Abstract
Reperfusion injury of ischemic tissue represents an acute inflammatory response that can cause significant morbidity and mortality. The mechanism of injury is not fully elucidated, but recent studies indicate an important role for natural antibody and the classical pathway of complement. To test the hypothesis that injury is initiated by specific IgM, we have screened a panel of IgM-producing hybridomas prepared from peritoneal cells enriched in B-1 cells. One clone, CM22, was identified that could restore pathogenic injury in RAG-1-/- mice in an intestinal model of ischemia/reperfusion (I/R). In situ activation of the classical pathway of complement was evident by deposition of IgM, complement C4, and C3 in damaged tissue after passive transfer of CM22 IgM. Sequence analysis of CM22 Ig heavy and light chains showed germ-line configurations with high homology to a VH sequence from the B-1 repertoire and a VK of a known polyreactive natural IgM. These data provide definitive evidence that I/R injury can be initiated by clonally specific natural IgM that activates the classical pathway of complement. This finding opens an avenue for identification of I/R-specific self-antigen(s) and early prevention of injury.
Ischemia/reperfusion (I/R) injury is a major complicating feature of many clinical disease entities. In general, I/R represents an acute inflammatory response after an ischemic event and subsequent restoration of blood flow (1). I/R is responsible for much of the severity of myocardial infarction, cerebral ischemic events, intestinal ischemia, and many aspects of vascular surgery, trauma, and transplantation (1). Intestinal I/R is a devastating syndrome. Approximately one-third of episodes are acute events and are responsible for most gastrointestinal ischemia-related deaths (mortality rate of 70-90%); this attests to the lethal nature of the disease if it is not recognized and treated promptly (2).
During ischemia, hypoxic cells undergo specific changes in enzymatic activities, mitochondrial function, cytoskeletal structure, membrane transport, and antioxidant defenses. One current model proposes that hypoxic cells activate neutrophils, which, in turn, contribute to vascular reperfusion injury by producing reactive oxygen species (3). However, additional mechanisms must be involved, because cellular injury can occur in the presence of a limited number of inflammatory cells (4-6).
Weisman et al. (7) proposed that acute inflammatory attack after reperfusion depended on activation of the serum complement system. They found that pretreatment with a soluble inhibitor of complement C3b (sCR1) can significantly reduce reperfusion injury in a rat model of myocardial infarction (7). Similar results were subsequently observed in a rat intestinal model (8) and a mouse model of skeletal muscle (9). The finding that an intact classical pathway of complement was required for I/R led to the suggestion that antibody might be involved in the mediation of inflammation (9). Indeed, mice deficient in classical pathway components, namely C4, C3, or total Ig (RAG-/-), are equally protected in the hind limb and intestinal models (9). Moreover, reconstitution of RAG-1-/- mice with IgM from WT mouse sera restores injury (10). These observations led to an alternative hypothesis that I/R injury is initiated by recognition and binding of preexisting or natural IgM to neoepitopes expressed by hypoxic cells (9, 10).
Recent studies extended this model, suggesting that a subset of B lymphocytes, in particular B-1 cells, are a major source of pathogenic IgM (11, 12). Notably, mice deficient in complement receptors 1 and 2 (Cr2-/-) have a pronounced reduction in injury in the intestinal I/R model (11, 12). The protection was due to a lack of specific IgM, because reconstitution of either strain with WT IgM (11, 12) or an enriched fraction of B-1 cells restores injury (12). Conversely, reconstitution of RAG-1-/- mice with IgM isolated from Cr2-/- mice failed to restore injury to a degree similar to WT IgM (12). Thus, these studies demonstrated that initiation of an I/R injury was not an inherent property of all IgM but suggested that it was specific and that B-1 cells were a potential important source (11, 12). B-1 cells, which reside primarily within the peritoneal and pleural cavities, represent a major source of preexisting or natural antibodies (13-15). B-1 cells are distinguished from conventional B-2 cells by their surface phenotype, i.e., CD11b+, CD43+, CD23lo, CD21int, and IgMhi, and their restricted germ-line repertoire (13-15). Circulating natural IgM acts as a first line of defense against foreign pathogens and may contribute to autoreactivity (16-19).
