Abstract
T cell numbers are maintained within narrow ranges in vivo. Introduction of naïve cells into lymphopenic environments results in proliferation and differentiation driven by the recognition of peptide/MHC complexes and by cytokine signaling. This process, often described as homeostatic proliferation, is here referred to as spontaneous proliferation. We show that, although the presence of memory CD4 T cells of broad repertoire efficiently inhibits proliferation/differentiation of naïve CD4 T cells, a memory population of similar size comprised of cells with a repertoire of limited diversity fails to do so, implying that cells of a given specificity prevent responses of cells of the same or related specificity. This finding suggests that the immune system has evolved mechanisms to attain a memory cell repertoire of great diversity independently of foreign antigens.
The immune system must control the numbers and state of activation of each of the major lymphocyte classes to ensure its efficient function. One mechanism through which this is achieved is homeostatic regulation (1, 2). Such regulation is crucial for functional competence and to control dangerous overreaction of the immune system. However, how lymphocyte homeostasis is regulated is not yet fully understood.
Homeostatic regulation has been studied by transferring T cells into lymphopenic recipients such as recently irradiated mice, mice with genetic defects affecting lymphocyte development, or neonatal mice that are physiologically lymphopenic (3-7). T cells proliferate in such environments without exogenous antigenic stimulation. These responses depend on T cell receptor (TCR)/MHC interactions, cytokine availability, and costimulation (3, 5, 7-14). Cells that undergo multiple rounds of division in lymphopenic settings also differentiate. These cells express memory cell markers and are capable of producing cytokines upon restimulation, mediating cytotoxicity, and preferentially migrating into nonlymphoid tissues (7, 15-18).
What causes such proliferation is not fully understood. Earlier studies have suggested that low-affinity self-antigens, mediating thymic positive selection, are responsible for the proliferation (3). Recently, it has been reported that TCR transgenic (Tg) T cells transferred into hosts expressing the same transgene fail to proliferate but that such cells do proliferate when transferred into mice expressing a different TCR transgene (19, 20). This finding suggests that T cells compete with each other for stimulation by low-affinity self-antigens. Whether such competition is a feature of physiologic (i.e., non-Tg) cell populations, in which cells of any given specificity are present at far lower frequency than is the case for cells in TCR Tg mice, has not been established, and the functional implications of “specificity competition” are uncertain.
Here, we show that monoclonal T cells, whether they are resting, recently activated, or in a memory state, fail to inhibit the proliferation of polyclonal naïve CD4 T cells, whereas polyclonal CD4 T cells that are cotransferred into lymphopenic hosts or have previously populated such hosts are fully capable of inhibiting the proliferation of naïve CD4 T cells. However, the degree of inhibition diminishes as the repertoire complexity of preexisting memory cells decreases, implying that proliferation of CD4 T cells is limited by competition with memory CD4 T cells specific for the same or related peptide/MHC complexes. We propose that this represents a mechanism by which the immune system attains a memory lymphocyte compartment with a diverse repertoire, independently of stimulation with exogenous antigen.
Materials and Methods
Mice. B10.A, Ly5.1 B10.A, B10.A 5C.C7 TCR Tg (Rag2-/-) (21), B10.A Rag2-/-, C57BL/10, C57BL/10 AND TCR Tg (Rag2-/-) (22), C57BL/10 Marilyn TCR Tg (Rag2-/-) (23), Ly5.1 C57BL/6, and C57BL/6 OT-I TCR Tg (Rag1-/-) (24) were obtained from National Institute of Allergy and Infectious Diseases contract facility at Taconic Farms. They were maintained under pathogen-free conditions at the National Institute of Allergy and Infectious Diseases animal facility.
Adoptive Transfer. Naïve lymph node (CD44dull) CD4 T cells were obtained by sorting on a FACSVantage SE (Becton Dickinson). Purity was >99%. Sorted naïve CD4 T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) at a final concentration of 1.25 μM and transferred i.v. into recipient mice. Total lymph node CD4 T cells were purified by incubation with FITC-labeled antibodies (anti-CD8, anti-B220, anti-I-A, anti-CD16/32, anti-NK1.1, and anti-HSA), followed by anti-FITC-conjugated microbeads (Miltenyi Biotech, Auburn, CA). Magnetic separation was performed by using Miltenyi LS columns. Purity of CD4 T cells was >98%. Antibodies were purchased from Pharmingen. In some experiments, BrdUrd (Sigma) was injected i.p. to measure proliferation rate. Six hours later, the mice were killed, and BrdUrd incorporation was measured by flow cytometry.
