic evidence for the importance of Vitamin D for immune health.
Multiple sclerosis is a complex trait in which allelic variation in the MHC class II region exerts the single strongest effect on genetic risk. Environmental factors act at a population level. Sunlight or Vitamin D are key environmental factors in etiology and might interact with inherited factors in the MHC class II region. A single MHC Vitamin D response element (VDRE) gene has been identified as HLA-DRB1*15 haplotypes. In a subgroup of individuals genetically predisposed to multiple sclerosis, deficiency of Vitamin D may cause non-activation of histocompatibility genes necessary for differentiating between self and foreign proteins.[94] Epidemiological evidence suggests that adults with high blood levels have the lowest risk of developing multiple sclerosis. Women who had the highest intake of Vitamin D had reduced risk of developing multiple sclerosis by 42%. Similar observations have been made in rheumatoid arthritis, and children born to mothers who were Vitamin D deficient had increased risk of wheezing disorders during early childhood. From the epidemiological evidence and some animal models, it is clear that Vitamin D plays an important role in immune health.
VITAMIN D AND INNATE IMMUNITY
The active form of Vitamin D has been shown to have anti-mycobacterial activity in vitro. Since the prevalence of Vitamin D concentration of <30 ng/mL was observed in 86% of patients with active tuberculosis, it had been used earlier to treat patients with tuberculosis. (Ref 127,128,129,130 up-to-date). However, Vitamin D supplementation cannot be recommended as treatment for tuberculosis unless more prospective studies come up with evidence-based criteria. In the case of HIV also, some in vitro studies have shown favorable effect on induction of autophagy. Direct correlation was observed between lower levels of 1,25(OH)2D with lower CD4+ T cell count, higher tumor necrosis factor level and speed of HIV disease progression (Ref 132,133,134).
Observations like high rates of influenza during winter have been correlated with lack of Vitamin D synthesis. Other factors like cold temperature and low relative humidity in indoor heating could be responsible.
VITAMIN D AND PSORIASIS
The keratinocytes in the skin are the major sources for 7 dehydrocholestrol, which is converted to Vitamin D3 in presence of sunlight. These cells also have VDR, which can convert 25(OH)D to 1,25(OH)2 D. Active Vitamin D is a potent inhibitor of keratinocytes and could be used safely for non-malignant hyper-proliferative skin disorders like psoriasis. Topically applied 1,25(OH)2D or some if its analogs could be used as a first-line therapy in psoriasis. Pooled data from randomized controlled trials show that calcipotriol is an effective and well-tolerated treatment for mild-to-moderate chronic plaque psoriasis. Although skin irritation is comparatively common, this rarely required withdrawal of calcipotriol treatment. Potent topical corticosteroids are equally effective with less short-term side effects. Future trials comparing the risk/benefit ratios from combined regimens of calcipotriol with other antipsoriatic agents are indicated. Observational studies also show a relationship between nutritionally poor 25(OH)D status and muscle weakness in children and elderly subjects. In most studies, the lowest Vitamin D concentrations (<20 ng/mL and especially <10 ng/ mL) were associated with the poorest muscle function, whereas higher levels of 25(OH)D (>20 ng/mL) were associated with better muscle function. There are several meta-analyses showing a reduction in risk of falls (relative risk reduction as high as 20%) following Vitamin D supplementation, particularly when the baseline Vitamin D status is poor. Supplementation with doses ranging from 700 to 1000 units/day is effective. In one meta-analysis, multi-component group exercise and Tai Chi were even more effective in reducing risk of falls than Vitamin D supplementation.
