Abstract
Background:
The 1μg cosyntropin test has some advantages over the 250μg test as a test of adrenal function. One of the concerns regarding the 1 μg test includes stability of the cosyntropin when reconstituted and stored. Classically the 5th percentile responses to cosyntropin in normal individuals have been used to define a normal response. Recent studies have shown that these normative values should be determined for individual assays.
Materials and Methods:
We performed a 1μg cosyntropin test using reconstituted and refrigerated (4-8° C) cosyntropin in saline solution in 49 non pregnant adults who were apparently healthy and had no exposure to exogenous glucocorticoids. The cosyntropin solution was stored for up to 60 days following reconstitution. We analysed the data for any association between duration of cosyntropin solution storage and the cortisol responses to cosyntropin administration.
Results:
The mean ± SD cortisol level at baseline, 30 and 60 min were-12.19 ± 3 μg/dl, 20.72 ± 2.63 μg/dl, 16.86 ± 3.33 μg/dl. The 5th percentile cortisol response at 30 min was 16.5 μg/dl (16.33 μg/dl rounded off). The correlation coefficients between number of days of cosyntropin solution storage and the cortisol responses at 30 and 60 min were (Spear mans rho = 0.06,-0.24 respectively) (P = 0.69 and 0.41). There were no differences in cortisol values whether the storage was for less than 30 days or more than 30 days (mean difference 0.25 μg/dl P = 0.44).
Conclusion:
The 5th percentile normative values determined for our assay is lower than what is currently being used clinically and in research publications. Prolonged refrigerated storage of cosyntropin solution does not affect the validity of the 1 μg cosyntropin test.
Keywords: 1 μg cosyntropin test, 1μg synacthen test, adrenal insufficiency
INTRODUCTION
The 250 μg cosyntropin test has been conventionally used as a test for impaired adrenal reserve. The Cosyntropin that is commercially available is a 250 μg preparation; it contains the 1-24 amino acids of Adrenocorticotropic hormone (ACTH). Earliest studies with cosyntropin showed that responses to cosyntropin were similar to surgical stress or trauma.[1] In a study comparing 250 μg, 0.5 μg, 1 μg cosyntropin with insulin tolerance test (ITT) in healthy volunteers, The 1 μg test was found to produce plasma 1-24ACTH levels of 120 pmol/l, approximately twice as high than that of the ITT but of the same order of magnitude. The cortisol responses at 30 minute were comparable to that of the 250 μg synacthen test.[2] The peak level of 1-24ACTH produced during 250 μg test is about 22000 pmol/l nearly 220 times that produced during Insulin tolerance test (ITT) or 1 μg synacthen. Moreover 1 μg synacthen test has been used in studies looking into milder degrees of HPA axis suppression, such as patients with asthma using Inhaled corticosteroids (ICS).[3,4] Receiver operator curve analyses which are classically used to assess the utility of clinical screening tests suggest that 1 μg test is superior to 250 μg test.[5,6]
There has been some criticism of the 1 μg test because the stability of reconstituted cosyntropin has been questioned and there has been one publication involving 8 normal individuals that suggests that cosyntropin if administered in small quantities produces false positive results presumably because it tends to adhere to plastic tubing used to administer the drug.[7] Conflicting this report there is evidence in literature for the stability of reconstituted cosyntropin in saline for up to four months.[8] Not withstanding these controversies 1 μg cosyntropin test has been used widely in research protocols to identify impaired adrenal reserve both internationally[9,10] and in India.[11] The cut offs for a clear pass response for the 1 and 250 μg tests have been classically derived as a response above the 5th percentile response in a group of normal individuals for each test.[12,13,14]
Studies have pointed out the need for determining normative responses for the cortisol assay because of variability between different assay methods.[15,16] Previous metanalyses have suggested a cut-off of 16 μg/dl for the 30 min value while using the 1 μg test when the test is used in patients with suspected adrenal insufficiency.[6] Despite this authors continue to use the empirical 18 μg/dl cut off in India.[11]
We performed a pilot study using the 1 μg cosyntropin test in 49 normal individuals in order to obtain the 5th percentile response for the test using an Immuno chemiluminometric assay. We also simultaneously tested the hypothesis that storage of synacthen in saline solution for up to 60 days does not affect cortisol responses.
