Abstract
Statins are an established class of drugs with proven efficacy in cardiovascular risk reduction. The concern over statin safety was first raised with the revelation of myopathy and rhabdomyolysis with the use of now withdrawn cerivastatin. Enhanced understanding of the mechanisms behind adverse effects of statins including an insight into the pharmacokinetic properties have minimised fear of statin use among clinicians. Studies reveal that occurrence of myopathy and rhabdomyolysis are rare 1/100000 patient-years. The risk of myopathy/rhabdomyolysis varies between statins due to varying pharmacokinetic profiles. This explains the differing abilities of statins to adverse effects and drug interaction potentials that precipitate adverse effects. Higher dose of rosuvastatin (80 mg/day) was associated with proteinuria and hematuria while lower doses were devoid of such effects. Awareness of drugs interacting with statins and knowledge of certain combinations such as statin and fibrates together with monitoring of altered creatine kinase activity may greatly minimise associated adverse effects. Statins also asymptomatically raise levels of hepatic transaminases but are not correlated with hepatotoxicity. Statins are safe and well tolerated including more recent potent statins such as, rosuvastatin. The benefits of intensive statin use in cardiovascular risk reduction greatly outweigh risks. The present review discusses underlying causes of statin-associated adverse effects including management in high risk groups.
Keywords: Myopathy, rhabdomyolysis, safety of statins, statins
INTRODUCTION
Statins or 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) inhibitors belong to the class of lipid-lowering agents that revolutionized pharmacotherapeutics of cardiovascular diseases, leading to a remarkable decline in cardiovascular death and disability in patients with or at risk of developing coronary heart disease (CHD).[1] Batteries of clinical trials have investigated the safety and efficacy of statins in reduction of cardiovascular risks. Most trials proclaimed statins safer and tolerable medicine having considerable risk/benefit ratio with the display of only mild and transient adverse effects such as gastrointestinal symptoms, headache, and rashes.[2] The present review discusses mechanisms and safety of statin-induced adverse effects and their management.
SEARCH STRATEGY USED
We identified electronic databases, mainly MEDLINE, HighWire, Cochrane, and Google Scholar for articles from 1990 through November 2011 using keywords “3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) inhibitors or Statins,” “Safety of Statins,” “Adverse Effects of Statins,” “Statin-associated Myopathy,” “Renal safety of statins,” “Mechanism of Statin-Induced Adverse effects,” “Management of Statin-induced Adverse effects.” In MEDLINE, we used Medical Subject Heading (MeSH) terms: “Hydroxymethylglutaryl-CoA Reductase Inhibitors”[Mesh] AND Safety,” “Hydroxymethylglutaryl-CoA Reductase Inhibitors”[Mesh] AND Adverse effects.”
UNDERSTANDING SAFETY OF STATINS
Accumulating clinical trial data on safety and efficacy of statins led to framing of guideline by the National Cholesterol Education Program and Adult Treatment Panel on use of statins in individuals at high-risk of CHD and other atherosclerotic vascular diseases. High risk patients are prescribed medications for diverse illnesses and often need to take them concurrently leaving enough scope for potential drug-drug interactions, a relevant factor determining the safety profile of statins.[3] Statin possesses differing pharmacological and pharmacokinetic properties and hence differ considerably in safety and has a potential to cause drug-drug interaction. The U.S. Food and Drug Administration (FDA) adverse event report expressed concern over incidence of side-effects, which could be much higher in real clinical situations where patients are not monitored as closely as in clinical trials.[4]
In 2001, the first statin, cerivastatin was withdrawn from market worldwide after confirmed reports of serious myopathy/rhabdomyolysis.[5] The withdrawal sent wave of panic among drug manufacturers and clinicians given the fact that statin by that time had established itself as first-line medicine for reduction of CVD risk. The most important adverse effects associated with statins were asymptomatic increase in hepatic enzymes and musculoskeletal disorders such as myalgia, myopathy, and rhabdomyolysis. Its use caused myalgia in 5% patients, myopathy in 0.1 to 0.2% patients, and rhabdomyolysis in 0.01% patients.[6]
Myopathy
Myopathy or myositis is defined as a diffuse muscle symptom that accompanies elevation of plasma creatine kinase (CK) concentration 10-times higher than the upper limit of normal.[7] It is generally marked by the presence of pain, tenderness, weakness due to severe pain and restriction in mobility. Patients with normal CK levels were also reported to develop myopathic symptoms with statin therapy, indicating that assessment of CK alone cannot adequately predict statin-associated myopathy. Muscle pain in patients taking statins could also occur due to the structural damage of muscle fibers in the absence of elevated CK levels.[8] Though myopathy is a class effect of statins, the potentiality to cause myopathy varies for each statin. In general, these muscular effects have been reported more with the use of synthetic, potent, and more lipophilic statins.[9]
Rhabdomyolysis
Rhabdomyolysis is characterized by marked elevation of CK activity >50-fold, myoglobinemia, myoglobinuria, and myoglobin-induced acute renal failure (oliguria, increased plasma creatinine, potassium, and phosphorus).[10] It is more aggressive and severe form of statin-induced myopathy, resulting in severe skeletal muscle injury, lysis, and excretion of dark brown urine (indicating presence of excess myoglobin release). Rhabdomyolysis alone has been accounted for approximately 10% risk of death due to hyperkalemia-induced arrhythmias or disseminated intravascular coagulation. The risk of rhabdomyolysis was extremely rare and was no more than 5/100000 patient-years.[11] However, considering the prevalence of statin use, even small AE reports would translate into huge health consequences. Patients on lovastatin, simvastatin, and atorvastatin therapy reported higher incidences of rhabdomyolysis. This was due to higher rate of statin metabolism by hepatic microsomal enzymes, cytochrome P3A450 (CYP) isoenzymes. Several commonly prescribed drugs are potent inhibitors of CYP3A4. Concurrent use of statins with these medications increase significantly the risk of rhabdomyolysis as opposed to monotherapy; the risk more often reported in statin-fibrate combination than in statin-niacin combination.[11,12]
Hepatotoxicity
Overall occurrence of statin-induced hepatotoxicity is extremely rare but may be present as asymptomatic elevation of serum transaminases, hepatitis, cholestasis, and acute liver failure (ALF). The mechanism of statin-induced hepatotoxicity is less well-elucidated. Induction of caspase activity, triggering of apoptosis, reduction of coenzyme Q10 (CoQ10), and generation of free radicals have been reported.[13,14] Asymptomatic elevation of hepatic transaminases has been observed in 0.5-2% of patients treated with statins. Statin-induced hepatitis, associated with high levels of transaminases (>3 times the upper limit of normal), hyperbilirubinemia, and clinical symptoms of liver dysfunction was rare and was estimated to be 1/100000 patients-years.[15] Statin-induced ALF was reported to be dose- and time-dependent as reported with other statins, hence making it virtually unpredictable. Potential risk of ALF in vulnerable patients on statin therapy remains unestablished since elevated serum transaminases has no predictive value clinically for ALF.[16] Recently, FDA has recommended revision of labeling instruction for statin and suggested removal of the need for routine periodic monitoring of liver enzymes in patients taking statins. The labels now recommend that liver enzyme tests should be performed before starting statin therapy and as clinically indicated thereafter. FDA reported serious liver injury with statins to be rare and unpredictable in individual patients, and that routine periodic monitoring of liver enzymes did not appear to be effective in detecting or preventing serious liver injury. [http://www.fda.gov/Drugs/DrugSafety].
