Abstract
We estimated life expectancy at birth for Gaucher disease type 1 (GD1) patients by comparing survival data from GD1 patients enrolled in ICGG Gaucher Registry to the U.S. population using standard life table methods. 2,876 GD1 patients had 102 reported deaths in 13,509 person-years of follow-up. Estimated life expectancy at birth was 68 y, compared with 77 y in reference population; splenectomized patients, 64 y; nonsplenectomized, 72 y. Causes of death for 63/102 patients were malignancy (17/63), cardiovascular (11/63), and cerebrovascular (8/63). Estimated life expectancy at birth for GD1 patients was ~9 y less than reference population. Malignancies did not contribute to shortened life expectancy.
Introduction
Gaucher disease type 1 (GD1) is an autosomal recessive lysosomal storage disease with highly variable penetrance and expressivity [1]. Although some patients from childhood to late adulthood are symptomatic due to combinations of hepatomegaly, splenomegaly, thrombocytopenia, anemia, skeletal pathology, growth retardation, and rarely, pulmonary disease, others have minimal evidence of disease with few to no symptoms throughout their lives [1]. Many physicians believe that even without specific treatment, predicted life expectancy in newly diagnosed patients should be no different than in nonaffected, healthy individuals. However, the chronic, usually progressive disease in symptomatic individuals and evidence for increased risk of eventual multiple myeloma, lymphoproliferative malignancies, hepatocellular carcinoma and, less certainly, other solid tumors [1–4] suggest that for some phenotypes, the natural course of disease may include shortened life expectancy as well. This study, using data from the International Collaborative Gaucher Group (ICGG) Gaucher Registry, [5] estimated life expectancy for GD1 patients who are phenotypically similar to the Registry population of more than 5,000 patients worldwide. Although 75–80% of the Registry patients are currently treated with enzyme therapy for Gaucher disease, most have been untreated for the greater part of their lives. The ICGG Gaucher Registry is supported by Genzyme Corporation, Cambridge, MA, and supervised by the ICGG scientific board of international physician experts in GD. Enrollment of individuals is contingent on informed patient consent subject to Institutional Review Board/Ethics Committee oversight.
Results
As of September 2006, 4,441 patients were enrolled in the Gaucher Registry of whom 3,879 were reported as having GD1. One thousand three (1,003) patients were excluded from this analysis because of a lack of at least one recorded assessment (i.e. no follow-up information) after their date of enrollment. Of the 2,876 remaining eligible patients, 102 have died since enrollment (Table I). Proportions of men and women were similar for all patients: alive and deceased. Compared to all patients, deceased patients were older at the time of diagnosis (35 y vs. 20 y) and were more likely to have had either total or partial splenectomy (48% vs. 27%). The majority of patients were from the United States (US), Europe, and Israel; 34% of the total and 32% of the deceased patients were Ashkenazi Jews. Patients were followed for a total of 13,509 person-years. The median age at death was 61 y; 75% of the deaths occurred after age 44 (Table I). Among the 102 deceased patients, 92 (90%) received enzyme therapy with either alglucerase or imiglucerase for a median 5.4 years. Among the other 2,448 patients in the study, in whom the median age at last follow-up is 35 y and 25% are younger than age 20 y, 2,356 (85%) received enzyme therapy for a median 6.1 y.
TABLE I.