MATERIALS AND METHODS
Forty nine non-pregnant volunteer normal adults were tested after Institutional ethical review board clearance and informed consent. Following were the exctlusion criteria:
Use of any oral/topical/inhaled glucocorticoid in the past 3 years for any duration
History of hypothalamo pituitaryadrenal disease
History of head injury in the past
History of snake bite
History of Disseminated intravascular coagulation in the past
Any history of diseases known to cause adrenal insufficiency.
Reconstitution and storage: Synthetic ACTH1–24 1ml of solution 250 μg/ml (Synacthen®, Novartis, Manufactured by Alliance Pharmaceutical ltd Chippenham, Wiltshire U.K) was diluted in 499 ml of 0.9% saline in plastic Intravenous fluid container. After reconstitution the solution was stored in refrigerator 2-8° for up to 60 days.
Performance of the test: An i.v access with 21 gauge scalp vein set was obtained. Blood was taken at 08:00 hrs for basal cortisol, Two ml of the reconstituted solution containing 1 μg co-syntropin was administered intravenously through the same cannula. The scalp vein set was flushed with 10 ml of normal saline following administration of cosyntropin. Blood was drawn 30 min later for cortisol from the same cannula. We performed a 60 min cortisol sampling in 14 patients. An interim analysis showed that the 60 min value was significantly lower than the 30 min value. Since our aim was to identify the peak response we did not perform the 60 min cortisol test in remaining patients, to optimize use of resources available to us. A note was made regarding the duration of storage of reconstituted co-syntropin at the time of the test.
Cortisol assay was done using the automated Immuno chemiluminometric Access2 assay system (Beckmann- Coulter Gallaway, Ireland). Cumulative Coefficient of variation was 3.68% at 16.9 μg/dl and 9.77% at 2.77 μg/dl. Internal Quality Control (IQC) material Lypocheck Immuno assay Plus control lot no. 40240 from Biorad laboratories with known cortisol concentrations were analyzed on the days of sample run. Internal Quality Control values were within cut off limits established by the laboratory.
RESULTS
The 1 μg cosyntropin test was performed in 49 subjects, 38 male (77.6%). The mean age of the subjects was 37.2 years, range (19-84 years). Majority of the subjects were young with 59.2% being 36 years or younger, 14.3% of subjects were 55 years or older[Figure 1].
Figure 1.
The age distribution of studied subjects
The Mean cortisol values at three time points are depicted in Table 1. The cortisol values at 30 min were significantly higher than the 0 min and 60 min value P < 0.001 for both. Figure 2 represents the same data graphically. The lowest and highest cortisol values at each time point and the fifth percentile cortisol value at each time point are depicted in Table 1. The lowest four responses at 30 min were 13.86 μg/dl, 16.07 μg/dl, 16.60 μg/dl and 17.47 μg/dl. All the remaining cortisol values were above 17.5 μg/dl. The 5th percentile response was 16.33 μg/dl-rounded off to 16.5 μg/dl.
Table 1.
Cortisol values at different time points for the group all values in μg/dl
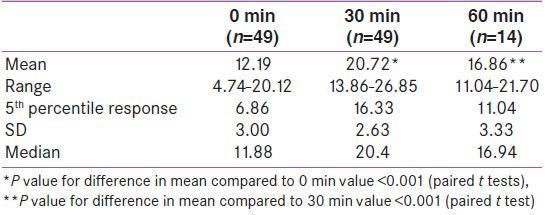
Figure 2.