To identify I/R-specific natural IgM-producing cells, a panel of hybridomas was generated from peritoneal cells enriched in B-1 cells, and their IgM product was screened in vivo for reconstitution of injury in RAG-1-/- mice. One clone, CM22, was identified in which its IgM restored significant I/R injury. Deposition of IgM, C4, and C3 within reperfused tissues correlated with pathogenesis after treatment of mice reconstituted with IgM isolated from CM22 but not from other hybridomas. Sequence analysis of pathogenic IgM identified an apparent usage of germ-line VH and VK genes in CM22 Ig heavy and light chains, respectively. Therefore, a single pathogenic IgM clone was identified, confirming a crucial link in the mechanism of classical pathway complement-mediated induction of I/R injury.
Materials and Methods
Animals and Generation of B-1 Cell Hybridomas. WT C57BL/6 and RAG-1-/- mice (C57BL/6 genetic background) were purchased from The Jackson Laboratory and bred under specific pathogen-free conditions. To generate hybridomas, peritoneal cells enriched in B-1 cells were obtained by means of peritoneal lavage of 8- to 12-week-old C57BL/6 mice. Recovered cells were pooled, washed, and activated with lipopolysaccharide overnight, then fused with S/P 20 myeloma cells followed by selection in hypoxanthine/aminopterin/thymidine medium (Cell Essential, Boston). IgM-producing hybridomas were screened by ELISA. Subcloning of hybridomas was carried out by limiting dilution, and clonality was confirmed by sequencing the VDJ regions of Ig. IgM from hybridoma supernatant or WT mouse serum (Accurate Chemicals) was isolated as described (10). The pentameric conformation of IgM was assessed by analysis on nonreducing SDS/PAGE compared with a high-molecular weight marker (Amersham Biosciences).
Sequencing of CM22 VH and VK cDNA. Total cellular RNA was extracted from 107 hybridoma cells by using TRIzol (GIBCO). First strand cDNA synthesis and PCR amplification were performed following the described method (20). Briefly, Ig heavy and light chain cDNA were amplified by using promiscuous 5′ VH and VK primers. The 3′ primers represented conserved sequences within the Cμ and Cκ regions, respectively. The amplified Ig heavy and light chains were purified on agarose gel and sequenced by using a PRISM BigDye mix and Genetic Analyzer (Applied Biosystems). Nucleotide sequence data were analyzed with the sequencher software package (Gene Codes, Ann Arbor, MI) and aligned with National Center for Biotechnology Information (NCBI) and Immunogenetics (http://imgt.cines.fr) databases.
Induction of Intestinal I/R Injury. Surgical protocol for I/R was performed as described (10). Briefly, after anesthesia, a laparotomy was performed, and the microclip (125 g of pressure; Roboz, Gaithersburg, MD) was applied to the superior mesenteric artery. After 40 min of ischemia, the microclip was removed, and all animals were kept warm for 3 h of reperfusion. At the end of reperfusion, the ischemic segment of the jejunum was harvested and the central 4 cm was excised for pathological analysis. Intestinal permeability index = (cpm-1·g-1 of jejunal loop)/(cpm-1·g-1 of blood) (10) after i.v. injection of radioalbumin.
Histopathology and Immunohistochemistry Analysis. Cryostat sections of intestinal tissues were stained by hematoxylin and eosin and examined by microscopy for mucosal damage. Pathology was scored based on the following criteria: % injury = (number of villi with subepithelial space/number of total villi) × 50% + (number of villi with epithelial disruption/number of total villi) × 100%. Villi with the following two morphological changes are considered pathologically damaged and were counted separately: (i) subepithelial space, defined as an acellular space under a continuous epithelial layer and milder form of damage; and (ii) epithelial disruption, defined as discontinuation of epithelial layer of villus and more severe form of damage.
For immunofluorescence, biotin-labeled anti-mouse IgM (Becton Dickinson) was incubated overnight on cryosections (fixed with 4% paraformaldehyde, Sigma) followed by staining for 1 h with streptavidin-Alexa 568 (Molecular Probes). Colocalization of C4 was achieved by staining with FITC-labeled rabbit anti-huC4c (DAKO), followed by anti-rabbit-Alexa 488 (Molecular Probes). The specificity of anti-C4c staining was confirmed by staining serial sections with biotin-labeled anti-mouse C4 for 1 h followed by streptavidin-FITC (Becton Dickinson). Colocalization of C3 was performed by using FITC-labeled anti-C3 (DAKO). Sections were mounted in Antifade Mounting Medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories). Fluorescent images were taken with a Leica digital imaging system.