Nephrotoxicity
Most clinical trials reported renoprotective effects of statins, and only few studies reported moderate proteinuria and hematuria with statins.[10,17,18] The dose of 80 mg/day rosuvastatin caused 12% incidence of proteinuria and occasionally hematuria, which led to subsequent withdrawal of this dose. However, a rosuvastatin dose of 10 mg/day for 12 weeks dosage had no effect on total urinary protein excretion, urinary excretion of albumin or immunoglobulin G. rosuvastatin dose of 20 mg/day showed increased α-1 macroglobulin with no deleterious effect along with enhanced glomerular filtration rate.[17] In a study involving 10,289 patients on rosuvastatin and 1,17,102 on other statins, García-Rodríguez and colleagues reported only 2 out of 14 cases of acute renal failures in patients using rosuvastatin. The relative risk of death associated with use of rosuvastatin compared with other statins was reported as 0.55 (95% CI: 0.44-0.68). The authors did not find any evidence of elevated risk of rosuvastatin-induced adverse effects, including nephrotoxicity when compared to other statins. They also did not find any evidence of increased mortality among patients taking rosuvastatin, even after adjustment of age, sex, and prior statin use.[18] Therefore, as a class, statins were reported to be well-tolerated with no known differences in safety. Though myalgia, myopathy, and rhabdomyolysis occur infrequently but were more common in patients with kidney transplant and with chronic kidney disease (CKD).[19] The effect was dose-related and may be precipitated by agents inhibiting CYP-450 isoenzymes. Hence, caution is warranted while co-administering any statin with drugs that metabolize through CYP3A4, particularly fibrates, cyclosporine, and azole antifungals. Given their demonstrated efficacy and safety record coupled with enhanced understanding, statins must be used in the management of patients with established coronary disease but their use in primary prevention of cardiovascular risk warrants caution in dialysis patients who are at greater risk of toxicity and drug interactions. Elderly patients with CKD are at greater risk of adverse drug reactions and, therefore, the lowest possible dose of statins has been suggested for the treatment of hyperlipidemia. The current guidelines state that statins may be used safely in patients with chronic renal diseases and hemodialysis and suggests dose reduction in severe renal impairment.
Other rare adverse effects
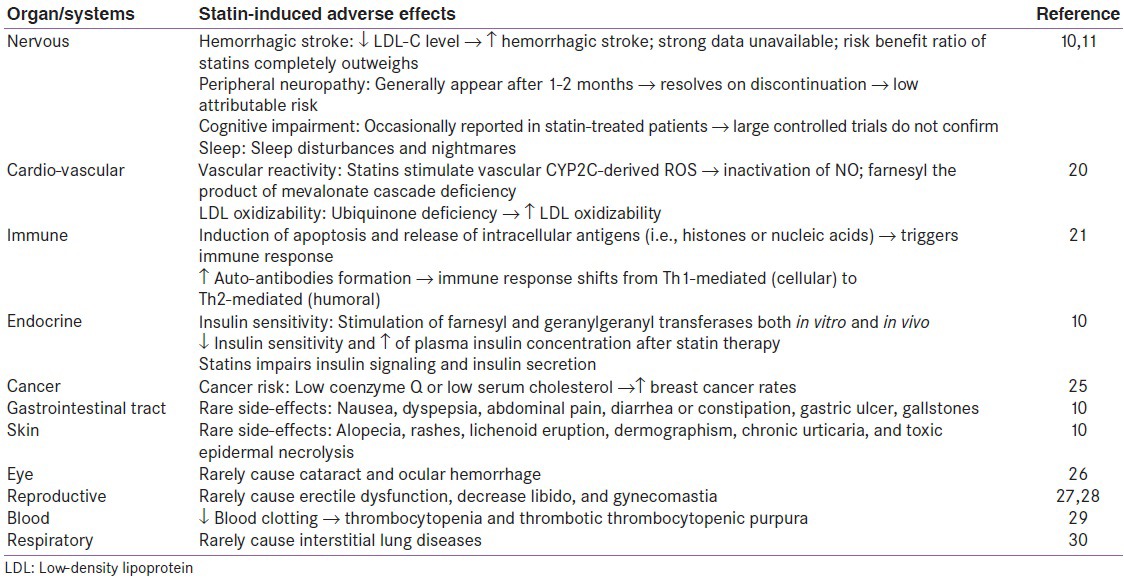
In addition to above statin-associated adverse effects, statin causes several other side-effects, which are comparatively insignificant and rare. They have been summarized in Table 1.[10,11,20,21,22,23,24,25,26,27]
Table 1.
Other rare adverse effects of statins*
MECHANISMS OF STATIN-INDUCED ADVERSE EFFECTS
Statin inhibits mevalonate synthesis by inhibiting enzyme HMG-CoA reductase that catalyzes conversion of HMG CoA to mevalonate [Figure 1]. Mevalonate not only acts as precursor of cholesterol but also serves as a precursor for non-steroid isoprenoids such as CoQ10, heme-A, and farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). These intermediates of mevalonate pathway impact the benefits as well as risk of statins.[28]
Figure 1.