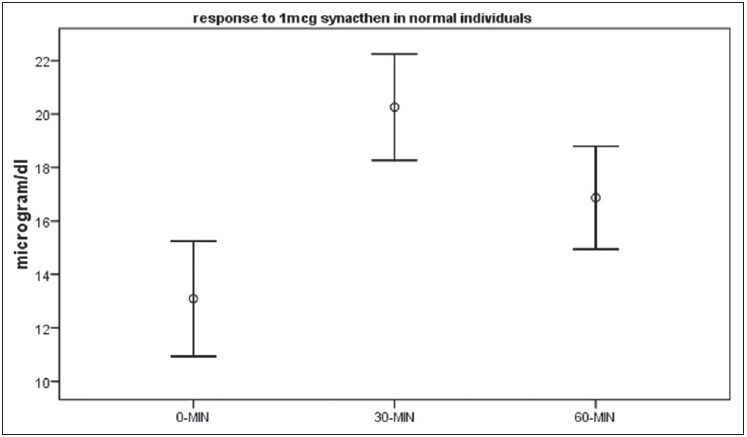
Mean and 95% CI values of coristol at baseline,30 min and 60 min
The incremental cortisol response was 8.53 ± 2.56 μg/dl (mean ± SD). ranging from 2.61-16.92 μg/dl. There was excellent correlation between the 30 min cortisol value and the incremental cortisol response pearsons R = 0.405 P = 0.004.
The cortisol values at 30 and 60 min had no relationship with number of days of refrigerated storage of the prepared cosyntropin solution. Spear mans rho = 0.06,-0.24 and P = 0.69, 0.41 for 30 min and 60 min cortisol respectively (data not shown). We divided the studied normal individuals into two groups one where the duration of reconstituted cosyntropin was stored <30 days and another where duration of storage ≥30 days. There was no difference in the mean 30 min cortisol response using the paired T test (mean difference 0.25 μg/dl P = 0.44) Table 2.
Table 2.
Comparison of 30 min cortisol responses to reconstituted cosyntropin stored for different durations

DISCUSSION
In our study we found that the 30 min cortisol response was higher than the 60 min cortisol response. The value of cortisol corresponding to 5th percentile cortisol response in our study population was 16.5 μg/dl. We also found that there was no association between duration of refrigeration of reconstituted synacthen and cortisol response for up to 60 days.
Classical comparative studies showed similar 30 min responses to cosyntropin for the 1 and 250 μg tests in the same individuals. In the 1 μg test the cortisol values quickly reduce and the 60 min value is always lower than the 30 min value.[2,14] The obvious clinical implication for this will be that while using the 1 μg LDSST test one needs to ensure that the 30 min sampling not be delayed by a few minutes as one can obtain a falsely low value for the cortisol response.
Classically the cut – offs for adrenal insufficiency have been derived by investigators as a 5th percentile response in normal individuals.[17] But this method has been disputed by some investigators who feel that the cut point for cortisol value in a stimulation test has to be derived by comparison with a gold standard – usually the ITT and doing a ROC analysis.[5,6] Unfortunately in the most common clinical situation where stimulation testing is necessary- Basal cortisol levels are equivocal with no clinical evidence of adrenal insufficiency and ITT is not feasible- there is no gold standard for diagnosis of adrenal insufficiency. Also in many cases the defect in HPA function could be transient and function may change over time. Some centers follow the protocol of using a response above the 5th percentile of normal response as a cut-off to rule out HPA axis suppression, They also use the 2.5th to 5th percentile values as abnormal requiring steroid supplementation as required.[12] The practice of using 5th percentile responses and using them clinically is continuing with the understanding that the cut points so derived are only a guide to clinical decision making.