Immunoprecipitation. Immunoprecipitation was performed by using a standard protocol (21). Briefly, intestinal tissues were flushed with sterile saline and immediately cryofrozen. Intestines were homogenized, and lysates were precleared with CNBr-activated Sepharose 4B (Amersham Biosciences) and Sepharose coupled with goat anti-rat IgG (Caltag, South San Francisco, CA). Postcleared supernatants were incubated with Sepharose conjugated with goat anti-mouse IgM (Caltag) to capture IgM-bound immunocomplexes. The bound samples were eluted and subsequently analyzed on SDS/PAGE (6%) under reducing conditions. Protein bands were visualized by staining with GelCode Blue (Pierce).
Results
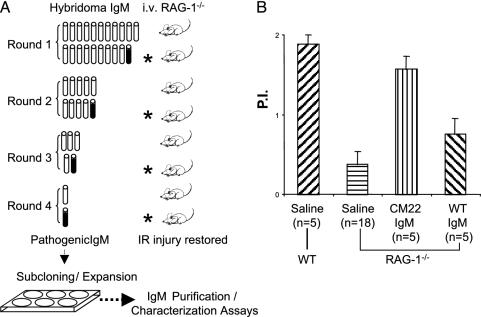
Recent studies suggest that reperfusion injury is initiated by specific natural IgM and that B-1 cells are a potential source (9-12). To test this hypothesis and to identify specific B-1 cell clones that might generate pathogenic I/R antibodies, IgM-producing hybridomas were prepared from WT peritoneal cells enriched in B-1 cells. Among an initial set of 80 hybridomas, 21 secreted appreciable levels of IgM. Ig was purified from the supernatant of each clone, and equal amounts were combined into a single pool for analysis by using the intestinal I/R model. RAG-1-/- mice were reconstituted with 0.5 mg of pooled IgM before surgery. Analysis of intestinal permeability index of treated mice indicated that the total pool of hybridoma IgM could restore injury comparable to serum IgM (data not shown). Thus, one or more of the 21 clones produced pathogenic IgM. After four rounds of subsequent pool restrictions (50 μg of IgM per clone in each pool), one hybridoma clone, CM22, was identified that, when injected alone, restored injury based on permeability index in RAG-1-/- mice (Fig. 1).
Fig. 1.
Identification of pathogenic hybridoma IgM from peritoneal B cells. (A) The strategy of assaying 21 hybridomas in groups in vivo. Intestinal vascular permeability is used as an index for reperfusion injury. The hybridoma group that tested positive (filled symbol) for induction of injury is further divided into two subgroups for next round test in animals. The positive hybridoma (CM22) is then subcloned by limiting dilution followed by expansion of the clone and purification of IgM. (B) Permeability indices of intestinal I/R comparing RAG-1-/- mice that received saline (negative control) or reconstituted with IgM from clone CM22 or WT control. Permeability index (P.I.) = (cpm-1·g-1 of jejunal loop)/(cpm-1·g-1 of blood).
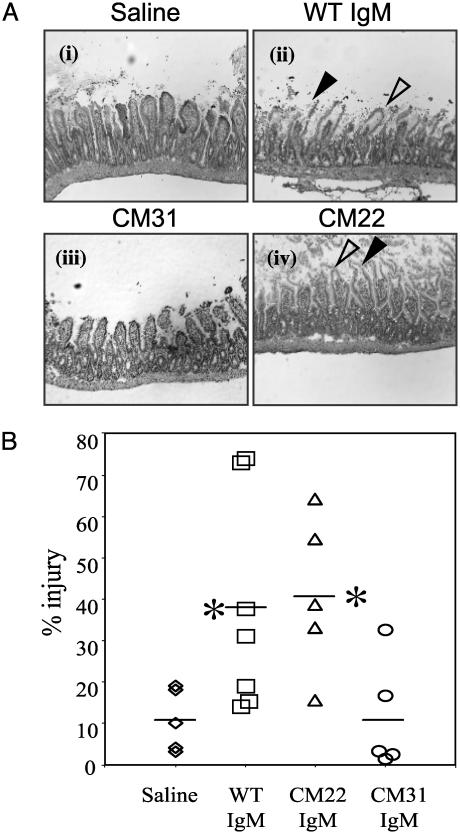
Histological examination of intestinal tissue confirmed the results from previous studies that pretreatment of RAG-1-/-mice with WT IgM led to I/R injury similar to that of WT mice. Injury was identified as frequent formation of subepithelial spaces and disruption of epithelial cells in the intestinal villi (Fig. 2 Ai and Aii). Quantification of injury using an integrated pathology score revealed that reconstitution with WT IgM caused an ≈4-fold increase in pathology score compared with mice receiving saline alone (38 ± 26%, n = 7, versus 11 ± 8%, n = 5; P = 0.017), respectively (Fig. 2B). Preischemic injection of RAG-1-/- mice with 200 μg of CM31 IgM, one hybridoma from the original pool, caused minimal injury similar to saline control (11 ± 13%, n = 5, versus 11 ± 8%, n = 5; P = 0.474) (Fig. 2 Aiii and B). In contrast, the same dose of IgM from hybridoma clone CM22 led to a 4-fold increase in tissue injury relative to saline control (41 ± 19%, n = 5, versus 11 ± 8%, n = 5; P = 0.01) (Fig. 2 Aiv and B). Therefore, CM22 specifically restores intestinal I/R injury in RAG-1-/- mice.