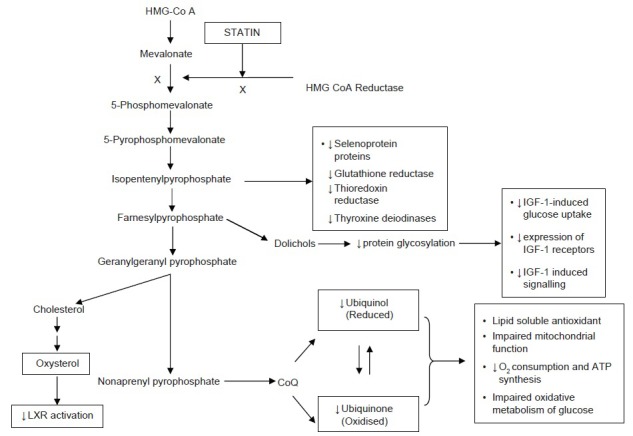
Mevalonate pathway depicting inhibition of downstream intermediate molecules resulting from statin inhibition of mevalonate synthesis. Mevalonate is not only precursor of cholesterol synthesis but also host of other molecules downstream such isopentenylpyrophosphate, farnesylpyrophosphate, geranylgeranyl pyrophosphate, dolichols etc., Statins inhibit HMG-CoA reductase, which catalyzes conversion HMG-CoA to mevalonate. Inhibition of these intermediates leads depletion of various essential molecules cause adverse effects of statins. CoQ: Coenzyme Q; IGF-1: Insulin-like growth factor-1; LXR: Liver X-receptor
Isoprenoid deficiency
Isoprenoids, FPP, and GGPP are important by-products of HMG-CoA pathway. These by-products are important component of protein isoprenylation or lipidation, a post-translational modification process where hydrophobic molecules are added to protein and activate them.[9,29] Inhibition of HMG-CoA reductase leads to decreased synthesis of these isoprenoid intermediates affecting protein isoprenylation. Alternatively, statins also promote dysprenylation (protein modification through alternate process). The 2 most important proteins affected are small GTPases and the lamins. Dysprenylation of GTPases ensue a slew of processes, including vacuolation of myofibrils, degeneration and swelling of cellular organelles and ultimately cell death. Reduction of protein isoprenylation also increases cytosolic calcium concentration and activates caspase-3 causing cell death. A role of isoprenoids in statin-induced myopathy was highlighted from the study that reported prevention of apoptosis by isoprenoid administration.[30]
Coenzyme Q
Coenzymes Q (CoQ) consists of 1,4-benzoquinone with a 50-carbon isoprenoid chain derived from FPP. Statin inhibits synthesis of mevalonate, precursor of FPP leading to inhibition of CoQ production. It has also been reported to decrease 20-40% of plasma CoQ10. CoQ10 is a lipid-soluble antioxidant synthesized by mammalian cells and is present as the reduced ubiquinol form and oxidized ubiquinone form (predominant form). It is the only antioxidant capable of regaining its active reduced form upon oxidation. This transition enables CoQ to function as electron carrier in mitochondrial respiratory chain. It acts as cofactor in mitochondrial oxidative phosphorylation and is important for adenosine triphosphate production. Statin-associated myopathy was suggested to result from inhibition of CoQ10 production in mitochondria. CoQ10 deficiency has led to several diseases, including infantile onset multi-systemic diseases, encephalomyopathies with recurrent myoglobinuria, cerebellar ataxia, myopathy, heart failure, Parkinson's disease, and malignancy.[31] It affects children more often than adults. One small clinical trial reported beneficial effect of CoQ10 supplementation in the treatment of statin-induced myopathy.[9]
Sarcolemal cholesterol deficiency
Though still debatable, a deficiency in the level of muscle cell membrane cholesterol has been suggested in alteration of the physical structure of muscle membrane, its integrity, and fluidity. These changes causes an imbalance in the dynamic equilibrium between sarcolemal membrane and plasma cholesterol and hence destabilizes muscle membrane.[32]
Selenoproteins
Selenocysteine synthesis utilizes isopentenylpyrophosphate derived from mevalonate pathway.[10,33] Selenocysteine is required to synthesize selenoproteins such as glutathione peroxidase and thioredoxin reductase (provides antioxidant defense), including thyroxine deiodinases, which catalyzes conversion of thyroxine to triiodothyronine. Statins reduces the availability of isopentenylpyrophosphate, leading to a decrease in production of selenoproteins. Selenium deficiency caused myopathy and cardiomyopathy resembling statin-induced myopathy. Hence, statins-induced deficiency of selenoproteins may impair antioxidant defense and thyroid function.
Dolichols
Dolichols are synthesized from farnesylpyrophosphate and act as carriers for oligosaccharide moiety for protein glycosylation (post-translational modification) required for protein trafficking and function. Statin impairs protein glycosylation by inhibiting dolichol production. One major consequence of statin-induced glycosylation is an impairment of insulin or insulin-like growth factor-1(IGF-1)-induced glucose uptake and proliferation of adipocytes along with reduced expression of glycosylated insulin and IGF-1 receptors and accumulation of unglycosylated receptors in endoplasmic reticulum.[34]
Drug interactions
Statin selectively inhibits HMG-CoA reductase and normally do not show any relevant affinity towards other enzymes or receptors (pharmacodynamic interaction). However, statins show significant pharmacokinetic interaction leading to potential drug-drug interactions.[35] All statins, except pravastatin, are extensively metabolized by liver that involve sets of hepatic microsomal cytochrome P450 isoenzymes. Lovastatin, simvastatin, and atorvastatin are metabolized by CYP3A4; rosuvastatin, and fluvastatin by CYP2C9 isoenzymes; pravastatin through sulfation; and pitavastatin by uridine diphosphate glucuronosyltransferase glucuronidation.[1,9] CYP3A4 isoenzymes are responsible for metabolizing most of the prescribed drugs in the liver. Concomitant use of drug and statin can alter the plasma levels of statins, leading to a risk of myopathy or rhabdomyolysis. However, about 1/3rd of prescriptions for statins are given in combination with drugs with side effects in only 3% of patients.