Flow Cytometry. Lymph nodes were taken at indicated period after transfer and analyzed for proliferation profile, phenotype, and cytokine production. The following antibodies (Pharmingen) were used: biotinylated anti-Ly5.1 (A20), phycoerythrin (PE)-anti-CD25 (PC61), cychrome-anti-CD44 (IM7), allophycocyanin (APC)-anti-CD4 (RM4-5), and streptavidin-PE. Flow cytometry was performed on FACSCalibur (Becton Dickinson), and data were analyzed with flowjo software (Treestar). For BrdUrd detection, cells were stained for surface markers before fixation. Fixed cells were then incubated with DNase I (Roche Diagnostics), followed by staining with FITC- or PE-labeled anti-BrdUrd antibody (Pharmingen).
Immunoscope Analysis. Total RNA was extracted from purified CD44low and CD44bright CD4 T cells by using the RNeasy procedure (Qiagen, Valencia, CA); cDNA was synthesized by using oligo(dT)12-18, SuperScript reverse transcriptase (Invitrogen), and RNase Out (Invitrogen) in the provided buffer. PCR runs to saturation (40 cycles) were carried out in 25-μl reaction volumes containing 0.6 unit of AmpliTaq Gold DNA polymerase (PE Applied Biosystems, Foster City, CA), 2.5 mM MgCl2, 0.2 mM dNTP, 0.25 μM of each primer (BV and BC), and normalized amounts of cDNA template in the provided buffer. Amounts of cDNA encoding TCRβ chain were determined by real-time quantitative PCR. Run-off reactions (five cycles) were then performed on 2 μl of PCR product with an internal BC-specific Fam-labeled primer at a final concentration of 0.1 μM in 10 μl final volume. Two-microliter volumes of run-off products were run on a 4.25% polyacrylamide 8 M urea gel in an automated 377 DNA sequencer (PE Applied Biosystems), together with size standard. The intensity of the various bands was then recorded and analyzed with immunoscope software.
Statistical Analysis. Distributions of CDR3 lengths in transferred cells and the T cell populations of adult mice were measured for different Vβs as described above. To quantify the degree of repertoire skewing relative to the TCR repertoire of standard adult T cell populations, first, the peak heights in all CDR3 length distributions were normalized to a sum of 1 for each Vβ. Then, for each peak, i.e., for each CDR3 length, the absolute difference between the normalized peak height of the standard population and that of the transferred cells was calculated and divided by the sum of the two normalized peak heights to make the comparison symmetrical with regard to the standard and the tested population. The sum of the absolute values of these scaled differences over all measured Vβs was taken as a measure of repertoire skewing of the transferred cells relative to the adult standard cells.
Results
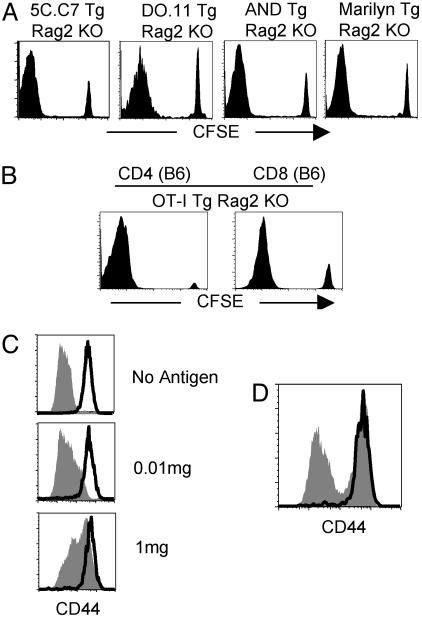
TCR Tg Mice Are Functionally Lymphopenic. When naïve T cells are transferred into lymphopenic hosts, some of the cells vigorously proliferate and acquire a memory cell phenotype (7, 15, 16). No such response occurred when the recipients were wild-type mice. Interestingly, when the recipients were 5C.C7 Tg Rag2-/- mice, a vigorous response was also observed (Fig. 1A). Proliferation of CD4 T cells was significantly inhibited by anti-MHC II mAb injection, indicating that it depends on MHC class II molecules and that it requires a TCR-MHC interaction (data not shown).
Fig. 1.
CD4 T cells of single specificity fail to block the proliferation of polyclonal naïve CD4 T cells. (A) A total of 2 × 106 CFSE-labeled naïve CD4 T cells from Ly5.1 B10.A, BALB/c, and C57BL/10 mice were transferred into 5C.C7 Tg Rag2-/-, DO.11 Tg Rag2-/-, and AND Tg Rag2-/- or Marilyn Tg Rag2-/- mice, respectively. Shown is CFSE profile of transferred cells (gated on Ly5.1+, KJ1.26+, Vβ3-, and Vβ6- CD4+, respectively) at 7 days after transfer. (B) CFSE-labeled naïve Ly5.1 CD4 and CD8 T cells were transferred into OT-I Tg Rag1-/- mice. CFSE profile was measured at 7 days after transfer (gated on Ly5.1+ CD4+). (C) 5C.C7 Tg Rag2-/- mice received 2 × 106 CFSE-labeled Ly5.1 CD4 T cells and were simultaneously implanted with a miniosmotic pump containing 0, 0.01, or 1 mg of cytochrome C protein. Lymph node CD4 T cells were analyzed for CD44 expression after 7 days of transfer. (D) Ly5.1 naïve CD4 T cells were transferred into 5C.C7 Tg Rag2-/- mice that had been immunized 60 days earlier by implantation of a miniosmotic pump containing 1 mg of cytochrome C protein. CD44 expression was measured 7 days after transfer. The bold line represents Ly5.1 cells and the filled area represents endogenous Tg cells. Experiments were repeated more than twice with similar results.