ACKNOWLEDGMENT
We acknowledge the help and secretarial assistance by Mr. Abraham Jacob.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Holick MF. Evolution and function of Vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Resurrection of Vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiesman EG. Massive doses of vitamin A and D in the prevention of common cold. Arch Otolaryn. 1941;34:789–91. [Google Scholar]
- 5.Cannel JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parathyroid function in post menopausal women with osteoporosis: Baseline data from the multiple outcomes of raloxifine evaluation clinical trial. J clin Endocrinol Metab. 2001;86:1212–21. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):S1678–88. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman FL. Appendix E. Newark (NJ): Prudential Press; 1915. The mortality of cancer throughout the world. [Google Scholar]
- 9.Apperly FL. The relation of solar radiation to cancer mortality in North America. Cancer Res. 1941;1:191–5. doi: 10.1158/0008-5472.CAN-15-3169. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:669–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 12.Dusso AS, Brown AJ, Slatopolsky Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D: A millennium perspective. J cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishii Y, et al. 1 alpha,25-Dihydroxycholecalciferol and human myeloid leukemia cell line(HL-60) Biochem J. 1982;204:713–9. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colston K, Colston MJ, Felsman D. 1,25 dihydroxyvitamin D 3 and malignant melanoma:the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–6. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 16.Cross HS, Bareis, Hofer H, Bischof MG, Bajna E, Kriwanek S, et al. 25 hydroxyvitamin D 3 1hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early carcinogenesis. Steroids. 2001;66:287–92. doi: 10.1016/s0039-128x(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 17.Feldman D, Zhao XY, Krishnan AV. Vitamin D and prostate cancer. Endocrinology. 2000;141:5–9. doi: 10.1210/endo.141.1.7341. [DOI] [PubMed] [Google Scholar]
- 18.Gknos PJ, London R, Hendler ED. Hypercalcemia and elevated 1,25 dihydroxy vitamin D levels in a patient with end stage renal disease and active tuberculosis. N Engl J Med. 1984;311:1683–5. doi: 10.1056/NEJM198412273112607. [DOI] [PubMed] [Google Scholar]
- 19.Adams JS, Singer FR, Gacad MA, Sharma OP, Hayes MJ, Vouros P, et al. Isolation and structural identification of 1,25 dihydroxy vitamin D 3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab. 1985;60:960–6. doi: 10.1210/jcem-60-5-960. [DOI] [PubMed] [Google Scholar]
- 20.Tangpricha V, Flanagan JN, Whitlateh LW, Tseng CC, Chen TC, Holt PR, et al. 25 hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357:1673–4. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- 21.Mawer EB, Hayes ME, Heys SE, Davies M, White A, Stewart MF, et al. Constitutive synthesis of 1,25 dihydroxy vitamin D 3 by a human small cell lung cell line. J Clin Endocrinol Metab. 1994;79:554–60. doi: 10.1210/jcem.79.2.8045976. [DOI] [PubMed] [Google Scholar]
- 22.Garland CF, Garland FC, Gorham ED. Can colon cancer incidence and death rates be reduced with calcium and vitamin D? Am J Clin Nutr. 1991;54:93S–201S. doi: 10.1093/ajcn/54.1.193S. [DOI] [PubMed] [Google Scholar]
- 23.DeLuca H. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6,suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. Non calcaemic actions of 1,25 dihydroxy vitaminD 3 and clinical applications. Bone. 1995;17:107S–11S. doi: 10.1016/8756-3282(95)00195-j. [DOI] [PubMed] [Google Scholar]
- 25.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25 dihydroxy D 3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986;78:557–66. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holick MF. Vitamin D: The underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes. 2002;9:87–98. [Google Scholar]
- 27.Hanchette CL, Scwartz GG. Geographic patterns of prostate cancer mortality. Cancer. 1992;70:2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25 hydroxy vitamin D and colon cancer: Eight year prospective study. Lancer. 1989;18:1176–8. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 29.Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110–7. doi: 10.1007/s11926-008-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagpal S, Na S, Rathachalam R. Non calcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–87. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 31.Bikle DD. Noncalcemic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holick MF. Vitamin D: Extraskeletal health. Endocrinol Metab Clin N Am. 2010;39:381–400. doi: 10.1016/j.ecl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 34.Cole CR, Grant FK, Tangpricha V, Swaby-Ellis ED, Smith JL, Jacques A, et al. 25-hydroxyvitamin D status of healthy, low income, minority children in Atlanta, Georgia. Pediatrics. 2010;125:633–9. doi: 10.1542/peds.2009-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razzaghy Azar M, Shakiba M. Assessment of vitamin D status in healthy children and adolescents living in Tehran and its relation to iPTH, gender, weight and height. Ann Hum Biol. 2010;37:692–701. doi: 10.3109/03014460903527348. [DOI] [PubMed] [Google Scholar]