Fig. 2.
IgM from a single B cell hybridoma (clone CM22) induces reperfusion injury in RAG-1-/- mice. (A) Representative cryosections were prepared from intestinal tissue of RAG-1-/- mice injected i.v. with saline (i), 400 μg of WT serum IgM (ii), 200 μg of IgM from clone CM31 (iii), or 200 μg of IgM from clone CM22 (iv) before treatment in the intestinal I/R model (see Materials and Methods). After surgery, intestinal tissues were harvested and stained with hematoxylin and eosin. Pathologic features of injury were indicated by arrowheads: filled arrowhead, epithelial disruption (disintegrated epithelium layer of villi); open arrowhead, subepithelial spaces (lack of cellular content beneath continuous epithelial layer). (Magnification, ×100). (B) Pathological scores of intestinal injury were calculated as described in Materials and Methods. *, Statistical significance determined by Student's t test of the Ig-treated versus saline-only groups.
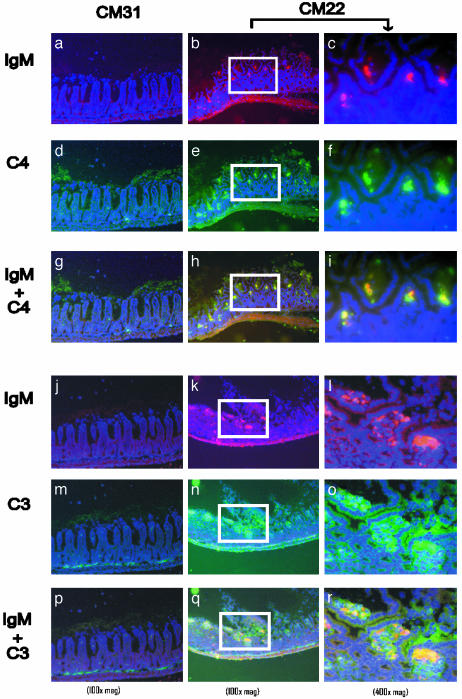
To examine activation in situ of the classical pathway of complement within injured villi, immunofluorescent staining for IgM and C4 was performed on intestinal sections. No specific deposition of IgM, C3, or C4 was observed in the intestinal villi of RAG-1-/- mice receiving CM31 IgM (Fig. 3 a, d, g, j, m, and p). In contrast, CM22 treatment led to the deposition of IgM and C4 in injured tissues of RAG-1-/- mice (Fig. 3 b, e, and h). As expected, colocalization of IgM and C3 was also detected in the injured villi (Fig. 3 k, n, and q). Analysis at high magnification revealed that deposition of IgM and complement was primarily within the subepithelial space, indicating specific accumulation and a correlation with pathogenesis (Fig. 3 c, f, i, l, o, and r). These results indicate that CM22 specifically binds to ischemic tissue and activates the classical pathway of complement.
Fig. 3.