Similar vigorous proliferation was also found in other TCR Tg mice. BALB/c CD4 T cells transferred into DO.11 Tg Rag2-/- mice, C57BL/10 CD4 T cells transferred into AND or Marilyn Tg Rag2-/- mice, and C57BL/6 CD4 or CD8 T cells transferred into OT-I Tg Rag1-/- mice (Fig. 1 A and B), all displayed a large proportion of cells that had fully diluted their CFSE at 7 days after transfer. Therefore, these results are consistent with previous reports (19, 20) that endogenous monoclonal Tg CD4 or CD8 T cells fail to inhibit the proliferation of transferred cells. This is true in instances in which the Tg cells themselves proliferate weakly when transferred to a Rag-/- environment (5C.C7, Marilyn, and DO11.10) or proliferate strongly upon such transfer (AND and OT-I). These results indicate that some polyclonal naïve CD4 T cells (or CD8 T cells) proliferate extensively upon transfer into an environment that is populated by T cells of a single specificity, implying that the capacity of naïve T cells to proliferate upon transfer into a lymphopenic environment is not simply due to a deficiency in the numbers of lymphocytes.
Memory and Effector CD4 T Cells of a Single Specificity Fail to Inhibit the Proliferation of Cotransferred Polyclonal CD4 T Cells. As reported (7, 25), cotransfer of 20 × 106 polyclonal Ly5.2 CD4 T cells diminished the proportion of Ly5.1 CD4 T cells that proliferated within Rag2-/- mice (Fig. 6, which is published as supporting information on the PNAS web site). The Ly5.1 cells and the cotransferred Ly5.2 cells proliferated to the same extent as judged by BrdUrd incorporation. By contrast, cotransfer of 20 × 106 5C.C7 CD4 T cells failed to inhibit proliferation of the Ly5.1 CD4 T cells as judged by both CFSE dilution and uptake of BrdUrd (Fig. 6). The cotransferred 5C.C7 cells showed only a minimal uptake of BrdUrd.
It has been proposed that the strength of TCR/MHC interaction in homeostatic expansion capacity plays a critical role in determining the degree of naïve cell proliferation in lymphopenic environments, but that the growth advantage of cells of the highest avidity is lost as the population reaches homeostatic equilibrium (26). Therefore, the failure of 5C.C7 cells to inhibit proliferation within lymphopenic recipient might be due to their naïve phenotype. To test this, we cotransferred 7 × 106 5C.C7 cells that had been primed in vitro with peptide for two rounds of 4 days each (effector cells). They failed to inhibit the proliferation of Ly5.1 polyclonal T cells within 5C.C7 Tg Rag2-/- mice (data not shown). Immunization of 5C.C7 Tg Rag2-/- mice with cytochrome C in a miniosmotic pump did not prevent cotransferred polyclonal CD4 T cells from dividing (data not shown) or up-regulating CD44 despite a strong response of the 5C.C7 cells (Fig. 1C). Finally, 5C.C7 resting memory cells that had been generated by previous immunization allowed subsequently transferred polyclonal Ly5.1 naïve CD4 T cells to proliferate (data not shown) and up-regulate CD44 expression (Fig. 1D). These results demonstrate that CD4 T cells of a single specificity whether in a resting, activated, or memory state fail to block the response of polyclonal cells.
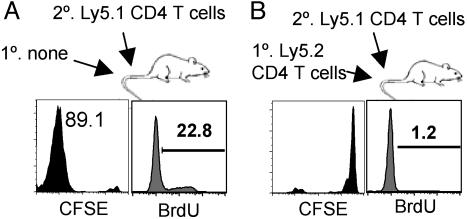
Polyclonal T Cells That Had Proliferated in Lymphopenic Hosts Are Fully Capable of Inhibiting the Proliferation of Newly Transferred Cells. Naïve cells transferred into Rag2-/- mice differentiate into CD44bright cells that populate the recipient's lymphoid organs to a stable level (27). To determine whether such cells control proliferation/differentiation of newly transferred naïve T cells, CFSE-labeled Ly5.1 naïve CD4 T cells were transferred into Rag2-/- mice that had received 2 × 106 CD4 T cells 60 days earlier. The total number of lymph node CD44bright CD4 T cells from the prior transfer was ≈1 × 106 cells; a similar number were present in the spleen. CD44bright cells constituted >80% of total recovered CD4 T cells. Naïve Ly5.1 T cells failed to proliferate when transferred into these mice (Fig. 2B), although they proliferated vigorously upon transfer into Rag2-/- mice that had not received a prior transfer (Fig. 2 A). Thus, polyclonal CD44bright CD4 T cells derived from the previous transfer is sufficient to inhibit the proliferation of newly transferred naïve CD4 T cells.
