Abstract
Background:
Gestational diabetes mellitus (GDM) is a metabolic disorder defined as glucose intolerance with the onset or first recognition during pregnancy. Women with GDM are at increased risk for adverse obstetric and perinatal outcome. Hence, it is imperative that an early detection and management of the disease is done to ensure better maternal and fetal outcomes.
Aims:
This study was done to evaluate the prevalence of gestational diabetes using diabetes in pregnancy Study Group India (DIPSI) criteria and further assess its feto-maternal outcome in western Rajasthan.
Materials and Methods:
This study was carried out in 500 patients between 24 and 28 weeks of gestation, attending the antenatal outdoor. These patients were given 75 g oral glucose irrespective of the meals and their plasma glucose was estimated at 2 h. Patients with plasma glucose values 140 mg/dl were labeled as GDM and the rest as the control or the non-GDM group. All GDM patients were followed up and treated with diet and/or insulin therapy till delivery to know maternal and fetal outcomes.
Results:
The prevalence of GDM in this study was 6.6%. Maternal and fetal complications in the GDM group were much higher than in the non-GDM group. Hypertension, vaginal candidiasis, and abruptio placentae were the common maternal complications, while macrosomia and stillbirths occurred in the fetuses.
Conclusion:
GDM as a disease entity adversely affects maternal and fetal outcomes. This also builds a strong case for following DIPSI guidelines in diagnosis and management of GDM.
Keywords: Diabetes in pregnancy study group india criteria, gestational diabetes mellitus, glucose intolerance, macrosomia
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with the onset or first recognition during pregnancy with or without remission after the end of pregnancy.[1] GDM is important in that it poses a risk to the pregnant woman and her baby. GDM is associated with higher incidence of maternal mellitus later in life.[2] The major morbidities associated with infants of diabetic mothers include respiratory distress, growth restriction, polycythemia, hypoglycemia, hypocalcemia, and hypomagnesemia, and congenital malformations.[3] Perinatal outcomes associated with poor glycemic control in mothers are associated with as high as 42.9% mortality.[4] Appropriate diagnosis and management of GDM can improve maternal and perinatal outcome. Many studies have been done in various parts of India on gestational diabetes, like Seshiah et al. in Chennai, Wahi et al. in Jammu, and Gajjar in Baroda, Gujarat.[5,6,7]
Very little data is available with regard to the prevalence of GDM from western Rajasthan which forms the Great Thar Desert. People here face living conditions different from their fellow countrymen, like arid climate, paucity of resources, and illiteracy. This is quite strange as a lot of research focus today is on the well-being of mother and the newborn child in general and the situation of GDM or diabetes in particular. The present study, therefore, has compiled data regarding the prevalence of GDM from western Rajasthan and its effect on pregnancy outcomes. In the present study, the Diabetes in Pregnancy Study Group India (DIPSI) guidelines have been followed for screening of subjects, so that a uniform protocol followed by similar groups in other parts of the country could enable a fair and judicious correlation with each other. Besides, DIPSI guidelines also facilitate both economical and feasible mode of evaluation. DIPSI diagnostic criterion of 2 h plasma glucose more than 140 mg/dl with 75 g oral glucose load is a modified version of WHO guidelines in that WHO procedure requires women to be in the fasting state, whereas DIPSI procedure is performed irrespective of the last meal timing.[1]
MATERIALS AND METHODS
The present study was conducted at the antenatal clinic in the Department of Obstetrics and Gynaecology at a medical college hospital, which is the largest public sector hospital in the western Rajasthan. A total of 500 patients were screened for GDM. The inclusion criteria included pregnant women at 24th-28th week of gestation, while all patients with h/o of DM prior to onset of pregnancy, major chronic diseases like carcinoma, tuberculosis, congestive cardiac failure (CCF), renal failure, and advanced liver failure were excluded from the present study. A standardized questionnaire was used, and details pertaining to family history, medical and obstetric history were collected. Body mass index (BMI) and blood pressure (BP) were also recorded. Informed consent was taken from the patients. Pregnant women were given 75 g oral glucose load irrespective of their last meal timing and venous blood sample was drawn at 2 h. The plasma glucose was estimated in the central laboratory by the glucose oxidase-peroxidase (GOD-POD) method.[18]
Diagnosis of GDM
The criterion used was if the 2 h venous plasma glucose measured after 75 g oral glucose load in non-fasting state was ≥140 mg/dl (DIPSI criteria) the patient was labeled as GDM.[19] The rest were classified as the normal glucose tolerant or the non-GDM group. GDM women were advised medical nutrition therapy (MNT) for 2 weeks. Those who did not respond by maintaining fasting plasma glucose (FPG) ≤90 mg/dl and peak post-meal glucose ≤120 mg/dl were advised insulin. All of them were followed until delivery. The antenatal and the postnatal course of the women and the perinatal outcome were studied.
