Abstract
Objective:
This study was undertaken to assess the efficacy and safetyof pioglitazone in combination with other oral antidiabetics (OADs) in Indian patients with type 2 diabetes mellitus (T2DM).
Materials and Methods:
This was an openlabel, prospective, no-randomized, single-center observational study conducted at a single center in India. A total of 958 adult patients with T2DM on OADs, with uncontrolled fasting (FBG) or postprandial blood glucose (PPG), were enrolled. Pioglitazone (7.5/15/30 mg) was added to existing therapy as a combination treatment with other OAD. Body weight (BW), body mass index (BMI), FBG and postprandial plasma glucose (PPPG) and glycosylated hemoglobin (HbA1c) were measured at the beginning (week 0) and at every follow-up treatment visit, i.e., 6 months (week 24), 1 year (week 48) and 2 years (week 96). Changes from baseline to each visit were analyzed using the Wilcoxon test. All patients also went through a urinalysis at baseline, and after 6 months, 1 year and 2 years of treatment, to assess for any abnormalities in the urine (pH, pus or protein), suggestive of bladder abnormalities.
Results:
The combined analysis was carried out on 958 completed patients in this study who were treated with pioglitazone 7.5 mg, 15 mg and 30 mg tablet and other OADs. The difference in mean value of FBG showed a highly significant decrease (P < 0.0001) from baseline to end of treatment, i.e., from 167.0, (59.16) 172.6 (58.51) and 171.0 (39.47) to 140.2, (26.46) 143.8 (22.04) and 138.5 (27.82) mg/dL. Similarly, PPG showed a significant (P < 0.0001, 0.002 and 0.008) decrease from baseline to end of the treatment, i.e., from 256.0, (61.79) 222.9 (67.88) and 223.6 (69.11) to 195.9, (46.92) 204.0 (48.03) and 187.6 (53.36) mg/dL, and there was a highly significant (P < 0.0001) decrease in HbA1c levels, i.e., from 8.46, 8.34 and 8.42% to 7.781, 7.78 and 7.73%, respectively. However, gain in mean BW was also observed from baseline to end of the treatment, i.e., from 69.90, (9.44) 68.29 (8.62) and 67.64 (7.75) kg to 71.69, (8.35) 70.08 (7.96) and 69.70 (7.99) kg, respectively, and BMI increased from 26.74 (14.18-53.04) kg/m2 at baseline to 27.45 (12.87-53.73) kg/m2 at the end of the treatment, respectively (P < 0.0001). No significant changes were found in urine in patients even after 2 years of treatment with pioglitazone. There was little variation in pH or presence of pus and proteins in the urine, indicating no increased risk of bladder-related abnormalities across all treated age groups even after 2 years of treatment with pioglitazone.
Conclusion:
Pioglitazone in combination with other OADs in Indian patients was an effective treatment protocol in glycemic control, reduction in FBG, PPPG and HbA1c and also helps in controlling weight gain in patients with T2DM. In this patient population, there was no increased risk of bladder-related abnormalities. Pioglitazone was therefore found to be a safe and efficacious addition to treatment in patients with poorly controlled diabetes.
Keywords: Pioglitazone, type 2 diabetes, oral antidiabetics, Indian patients
INTRODUCTION
Many patients with type 2 diabetes mellitus (T2DM) have poorly controlled disease, even with intensive therapy.[1] New agents with the capability of improving glycemic control when used in addition to, or instead of, existing drugs would be useful, particularly if they also have beneficial effects in lowering the risk of microvascular and macrovascular complications.
The thiazolidinediones are a recently developed class of oral glucose-lowering drugs used in the treatment of T2DM.[2] They are thought to work through activation of the peroxisome proliferator-activated receptor gamma thus reducing insulin resistance.[3] The US food and drug administration (FDA) has approved 2 thiazolidinediones, rosiglitazone and pioglitazone; a third, troglitazone, was withdrawn because of reports of severe and unpredictable hepatotoxicity. In the United States, pioglitazone is approved for use both as monotherapy and in combination with metformin, a sulfonylurea or insulin. In Europe, pioglitazone is approved for use in combination with metformin or a sulfonylurea but not insulin.[4]
The purpose of the present open-label, prospective, non-randomized, single-centric observational study was to assess the efficacy and safety of pioglitazone in combination with other antidiabetics (ADs) in Indian patients with T2DM.