Both CYP450 inhibitors and inducers play an important role in disposition of statin, in terms of their plasma levels and the risk of statin-induced adverse effects [Table 2].[36] Cytochrome P450 inhibitors are defined as the agents that inhibits the production of the hepatic microsomal enzymes, leading to high plasma levels of statins and greater risk of statin-induced adverse effects like myositis and rhabdomyolysis. Cytochrome P450 inducers are defined as the agents that causes induction of hepatic microsomal enzymes, leading to decrease plasma levels of statins, and hence decreased bioavailability of stain.[1] The common inducers of CYP3A4 isoenzyme were barbiturates, phenytoin, phenobarbital, barbiturates, rifampin, dexamethasone, cyclophosphamide, carbamazepine, omeprazole, and troglitazone, and the common inhibitors were ketoconazole, itraconazole, fluconazole, erythromycin, clarithromycin, tricyclic anti-depressants, nefazodone, venlafaxine, fluvoxamine, fluoxetine, sertraline, cyclosporine A, tacrolimus, mibefradil, diltiazem, verapamil, protease inhibitors, midazolam, corticosteroids, grapefruit juice, tamoxifen, and amiodarone. The common inducers for CYP2C9 were rifampin, phenobarbital, phenytoin, and troglitazone, and the common inhibitors were ketoconazole, fluconazole, and sulfaphenazole. It is well-known that lipophilic nature of a drug influences its absorption and hydrophilic nature helps in excretion. Most statins are lipophilic in nature, except pravastatin and rosuvastatin; explaining their high safety profile over other statins.
Table 2.
Safety profiles of statins
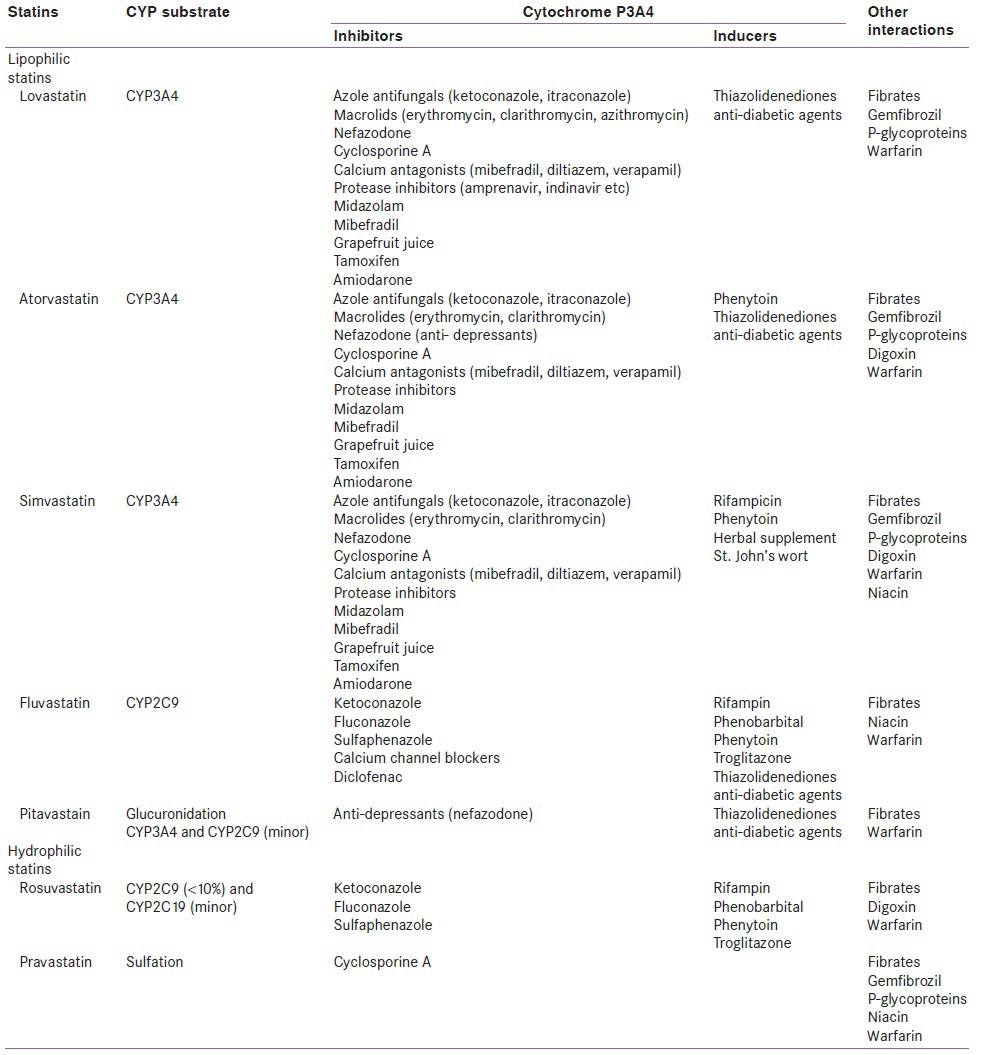
Of CYP450 inhibitors, protease inhibitors (amprenavir, indinavir, nelfinavir, ritonavir, saquinavir) are the potent inhibitors of CYP3A4, and its concurrent administration increased plasma statin concentration up to 30-fold; it causes myalgia, rhabdomyolysis, and transaminases elevations.[37] Hence, lovastatin and simvastain are not recommended with PI.
As fluvastatin, pravastatin, rosuvastatin are primarily metabolized by CYP2C9, they are less subject to drug interaction than other statins. In the presence of cyclosporine A, there is 5-23-fold increase in pravastatin bioavailability, leading to reduced biliary clearance of pravastatin and hence increased risk of myopathy. With fluvastatin, cyclosporine A shows milder interaction, which may be due to fluvastatin interaction with CYP2C9.