Deposition of CM22 IgM, C4,and C3 on injured intestinal tissues. Representative cryosections of intestinal tissues were harvested after intestinal I/R from RAG-1-/- mice pretreated with either IgM from CM31 (a, d, g, j, m, and p) or CM22 (b, c, e, f, h, i, k, l, n, o, q, and r). All sections were stained with anti-IgM-biotin followed by streptavidin-Alexa 568 (red) and counterstained with 4′,6-diamidino-2-phenylindole (violet). Cryosections were costained with anti-C4-FITC (green in a-i) and anti-C3-FITC (green in j-r). The colocalization of IgM and C4 (red + green = yellow) is represented in g-i. Colocalization of IgM and C3 is represented in p-r (red + green = yellow). High-magnification (×400) images of CM22 treatment (c, f, i, l, o, and r) were taken from the same region as ×100 magnification marked by white boxes.
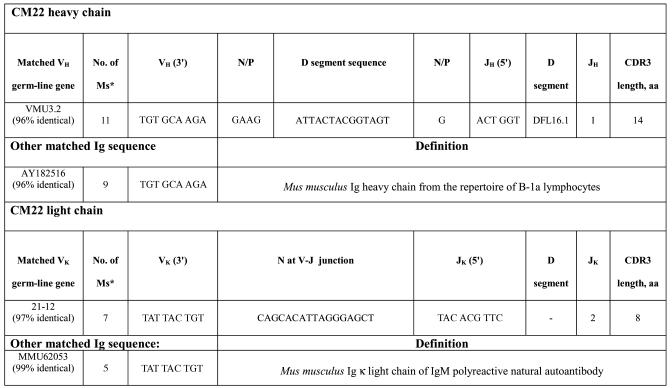
To characterize the nucleotide sequence of CM22 Ig variable regions, cDNA was generated from hybridoma cell total RNA and amplified by using promiscuous 5′ VH or VK in combination with 3′ CH or CK oligonucleotide primers. Nucleotide sequence of amplified VDJ was determined by using an automated sequencer (Applied Biosystems). Comparison of respective nucleotides with the Ig database indicated that CM22 VH sequence was 96% identical to that of germ-line VH3.2, a member of the J558 family (Table 1). The DH and JH regions were identified as DFL1.6 and JH1, respectively. Limited N-nucleotide additions were evident at both the V-D or D-J junction, and complementarity-determining region 3 (CDR3) consisted of 14 aa, a typical feature of CDR3 sequences generated by B-1 cells (2-20 aa with few N-additions) (20). A murine Ig heavy chain sequence from the repertoire of B-1a lymphocytes (GenBank accession no. AY182516) was identified with 96% homology to CM22. The light chain sequence of CM22 matched germ-line VK 21-12 gene with 97% identical nucleotides (Table 1); it contained a JK2 region with 8 aa in the CDR3. In addition, CM22 light chain includes 17 nt between framework region 3 (FWR3) and JK2, but they are identical to the VK21-12 germ-line gene (except one G-to-T mutation at the eighth nucleotide after FWR3). The CM22 light chain sequence is 99% identical to the κ light chain of an IgM polyreactive natural autoantibody (GenBank accession no. MMU62053). These results indicate that CM22 has the major features of natural IgM derived from the B-1 repertoire.
Table 1. CM22 heavy and light chain sequences.
* Number of mismatched nucleotides in VH or VK with the GenBank sequences.
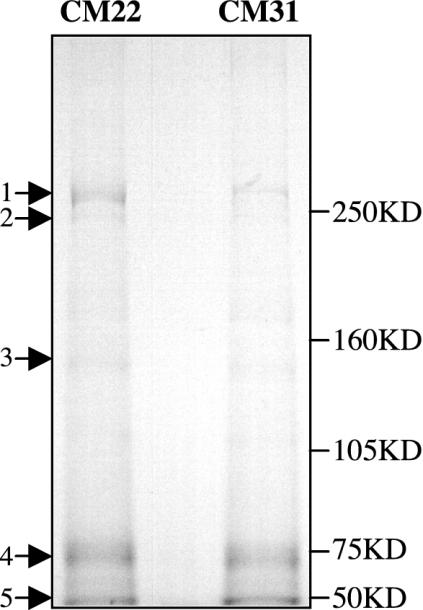
The identification of a single monoclonal IgM that initiates injury in RAG-1-/- mice suggests that binding to ischemic tissue is specific. It was hypothesized that such antigen(s) was expressed or exposed during ischemia and recognized by pathogenic IgM during reperfusion. To test this possibility, intestinal tissues were harvested after 15 min of reperfusion from RAG-1-/- mice reconstituted with either CM22 or CM31. Tissues were homogenized, and IgM complexes were isolated and analyzed on 6% SDS/PAGE gels as described in Materials and Methods. Comparison of immune precipitates from the two groups of treated mice identified a 75-kDa band of similar intensity that represents IgM heavy chain (Fig. 4, arrow 4). The 50-kDa band most probably represents heavy chain of the goat IgG (anti-mouse IgM) (Fig. 4, arrow 5). Moreover, several minor bands (arrows 2 and 3) also appeared in common. By contrast, CM22 precipitated a prominent band at ≈250 kDa that was only weakly present in the CM31 lysate. Thus, CM22 appears to recognize a specific high molecular weight protein in I/R-treated mice.