Patient Demographics and Characteristics
| Deceased patients N = 102 | Total patients N = 2,876 | |
|---|---|---|
| Age at diagnosis (n) | 92 | 2,771 |
| Median (25th, 75th) | 30 (11, 62) | 15 (5, 30) |
| Mean (SD) | 35.0 (26.34) | 19.9 (17.72) |
| Range | 0–81 | 0–82 |
| Year of diagnosis (n) | 92 | 2,771 |
| Median (25th, 75th) | 1985 (1967, 1993) | 1991 (1979, 1997) |
| Mean (SD) | 1978.6 (17.62) | 1986.9 (13.63) |
| Range | 1928–2002 | 1928–2006 |
| Age at death or last follow-up (n) | 99 | 2,876 |
| Median (25th, 75th) | 61 (44, 75) | 35 (20, 51) |
| Mean (SD) | 57.6 (23.11) | 36.5 (19.95) |
| Range | 2–91 | 0–92 |
| Year of death or last follow-up (n) | 99 | 2,876 |
| Median (25th, 75th) | 2000 (1998, 2002) | 2005 (2002, 2006) |
| Mean (SD) | 1999.9 (3.4) | 2003.4 (3.4) |
| Range | 1993–2006 | 1991–2006 |
| Gender, n (%) | ||
| Male | 52 (51) | 1,344 (47) |
| Female | 50 (49) | 1,532 (53) |
| Genotype, n (%) | ||
| N370S/N370S | 26 (25) | 699 (24) |
| All other known genotypes | 29 (28) | 1,487 (52) |
| Unknown/genotype not reported | 47 (46) | 690 (24) |
| Spleen status, n (%) | ||
| Total splenectomy | 47 (46) | 726 (25) |
| Partial splenectomy | 2 (2) | 57 (2) |
| Non-splenectomized | 53 (52) | 2,088 (73) |
| Not reported | 0 (0) | 5 (0) |
| Ethnicity, n (%) | ||
| Jewish, Ashkenazi | 33 (32) | 991 (34) |
| Caucasian, non-Jewish | 14 (14) | 782 (27) |
| African-American/Caribbean | 0 (0) | 160 (6) |
| Hispanic | 5 (5) | 106 (4) |
| Arab | 1 (1) | 66 (2) |
| Asian | 1 (1) | 26 (1) |
| Others | 1 (1) | 69 (2) |
| Not reported | 47 (46) | 676 (24) |
| Geographic Region, n (%) | ||
| USA | 67 (66) | 1,227 (43) |
| Europe | 21 (21) | 1,056 (37) |
| Rest of World | 14 (14) | 593 (21) |
Table II shows the age categories of deceased patients and the number of person-years. Fifty-three deaths (52%) occurred in patients older than age 60 y. However, 39 deaths (38%) were reported in patients between 20 and 59 years of age who may have had clinically more severe phenotypes. Although no patient died during the first year of life, 10 patients died before age 20 y. Among these, GBA genotype is unknown for 9 patients. The cause of death was reported for seven of these patients:
Complications and diseases other than Gaucher
Pulmonary hypertension (genotype L444P/?)
Seizures with respiratory failure
Complications of a bone marrow transplantation
Complications of cardiac surgery
Acute lymphocytic leukemia
Death due to an accident
TABLE II.
Mortality Risk by Age in Patients with Type 1 Gaucher Disease
| Age | No. of deaths | Person-years of follow-up | Deaths per 100 person-years |
|---|---|---|---|
| 0 to <1 | 0 | 3 | 0.0 |
| 1 to <5 | 2 | 221 | 0.9 |
| 5 to <10 | 4 | 869 | 0.5 |
| 10 to <15 | 2 | 1,230 | 0.2 |
| 15 to <20 | 2 | 1,257 | 0.2 |
| 20 to <25 | 5 | 1,143 | 0.4 |
| 25 to <30 | 0 | 1,055 | 0.0 |
| 30 to <35 | 1 | 1,040 | 0.1 |
| 35 to <40 | 7 | 1,072 | 0.7 |
| 40 to <45 | 4 | 1,064 | 0.4 |
| 45 to <50 | 7 | 1,146 | 0.6 |
| 50 to <55 | 7 | 1,035 | 0.7 |
| 55 to <60 | 8 | 781 | 1.0 |
| 60 to <65 | 7 | 520 | 1.3 |
| 65 to <70 | 8 | 423 | 1.9 |
| 70 to <75 | 12 | 327 | 3.7 |
| 75 to <80 | 12 | 175 | 6.9 |
| 80 to <85 | 2 | 96 | 2.1 |
| 85 to <90 | 10 | 46 | 21.6 |
| ≥90 | 2 | 6 | 34.7 |
| Total | 102 | 13,509 | 0.8 |
It is therefore possible that the underlined patients were misclassified and indeed had variants of type 3 GD.