Intake of grapefruit juice (≥ one liter per day) also increased bioavailability of statins because of the inhibition of intestinal CYP3A4 isoenzyme. The recommended dose for simvastatin and atorvastatin was 10 mg/day, 20 mg/day for lovastatin and 5 mg/day for rosuvastatin due to competition for CYP3A4 when used concurrently with cyclosporine. Similarly, amiodarone dramatically elevated plasma levels of simvastatin levels and, therefore, dose was restricted to 20 mg/day.[9,38] Restrain is warranted in co-prescribing warfarin with statins since fluvastatin and to a lesser extent rosuvastatin are substrates for CYP2C9, which metabolizes warfarin.[38]
Interactions with other agents
Combination of statins and fibrates impairs liver functions, leading to higher levels of statins and hence myopathy. In a study, about 0.12% prevalence of myopathy associated with CK elevations has been found with combination of statins and fibrates.[39] Concurrent gemfibrozil use increased plasma levels of statins by 2-folds.[39] The risk of rhabdomyolysis with gemfibrozil was found to be 10- to 15-fold higher compared to fenofibrate because of differences in fibrate metabolism.[40]
Gemfibrozil-mediated enhancement of myopathic effects was due to competitive inhibition of specific CYP450 and UDP-glucuronosyltransferase (UGT) isoenzymes causing reduced statin clearance. The decrease in statin clearance was due to the competition for glucuronidation, which was required by both statins and fibrates for their metabolism. Statin glucuronidation is an intermediate step in the conversion of active acid forms to lactones and subsequent metabolism by the hepatic CYP450 system.[9]
There was no evidence that niacin and statin combination caused adverse effects greater than risk from individual agents.[1] However, increased risk of myopathy were reported in Chinese population given simvastatin 80 mg/day concurrently with the lipid lowering dose of niacin ≥1 g/day, leading to restriction of simvastatin dose of 40 mg/day in Chinese population on niacin therapy.[41] Statin when given along with ezetimibe increased myopathy.
Transport proteins, P-glycoproteins leads to low bioavailability of atorvastatin, lovastatin, simvastatin, pravastatin, leading to rhabdomyolysis.[1] Co-administration of atorvastatin (80 mg/day) and digoxin (0.25 mg/day) for 20 days increased exposure to digoxin by inhibition of P-glycoproteins.
Management of statin-induced adverse effects
Literature clearly documented increased risk with higher doses and serum concentrations of statins. The reported prevalence of statin-associated adverse effects are less, and among all the available statins, rate of fatal rhabdomyolysis was reported to be less than 1 death/million prescriptions.[42] The National Lipid Association (NLA) Statin Safety Task Force[5] published guidelines regarding the management of statin-associated adverse effects briefly summarized in Table 3.
Table 3.
Management of statin-associated adverse effects

HIGH RISK/VULNERABLE POPULATION TO STATIN ADVERSE EFFECTS
Statins are mostly safe, but certain population groups are at an elevated risk of developing statin-associated adverse effects and in whom careful monitoring of statins is recommended.
Alcoholics
There is lack of literature documenting prevalence of statin myopathy among alcoholics; however, excess alcohol intake has been a risk factor for rhabdomyolysis induced by pressure necrosis.[43] In the Heart Protection Study, no upper limit for alcohol consumption was set till the time liver function tests remained within an acceptable range.[44]
Pregnant women
Statins have been contraindicated in pregnancy.[44] Premenopausal women treated with statins were asked to avoid pregnancy or if they so intend, should to stop statin therapy. There have been reports of statins inducing teratogenicity and have caused congenital abnormalities in the babies of women who took statins during early pregnancy. However, further prospective clinical trial collection of data could ascertain further teratogenic potentials of statins.[45,46]
Patients on warfarin
Statins such as simvastatin, fluvastatin, and rosuvastatin have been reported to potentiate the anticoagulant effect of warfarin.[47] People requiring warfarin should check their anticoagulation control while initiating, stopping, or modifying statin therapy. However, the change in the required dose of warfarin is small, but occasionally patients may experience clinically relevant changes to their anticoagulant control.
Geriatric patients
Statins have demonstrated benefits in geriatrics in those with CHD and diabetes mellitus.[48] Future studies exploring statin efficacy in primary prevention for patients older than 75-80 years are needed along with better risk assessment tools. From a benefit risk perspective, the benefits of statin therapy in the elderly clearly outweighed the low risk of serious side effects. However, randomized trial data have shown that lowering cholesterol no longer extended life in the elderly, even those at high risk of heart disease. The elderly may be more vulnerable to known adverse effects, and evidence provides cause for concern that new risks may supervene, including cancer, neurodegenerative disease, and heart failure. The impact of statin adverse effects (e.g., muscle and cognitive problems) may be amplified in elderly, and even modest lowering of cognitive and physical function in older elderly may portend increased disability, hospitalization, institutionalization, and mortality.[49] No dose adjustment was recommended despite the fact that geriatrics may be at higher risk of developing myopathy. In randomized trials that included people above 80 year of age, the safety profile and relative benefits of statin treatment have been reported to be similar to those in young adult people. Recent literatures indicate the benefits of statin therapy in the elderly, which outweigh the low risk of serious side effects, still the use of statins in the elderly should be undertaken with circumspection and close scrutiny for any possible adverse effects.
Pediatric patients
There are limited, short-term data demonstrating that statins are apparently safe in children, though long-term follow-up is completely lacking.[50] At an 8 years of age, a child's brain and other organ systems remain in dynamic stages of growth and development, which considerably raise concern that long-term pharmacotherapy initiated at this age may adversely affect the central nervous system, immune function, hormones, energy metabolism, or other systems in unanticipated ways. Recent research suggested that increasing body weight in childhood, even within the range considered normal, was strongly associated with the risk of cardiovascular disease in adulthood.[51] The PLUTO (Pediatric Lipid-redUction Trial of rOsuvastatin) study involving adolescents, age 10 to 17 years along with other studies in nearly 1,000 pediatric patients confirmed that LDL-C lowering with statins was well tolerated in adolescents with familial hypercholesterolemia (FH).[52]
The present body of literatures on statin use in pediatric patients revealed that statins are effective at lowering LDL and TC levels and are fairly well-tolerated for the short-term period in children; therefore, currently an appropriate choice for use in FH as outlined by the clinical report and possibly for other childhood dyslipidemia with elevated TC and LDL levels after lifestyle modifications have been unsuccessful. However, appropriate monitoring of drug adverse effects and growth and development should occur in all patients.[53]
Cardiac patients
Some reports noted harmful effects of statins in patient with cardiac failure since it was observed that low cholesterol are associated with poor outcome in such patients.[54] One large study showed high levels of N-terminal pro-B type natiuretic peptide (N-BNP), which was predictive of cardiac failure, received similar cardiovascular benefits with simvastatin compared with patients without cardiovascular hazard.[55]
Kidney function
Although statins are considered safe in moderate renal impairment, but patients having glomerular filtration rate in the range of 30-60 mL/min were at a higher cardiovascular risk. Data suggests statins beneficial in these subgroups, but they may be at a higher risk of myopathy. One trial showed no cardiovascular benefits with atorvastatin 20 mg/day in patients with diabetes on maintenance hemodialysis; therefore, role of statins for the prevention of cardiovascular disease in patients with chronic kidney disease is less well-understood).[56]
A meta-analysis of 36 studies that included 40,600 participants assessed the effects of rosuvastatin on the renal safety. The study suggested that intensive LDL-C-lowering treatment with rosuvastatin did not affect the risk of developing renal insufficiency or renal failure in patients who do not have advanced, pre-existing renal disease.[57] The study supported that rosuvastatin may be safely used in renal-compromised patients.