Fig. 2.
Polyclonal CD4 T cells that had undergone proliferation in lymphopenic mice are able to regulate the proliferation of newly transferred CD4 T cells. (A) B10.A Rag2-/- mice received 2 × 106 CFSE-labeled Ly5.1 naïve CD4 T cells. (B) B10.A Rag2-/- mice that had received 2 million Ly5.2 CD4 T cells 60 days before were given 2 × 106 CFSE-labeled naïve Ly5.1 CD4 T cells. Lymph nodes were taken 7 days after the second transfer, and CFSE profile and BrdUrd uptake of Ly5.1 CD4 T cells were measured. Similar results were observed when 5C.C7 Tg Rag2-/- mice were used as recipients (data not shown). Similar results were obtained from three independent experiments.
Complexity of Repertoire Is Determined by the Number of Transferred Cells. Because CD44bright CD4 T cells of single specificity failed to inhibit proliferation of polyclonal CD4 T cells, whereas cells of diverse specificity did inhibit, we asked whether the repertoire diversity of the initially transferred cells plays a role in regulating the proliferation of newly transferred cells. An approach to prepare mice with differing degrees of repertoire diversity might be to vary the number of T cells used to populate lymphopenic animals. We first wished to determine whether populating Rag2-/- mice with varying numbers of CD4 T cells would lead to animals that expressed similar or different numbers of CD44bright CD4 T cells when they had achieved stable numbers at 2 months after transfer. Rag2-/- mice received from 104 to 107 CD4 T cells from Ly5.2 mice. Two months later, we observed that the numbers of CD44bright CD4 cells in the recipients were essentially similar (lymph node, Fig. 3A; spleen, data not shown).
Fig. 3.
Repertoire diversity, but not cell number, depends on the number of transferred cells. (A) Different numbers (104 to 107) of Ly5.2 CD4 T cells were transferred into Rag2-/- mice. Total lymph node CD44bright CD4 T cell numbers from each recipient were calculated by FACS analysis 60 days after transfer. Shown is one representative (two to three mice) from three independent experiments. (B) CD44bright CD4 T cells were sorted from individual Rag2-/- recipients that had received different numbers of CD4 T cells 2 months earlier. Repertoires of selected Vβs of CD44bright CD4 T cells were analyzed by immunoscope technique. (C) Statistical analysis of repertoire incompleteness. Each point represents an individual mouse.
An immunoscope analysis of TCR complexity in sorted CD44bright cells obtained from individual mice that had been populated with varying numbers of CD4 T cells revealed a striking decrease in complexity (increase in repertoire incompleteness) as the frequency of initially transferred cells decreased (Fig. 3B). Repertoire incompleteness was assessed by calculating, for each Vβ, the differences between the normalized distribution of Ly5.2 TCR CDR3 lengths in CD44bright cells from each individual and those from the mean of two normal adult mice. We summed the absolute values of these differences for all Vβ's and plotted the sum against the log of the number of transferred cells. The correlation coefficient, R2, was 0.85, indicating that repertoire incompleteness increased as the number of initially transferred cells diminished (Fig. 3C).
Mice with a Limited Repertoire of Polyclonal CD4 Memory T Cells Fail to Inhibit Proliferation of Newly Transferred Cells. We transferred 2 × 106 CFSE-labeled Ly5.1 naïve CD4 T cells into Rag2-/- animals that had previously received 105 to 107 Ly5.2 cells (as shown in Fig. 3) and observed a striking difference in the numbers and proportion of CD44bright Ly5.1 cells and of Ly5.1 cells that had diluted their CFSE (Fig. 4 A and B). In mice that had previously received 107 cells, only ≈103 Ly5.1 cells in the lymph node were CD44bright, and only ≈1% of the Ly5.1 cells were CFSElow. By contrast, in mice that had initially received 105 Ly5.2, ≈2 × 105 Ly5.1 cells in the lymph node were CD44bright and ≈62% of the Ly5.1 cells were CFSElow. This could not be accounted for by a different localization of the cells in each recipient; essentially similar results were seen in the liver (Fig. 4A) and spleen (data not shown). Although the proportion of the secondarily transferred Ly5.1 cells that had undergone proliferation was inversely related to the number of initially transferred cells, those newly transferred cells that did proliferate did so in a manner similar to cells in an “empty” mouse (Fig. 4C); they diluted their CFSE equally and the uptake of BrdUrd on day 7 among the CFSEbright cells was similar (20-40%). By contrast, the proliferation rate of preexisting CD44bright cells, derived from the previous transfer, was relatively low (5-10% measured by in vivo BrdUrd uptake).