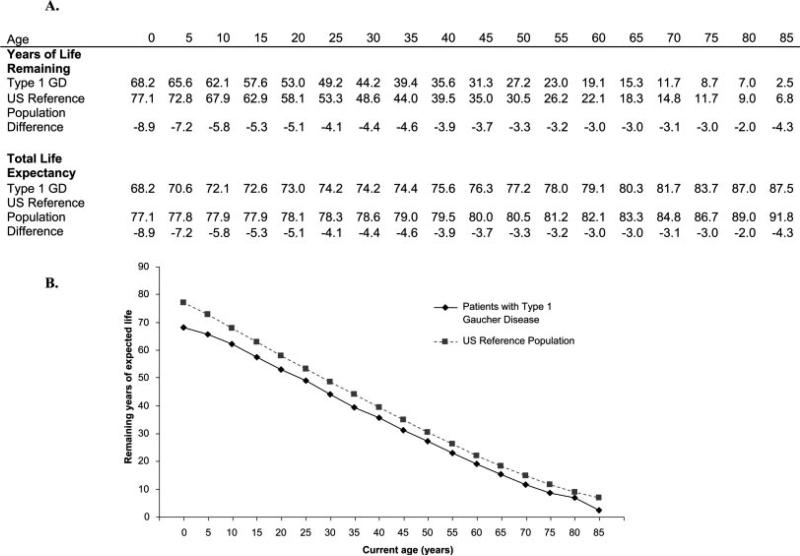
Estimated life expectancy at birth was shorter for the Registry population than for the reference population by 8.9 years (68.2 y vs. 77.1 y) (Table III). Compared to gender-specific reference populations, life expectancy was shortened in both men and women by approximately 7–10 y. Splenectomized patients had an associated 13.2 y decrease in calculated life expectancy from birth (Table III) whereas for nonsplenectomized patients, life expectancy was only 5.1 y less than for the reference population. At any age, patients with GD1 had a greater predicted risk of death compared with the general reference population (see Fig. 1).
TABLE III.
Life Expectancy in Gaucher Disease Type 1 Patients and the US Reference Population
| Population | Deaths | Person-years | Average life expectancy at birth (years) | Difference from U.S. population |
|---|---|---|---|---|
| US Reference population | 77.1 | |||
| Male | 74.3 | |||
| Female | 79.9 | |||
| Gaucher Disease type 1 | 102 | 13,509 | 68.2 | –8.9 |
| Spleen status | ||||
| Partial/total splenectomy | 49 | 4,195 | 63.9 | –13.2 |
| Non-splenectomized | 53 | 9,305 | 72.0 | –5.1 |
| Gender | ||||
| Male | 52 | 6,081 | 66.9 | –7.4 |
| Female | 50 | 7,428 | 70.0 | –9.9 |
Figure 1.
Years of life remaining and total life expectancy by age compared with the US reference population.
For the US subset of GD1 patients (67 deaths in 6,404 person-years), the estimated life expectancy at birth was not substantially different from that of the global Registry population (70.9 y vs. 68.2 y).
The causes of death for patients with GD1 varied widely, as listed in Table IV. The most frequent causes were malignancy, cardiovascular and cerebrovascular diseases and the median age of death from these causes was 72, 58, and 64 y, respectively.
TABLE IV.
Cause of Deaths and Median Ages of Deaths
| Reported cause of death | Number of cases | Median age (yrs) (25th, 75th) |
|---|---|---|
| Malignancy | 17 | 72 (63, 74) |
| Malignancy (multiple myeloma) | 2 | |
| Malignancy (nonhematologic) | 10 | |
| Malignancy (other [nonmyeloma] hematologic malignancies) | 5 | |
| Cardiovascular | 11 | 58 (37, 85) |
| Cardiovascular (ischemic heart disease) | 2 | |
| Cardiovascular (other) | 7 | |
| Cardiovascular (pulmonary hypertension) | 2 | |
| Cerebrovascular | 8 | 64 (51, 77) |
| Cerebrovascular (bleeding/hemorrhage) | 2 | |
| Cerebrovascular (other/unknown) | 6 | |
| Neurological disease | 5 | 67 (63, 89) |
| Liver disease | 4 | 48 (36, 74) |
| Infectious disease | 4 | 65 (61, 69) |
| Chronic lung/respiratory disease | 2 | 82 (77, 88) |
| Accident/injury | 1 | 19 (19, 19) |
| Othera | 11 | 68 (14, 77) |
| Cause not reported | 39 | 54 (42, 73) |
| Total | 102 | 61 (44, 75) |
Other denoted deaths from the following causes: intra-abdominal hemorrhage, multiple organ failure, pulmonary edema, pancytopenia, renal failure, dehydration, and complications from bone marrow transplant or coronary artery bypass surgery.