INCREASING SAFETY OF STATINS
Statins may be classified into 3 categories based on their increasing potency and efficacy in lowering plasma low-density lipoprotein cholesterol (LDL-C) concentration. The first generation statins included lovastatin, pravastatin, and fluvastatin; simvastatin and atorvastatin among second generation; and rosuvastatin and pitavastatin among third generation statins.
First generation statins
The first generation statins (FGS) were introduced during the late 1980s and 1990s, and this class of statins had the lowest potency. Among FGS, pravastatin was the most studied statin, and several clinical trials showed reduction in LDL-C levels, cardiac mortality, and coronary events.[58] In secondary prevention and symptomatic coronary disease patients too, pravastatin was proved to be effective. Though the adequate evidence is lacking, lovastatin and fluvastatin also demonstrated benefited cardiovascular risk reduction. In the FGS, pravastatin and fluvastatin commanded much attention because of their low drug interaction as they are not metabolized by CYP450 isoenzyme systems. Hence, in spite of their low potency, they are used as an alternative in patients who are intolerant to potent statins.
Second generation statins
The second generation of statins (SGS) was marked by introduction of atorvastatin and simvastatin. Even today, they are considered as the best selling statins. These statins had superior efficacy in lowering plasma LDL-C levels than FGS. The daily doses of only 10 mg atorvastatin and 20 mg simvastatin caused greater than 30% lowering of LDL compared with 20-40 mg daily doses of FGS. Battery of trials demonstrated their use in both primary and secondary trials. Trials to study intensive versus moderate statin therapy for maximizing LDL-C lowering and to achieve better cardiovascular outcomes would be possible only with the availability of more potent and superior SGS. Intensive statin therapy was mostly directed at secondary prevention patients who mostly were benefited from aggressive lipid-lowering agents. The pharmacological demonstration of atorvastatin and simvastatin drug-drug interaction is now well-established and had raised many eyebrows in the use of SGS in high-risk patients. However, various clinical trials demonstrated adequate safety and efficacy of aggressive lipid-lowering in high-risk patients with SGS.[59,60,61,62] Wider information on statin drug interactions and monitoring of the statin adverse effects would further help in minimizing statin-induced myopathy.
Third generation statins (rosuvastatin)
Third generation statins (TGS) included rosuvastatin and pitavastatin, which had high potency and efficacy and thus termed as super statins.[58] Rosuvastatin owes remarkable potency and efficacy due to its fluorinated phenyl group and hydrophilic methane sulphonamide group in addition to the common dihydroxyheptenoic acid side chain. Its unique chemical structure enables multiple and strong binding with HMG-CoA reductase enzyme. It has low drug interaction potential due to its hydrophilic nature, which avoids biotransformation for conversion into water-soluble intermediates for elimination.[62] Pitavastatin also have several clinical advantages over FGS and SGSs. It's lowering potentiality of serum LDL-C was greater than pravastatin but was similar to atorvastatin. It is primarily metabolized through glucuronidation, and only minor fractions are metabolized by CYP2C9 and CYP3A4. Therefore, pitavastatin is hardly metabolized by microsomal cytochrome P450 system compared to other statins and hence has an advantage of not having unexpected interactions with other drugs. These TGSs are used as an alternative to other statins in high-risk patients who more often develop statin intolerance.
CONCLUSION
Almost all the statin trials reported statins to be safe and tolerable. However, in the August 2001, withdrawal of cerivastatin caused widespread ripples among clinicians because of their wide usage in reduction of cardiovascular morbidity and mortality. The revelation that statins may cause fatal rhabdomyolysis raised questions on the safety of statin. Later, several clinical trials dispelled this notion, and the current guidelines suggested dose reduction and halting of statin therapy only in extreme conditions. Subsequent to the rise of safety issues, new potent statins such as rosuvastatin has been scrutinized regarding high dose (80 mg/day) that caused proteinuria and hematuria. However, these effects were transient and reversible, requiring just the reduction of the dosage. The understanding of relatively common statin-associated adverse effects will enable clinicians in making decision in choosing out appropriate statin for their patients giving due consideration to the fact that benefits of statins greatly outweigh its risks.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bellosta S, Paoletti R, Corsinin A. Safety of statins: Focus on clinical pharmacokinetics and drug interaction. Circulation. 2004;109(III):50–7. doi: 10.1161/01.CIR.0000131519.15067.1f. [DOI] [PubMed] [Google Scholar]
- 2.Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: A systematic overview of randomized clinical trials. Circulation. 2006;114:2788–97. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 3.National cholesterol education program (NCEP) expert panel on detection, Evaluation and treatment of high cholesterol in adults (Adult Treatment Panel III). Third report of the national cholesterol education program expert panel on Detection, Evaluation and treatment of high cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 4.Omar MA, Wilson JP. FDA adverse event reports on statin associated rhabdomyolysis. Ann Pharmacother. 2002;36:288–95. doi: 10.1345/aph.1A289. [DOI] [PubMed] [Google Scholar]