MATERIALS AND METHODS
Patients and study design
The study was conducted in a single center in India, viz. Chennai. Male and female patients with type 2 diabetes, aged between 16 and 80 years with body mass index (BMI) ≥23 kg/m2 and ≤35 kg/m2 , were included in this study. This open-label, prospective, non-randomized, single-center observational study was carried out in patients suffering with T2DM who were not achieving glycemic control on current insulin therapy. Patients were enrolled into one of two 2-year prospective observational trials. In this study, concomitant therapy of pioglitazone (7.5, 15 and 30 mg/day) in combination with other ADs, i.e., insulin (I), Sulfonylurea + Metformin + Insulin (S + M + I), Sulfonylurea + Metformin + Glitazones (S + M + G), Sulfonylureas + Metformin + Acarbose (S + M + A), Sulfonylureas + Metformin + Glitazones + Insulin (S + M + G + I) and Sulfonylureas + Metformin + Acarbose + Insulin (S + M + A + I) was administered in patients who were suffering with T2DM.
For this study, uncontrolled glycemia was defined by the following criteria: Hemoglobin (HbA1c) ≥7.0% or fasting plasma glucose ≥140 mg/dL and/or post-prandial plasma glucose (PPPG) ≥200 mg/dL.
Patients with evidence of any renal/hepatic de-compensation, positive test of pregnancy, failure to adhere to study regimen or significant comorbid conditions were excluded from the study.
All patients were screened according to protocol by clinical evaluation, medication history, physical examination and lab chemistry.
The study endpoints included evaluation of overall glycemic control and change in Body weight (BW) by addition of pioglitazone in combination with other ADs. The safety of pioglitazone in the Indian population was also analyzed.
Treatment
Patients received 7.5/15/30 mg pioglitazone in combination with other ADs. BW, BMI, fasting blood glucose (FBG) and PPPG estimation and glycosylated HbA1c were measured at the beginning (week 0) and at every treatment visit, i.e., 6 months (week 24), 1 year (week 48) and 2 years (week 96). At Visit 1 (Day 0), inclusion and exclusion criteria were reviewed and study medication was prescribed only to the eligible patients. Detailed instructions about the dose administration were given to each patient. The dose of the study medication was titrated as per the targeted glycemic levels.
At visit 2 (6 months post-treatment), visit 3 (1 year post-treatment) and visit 4 (2 years post-treatment), the dose of the study medication was reviewed and titrated appropriately as per the results of fasting and PPPG and glycosylated HbA1c. Adverse events were noted in the case record form.
Statistical analysis
Statistical analysis was performed on the basis of efficacy parameters compared with the baseline value for the same patient. Data were reported as means and SDs. Student's t-test (paired) and t-test (Wilcoxon Rank Sum Test) were used to assess the statistical significance. Value of P < 0.0001 was considered to be highly significant and P < 0.05 was considered to be significant. The statistical analysis system (SAS) Version 9.1.3 statistical package was used for analysis of the study outcome data.
RESULTS
A total of 958 adult patients were recruited and randomized to treatment with pioglitazone 7.5 mg + ADs (n = 261) or pioglitazone 15 mg + ADs (n = 590) or pioglitazone 30 mg + ADs (n = 107).
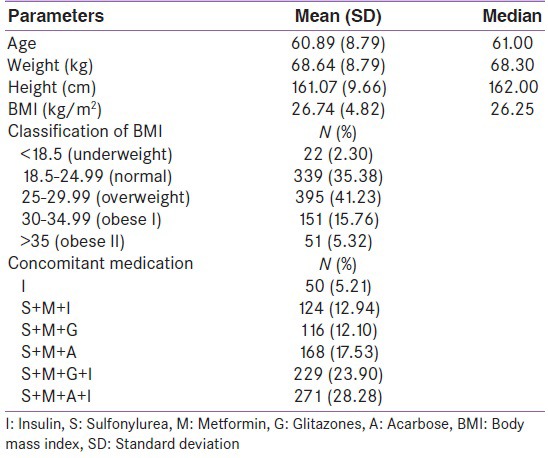
Table 1 shows the demographic characteristics of the patient. About 62.32% were categorized as overweight or obese, 35.38% normal weight and only 2.30% were categorized as underweight in this study.