Fig. 4.
Repertoire diversity of preexisting CD44bright CD4 T cells determines the behavior of newly transferred cells. (A and B) Rag2-/- recipients as described in Fig. 3A received 2 × 106 CFSE-labeled Ly5.1 naïve CD4 T cells. Seven days after transfer, total numbers of CD44bright Ly5.1 CD4 T cells and CFSE profiles from lymph nodes and liver were determined. The proportion of cells that had undergone more than seven divisions is indicated. Similar results were obtained when 5C.C7 Tg Rag2-/- mice were used as recipients (data not shown). (C) Proliferation rates of both Ly5.1 and Ly5.2 CD44bright T cells were also measured after an in vivo BrdUrd injection. Groups consisted of four to six individual mice from two independent experiments. (D) Rag2-/- mice received 0.1 × 106 Ly5.2 CD4 T cells. Fifty days later, these mice received 2 × 106 CD44dull Ly5.1 CD4 T cells. Ly5.2 and Ly5.1 CD44bright CD4 T cells were sorted from individual mice at 7 days after Ly5.1 cell transfer. Shown are immunoscope profiles of a set of Vβs from Ly5.1 (red) and Ly5.2 (blue) cells from two individual mice.
The association of the degree of proliferation/differentiation of secondarily transferred cells with the repertoire complexity of the already resident memory cells strongly suggests that the capacity of a cell to expand upon transfer could be determined by whether there exist, in the recipient, cells of similar or cross-reactive specificity. That is, cells will expand upon secondary transfer only if they find a “specificity hole” in the repertoire of the recipient. Therefore, we examined the immunoscope patterns of the primarily (Ly5.2) and secondarily (Ly5.1) transferred cells in two animals that had received a primary transfer of 105 cells (Fig. 4D). As can be seen, the position of the dominant peaks for each of the analyzed Vβ's of the Ly5.1 and Ly5.2 cells is different. This is particularly dramatic for Vβ1, 2, 5.1, 5.2, 6, 8.1, 13, and 14. This is consistent with the specificity of the initially transferred cells influencing which secondarily transferred cells can expand and differentiate.
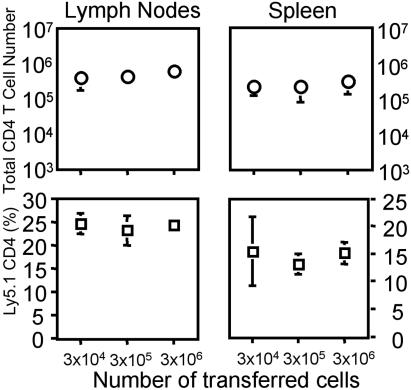
Absolute Numbers of CD25+ CD4 Regulatory T Cells Are Not Responsible for the Regulation of Homeostasis. Regulatory T cells have been proposed to play a role in the regulation of lymphocyte homeostasis in a number of experimental systems, particularly in autoimmunity (28). To determine whether the proliferation of transferred cells within the mice that had been populated with limited numbers of CD4 T cells might be due to a relative lack of CD25+ regulatory T cells, we transferred 3 × 104 to 3 × 106 cells consisting of a mixture of Ly5.1 CD25+ CD4 T cells and Ly5.2 CD25- CD4 T cells in a 1:9 ratio into Rag2-/- recipients. These cells had been obtained at a purity of ≈99% by cell sorting. One month later, the number and frequency of Ly5.1 cells was observed to be independent of the number of initially transferred cells (Fig. 5), indicating that any difference in the capacity of mice that received different numbers of CD4 T cells to support proliferation/differentiation of subsequently transferred cells could not be ascribed to differences in the numbers of regulatory T cells present at the time of the second transfer.
Fig. 5.
CD25+ regulatory T cells are not responsible for the homeostatic regulation. FACS-sorted Ly5.1 CD25+ CD4 T cells and Ly5.2 CD25- CD4 T cells were mixed at a 1:9 ratio, and different numbers (3 × 104 to 3 × 106) were transferred into Rag2-/- mice. One month after transfer, the proportion and total number of Ly5.1 CD4 T cells were calculated from seven to eight individual mice per each group.