- 36.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25OHD levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics. 2009;124:1404–10. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rovner AJ, O’Brien KD. Hypovitaminosis D among healthy children in the United States: A review of the current evidence. Arch Pediatr Adolesc Med. 2008;162:513–9. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 38.Hodgkin P, Kay, Liumb GA, Stranbury SW. Vitamin D deficiency in Asians at home and in Britain. Lancet. 1973;2:167–72. doi: 10.1016/s0140-6736(73)93004-3. [DOI] [PubMed] [Google Scholar]
- 39.Harinarayan CV, Gupta N, Kochupillai N. Vitamin D status in primary hyperparathyroidism. Clin Endocrinol. 1995;43:351–8. doi: 10.1111/j.1365-2265.1995.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 40.Goswami R, Gupta N, Goswami D, Marwala RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentration in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–5. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 41.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D, Srinivasarao PV, Sarma KV, et al. High prevalence of low dietary calcium, high phytate consumption and vitamin D deficiency in healthy south Indians. Am J Clin Nutr. 2007;85:1062–7. doi: 10.1093/ajcn/85.4.1062. [DOI] [PubMed] [Google Scholar]
- 42.Harinarayan CV, Joshi SR. Vitamin D status in India--Its implications and remedial measures. J Assoc Physicians India. 2009;57:40–8. [PubMed] [Google Scholar]
- 43.Holick MF. Vitamin D: Importance in the prevention of cancers, type I diabetes, heart disease and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 44.Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67:644–50. doi: 10.1210/jcem-67-4-644. [DOI] [PubMed] [Google Scholar]
- 45.Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(6 Suppl):1706S–9S. doi: 10.1093/ajcn/80.6.1706S. [DOI] [PubMed] [Google Scholar]
- 46.Gloth FM, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–6. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 47.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25 hydroxy vitamin D. J Am Coll Nutr. 2003;22:142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 48.om D Thacher, Bart L Clarke. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiscella K, Franker P. Vitamin D, race and cardiovascular mortality with findings from a national US sample. Ann Fam Med. 2010;8:11–8. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giovannucci E, Lui Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fleck A. Latitude and ischemic heart disease. Lancet. 1989;1:613. doi: 10.1016/s0140-6736(89)91634-6. [DOI] [PubMed] [Google Scholar]
- 52.Grimes DS, Hindle E, Dyer T. Sunlight, cholesterol and coronary heart disease. QJM. 1996;89:579–89. doi: 10.1093/qjmed/89.8.579. [DOI] [PubMed] [Google Scholar]
- 53.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilz S, Marz W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–35. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 55.Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, et al. Low Vitamin D levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39:2611–3. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 56.Wolf M, Shah A, Guterraz O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early motality among incident hemodialysis patients. Kidney Int. 2007;72:1004–13. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 57.Rajasree S, Rajpal K, Kartha CC, Sarma PS, Kutty VR, Iyer CS, et al. Serum 25-hydroxyvitamin D 3 levels are elevated in South Indian patients with ischemic heart disease. Eur J Epidemiol. 2001;17:567–71. doi: 10.1023/a:1014559600042. [DOI] [PubMed] [Google Scholar]
- 58.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 59.Forman JP, Curhan GC, Taylor EM. Plasma 25-hydroxyvitamin D levels and risk of hypertension among young women. Hypertension. 2008;52:828–32. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short term vitamin D (3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2007;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 61.Krause R, Bühring M, Hopfenmüller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–10. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 62.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D 3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49:640–6. [PubMed] [Google Scholar]
- 63.Lacroix AZ, Kotchen J, Anderson G, Brzyski R, Cauley JA, Cummings SR, et al. Calcium plus vitamin D supplementation and mortality in post menopausal women: The women's health initiative calcium-vitamin D randomized control trial. J Gerontol A Biol Sci Med Sci. 2009;64:559–67. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009;338:40–4. doi: 10.1097/MAJ.0b013e3181aaee91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Triwedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D 3 supplementation on fractures and mortality in men and women living in the community: Randomized double blind control trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Motiwala SR, Wang TJ. Vitamin d and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:345–53. doi: 10.1097/MNH.0b013e3283474985. [DOI] [PubMed] [Google Scholar]