Table 1.
Demographic characteristics of study participants (N=958)
Efficacy analysis
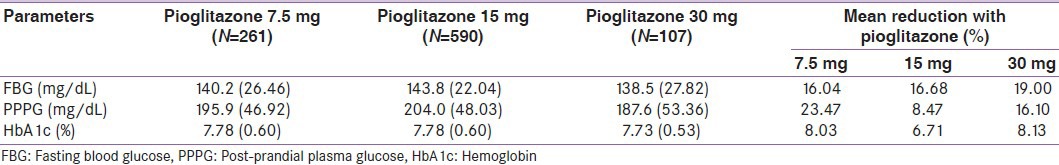
The efficacy analysis was carried out on 958 patients who completed the study. The benefits of addition of different dosages of pioglitazone to existing treatment are represented in Table 2 (7.5 mg [Table 2a], 15 mg [Table 2b] and 30 mg tablet [Table 2c]). The difference in mean value of FBG showed a highly significant decrease (P <0.0001) from baseline to end of treatment, i.e., from 167.0, (59.16) 172.6 (58.51) and 171.0 (39.47) to 140.2, (26.46) 143.8 (22.04) and 138.5 (27.82) mg/dL.
Table 2a.
Effect of pioglitazone 7.5 mg on fasting blood glucose, post-prandial plasma glucose and hemoglobin before and during 96 weeks of treatment
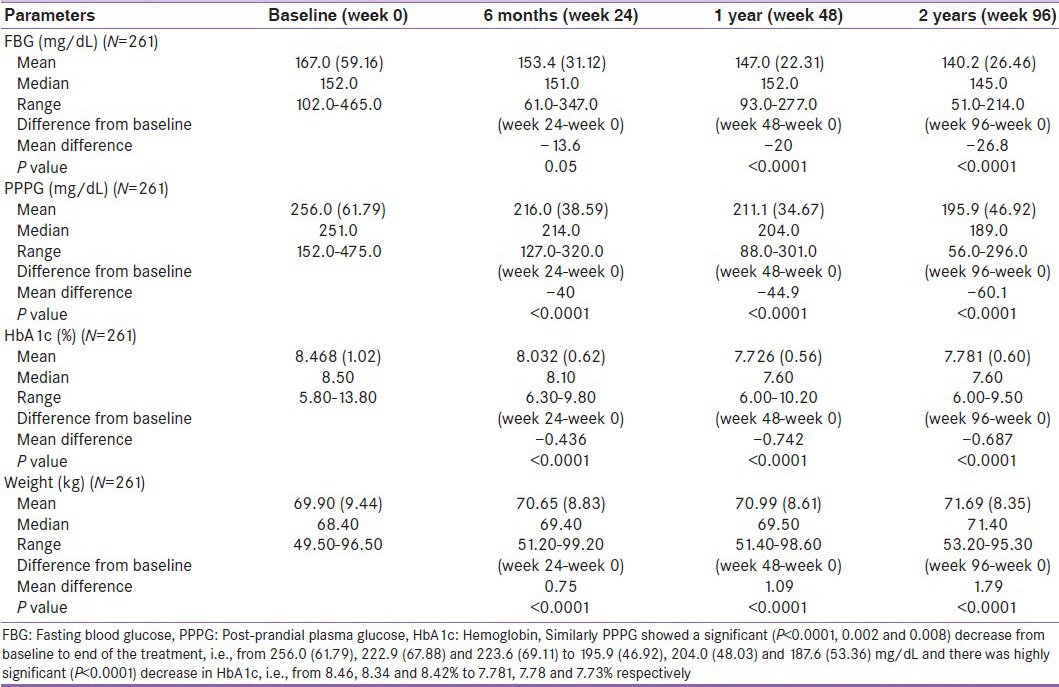
Table 2b.