Discussion
Although the importance of TCR/MHC interactions in T cell proliferation within lymphopenic environments has been demonstrated, the cellular mechanisms driving such proliferative responses are not fully understood. Two recent reports have proposed that antigen-specific competition could be important in controlling proliferation/differentiation stimulated by lymphopenia (19, 20). Both experiments used two sets of TCR Tg cells, which were transferred into homologous or heterologous TCR Tg recipients. Only the homologous recipients limited the proliferation of the transferred cells. Shen and colleagues (19) used two sets of CD8 TCR Tg cells, whereas Khoruts and colleagues (20) used CD4 TCR Tg T cells. Although these results are consistent with the concept that competition for a mutually recognized antigen (peptide/MHC complex) limits proliferation, they involve very large numbers of cells with a single specificity, providing limited information on the potential physiologic significance of specificity-based inhibition. Thus, their significance for homeostasis in normal settings is not clear. Here, we addressed this issue by using polyclonal T cell populations in which cells of any given specificity should be represented at a relatively low frequency. Our results show that repertoire complexity of resident memory CD4 cells correlates with the capacity of naïve cells to proliferate. It implies that memory cells of specificity similar to that of the introduced naïve cells can prevent those cells from undergoing proliferation, even when the memory cells are present at a relatively low frequency. Because competition for stimulatory self-peptide/MHC complexes is a likely mechanism for inhibition, this implies that even the numbers of memory cells resident in a normal mouse as a result of prior homeostatic proliferation are sufficient to efficiently inhibit the proliferation of newly introduced naïve cells of the same or related specificity.
Consistent with this finding, we showed that polyclonal CD4 or CD8 T cells proliferated vigorously after transfer into several different Tg mice, implying that the resident Tg cells, even though present in substantial numbers, could not inhibit the proliferation of naïve CD4 T cells, presumably because they can only compete with the limited set of naïve cells that cross-reacts with the TCR Tg cells. The failure of Tg cells to inhibit polyclonal cells cannot be explained simply on the basis that the Tg cells, themselves, are unable to proliferate. For example, OT-I and AND Tg T cells proliferate vigorously upon transfer into a lymphopenic environment (10, 12, 19, 26). 5C.C7 and DO.11 Tg T cells show little proliferation in the period immediately after transfer into a lymphopenic setting (4, 7). It has been suggested that CD5 levels reflect the “strength” with which the T cell has been signaled and in turn the avidity of TCR for self-antigens (30). However, in some cases, the level of CD5 is not necessarily correlated with expansion capacity in lymphopenic environments (26). Although the role of signal strength in lymphocyte homeostasis remains in doubt, it seems reasonable that those polyclonal cells that do divide upon transfer into a lymphopenic environment have a higher avidity for a set of expressed self peptide/MHC complexes than do the cells that fail to divide. Recent observations have shown that, in a lymphopenic environment, viral antigen-specific memory CD8 T cells proliferate to a lesser extent than polyclonal CD8 T cells (29). This finding suggests that T cells that have responded to authentic exogenous antigens are not necessarily capable of undergoing spontaneous proliferation, whereas among the polyclonal cells some can recognize endogenous-peptides sufficiently well to proliferate. We do not yet have an estimate of the proportion of naïve cells that undergo such proliferation. We are hampered in this regard because we cannot precisely measure the rate of cell death nor do we know the precise number of cell divisions that a cell that fully diluted its CFSE has gone through. If only 1% of the cells responded upon transfer and went through seven divisions without any cell death, they would constitute >99% of the cells present at 7 days. Immunoscope analysis clearly revealed that the cells that did respond have a very diverse repertoire, suggesting that they do not arise from a very limited number of clonal precursors. Whether this could be generated from 1% of the naïve population or requires that the frequency of responding cells is substantially greater and that there is considerable cell death during the process of proliferation in lymphopenic environments remains to be established.
It has been recently demonstrated that homeostasis-driven proliferation in which cells have undergone seven or more divisions within 1-2 weeks mainly contributes to the memory cell compartment even though the transferred cells were naïve (7, 27). Thus, this proliferation is not likely to be homeostatic for naïve cells; rather, it appears more involved in maintaining homeostasis of memory cells by controlling the rate of cell entry into the memory pool. However, it can be argued that this proliferation is not homeostatic for the total number of memory CD4 T cells. Our data show that the capacity of equivalent numbers of CD44bright CD4 T cells resident in a populated host to limit proliferation of newly introduced cells depends on the diversity of their repertoire. Because this proliferation appears to be stimulated by endogenous-peptide/MHC complexes and is probably not regulating the total number of CD4 T cells, we would propose that it be designated spontaneous proliferation rather than homeostatic proliferation. Interestingly, there clearly is a robust mechanism that does regulate the numbers of memory (CD44bright) cells. Thus, when we transfer numbers of CD4 T cells ranging from 104 to 107 into Rag2-/- recipients, the numbers of cells with a memory phenotype found in lymph nodes and spleen 1-2 months later is essentially the same and relatively similar to that in normal mice. Because the complexity of the repertoire of cells derived from the 107 populating dose is far greater than from the 104 repopulating does, it follows that “clone” sizes must be much larger in mice that received 104 cells than in those mice that received 107 cells. Indeed, the proliferative rate of the CD44bright cells at 2 months after transfer is the same in the different groups; ≈5% of the cells take up BrdUrd after a 6-h “pulse” of nucleotide.