- 5.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusion and recommendations of the National lipd association statin safety assessment task force. Am J Cardiol. 2006;97:89–94. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson TA. Toward pain free statin prescribing clinical algorithm for diagnosis and management of myalgia. Mayo Clinic Proceedings. 2008;83:87–700. doi: 10.4065/83.6.687. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton-Craig I. Statin-associated myopathy. Med J Aust. 2001;175:486–9. doi: 10.5694/j.1326-5377.2001.tb143683.x. [DOI] [PubMed] [Google Scholar]
- 8.Mohaupt MG, Karas RH, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L, et al. Association between statin-associated myopathy and skeletal muscle damage. CMAJ. 2009;181:E11–8. doi: 10.1503/cmaj.081785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abd TT, Jacobson TA. Statin induced myopathy: A review and myopathy. Expert Opin Drug Saf. 2011;10:373–87. doi: 10.1517/14740338.2011.540568. [DOI] [PubMed] [Google Scholar]
- 10.Beltowski J, Wojcicka G, Jamroz-Wisniewska A. Adverse effects of statins-mechanisms and consequences. Curr Drug Saf. 2009;4:209–28. doi: 10.2174/157488609789006949. [DOI] [PubMed] [Google Scholar]
- 11.Law M, Ridnicka AR. Statin safety: Systematic review. Am J Cardiol. 2006;97:52–60. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Linares LA, Golomb BA, Jaojoco JA, Sikand H, Phillips PS. The modern spectrum of rhabdomyolysis: Drug toxicity revealed by creatine kinase screening. Curr Drug Saf. 2009;4:181–7. doi: 10.2174/157488609789007010. [DOI] [PubMed] [Google Scholar]
- 13.Kubota T, Fujisaki K, Itoh Y, Sendo T, Oishi R. Apoptotic injury in cultured human hepatocytes induced by HMG CoA reductase inhibitors. Biochem Pharmacol. 2004;67:2175–86. doi: 10.1016/j.bcp.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 14.Nakad A, Bataille L, Hamoir V, Sempoux C, Horsmans Y. Atorvastatin-induced acute hepatitis with absence of cross-toxicity with simvastatin. Lancet. 1999;353:1763–4. doi: 10.1016/S0140-6736(99)00569-3. [DOI] [PubMed] [Google Scholar]
- 15.de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: A meta-analysis. Pharmacotherapy. 2004;24:584–91. doi: 10.1592/phco.24.6.584.34738. [DOI] [PubMed] [Google Scholar]
- 16.Brewer HB., Jr Benefit risk assessment of rosuvastatin 10 to 40 miligrams. Am J Cardiol. 2003;92:23–9. doi: 10.1016/s0002-9149(03)00779-3. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748–55. doi: 10.1016/j.amjcard.2005.09.110. [DOI] [PubMed] [Google Scholar]
- 18.García-Rodríguez LA, Massó-González EL, Wallander MA, Johansson S. The safety of rosuvastatin in comparison with other statins in over 100,000 statin users in UK primary care. Pharmacoepidemiol Drug Saf. 2008;17:943–52. doi: 10.1002/pds.1603. [DOI] [PubMed] [Google Scholar]
- 19.Olyaei A, Greer E, Delos Santos R, Rueda J. The efficacy and safety of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors in chronic kidney disease, dialysis, and transplant patients. Clin J Am Soc Nephrol. 2011;6:664–78. doi: 10.2215/CJN.09091010. [DOI] [PubMed] [Google Scholar]
- 20.Lankin VZ, Tikhaze AK, Kukharchuk VV, Tutunov VS, Medvedeva NV, Kotkina TI, et al. Antioxidants decrease the intensification of low density lipoprotein in vivo peroxidation during therapy with statins. Mol Cell Biochem. 2003;249:129–40. [PubMed] [Google Scholar]
- 21.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, Numabe A, Saito K, Negoro H, et al. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93:948–56. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 22.Portakal O, Ozkaya O, Erden IM, Bozan B, Kosan M, Sayek I. Coenzyme Q10 concentrations and antioxidant status in tissues of breast cancer patients. Clin Biochem. 2000;33:279–8. doi: 10.1016/s0009-9120(00)00067-9. [DOI] [PubMed] [Google Scholar]
- 23.Fraunfelder FW. Ocular hemorrhage possibly the result of HMG CoA reductase inhibitors. J Ocul Pharmacol Ther. 2004;20:179–82. doi: 10.1089/108076804773710858. [DOI] [PubMed] [Google Scholar]
- 24.de Graaf L, Brouwers AH, Diemont WL. Is decreased libido associated with the use of HMG-CoA-reductase inhibitors? Br J Clin Pharmacol. 2004;58:326–8. doi: 10.1111/j.1365-2125.2004.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiortsis DN, Filippatos TD, Mikhailidis DP, Elisaf MS, Liberopoulos EN. Statin-associated adverse effects beyond muscle and liver toxicity. Atherosclerosis. 2007;195:7–16. doi: 10.1016/j.atherosclerosis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Szczeklik A, Undas A, Musial J, Gajewski P, Swadzba J, Jankowski M. Antithrombotic actions of statins. Med Sci Monit. 2001;7:138–5. [PubMed] [Google Scholar]
- 27.Fernández AB, Karas RH, Alsheikh-Ali AA, Thompson PD. Statins and interstitial lung disease: A systematic review of the literature and of food and drug administration adverse event reports. Chest. 2008;134:824–30. doi: 10.1378/chest.08-0943. [DOI] [PubMed] [Google Scholar]
- 28.Buhaescu I, Izzedine H. Mevalonate pathway: A review of clinical and therapeutical implications. Clin Biochem. 2007;40:575–84. doi: 10.1016/j.clinbiochem.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Vaklavas C, Chatzizsis YS, Ziakas A, Zamboulis C, Giannoglou GD. Molecular basis statin associated myopathy. Atherosclerosis. 2009;202:18. doi: 10.1016/j.atherosclerosis.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Guijarro C, Blanco-Colio L, Ortego M, Alonso C, Ortiz A, Plaza JJ, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase and isoprenylation inhibitors induce apoptosis of vascular smooth muscle cells in culture. Circ Res. 1998;83:490. doi: 10.1161/01.res.83.5.490. [DOI] [PubMed] [Google Scholar]