Discussion
Currently, many physicians believe that GD1-associated morbidity affects the quality of patients’ lives, [6] but is unlikely to affect overall life expectancy. We used the ICGG Gaucher Registry database to investigate this previously untested supposition. This epidemiological analysis is not intended to guide individual treatment decisions which should be based on the patient's past history, current symptomatology, assessment of risk of future complications and morbidity. Our results do neither elucidate any possible influence of enzyme therapy on life expectancy nor was this analysis designed to evaluate any such effect. It is possible that the survival of some patients who, prior to the advent of enzyme therapy, might have died at a relatively young age because of skeletal, hepatic, or pulmonary complications, has been extended because of treatment. Such patients eventually succumb either to unrelated co-morbidities or to not yet fully delineated complications of GD including late neurological manifestations, cardiovascular events and malignancies. However, it is difficult to test this hypothesis at this time because (1) comprehensive life expectancy and cause of death statistics from the pre-enzyme therapy era are lacking; (2) the recent advent of treatment has led to changes in clinical practice regarding splenectomy; and (3) the chronicity and longevity associated with GD1. We hope that our results will serve as a baseline for future analyses of survival trends in GD1, particularly as the effects of long term enzyme therapy and other proposed treatments are increasingly identified.
The ICGG Gaucher Registry is the largest dataset on patients with GD worldwide, and represents a sizeable portion of the total known symptomatic patients with GD1. Among patients currently on enzyme therapy, who are heavily represented in the Registry, the majority have been treated for less than half of their lives, and many, for substantially less. Consequently, we believe the Registry population approximates to the greatest extent possible a model for studying life expectancy in GD1 as if prior to the advent of enzyme therapy. A population of alglucerase/imiglucerase-naïve patients that is representative of all GD1 phenotypes worldwide and sufficiently large for life expectancy analysis has never been assembled, and, because enzyme therapy has proven effective in controlling symptoms of GD, [7] likely never will.
Limitations of this study include different sources of bias, which may influence our reported results. The number of patient deaths is insufficient for subgroup analysis of so-called nonsevere genotypes such as N370S/N370S, or of particular ethnic populations. Patients with clinically aggressive phenotypes may have died relatively young without the opportunity to be enrolled in the Registry. Additionally, because all data to the Registry are provided voluntarily, some deaths among enrolled patients may not have been reported. The impact of under-representation of deaths among Registry enrollees may cause our calculation to overestimate the true life expectancy among patients with GD1.
Because the Registry primarily enrolls patients with manifestations severe enough to come to medical attention and receive a diagnosis, our finding of shortened estimated life expectancy at birth may not pertain to all GD1 phenotypes. Worldwide, there are likely several thousand minimally affected individuals who reside in countries with large Ashkenazi Jewish populations such as the United States and Israel. Were all people with asymptomatic or apparently mild disease included in the database, our estimation of life expectancy would likely increase. However, because the large majority of these patients are unrecognized and therefore unstudied, we also do not know with certainty that their life expectancy is indeed normal. For example, we do not yet know whether individuals with asymptomatic or mild disease share the increased risk for myeloma, other lymphoproliferative malignancies and hepatocellular carcinoma that has been observed in the Gaucher Registry as well as other GD1 populations [2–4]. Additionally, unanticipated findings such as the inordinately high proportion of even heterozygous Gaucher (GBA) mutations found worldwide among patients with Parkinson's disease [8,9] suggest the need for further examination of factors that may influence outcomes including life expectancy in patients with GD1.
Because the phenotypic classification of Registry patients by the referring physician is not subject to independent verification, and because genotype data is lacking in 46% of the deceased patients and in 24% of the remaining cohort, we cannot be absolutely certain that our population is totally free of patients with type 3 GD. Erroneous inclusion of these patients into this study would confound our life expectancy calculation. Although none of the patients included in this study were classified as having type 3 GD by their physicians, three deceased patients younger than age 20 y did have findings possibly indicative of type 3 GD. However, when analysis was confined to US patients only, in whom there were no deaths in patients younger than age 20 y (the group in whom erroneous inclusion of patients with neuronopathic disease is most likely), life expectancy was still substantially shorter than in the reference population.