Statistical analysis
Results were expressed as number and percentages. Chi-square test for proportions was used for comparing GDM and control. P < 0.05 were considered to be significant.
RESULTS
A total of 500 subjects at 24-28 weeks of gestation were evaluated for GDM using the DIPSI criteria. Out of 500 subjects, 33 (6.6%) were diagnosed as GDM. The remaining formed the non-GDM group. The mean age of the patients was 25.33 ± 3.17 years. Table 1 shows the comparison of prevalence of risk factors between GDM and non-GDM population. Family history of diabetes mellitus, age ≥25 years, past history of GDM, and BMI ≥25 kg/m2 were significantly associated with GDM group (P < 0.001).
Table 1.
Comparison of prevalence of risk factors in GDM and non-GDM population
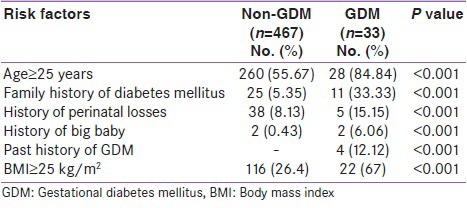
Table 2 shows the distribution of associated complications such as pregnancy-induced hypertension (PIH), vaginal candidiasis, and abruption placentae. The prevalence of all these complications was higher in the GDM group than in the non-GDM group, with statistical significance (P < 0.01).
Table 2.
Distribution of associated complications between non-GDM and GDM population
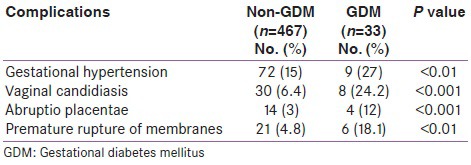
Table 3 shows statistical correlation between delivery outcomes in women with and without GDM. Prevalence of Cesarean delivery and assisted vaginal delivery was statistically higher in non-GDM group than in GDM group (P < 0.001). Although the percent prevalence of shoulder dystocia and postpartum hemorrhage (PPH) was higher in GDM than in non-GDM group, it did not reach statistical significance.
Table 3.
Delivery outcomes in non-GDM and GDM population
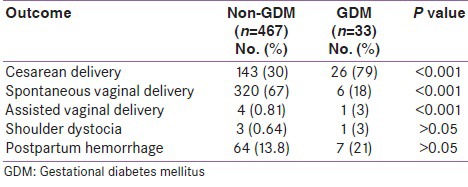
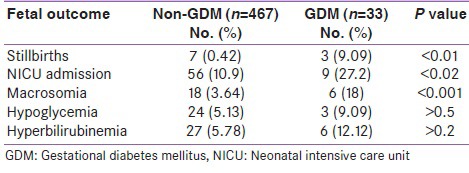
Table 4 shows the fetal outcomes in the study group. The prevalence of stillbirths, macrosomia, and neonatal intensive care unit (NICU) admissions was higher in the GDM group than in the non-GDM group, with statistical significance (P < 0.05). The prevalence of hypoglycemia and hyperbilirubinemia was higher in GDM than in non-GDM group, but it did not reach statistical significance.
Table 4.
Fetal outcomes in non-GDM and GDM population
In the present study, out of 33 GDM patients, 14 (42.4%) patients were managed on both diet and insulin, while 19 (57.57%) were managed on diet alone. Premix insulin (30/70) was given starting with an initial dose of 4U subcutaneously and was increased according to the fasting and 2hr pp plasma glucose levels.
DISCUSSION
The present prospective hospital-based study, which is the first of its kind to be undertaken in this part of the country, showed the prevalence of GDM as 6.6%. GDM prevalence has been reported variably from 1.4 to 14% worldwide and differently among racial and ethnic groups. Prevalence is higher in Blacks, Latino, Native Americans, and Asian women than in White women. Compared to European women, the prevalence of gestational diabetes has increased 11-fold in women from the Indian subcontinent. Nilofer in Davengere, Karnataka, performed a similar study and found a prevalence rate of 6%.[8] Wahi et al. from Jammu found a prevalence rate of 6.94%.[6] DIPSI guidelines having suggested one-time plasma sugar level as a measure to detect GDM is an attempt to predict future possibility and predisposition for diabetes mellitus.