Fig. 4.
Immunoprecipitation of I/R-specific antigen(s). Intestinal lysates were obtained from CM22- or CM31-reconstituted RAG-1-/- mice after 15 min of reperfusion. Immunoprecipitation was performed as described in Materials and Methods, and IgM complexes were analyzed by SDS/6% PAGE under reducing conditions. Molecular mass markers are indicated on the right.
Discussion
The existence of I/R-specific natural antibodies was suggested by earlier studies (9-12). In the present study, we find that IgM from a single hybridoma clone, CM22, was sufficient to restore reperfusion injury in RAG-1-/- mice in the intestinal I/R model. This effect was highly specific, because IgM prepared from 20 other hybridomas did not restore injury. CM22 treatment in RAG-1-/- animals led to the activation of the classical complement pathway as evidenced by the colocalization of C4 and C3 with IgM in the injured tissue. Moreover, analysis of IgM immune precipitates from I/R-treated mice suggests that CM22 binds a specific band of ≈250 kDa. These findings support the model that natural IgM recognizes novel neo-antigens expressed or exposed on damaged tissue and, upon binding to the cell surface, initiates inflammation by activating complement in the classical pathway.
These results suggest that the cellular origin of I/R antibody is most likely B-1 cells. Hybridoma clone CM22 was generated from peritoneal cells that include an enriched fraction of B-1, and its Ig heavy and light chain sequences display germ-line configuration, a typical feature of a B-1 cell antibody. In addition, the CM22 VH sequence matches a sequence from the B-1a repertoire, and its light chain matches a natural IgM κ sequence. The finding that one purified IgM hybridoma, CM31 (as well as the pool of other 20 hybridomas), did not restore injury confirms that pathogenic IgM is clonally specific for self-antigen in I/R. In addition, a previous study showed that natural IgM from Cr2-/- mice did not restore injury in RAG-1-/-animals, suggesting a deficiency in a specific B-1 subset (12). Nevertheless, additional studies are needed to determine whether additional B-1 or B-2 cells can produce I/R-specific self-reactive IgM.
A hallmark of natural IgM is specificity for highly conserved self-antigens often shared by pathogens. For example, murine IgM binds highly conserved nuclear antigens, such as histones, DNA, and ribosomal protein (22, 23). Moreover, natural IgM binds carbohydrate antigens expressed on red cell surface that often crossreact with bacterial products. In one anti-erythrocyte Ig transgenic model (Tg), expression of the IgM Tg directs a predominant B-1 cell phenotype (24). In this model, the development and maintenance of the autoreactive B-1 cells, which leads to spontaneous autoimmune hemolytic anemia, depends on the presence of enteric bacteria, suggesting a crossreaction of self-antigen with bacteria (25). It is possible that CM22-specific B cells are also naturally preserved in normal animals by self-antigen(s) and may be also maintained by similar epitopes on enteric bacteria.
The nature of neoantigen(s) recognized by the I/R-specific antibody needs further studies. Current evidence suggests that these antigens are common or conserved, because CM22 also appears to restore injury in the hind limb model (W.G.A., M.Z., M.C.C., and F.D.M., unpublished results). One possibility is that conserved neoantigens are located on endothelial cells, which are universal among different organs and underlying parenchyma. Conserved neoantigens may be self-molecules that translocate from cytoplasm onto the cell surface or membranous molecules that undergo conformational changes after hypoxic stress. The finding of a prominent band at ≈250 kDa in immune precipitates isolated from intestinal tissue of CM22-treated mice suggests this approach can be used in future studies to identify the ischemic antigen(s).