Splenectomy is heavily weighted in the Gaucher Severity Score Index, [10] and is sometimes associated with hepatic [11], skeletal [12,13], and pulmonary [14] complications of GD. We found a significantly shorter life expectancy in splenectomized patients. Our data are insufficient to determine whether the shorter estimated life expectancy observed in patients with GD1 and splenectomy is related to a more aggressive phenotype that dictated the need for removal of the spleen, to acceleration of hepatic [11], pulmonary [14], and skeletal [12,13] manifestations after splenectomy, or to increased risk of mortality attributable to splenectomy per se and independent of GD. Past management of GD often included splenectomy for life-threatening thrombocytopenia or hypersplenism. This practice is now quite rare as management of the disease has changed with the advent of enzyme therapy that generally reverses symptomatic splenomegaly [7]. Nevertheless, even among nonsplenectomized patients, estimated life expectancy is shorter (72 y) than in the US Reference population (77 y).
Our analysis of causes of death was not confirmed by death certificates or other official records, and is further limited by substantial missing data. With this caveat in mind, we found 7 hematological malignancies (2 multiple myeloma) and 10 solid tumor malignancies, with a median age at death of 72 y. This is consistent with the median age (73 y) of cancer-related mortality in the general population, [15] and with other studies showing that except for multiple myeloma, lymphoproliferative disorders, and hepatocellular carcinoma, there is no definite association between GD1 and risk of malignancy [2–4].
The percentage of cerebrovascular deaths (13%) was higher than expected based on US statistics (6%) [16] and the median age at death for both cardiovascular (58 y) and cerebrovascular disease (64 y) was younger than expected in the general population (81 y and 82 y, respectively) [15]. This preliminary finding is of interest in the context of metabolic abnormalities associated with GD1 including decreases in serum lipoprotein concentrations [17], abnormal glucose metabolism [18], and insulin resistance [19], decreases in serum adiponectin, [20] and increases in serum inflammatory cytokines [21].
We conclude that estimated life expectancy at birth for GD1 patients phenotypically similar to the Gaucher Registry population is about 9 y less than for the reference population. This reduced life expectancy difference persists but decreases with age. Mortality is greater in patients who have had splenectomy. At the present time, we have insufficient data to allow us to apply these general results to specific subgroups whether on the basis of genotype or ethnicity. Because these results reflect past medical practice in an evolving field, they are not likely to be directly relevant to reproductive and medical decisions that depend on estimates of future risk.
Methods
All patients with GD1 enrolled in the ICGG Gaucher Registry as of September 2006 with at least one recorded assessment after their date of enrollment were included (n = 2,876). Patients enrolled posthumously were excluded (n = 21). Most (n = 2,448) patients had some exposure to enzyme therapy (alglucerase or imiglucerase); 428 patients were never treated. The diagnosis of GD and assignment of subtype was physician-reported and based on determination of glucocerebrosidase activity, DNA analysis, or minimally, on tissue histopathology (bone marrow, liver, or spleen).
A standard life table approach [22,23] was used to estimate life expectancy for all patients and for subgroups of patients according to gender and splenectomy status. Follow-up for each patient extended from the date of enrollment until the last recorded assessment submitted, or date of death for deceased patients. Deaths and person-years of follow-up time were summarized to calculate mortality rates within 5 year age categories. Life expectancy at birth was computed by simulating survival for a hypothetical cohort of 100,000 patients with mortality rates equal to those of the population with GD1. This was compared with the life expectancy for the US reference population for the year 2002, which is representative of all developed countries as defined by the United Nations [24]. To validate the international applicability of the US reference population, analyses were additionally performed solely for the subset of US patients. Causes of death were identified by physician reports entered in the ICGG Gaucher Registry or from Genzyme's Pharmacovigilance department database. Where available, cases were classified by consensus of 3 authors as to the proximal cause of death.