Our findings of this study are largely at tandem with those of literature at the national as well as international level. We, therefore, infer from the above study that western Rajasthan, despite its hot and arid climate, varying ethnicity, food habits, and living standards is very much a part of diabetes spectrum the world over.
Compared with non-GDM subjects, GDM patients were older, with the mean ages of the two groups being 24.7 ± 3.11 years and 27.1 ± 2.44 years, respectively. Similar study from South India showed age > 25 years as a risk factor for GDM.[9] Obesity as a significant risk factor for GDM is supported by several studies finding that overweight or obesity at the start of pregnancy predisposes to GDM. Das et al. and Gomez et al. found that 25% and 50% of women with GDM, respectively, had obesity.[10,11] This may be due to increased demands on maternal metabolism during pregnancy from excess weight, resulting in imbalances in hormonal carbohydrate regulation mechanisms, and insulin sensitivity. Nilofer found obesity as a risk factor in 88.89% of GDM patients.[8] In our study, a significant proportion of subjects with GDM were overweight [22 (66.67%)] and obese [6 (18.18%)]. Similar results were also found by Garshasbi in Iran.[12] Family history of diabetes mellitus was found in 33.3% of our GDM women. Interestingly, history of diabetes in mother was twice as common as history of diabetes in father (18.18% vs. 9.09%). Probably the mothers of GDM women might also have had suffered from GDM in their pregnancies but remained undetected, hence supporting the familial association of GDM.
Our study shows that 15.15% of GDM mothers had history of previous fetal or early neonatal deaths. Hoseini conducted a similar study in Iran on 227 patients and found that 12.3% of the GDM women had history of previous fetal or early neonatal deaths.[13] Wahi et al. also found 24.9% of their GDM patients with a positive family history of perinatal losses.[6] Insulin being a potent growth factor promotes lipogenesis, protein synthesis, and therefore growth of the fetus. Hence, history of prior delivery of a big baby or a macrosomic baby (birth weight > 4 kg) is also indicative of existence of GDM in previous pregnancies. In our study, 6.06% of GDM women gave history of previous delivery of big baby.
Our study revealed that the most common complications seen in GDM mothers were gestational hypertension (36.4%) followed by vaginal candidiasis (24.2%), premature rupture of membranes (PROM; 18.1%), and abruptio placentae (12.12%). Gajjar found that most common maternal complication seen in GDM mothers was gestational hypertension (36.4%) followed by abruptio placentae (20%).[7] Another study of 972 GDM mothers in Saudi Arabia showed that the common complications were perineal tear (18%) that caused postpartum hemorrhage, followed by gestational hypertension (2%).[14]
Gajjar found a Cesarean rate of 19.5% in the GDM patients.[7] Cesarean delivery rate in our study was 78.8% amongst the GDM patients, with the most common indication being arrest of labor. This is quite high probably because in our setup, there is lack of adequate intrapartum fetal monitoring and surveillance techniques due to less infrastructure and greater patient load. Hence, lesser number of high-risk patients are given trial of labor andhence mor number of patients are delivered by Lower Segment C esarean Section. Our study demonstrated that 18.1% of newborns of GDM mothers were macrosomics as opposed to 3.64% in the non-GDM group. Hong et al. also found an incidence of 6.5% of macrosomia in the GDM group.[15] Our study showed that prevalence of stillbirth was 9.09% in GDM deliveries. In a study conducted by Odar in Uganda, a stillbirth rate of 16.7% was found.[16] The incidence of hypoglycemia and hyperbilirubinemia were 9.09% and 12.12%, respectively, which were in concordance with the observations of a case control study done in Brazil.[17] The incidence of hypoglycemia and hyperbilirubinemia in that study were 16.3% and 6.1%, respectively.
CONCLUSION
Women with GDM are at an increased risk for adverse obstetric and perinatal outcomes, and western Rajasthan, in spite of its unique geographic and socioeconomic features, is no exception to it. Although eradication of GDM is impossible, we can definitely prevent its adverse effects on pregnancy outcome.