In a recent study Klose et al.,[15] showed that the 2.5th percentile response varied from 17.2 μg/dl (475 nmol/l) to 18.9 μg/dl (523 nmol/l) in three different contemporary assays and the stimulated cortisol values varied up to 110 nmol/l (3.98 μg/dl) in the same sample when assayed by different methods. The tendency for a downward shift in cortisol values has been attributed to higher specificity for cortisol and lesser cross reactivity with intermediaries of cortisol metabolism. The use of 550 nmol/l (20 μg/dl) or 500 nmol/l (18 μg/dl) as a cut off would lead to substantial number of false positives. This means that separate cut offs are needed for different cortisol assays.
Our study we found that the 5th percentile cut-off was 16.5 μg/dl this value is substantially lower than 18 μg/dl being used clinically. This figure is similar to 16 μg/dl identified by ROC curve analysis of patient level data of different published studies–while using the 1 μg cosyntropin test.[6] The contribution of our study is that we have demonstrated that stimulated cortisol cut-offs lower than the currently used cut point is probably warranted with our assay. In order to arrive at a clinically useful cut off we need to study cortisol values at 30 min in patients with clinically suspected cortisol insufficiency with a longitudinal follow-up. We suggest that any cortisol value below 16.5 μg/dl at 30 min while using the 1 μg cosyntropin test will be suspicious of adrenal insufficiency based on our data alone.
One of the main criticisms against the 1 μg cosyntropin test is that storage of prepared cosyntropin solution is not possible and it has to be used immediately upon reconstitution. A previous study has shown stability of cosyntropin after storage for four months at 4°C.[8] Our study also had similar findings when we used the 30 min cortisol value as an indirect bioassay of the 1-24 ACTH levels, we found no difference in cortisol levels on storage up to 30 days or even longer. There was no linear relationship between number of days stored and cortisol response. This finding is of importance in a resource constrained country like ours where reconstituted cosyntropin can be used stored for up to two months and used in multiple patients. Measurement of 1-24 cosyntropin in the reconstituted solution and/or retesting in the same individuals after duration of storage could have better supported our hypothesis and these are shortcomings in our study.
Footnotes
Source of Support: Partially funded by Dr Reddy's laboratories ltd and Beckmanncoulter Ltd for procurement of kits
Conflict of Interest: None declared
REFERENCES
- 1.Harris MJ, Baker RT, McRoberts JW, Mohler JL. The adrenal response to trauma, operation and cosyntropin stimulation. Surg Gynecol Obstet. 1990;170:513–6. [PubMed] [Google Scholar]
- 2.Nye EJ, Grice JE, Hockings GI, Strakosch CR, Crosbie GV, Walters MM, et al. Comparison of adrenocorticotropin (ACTH) stimulation tests and insulin hypoglycemia in normal humans: Low dose, standard high dose, and 8-hour ACTH-(1-24) infusion tests. J Clin Endocrinol Metab. 1999;84:3648–55. doi: 10.1210/jcem.84.10.6062. [DOI] [PubMed] [Google Scholar]
- 3.Grebe SK, Feek CM, Durham JA, Kljakovic M, Cooke RR. Inhaled beclomethasone dipropionate suppresses the hypothalamo-pituitary-adrenal axis in a dose dependent manner. Clin Endocrinol (Oxf) 1997;47:297–304. doi: 10.1046/j.1365-2265.1997.2391059.x. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer EO, Berger WE, Berkowitz RB, Bronsky EA, Dvorin DJ, Finn AF, et al. A dose-ranging study of mometasone furoate aqueous nasal spray in children with seasonal allergic rhinitis. J Allergy Clin Immunol. 1999;104:107–14. doi: 10.1016/s0091-6749(99)70121-1. [DOI] [PubMed] [Google Scholar]