- 31.Quinzii CM, Hirano M, Mauro S. CoQ10 deficiency diseases in adults. Mitochondrion. 2007;7:S 122–6. doi: 10.1016/j.mito.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita I, Sato I, Ma L, Murota S. Enhancement of membrane fluidity in cholesterol-poor endothelial cells pre-treated with simvastatin. Endothelial. 1997;5:107–13. doi: 10.3109/10623329709079868. [DOI] [PubMed] [Google Scholar]
- 33.Moosmann B, Behl C. Selenoprotein synthesis and side effects of statins. Lancets. 2004;363:892–4. doi: 10.1016/S0140-6736(04)15739-5. [DOI] [PubMed] [Google Scholar]
- 34.Skorupinska-Tudek K, Wojcik J, Swiezewska E, Danikiewicz W. Polyisoprenoids: Structure, biosynthesis and function. Prog Lipid Res. 2005;44:235–58. doi: 10.1016/j.plipres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Bottorff M. Statin safety and drug interactions: Clinical implications. Am J Cardiol. 2006;97:27C–31. doi: 10.1016/j.amjcard.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Ray GM. Antiretroviral and statin drug-drug interactions. Cardiol rev. 2009;17:44. doi: 10.1097/CRD.0b013e3181903b7f. [DOI] [PubMed] [Google Scholar]
- 37.Becquemont L, Neuvonen M, Verstuyft C, Jaillon P, Letierce A, Neuvonen PJ, et al. Amiodarone interacts with simvastatin but not with pravastatin disposition kinetics. Clin Pharmacol Ther. 2007;81:679–84. doi: 10.1038/sj.clpt.6100098. [DOI] [PubMed] [Google Scholar]
- 38.Andrus MR. Oral anticoagulant drug interactions with statins: Case report of fluvastatin and review of the literature. Pharmacotherapy. 2004;24:285–90. doi: 10.1592/phco.24.2.285.33137. [DOI] [PubMed] [Google Scholar]
- 39.Shek A, Ferrill MJ. Statin – fibrate combination therapy. Ann Pharmacother. 2001;35:908–17. doi: 10.1345/aph.10315. [DOI] [PubMed] [Google Scholar]
- 40.Jones P, Davidson M. Reporting rate of rhabdomyolysis with fenofibrate+statin versus gemfibrozil+any statin. Am J Cardiol. 2005;95:120–32. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 41.Bays H. Safety of niacin and simvastatin combination therapy. Am J Cardiol. 2008;101:53–8. doi: 10.1016/j.amjcard.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Guthrie RM. How safe is aggressive statin therapy? Prog Cardiovasc Nurs. 2006;21:140–5. doi: 10.1111/j.0889-7204.2006.05616.x. [DOI] [PubMed] [Google Scholar]
- 43.Lane R, Phillips M. Rhabdomyolysis. BMJ. 2003;327:115–6. doi: 10.1136/bmj.327.7407.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melli G, Chaudhary V, Cornblath DR. Rabdomyolysis an evaluation of 475 hospitalised patients. Medicine (Baltimore) 2005;84:377–85. doi: 10.1097/01.md.0000188565.48918.41. [DOI] [PubMed] [Google Scholar]
- 45.Edison RJ, Meunke M. Central nervous system and limb anomalies in case reports of first trimester statin exposure. N Engl J Med. 2004;350:1579–82. doi: 10.1056/NEJM200404083501524. [DOI] [PubMed] [Google Scholar]
- 46.Lankas GR, Cukierski MA, Wise LD. The role of maternal toxicity in lovastatin induced developmental toxicity. Birth Defects Res B Dev Reprod Toxicol. 2004;71:111–23. doi: 10.1002/bdrb.20005. [DOI] [PubMed] [Google Scholar]
- 47.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–90. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 48.Golomb BA. Implications of statin adverse effects in the elderly. Expert Opin Drug Saf. 2005;4:389–97. doi: 10.1517/14740338.4.3.389. [DOI] [PubMed] [Google Scholar]
- 49.Walker DB, Jacobson TA. Initiating statins in the elderly: The evolving challenge. Curr Opin Endocrinol Diabetes Obes. 2008;15:182–7. doi: 10.1097/MED.0b013e3282f7cd6d. [DOI] [PubMed] [Google Scholar]
- 50.de Ferranti S, Ludwig DS. Storm over Statins: The Controversy Surrounding Pharmacologic Treatment of Children. N Engl J Med. 2008;359:1309–12. doi: 10.1056/NEJMp0805953. [DOI] [PubMed] [Google Scholar]
- 51.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avis HJ, Hutten BA, Gagné C, Langslet G, McCrindle BW, Wiegman A, et al. Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. J Am Coll Cardiol. 2010;55:1121–6. doi: 10.1016/j.jacc.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 53.Eiland LS, Luttrell PK. Use of statins for dyslipidemia in the pediatric population. J Pediatr Pharmacol Ther. 2010;15:160–2. [PMC free article] [PubMed] [Google Scholar]
- 54.Ashton E, Liew D, Krum H. Should patients with chronic heart failure be treated with “statins”? Heart Fail Monit. 2003;3:82–6. [PubMed] [Google Scholar]
- 55.Khush KK, Waters DD, Bittner V, Deedwania PC, Kastelein JJ, Lewis SJ, et al. Effect of high-dose atorvastatin on hospitalizations for heart failure: Subgroup analysis of the Treating to New Targets (TNT) study. Circulation. 2007;115:576–83. doi: 10.1161/CIRCULATIONAHA.106.625574. [DOI] [PubMed] [Google Scholar]
- 56.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 57.Stein EA, Vidt DG, Shepherd J, Cain VA, Anzalone D, Cressman MD. Renal safety of intensive cholesterol-lowering treatment with rosuvastatin: A retrospective analysis of renal adverse events among 40,600 participants in the rosuvastatin clinical development program. Atherosclerosis. 2012;221:471–7. doi: 10.1016/j.atherosclerosis.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Kapur NK, Mususnuru K. Clinical efficacy and safety of statins in managing cardiovascular risk. Vasc Healt Risk Manag. 2008;4:341–53. doi: 10.2147/vhrm.s1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 60.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, et al. High-dose atorvastatin versus usual dose simvastatin for secondary prevention after myocardial infarction: The IDEAL study: A randomised controlled trial. JAMA. 2005;294:2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 61.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndrome. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 62.Holdgate GA, Ward WH, Mc Taggert F. Molecular mechanism for inhibition of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase by rosuvastatin. Biochem Soc Trnans. 2003;31:521–31. doi: 10.1042/bst0310528. [DOI] [PubMed] [Google Scholar]