Thus, specificity competition appears to determine whether a naïve cell can enter the pool of rapidly replicating cells differentiating into memory cells. On the other hand, replicative rates of memory cells of a given specificity appear similar whether they are members of large or small clones. This suggests that competition for access to antigen, if that is the mechanism underlying inhibition of proliferation, is much more important in the early proliferative response, when naïve cells are beginning to divide and differentiate, than in the established memory cell pool. Regulation of total cell number is probably at the level of competition for a soluble factor. The relatively rapid rate of proliferation of the established memory cells implies that there must be a significant rate of cell death because the memory cell population appears to be in equilibrium.
The simplest explanation for the difference in the frequency of naïve cells that proliferate and differentiate into memory cells in the presence of memory cell populations of different repertoire complexity is that naïve cells proliferate and differentiate into memory cells only when they see a “repertoire hole” in the pool of memory T cells. In other words, memory cells will limit the proliferation of naïve cells that express receptors of same or related specificity.
How would competition or inhibition of naïve cells by memory cells be manifest? Conventional memory CD4 T cells may compete with naïve CD4 T cells of similar specificity for the same peptide/MHC complexes on the surface of relevant APC (20). This could take the form of a physical competition in which the memory cells would limit the access of newly transferred naïve cells to their cognate peptide/MHC complexes. Recently, it has been demonstrated that the interaction of antigen-specific T cells with dendritic cells expressing an ovalbumin peptide/Kb complex diminishes further immune responses by limiting expression of the complex on the surface of the dendritic cells (31). Thus, memory cells may inhibit naïve cells not by competing for antigen but by limiting the amount of antigen. An alternative possibility is that interaction of memory T cells with their relevant peptide/MHC complex may eliminate or inactivate the APC or that a regulatory T cell activated on a given APC would inactivate naïve cells interacting with that same APC (28, 32). Thus, naïve cells recognizing ligands expressed on the same APC that the competitor or regulator was also interacting with would fail to be stimulated. Repertoire-dependent regulation would require that the pattern of peptides expressed on individual APC not be uniform. The degree of overlap in peptide/MHC complex expression on individual dendritic cells would determine the degree to which inhibition would appear to be antigen-specific or antigen-nonspecific.
CD25+ regulatory T cells have been proposed as key regulators of homeostatic proliferation (28). Because the number of regulatory T cells in repopulated Rag2-/- mice was independent of the number of transferred cells, the dependence of the capacity of secondarily transferred cells to expand on the number of initially transferred cells cannot be explained by differences in the numbers of regulatory T cells in these mice. However, it could be explained by the specificity of those cells so that either competition by conventional memory cells of a given specificity or inhibition by regulatory cells of similar specificity, or both may determine the capacity of newly transferred cells to expand. In fact, it has been shown that disease-preventing T regulatory cells are generated by the presence of peripheral autoantigen (33).
Overall, our data indicate that such spontaneous proliferation, occurring most prominently during an early stage in the development of the immune system (7), leads to the generation of a memory cell repertoire of broad specificity that selectively inhibits further proliferation of naïve populations having the same or related specificities, but not the proliferation and differentiation of newly emerging naïve cells with sufficiently unrelated specificities. This implies that there is a very strong pressure on the host to diversify the repertoire of memory cells in the absence of overt exogenous antigenic stimulation. An important challenge is to understand the biologic importance of a diverse repertoire in the memory CD4 T cell population, taking into account that the frequency of cells of any given specificity may be relatively low. A corollary is the need to determine the relative contribution of spontaneous proliferation and of conventional, antigen-driven proliferation and differentiation to the memory cell pool of individuals of various ages and of distinct antigenic experience.
Supplementary Material
Acknowledgments
We thank Dr. Zvi Grossman for helpful discussion and Sarah Tanksley for cell sorting.
Abbreviations: TCR, T cell receptor; CFSE, carboxyfluorescein diacetate succinimidyl ester; Tg, transgenic; APC, allophycocyanin.