- 67.Elamin MB, Abu Ehnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin d and cardiovascular outcomes: A systematic review and meta analysis. J Clin Endocrinol Metab. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 68.Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35:11–7. doi: 10.1007/s12020-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 69.Mathieu C, Waer M, Carteels K, Laureys J, Bouillon R. Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin D3, KH1060. Endocrinology. 1995;136:866–72. doi: 10.1210/endo.136.3.7867594. [DOI] [PubMed] [Google Scholar]
- 70.Gregori S, Giarratana N, Smiroldo S, Uskokovie M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–74. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 71.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch Dis Child. 2008;93:512–7. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 72.Vitamin D supplement in early childhood and risk for Type I (insulin- dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia. 1999;42:51–4. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]
- 73.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 74.Fronczak CM, Baron AE, Chase HP, Ross C, Brady HL, et al. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26:3237–92. doi: 10.2337/diacare.26.12.3237. [DOI] [PubMed] [Google Scholar]
- 75.Ishii H, Suzuki H, Baba T, Nakamura K, Watanabe T. Seasonal variation of glycemic control in type 2 diabetic patients. Diabetes Care. 2001;24:1503. doi: 10.2337/diacare.24.8.1503. [DOI] [PubMed] [Google Scholar]
- 76.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 77.de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes care. 2008;31:701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scragg R, Sowers M, Bell C. Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 79.Pietschmann P, Schernthaner G, woloszczuk W. Serum osteocalcin levels in diabetes mellitus: Analysis of the type of diabetes and microvascular complications. Diabetologia. 1988;31:892–5. doi: 10.1007/BF00265373. [DOI] [PubMed] [Google Scholar]
- 80.Isaia G, Giorgino R, Adani S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24:1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 81.Mattila C, Knelct P, Mannisto S, Rissanen H, Laaksonen MA, Montonen J. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 82.Knelct P, Laaksonen M, Mattila C, Harkanen T, Marniemi J, Heliövaara M, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–71. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 83.Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38:1239–45. doi: 10.1007/BF00422375. [DOI] [PubMed] [Google Scholar]
- 84.Fliser D, Stefanski A, Frank E, Fode P, Gudarzi A, Ritz E. No effect of calcitriol on insulin-mediated glucose uptake in healthy subjects. Eur J Clin Invest. 1997;27:629–33. doi: 10.1046/j.1365-2362.1997.1520699.x. [DOI] [PubMed] [Google Scholar]
- 85.Pittas AG, Lau J, Hu B, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes: A systematic review and metaanalysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guy M, Lowe LC, Bretherton-Watt D, Mansi JL, Peckitt C, Bliss J, et al. Vitamin D Receptor Gene Polymorphisms and Breast Cancer Risk. Clin Cancer Res. 2004;10:5472–81. doi: 10.1158/1078-0432.CCR-04-0206. [DOI] [PubMed] [Google Scholar]
- 87.Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. Vitamin D and reduced risk of breast cancer: A population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:422–99. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- 88.Grant WB. An ecologic study of dietary and solar ultraviolet-B links to breast carcinoma mortality rates. Cancer. 2002;94:272–81. doi: 10.1002/cncr.10196. [DOI] [PubMed] [Google Scholar]
- 89.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qui H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J Clin Oncol. 2011;29:3775–82. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- 90.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 91.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 92.Koeffler HP, Hirjik J, Iti L. 1,25-Dihydroxyvitamin D3: In vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat Rep. 1985;69:1399–407. [PubMed] [Google Scholar]
- 93.Pálmer HG, Larriba MJ, García JM, Ordóñez-Morán P, Peña C, Peiró S, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;9:917–9. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 94.Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton SM, Dyment DA, et al. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009;5:e1000369. doi: 10.1371/journal.pgen.1000369. Epub 2009 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]