Effect of pioglitazone 15 mg on fasting blood glucose, post-prandial plasma glucose and hemoglobin before and during 96 weeks of treatment
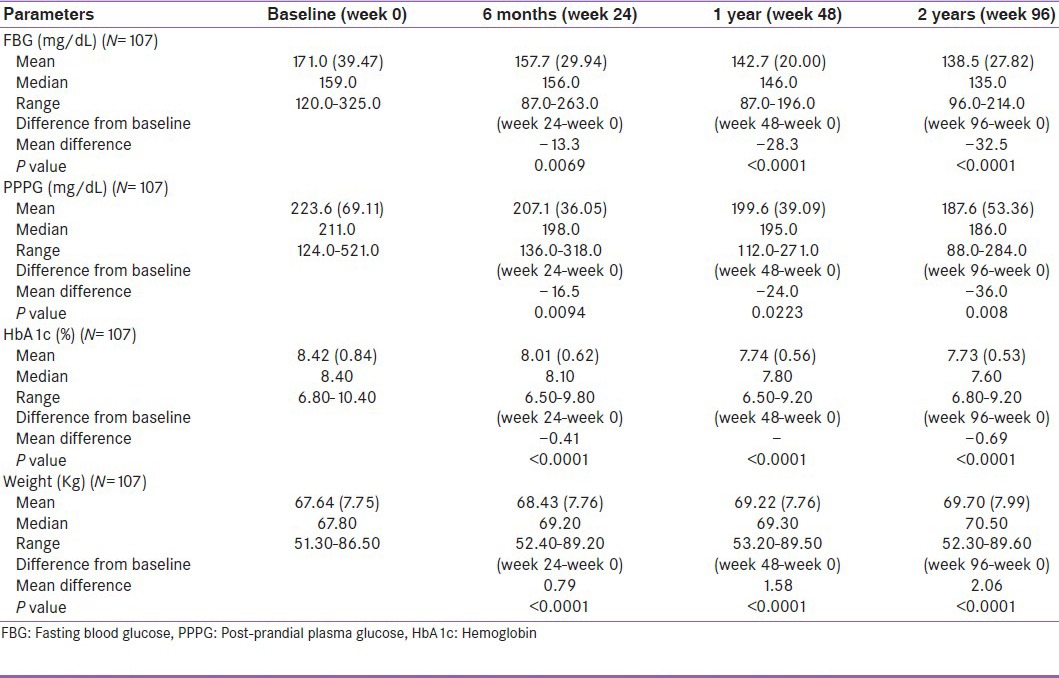
Table 2c.
Effect of pioglitazone 30 mg on fasting blood glucose, post-prandial plasma glucose and hemoglobin before and during 96 weeks of treatment
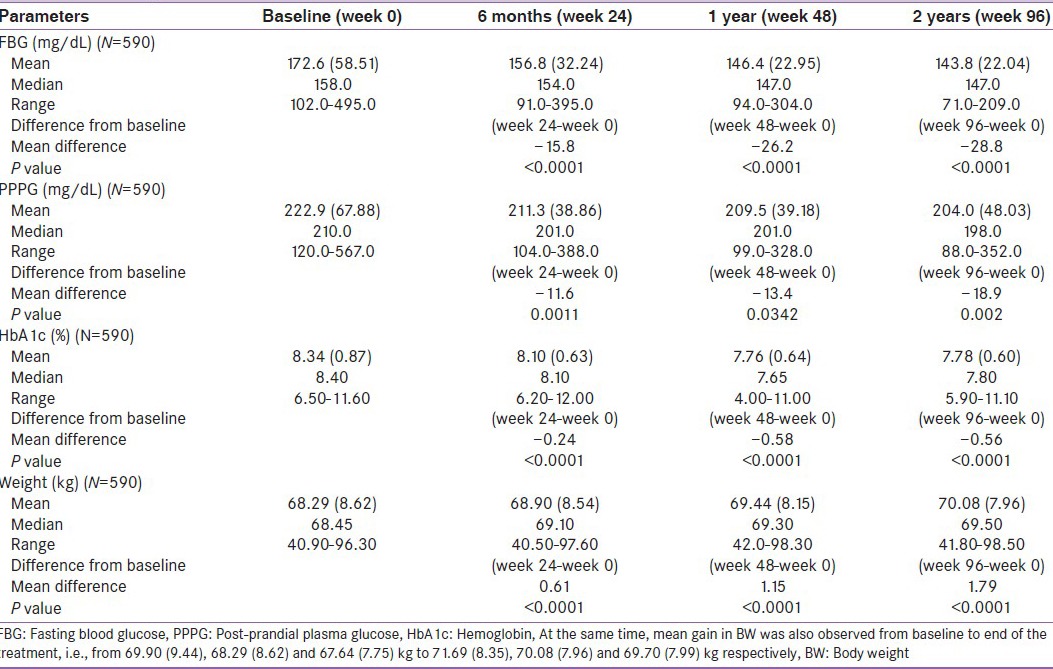
Similarly, PPPG showed a significant (P < 0.0001, 0.002 and 0.008) decrease from baseline to end of the treatment, i.e., from 256.0, (61.79) 222.9 (67.88) and 223.6 (69.11) to 195.9, (46.92) 204.0 (48.03) and 187.6 (53.36) mg/dL, and there was a highly significant (P < 0.0001) decrease in HbA1c, i.e., from 8.46, 8.34 and 8.42% to 7.781, 7.78 and 7.73%, respectively.
At the same time, mean gain in BW was also observed from baseline to end of the treatment, i.e., from 69.90, (9.44) 68.29 (8.62) and 67.64 (7.75) kg to 71.69, (8.35) 70.08 (7.96) and 69.70 (7.99) kg, respectively.
Table 2d shows a dose-dependent reduction in glycemic parameters from baseline to end of treatment. This study confirmed the effectiveness of pioglitazone on glycemic control as there was a significant improvement of HbA1c, PPPG and FBG levels after 2 years’ use of 7.5 mg, 15 mg and 30 mg doses of pioglitazone.