The establishment of a crucial role for classical pathway of complement in I/R injury does not necessarily exclude the involvement of other innate immune components. For instance, C-reactive protein has been shown to play a role in cardiac I/R injury (26). It is likely that multiple components of innate immunity participate in different phases of injury. Self-reactive natural antibodies may participate in the early phase of attack by recognizing novel ischemic antigens, which subsequently activate complement. Release of chemotaxis mediators such as C3a or C5a can enhance the migration of inflammatory cells to the site, which will amplify injury at later phases. Supporting evidence for this model includes the observations that neutrophil activation is complement-dependent during intestinal I/R (27) and that anti-C5 treatment can attenuate tissue injury and neutrophil recruitment in the intestinal and heart models (28, 29). In addition, mast cells could participate in exacerbation of injury after complement activation, as observed in acute septic peritonitis (30). Mast cells and mast cell-induced reactions appear to contribute to an increase in mucosal permeability during reperfusion, but their role in pathological injury remains unclear (6, 31, 32).
In summary, the identification of a single IgM clone that restores I/R injury provides a crucial link between initiation and activation of complement after reperfusion of ischemic tissue. The availability of an ischemia-specific IgM should lead to identification of neoantigens formed during I/R and potential therapy for intervention in this common clinical disorder.
Acknowledgments
We thank Jurong Xia, Grace Ding, and other members of our respective laboratories (M.C.C. and F.D.M.) for technical help and critical reading of the manuscript. This work was supported in part by National Institutes of Health Grants PO1-GM52585 (to F.D.M., M.C.C., and H.B.H.) and R43-AI51045-01A1 (to E.M.A.).
Abbreviations: I/R, ischemia/reperfusion; CDR3, complementarity-determining region 3.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY547436 and AY547437).
References
- 1.Cotran, R. S. (1999) in Robbins Pathologic Basis of Disease (Saunders, St. Louis), pp. 7-12.
- 2.Lawrence, J. B. (2003) in Current Diagnosis & Treatment in Gastroenterology, ed. Friedman, S. L. (McGraw-Hill, New York), Section I.9.
- 3.Li, C. & Jackson, R. M. (2002) Am. J. Physiol. 282, C227-C241. [DOI] [PubMed] [Google Scholar]
- 4.Koike, K., Moore, E. E., Moore, F. A., Franciose, R. J., Fontes, B. & Kim, F. J. (1995) J. Trauma 39, 23-27; discussion, 27-28. [DOI] [PubMed] [Google Scholar]
- 5.Simpson, R., Alon, R., Kobzik, L., Valeri, C. R., Shepro, D. & Hechtman, H. B. (1993) Ann. Surg. 218, 444-453; discussion, 453-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo, A., Boros, M., Kaszaki, J. & Nagy, S. (1997) Shock 8, 284-291. [DOI] [PubMed] [Google Scholar]
- 7.Weisman, H. F., Bartow, T., Leppo, M. K., Marsh, H. C., Jr., Carson, G. R., Concino, M. F., Boyle, M. P., Roux, K. H., Weisfeldt, M. L. & Fearon, D. T. (1990) Science 249, 146-151. [DOI] [PubMed] [Google Scholar]
- 8.Hill, J., Lindsay, T. F., Ortiz, F., Yeh, C. G., Hechtman, H. B. & Moore, F. D., Jr. (1992) J. Immunol. 149, 1723-1728. [PubMed] [Google Scholar]
- 9.Weiser, M. R., Williams, J. P., Moore, F. D., Kobzik, L., Ma, M., Hechtman, H. B. & Carroll, M. C. (1996) J. Exp. Med. 183, 2343-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams, J. P., Pechet, T. T., Weiser, M. R., Reid, R., Kobzik, L., Moore, F. D., Carroll, M. C. & Hechtman, H. B. (1999) J. Appl. Physiol. 86, 938-942. [DOI] [PubMed] [Google Scholar]