References
- 1.Freitas, A. A. & Rocha, B. (2000) Annu. Rev. Immunol. 18, 83-111. [DOI] [PubMed] [Google Scholar]
- 2.Jameson, S. C. (2002) Nat. Rev. Immunol. 2, 547-556. [DOI] [PubMed] [Google Scholar]
- 3.Ernst, B., Lee, D.-S., Chang, J. M., Sprent, J. & Surh, C. D. (1999) Immunity 11, 173-181. [DOI] [PubMed] [Google Scholar]
- 4.Bender, J., Mitchell, T., Kappler, J. & Marrack, P. (1999) J. Exp. Med. 190, 367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudmundsdottir, H. & Turka, L. A. (2001) J. Immunol. 167, 3699-3707. [DOI] [PubMed] [Google Scholar]
- 6.Le Campion, A., Bourgeois, C., Lambolez, F., Martin, B., Leaument, S., Dautigny, N., Tanchot, C., Penit, C. & Lucas, B. (2002) Proc. Natl. Acad. Sci. USA 99, 4538-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Min, B., McHugh, R., Sempowski, G. D., Mackall, C., Foucras, G. & Paul, W. E. (2003) Immunity 18, 131-140. [DOI] [PubMed] [Google Scholar]
- 8.Viret, C., Wong, F. S. & Janeway, C. A., Jr. (1999) Immunity 10, 559-568. [DOI] [PubMed] [Google Scholar]
- 9.Ge, Q., Rao, V. P., Cho, B. K., Eisen, H. N. & Chen, J. (2001) Proc. Natl. Acad. Sci. USA 98, 1728-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kieper, W. C. & Jameson, S. C. (1999) Proc. Natl. Acad. Sci. USA 96, 13306-13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. (2000) Science 288, 675-678. [DOI] [PubMed] [Google Scholar]
- 12.Goldrath, A. W., Sivakumar, P. V., Glaccum, M., Kennedy, M. K., Bevan, M. J., Benoist, C., Mathis, D. & Butz, E. A. (2002) J. Exp. Med. 195, 1515-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. (2000) Nat. Immunol. 1, 426-432. [DOI] [PubMed] [Google Scholar]
- 14.Tan, J. T., Ernst, B., Kieper, W. C., LeRoy, E., Sprent, J. & Surh, C. D. (2002) J. Exp. Med. 195, 1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murali-Krishna, K. & Ahmed, R. (2000) J. Immunol. 165, 1733-1737. [DOI] [PubMed] [Google Scholar]
- 16.Oehen, S. & Brduscha-Riem, K. (1999) Eur. J. Immunol. 29, 608-614. [DOI] [PubMed] [Google Scholar]
- 17.Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. (2001) Science 291, 2413-2417. [DOI] [PubMed] [Google Scholar]
- 18.Dummer, W., Niethammer, A. G., Baccala, R., Lawson, B. R., Wagner, N., Reisfeld, R. A. & Theofilopoulos, A. N. (2002) J. Clin. Invest. 110, 185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troy, A. E. & Shen, H. (2003) J. Immunol. 170, 672-676. [DOI] [PubMed] [Google Scholar]
- 20.Moses, C. T., Thorstenson, K. M., Jameson, S. C. & Khoruts, A. (2003) Proc. Natl. Acad. Sci. USA 100, 1185-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seder, R. A., Paul, W. E., Davis, M. M. & Fazekas de St. Groth, B. (1992) J. Exp. Med. 176, 1091-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaye, J., Hsu, M. L., Sauron, M. E., Jameson, J. C., Gascoigne, R. J. & Hedrick, S. M. (1989) Nature 341, 746-749. [DOI] [PubMed] [Google Scholar]
- 23.Lantz, O., Grandjean, P., Matzinger, P. & Di Santo, J. P. (2000) Nat. Immunol. 1, 54-58. [DOI] [PubMed] [Google Scholar]
- 24.Hogquist, K. A., Jameson, S. C., Heath, W. R., Howard, J. L., Bevan, M. J. & Carbone, F. R. (1994) Cell 76, 17-27. [DOI] [PubMed] [Google Scholar]
- 25.Dummer, W., Ernst, B., LeRoy, E., Lee, D.-S. & Surh, C. D. (2001) J. Immunol. 166, 2460-2468. [DOI] [PubMed] [Google Scholar]
- 26.Kassiotis, G., Zamoyska, R. & Stockinger, B. (2003) J. Exp. Med. 197, 1007-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge, Q., Hu, H., Eisen, H. N. & Chen, J. (2002) Proc. Natl. Acad. Sci. USA 99, 2989-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shevach, E. M. (2002) Nat. Rev. Immunol. 3, 33-41. [DOI] [PubMed] [Google Scholar]
- 29.Peacock, C. D., Kim, S.-K. & Welsh, R. M. (2003) J. Immunol. 171, 655-663. [DOI] [PubMed] [Google Scholar]
- 30.Kieper, W. C., Burghardt, J. T. & Surh, C. D. (2004) J. Immunol. 172, 40-44. [DOI] [PubMed] [Google Scholar]
- 31.Kedl, R. M., Schaefer, B. C., Kappler, J. & Marrack, P. (2002) Nat. Immunol. 3, 27-32. [DOI] [PubMed] [Google Scholar]
- 32.Wong, P. & Pamer, E. G. (2003) Immunity 18, 499-511. [DOI] [PubMed] [Google Scholar]
- 33.Seddon, B. & Mason, D. (1999) J. Exp. Med. 189, 877-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.