Table 2d.
Pioglitazone dose-dependent reduction in glycemic parameters from baseline to 2 years
Safety analysis
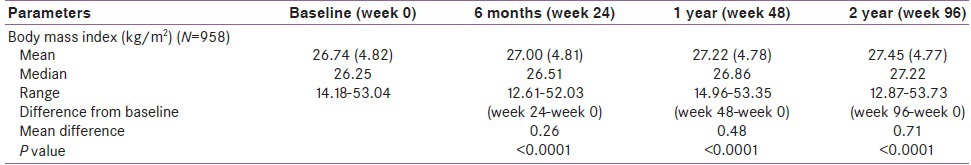
The overall mean of BMI at baseline was 26.74 kg/m2 (range: 14.18-53.04 in patient under oral antidiabetics [OADs]). Addition of pioglitazone results in statistically significant increases in BMI only from Week 96 (P < 0.0001) [Table 3], i.e., at 2 years of therapy.
Table 3.
Safety parameters of patients related to pioglitazone treatment
Pioglitazone was also found to be safe and efficacious across all treated age groups, i.e., from 18 to > 60 years.
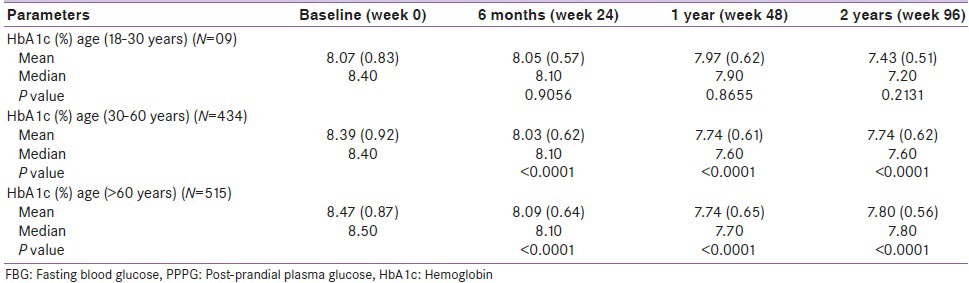
Table 4 shows an age-wise reduction in HbA1c, i.e., 18-30 years (N = 09), 30-60 years (N = 434) and > 60 years (N = 515). The difference in mean value of HbA1c (age 18–30 years) showed a non-significant decrease (P = 0.21) from baseline to end of treatment, i.e., from 8.07% (0.83) to 7.43% (0.51). Similarly, HbA1c (age 30-60 years) showed a significant (P < 0.0001, 0.002 and 0.008) decrease from baseline to end of the treatment, i.e., from 8.39% (0.92) to 7.74%, (0.62) and there was a highly significant (P < 0.0001) decrease in HbA1c (age > 60 years), i.e., from 8.47% (0.87) to 7.80%, (0.56) respectively.