- 5.Abdu TAM, Elhadd TA, Neary R, Clayton RN. Response to Dr. Oelkers: There Is Enough Evidence in Favor of Low Dose ACTH Test – Authors’ Response. J Clin Endocrinol Metab. 1999;84:2973. [Google Scholar]
- 6.Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, et al. Corticotropin Tests for Hypothalamic-Pituitary-Adrenal Insufficiency: A Metaanalysis. J Clin Endocrinol Metab. 2008;93:4245–53. doi: 10.1210/jc.2008-0710. [DOI] [PubMed] [Google Scholar]
- 7.Murphy H, Livesey J, Espiner EA, Donald RA. The Low Dose ACTH Test: A further word of caution. J Clin Endocrinol Metab. 1998;83:712-a-3. doi: 10.1210/jc.83.2.712-a. [DOI] [PubMed] [Google Scholar]
- 8.Dickstein G, Shechner C, Nicholson We, Rosner I, Shen-Orr Z, Adawi F, et al. Adrenocorticotropin Stimulation Test: Effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab. 1991;72:773–8. doi: 10.1210/jcem-72-4-773. [DOI] [PubMed] [Google Scholar]
- 9.Nyunt O, Cotterill AM, Archbold SM, Wu JY, Leong GM, Verge CF, et al. Normal Cortisol Response on Low-Dose Synacthen (1 μg) Test in Children with Prader Willi Syndrome. J Clin Endocrinol Metab. 2010;95:E464–7. doi: 10.1210/jc.2010-0647. [DOI] [PubMed] [Google Scholar]
- 10.Poomthavorn P, Isaradisaikul B, Chuansumrit A, Khlairit P, Sriphrapradang A, Mahachoklertwattana P. High Prevalence of “Biochemical” Adrenal Insufficiency in Thalassemics: Is It a Matter of Different Testings or Decreased Cortisol Binding Globulin? J Clin Endocrinol Metab. 2010;95:4609–15. doi: 10.1210/jc.2010-0205. [DOI] [PubMed] [Google Scholar]
- 11.Shashikala G, Shashidhar P. Low dose adrenocorticotropic hormone test and adrenal insufficiency in critically ill acquired immunodeficiency syndrome patients. Indian J Endocrinol Metab. 2012;16:389–94. doi: 10.4103/2230-8210.95680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha A, Tomlinson JW, Clark PM, Holder G, Stewart PM. The long-term predictive accuracy of the short synacthen (Corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2006;91:43–7. doi: 10.1210/jc.2005-1131. [DOI] [PubMed] [Google Scholar]
- 13.Bhansali A, Subrahmanyam KA, Talwar V, Dash RJ. Plasma cortisol response to 1 microgram adrenocorticotropin at 0800 h and 1600 h in healthy subjects. Indian J Med Res. 2001;114:173–6. [PubMed] [Google Scholar]
- 14.Gonzalbez J, Villabona C, Ramon J, Navarro MA, Gimenez O, Ricart W, et al. Establishment of reference values for standard dose short synacthen test (250 microgram), low dose short synacthen test (1 microgram) and insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in normal subjects. Clin Endocrinol (Oxf) 2000;53:199–204. doi: 10.1046/j.1365-2265.2000.01028.x. [DOI] [PubMed] [Google Scholar]
- 15.Klose M, Lange M, Rasmussen AK, Skakkebæk NE, Hilsted L, Haug E, et al. Factors influencing the adrenocorticotropin test: Role of contemporary cortisol assays, body composition, and oral contraceptive agents. J Clin Endocrinol Metab. 2007;92:1326–33. doi: 10.1210/jc.2006-1791. [DOI] [PubMed] [Google Scholar]
- 16.Clark PM, Neylon I, Raggatt PR, Sheppard MC, Stewart PM. Defining the normal cortisol response to the short Synacthen test: Implications for the investigation of hypothalamic-pituitary disorders. Clin Endocrinol (Oxf) 1998;49:287–92. doi: 10.1046/j.1365-2265.1998.00555.x. [DOI] [PubMed] [Google Scholar]
- 17.Mayenknecht J, Diederich S, Bähr V, Plöckinger U, Oelkers W. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab. 1998;83:1558–62. doi: 10.1210/jcem.83.5.4831. [DOI] [PubMed] [Google Scholar]