ACKNOWLEDGMENT
We would like to thank Dr. Sumitra Bora, Professor and Head, Dept. Of Obstetrics and Gynaecology and Dr. S. L. Mathur, Professor and Consultant Diabetologist, Dr. S. N. Medical College, Jodhpur, Rajasthan, for providing their support for the study. We also thank Mr. Sunil Bhati, Statistician, for helping with the statistics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Seshiah V, Das AK, Balaji V, Joshi SR, Parikh MN, Gupta S Diabetes in Pregnancy Study Group. Gestational diabetes mellitus-guidelines. J Assoc Physicians India. 2006;54:622–8. [PubMed] [Google Scholar]
- 2.Davey RX, Hamblin PS. Selective versus universal screening for gestational diabetes mellitus: An evaluation of predictive risk factors. Med J Aust. 2001;174:118–21. doi: 10.5694/j.1326-5377.2001.tb143181.x. [DOI] [PubMed] [Google Scholar]
- 3.Opara PI, Jaja T, Onubogu UC. Morbidity and mortality amongst infants of diabetic mothers admitted into a special care baby unit in Port Harcourt, Nigeria. Ital J Pediatr. 2010;36:77. doi: 10.1186/1824-7288-36-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otolorin EO, Famuyiwa OO, Bella AF, Dawodu AH, Adelusi B. Reproductive performance following active management of diabetic pregnancies at the university college hospital, Ibadan, Nigeria. Afr J Med Med Sci. 1985;14:155–60. [PubMed] [Google Scholar]
- 5.Balaji V, Balaji M, Anjalakshi C, Cynthia A, Arthi T, Seshiah V. Diagnosis of gestational diabetes mellitus in Asian-Indian women. Indian J Endocrinol Metab. 2011;15:187–90. doi: 10.4103/2230-8210.83403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of gestational diabetes mellitus and its outcomes in Jammu region. J Assoc Physicians India. 2011;59:227–30. [PubMed] [Google Scholar]
- 7.Gajjar F, Maitra K. Intrapartum and perinatal outcomes in women with gestational diabetes and mild gestational hyperglycemia. J Obstet Gynaecol India. 2005;55:135–7. [Google Scholar]
- 8.Nilofer AR, Raju VS, Dakshayini BR, Zaki SA. Screening in high-risk group of gestational diabetes mellitus with its maternal and fetal outcomes. Indian J Endocrinol Metab. 2012;16:74–8. doi: 10.4103/2230-8210.94268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seshiah V, Balaji V, Balaji MS. Prevalence of gestational diabetes mellitus in south India (Tamil Nadu) – a community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 10.Das V, Kamra S, Mishra A. Screening for gestational diabetes and maternal and fetal outcome. J Obstet Gynaecol India. 2004;54:449–51. [Google Scholar]
- 11.Gómez HL, Martínez ML, Rodríguez ZM. Clinical and epidemiological profile of diabetes mellitus in pregnancy, Isle of youth, 2008. MEDICC Rev. 2011;13:29–34. doi: 10.37757/MR2011V13.N1.8. [DOI] [PubMed] [Google Scholar]
- 12.Garshasbi A, Faghihzadeh S. The prevalence of gestational diabetes mellitus and its risk factors in Tehran. J Fam Reprod Health. 2008;2:75–80. [Google Scholar]
- 13.Hoseini S, Hantoushzadeh S, Shoar S. Evaluating the extent of pregravid risk factors of gestational diabetes mellitus in women in Tehran. Iran Red Crescent Med J. 2011;13:407–14. [PMC free article] [PubMed] [Google Scholar]
- 14.El-Mallah KO, Narchi H, Kulaylat NA, Shaban MS. Gestational and pre-gestational diabetes: Comparison of maternal and fetal characteristics and outcome. Int J Gynaecol Obstet. 1997;58:203–9. doi: 10.1016/s0020-7292(97)00084-2. [DOI] [PubMed] [Google Scholar]
- 15.Hong JU, Rumbold AR, Wilson KJ, Crowther CA. Borderline gestational diabetes mellitus and pregnancy outcomes. BMC Pregnancy Childbirth. 2008;8:31. doi: 10.1186/1471-2393-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odar E, Wandabwa J, Kiondo P. Maternal and fetal outcome of gestational diabetes mellitus in Mulago hospital, Uganda. Afr Health Sci. 2004;4:9–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Madi JM, Viecceli C, Barazzetti DO, Pavan G, Triches CB, Araújo BF. Gestational diabetes and perinatal outcomes: A case control study. Journal of Medicine and Medical Science. 2011;2:1022–7. [Google Scholar]
- 18.John A. Lott and Kathie Turner. Evaluation of Trinder's glucose oxidase method for measuring glucose in serum and urine. Clinical Biochemistry, Penguin books; 1975:1754–60. [PubMed] [Google Scholar]
- 19.Anjalakshi C, Balaji V, Balaji MS, Ashalata S, Suganthi S, Arthi T, et al. A single test procedure to diagnose gestational diabetes mellitus. Acta Diabetol. 2009;46:51–4. doi: 10.1007/s00592-008-0060-9. [DOI] [PubMed] [Google Scholar]