Table 4.
Age wise reduction in hemoglobin with pioglitazone before and during 2 years
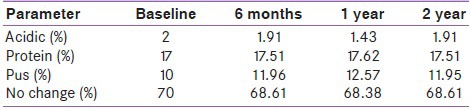
In addition, there was no significant change in the pH of urine or in the presence of pus and protein in the urine from baseline to 2 years of treatment [Table 5]. Also, a greater percentage of patients showed no significant abnormalities in urine during the entire treatment period.
Table 5.
% patients with changes in pH or indicating presence of protein/pus in urine, compared to those with no abnormalities in urine
DISCUSSION
In this study, pioglitazone was effective in reducing FBG, PPPG levels and HbA1c in patients with poorly controlled type 2 diabetes, both as monotherapy and in combination with metformin, glitazones, acarbose, a sulfonylurea or insulin. With pioglitazone monotherapy, HbAlc decreased by up to 2.6% compared with placebo in a dose-response fashion. The effect was sustained for up to 26 weeks. With pioglitazone combination therapy, HbAlc decreased up to 1.58% compared with placebo, again in a dose-response fashion. In light of evidence from PNFP-014,[5,6,7,8,9,10,11,12,13,14,15,16,17] that the combination of pioglitazone with insulin is effective in reducing hyperglycemia, the reasons for the differences in the US and European indications for the drug are unclear. Triglyceride levels decreased ~30-70 mg/dL with pioglitazone combination therapy and High-density lipoprotein - Cholesterol (HDL-C) levels increased by ~4-5 mg/dL. The evidence of an effect on triglyceride levels was less consistent for pioglitazone monotherapy.
An increase in BW was associated with pioglitazone treatment in all studies. Although this increase was greater in the US studies[17] than in the Japanese studies,[18,19,20,21] given the differences in baseline weights, the proportional increase was not dissimilar. A review of the safety and tolerability of pioglitazone[22] includes a figure combining data from the studies of monotherapy and combination therapy and showing weight changes up to 60 weeks (European and Japanese studies) and 96 weeks (US studies). Weight continued to increase for as long as data were recorded (60 weeks) in the European and Japanese studies and up to 84 weeks in the US studies. Whether the weight gain associated with pioglitazone therapy reaches a plateau cannot be determined without studies of a longer duration.
However, despite a consistent and significant increase in BW and total body fat mass, glycemic control and both hepatic and peripheral tissue sensitivity to insulin improved after pioglitazone treatment. Earlier studies have shown that pioglitazone was able to improve insulin sensitivity through a reduction in muscle lipid along with its redistribution into subcutaneous adipose tissue. Indeed, treatment with pioglitazone not only results in improvement in glucose homeostasis but simultaneously also alters fat metabolism and/or fat topography. Indeed, studies show that pioglitazone induces decrease in visceral fat, a shift of fat distribution from visceral to subcutaneous adipose depots consequently leading to improvements in both hepatic and peripheral tissue sensitivity to insulin.[23]
There are no randomized, controlled trials in the literature that directly compare pioglitazone with any other antidiabetic agent (including other thiazolidinediones). Results of a non-randomized study[24] suggest that rosiglitazone and pioglitazone may have a similar effect on blood glucose levels, and that pioglitazone may have a greater beneficial effect on lipid levels while leading to greater weight gain.