- 11.Fleming, S. D., Shea-Donohue, T., Guthridge, J. M., Kulik, L., Waldschmidt, T. J., Gipson, M. G., Tsokos, G. C. & Holers, V. M. (2002) J. Immunol. 169, 2126-2133. [DOI] [PubMed] [Google Scholar]
- 12.Reid, R. R., Woodcock, S., Shimabukuro-Vornhagen, A., Austen, W. G., Jr., Kobzik, L., Zhang, M., Hechtman, H. B., Moore, F. D., Jr., & Carroll, M. C. (2002) J. Immunol. 169, 5433-5440. [DOI] [PubMed] [Google Scholar]
- 13.Kantor, A. B. & Herzenberg, L. A. (1993) Annu. Rev. Immunol. 11, 501-538. [DOI] [PubMed] [Google Scholar]
- 14.Herzenberg, L. A., Baumgarth, N. & Wilshire, J. A. (2000) Curr. Top. Microbiol. Immunol. 252, 3-13. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa, K., Shinton, S. A., Asano, M. & Hardy, R. R. (2000) Curr. Top. Microbiol. Immunol. 252, 15-22. [DOI] [PubMed] [Google Scholar]
- 16.Hamano, Y., Hirose, S., Ida, A., Abe, M., Zhang, D., Kodera, S., Jiang, Y., Shirai, J., Miura, Y., Nishimura, H. & Shirai, T. (1998) Blood 92, 3772-3779. [PubMed] [Google Scholar]
- 17.Murakami, M. & Honjo, T. (1996) Semin. Immunol. 8, 3-9. [DOI] [PubMed] [Google Scholar]
- 18.Ochsenbein, A. F. & Zinkernagel, R. M. (2000) Immunol. Today 21, 624-630. [DOI] [PubMed] [Google Scholar]
- 19.Baumgarth, N., Herman, O. C., Jager, G. C., Brown, L. E., Herzenberg, L. A. & Chen, J. (2000) J. Exp. Med. 192, 271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidl, K. J., MacKenzie, J. D., Wang, D., Kantor, A. B., Kabat, E. A. & Herzenberg, L. A. (1997) Int. Immunol. 9, 689-702. [DOI] [PubMed] [Google Scholar]
- 21.Bonifacino, J. S. & Morgan, K., eds. (2003) Current Protocols in Cell Biology (Wiley, Indianapolis).
- 22.Lydyard, P. M., Quartey-Papafio, R., Williams, W., Feldman, R. F., Mackenzie, L., Youinou, P. Y. & Isenberg, D. (1990) J. Autoimmun. 3, 37-42. [DOI] [PubMed] [Google Scholar]
- 23.Underwood, J. R., Cartwright, G. A., McCall, A. M., Tribbick, G., Geysen, M. H. & Hearn, M. T. (1994) J. Autoimmun. 7, 291-320. [DOI] [PubMed] [Google Scholar]
- 24.Murakami, M., Tsubata, T., Okamoto, M., Shimizu, A., Kumagai, S., Imura, H. & Honjo, T. (1992) Nature 357, 77-80. [DOI] [PubMed] [Google Scholar]
- 25.Murakami, M., Nakajima, K., Yamazaki, K., Muraguchi, T., Serikawa, T. & Honjo, T. (1997) J. Exp. Med. 185, 791-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagrand, W. K., Niessen, H. W., Wolbink, G. J., Jaspars, L. H., Visser, C. A., Verheugt, F. W., Meijer, C. J. & Hack, C. E. (1997) Circulation 95, 97-103. [DOI] [PubMed] [Google Scholar]
- 27.Xiao, F., Eppihimer, M. J., Willis, B. H. & Carden, D. L. (1997) J. Appl. Physiol. 82, 1459-1465. [DOI] [PubMed] [Google Scholar]
- 28.Zhao, H., Montalto, M. C., Pfeiffer, K. J., Hao, L. & Stahl, G. L. (2002) J. Appl. Physiol. 93, 338-345. [DOI] [PubMed] [Google Scholar]
- 29.Riley, R. D., Sato, H., Zhao, Z. Q., Thourani, V. H., Jordan, J. E., Fernandez, A. X., Ma, X. L., Hite, D. R., Rigel, D. F., Pellas, T. C., et al. (2000) J. Thorac. Cardiovasc. Surg. 120, 350-358. [DOI] [PubMed] [Google Scholar]
- 30.Prodeus, A. P., Zhou, X., Maurer, M., Galli, S. J. & Carroll, M. C. (1997) Nature 390, 172-175. [DOI] [PubMed] [Google Scholar]
- 31.Kanwar, S., Hickey, M. J. & Kubes, P. (1998) Am. J. Physiol. 275, G212-G218. [DOI] [PubMed] [Google Scholar]
- 32.Kimura, T., Andoh, A., Fujiyama, Y., Saotome, T. & Bamba, T. (1998) Clin. Exp. Immunol. 111, 484-490. [DOI] [PMC free article] [PubMed] [Google Scholar]