In the US monotherapy studies reported on the FDA Web site,[17] patients who had been taking another antidiabetic drug had poorer glycemic control after the switch to pioglitazone. Although this suggests that pioglitazone may be less-effective than the other drugs, this finding may have been the result of follow-up periods being too short for HbAlc values to fully reflect decreases in blood glucose levels.
To the best of our knowledge, this is the first prospective, observational study on the efficacy and safety of pioglitazone in Indian patients. In this study, the efficacy of different strengths of pioglitazone combination on top of other antidiabetic drugs (s) has been shown, as both fasting and postprandial glucose better controlled after addition of pioglitazone. The significant improvement of glycemic parameters had been observed since every treatment visits.
Regarding safety evaluation, the results of the study showed increases in BW and BMI. Although the increase of BW and BMI were indeed very small compared with those found by another study,[25] they were, however, statistically significant.
Apart from BW and BMI, we also would be like to evaluate the effect of pioglitazone on age-wise reduction in HbA1c [Table 4]. Age range of 18-30 years was statistical non-significant, but clinical reduction in HbA1c was significant. Similarly, age range of 30-60 years and age >60 years was statically significant when adding pioglitazone with current ODAs therapy.
Urinalysis of all patients on piogliotazone in the study revealed that there was no significant change in urine pH, proteinuria or pyuria and hematuria from baseline up to 2 years of treatment with pioglitazone across all dosages studied, and across all age groups, indicating the absence of any bladder-related abnormalities such as bladder cancer in the treated patients. This is significant in light of the current controversy related to increased risk of bladder cancer in patients on pioglitazone. Our results are similar to those recently reported by Li et al., suggesting that pioglitazone may not be significantly associated with an increased risk of bladder cancer in patients with type 2 diabetes.[26]
Limitations
Our present study has some limitations, including a low patients numbers, the lack of control group, a relatively short period of observation (2 years) and lack of other safety parameters such as urine analysis. However, our finding concerning efficacy and safety parameters seem to be in accordance with other studies.
CONCLUSION
It is concluded from the present study that combination therapy with pioglitazone as add-on therapy to existing OADs therapy is an effective, well-tolerated therapeutic approach to improve glycemic control in patients with type 2 diabetes. It was found to be safe and well tolerated across the age group studied and in combination with other ADs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.UK Prospective Diabetes Study Group. UK Prospective Diabetes Study 16.Over-view of 6 years’ therapy of type II diabetes: A progressive disease. Diabetes. 1995;44:1249–58. [PubMed] [Google Scholar]
- 2.Bailey CJ. Rosiglitazone and pioglitazone: Two new thiazolidinediones. Pract Diabetes Int. 2000;17:1–3. [Google Scholar]
- 3.Petrie J, Small M, Connell J. “Glitazones”, a prospect for non-insulin-dependent diabetes. Lancet. 1997;349:70–1. doi: 10.1016/S0140-6736(97)22002-7. [DOI] [PubMed] [Google Scholar]
- 4.Summary of product characteristics, February 2001. European Agency for the Evaluation of Medicinal Products Web site. [Last accessed 2001 Mar 1]. Available from: http://www.emea.eu.int .
- 5.Rubin C, Egan J, Schneider R. Combination therapy with pioglitazone and insulin in patients with type 2 diabetes. Diabetes. 1999;48:A110. Abstract. [Google Scholar]
- 6.Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on glucose metabolism in sulfonylurea treated patients with type 2 diabetes. Diabetes. 2000;49:A117. Abstract. [Google Scholar]
- 7.Schneider R, Lessem J, Lekich R. Pioglitazone is effective in the treatment of patients with type 2 diabetes. Diabetes. 1999;48:A109. Abstract. [Google Scholar]
- 8.Egan JW, Mathisen AL. The effect of pioglitazone on glucose control and lipid profile in patients with type 2 diabetes. Diabetes. 2000;49:A105. Abstract. [Google Scholar]
- 9.Mathisen AL, Schneider R, Rubin C, Houser V. The effect of pioglitazone on glucose control and lipid profile in patients with type 2 diabetes. Diabetologia. 1999;42:A227. Abstract. [Google Scholar]
- 10.Mathisen A, Geerlof J, Houser V. The effect of pioglitazone on glucose control and lipid profile in patients with type 2 diabetes. Diabetes. 1999;48:A102–3. Abstract. [Google Scholar]
- 11.Egan J, Rubin C, Mathisen A. Combination therapy with pioglitazone and metformin in patients with type 2 diabetes. Diabetes. 1999;48:A117. Abstract. [Google Scholar]
- 12.Mathisen A, Egan J, Schneider R. The effect of combination therapy with pioglitazone and sulfonylurea on the lipid profile in patients with type 2 diabetes. Diabetes. 1999;48:A106. Abstract. [Google Scholar]
- 13.Schneider R, Egan J, Houser V. Combination therapy with pioglitazone and sulfonylurea in patients with type 2 diabetes. Diabetes. 1999;48:A106. Abstract. [Google Scholar]
- 14.Lebrizzi R, Egan JW. The HbAlc and blood glucose response to pioglitazone in combination with another antidiabetic agent in patients with type 2 diabetes. Diabetes. 2000;49:All4. Abstract. [Google Scholar]
- 15.Egan J, Rubin C, Mathisen A. Adding pioglitazone to metformin therapy improves the lipid profile in patients with type 2 diabetes. Diabetes. 1999;48:A106. Abstract. [Google Scholar]
- 16.Hanefeld M, Goke B. Combining pioglitazone with a sulphonylurea or mefformin in the management of type 2 diabetes. Exp Clin Endocrinol Diabetes. 2000;108:256–266. [Google Scholar]
- 17.Center for Drug Evaluation and Research [US Food and Drug Administration Web site]. New and generic drug approvals 1998-2000. Medical and Statistical Reviews. 2000. [Last accessed 2000 Aug 2]. Available from: http://www.fda.gov/cder/foi/nda/99/021073A Actos.htm .
- 18.Kaneko T, Baba S, Toyota T. Dose finding study of AD-4833 in patients with non-insulin dependent diabetes mellitus (NIDDM) on treatment with a sulfonylurea drug. Single blind comparative study on four dosages. Jpn J Clin Exp Med. 1997;74:1278–306. [Google Scholar]
- 19.Kaneko T, Baba S, Toyota T. Dose finding study of AD-4833 in patients with non-insulin dependent diabetes mellitus (NIDDM) on diet therapy alone. Double-blind comparative study on four dosages. Jpn J Clin Exp Med. 1997;74:1250–77. [Google Scholar]
- 20.Kaneko T, Baba S, Toyota T. Clinical evaluation of an insulin-resistance improving agent, AD-4833, in patients with non-insulin dependent diabetes mellitus (NIDDM) on diet therapy alone. A placebo controlled double blind clinical study. Jpn J Clin Exp Med. 1997;74:1491–514. [Google Scholar]
- 21.Kaneko T, Baba S, Toyota T. Clinical evaluation of an insulin-resistance improving agent, AD-4833, in patients with non-insulin dependent diabetes mellitus (NIDDM) on treatment with an SU drug. A placebo controlled double blind clinical study. Jpn J Clin Exp Med. 1997;74:1515–39. [Google Scholar]
- 22.Belcher G, Matthews DR. Safety and tolerability of pioglitazone. Exp Clin Endocrinol Diabetes. 2000;108(suppl 2):S267–S273. [Google Scholar]
- 23.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–91. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 24.King AB. A comparison in a clinical setting of the efficacy and side effects of three thiazolidinediones. Diabetes Care. 2000;23:557. doi: 10.2337/diacare.23.4.557b. [DOI] [PubMed] [Google Scholar]
- 25.Pendsey SP, Dhanvijay VP, Joshi PP. Efficacy of pioglitazone as on add on drug with insulin, glibenclamide and metformin in patients with uncontrolled type 2 diabetes mellitus. Diabetologia Croatia. 2002;31:51–7. [Google Scholar]
- 26.Li W, Macdonald TM, Mackenzie IS. Pioglitazone and bladder cancer: A propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254–9. doi: 10.1111/j.1365-2125.2012.04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