Acknowledgments
We would like to thank the patients with type 1 (nonneuronopathic) Gaucher disease and their physicians and health care personnel who submit data to the International Collaborative Gaucher Group (ICGG) Gaucher Registry; the ICGG Gaucher Registry support team at Genzyme Corporation; J. Alexander Cole, D.Sc., M.P.H. (Genzyme Corporation) for assistance with the data analysis; Andrea Gwosdow, Ph.D. for assistance in preparing the manuscript; Robert Brown (Genzyme Corporation) and Trent Richardson (Genzyme Corporation) for graphic design of the figure. This manuscript was supported, in part, by Genzyme Corporation.
Conflict of interest: Financial and logistical support for this work was provided by Genzyme Corporation (Cambridge, MA). The database for the ICGG Gaucher Registry is housed at Genzyme Corporation and supported by Genzyme employees. The biostatistician and epidemiologist authors of this manuscript (K. Kacena and P. Velentgas) were employed by Genzyme Corporation during the data analysis phase of the study. However, at the present time, both are employed elsewhere, but have continued to participate in the development of the manuscript. Other author disclosures: Neal Weinreb and Gregory Pastores receive educational grants from Genzyme Corporation; Pramod Mistry receives a research grant from Genzyme Corporation; Stephan vom Dahl receives consulting fees from Genzyme Corporation. Patrick Deegan, Pramod Mistry, Stephan vom Dahl and Neal Weinreb have received speaking fees from Genzyme Corporation.
Contract grant sponsor: Genzyme Corporation (Cambridge, MA).
Footnotes
Katherine A. Kacena is currently at Molecular Insight Pharmaceuticals, Cambridge, MA, USA.
Priscilla Velentgas is currently at Department of Ambulatory Care and Prevention, Harvard Medical School and Harvard Pilgrim Health Care, Boston, MA.
References
- 1.Grabowski G, Kolodny E, Weinreb N, et al. Gaucher disease: Phenotypic and genetic variation. In: Scriver CR, Beaudet A, Valle D, Slye WS, editors. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2006. [March 31, 2008]. Chapter 146.1. Available from: http://genetics.accessmedicine.com/serverjava/Arknoid/amed/mmbic/co_chapters/ch146.1/ch146.1_p01.html. [Google Scholar]
- 2.Rosenbloom BE, Weinreb NJ, Zimran A, et al. Gaucher disease and cancer incidence: A study from the Gaucher Registry. Blood. 2005;105:4569–4572. doi: 10.1182/blood-2004-12-4672. [DOI] [PubMed] [Google Scholar]
- 3.Zimran A, Liphshitz I, Barchana M, et al. Incidence of malignancies among patients with Gaucher disease type 1 from a single referral clinic. Blood Cells Mol Dis. 2005;34:197–200. doi: 10.1016/j.bcmd.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 4.de Fost M, vom Dahl S, Weverling GJ, et al. Increased incidence of cancer in adult Gaucher disease in Western Europe. Blood Cells Mol Dis. 2006;36:53–58. doi: 10.1016/j.bcmd.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Charrow J, Andersson HC, Kaplan P, et al. The Gaucher Registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160:2835–2843. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- 6.Giraldo P, Solano V, Perez-Calvo J-I, et al. Spanish Group on Gaucher disease. Quality of life related to Gaucher disease type 1: Spanish experience. Qual Life Res. 2005;14:453–461. doi: 10.1007/s11136-004-0794-y. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb NJ, Charrow J, Andersson HC, et al. Effectiveness of enzyme replacement therapy in 1028 patients with Gaucher disease type 1 after 2 to 5 years of treatment: a report from the Gaucher Registry. Am J Med. 2002;113:112–119. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- 8.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 9.Itokawa K, Tamura N, Kawai N, et al. Parkonsinism in type 1 Gaucher's disease. Intern Med. 2006;45:1165–1167. doi: 10.2169/internalmedicine.45.1790. [DOI] [PubMed] [Google Scholar]
- 10.Zimran A, Kay A, Gelbart T, et al. Gaucher disease: Clinical, laboratory, radiologic, and genetic features of 53 patients. Medicine (Baltimore) 1992;71:337–353. [PubMed] [Google Scholar]
- 11.Lachmann RH, Wight DG, Lomas DJ, et al. Massive hepatic fibrosis in Gaucher's disease: Clinico-pathological and radiological features. Q J Med. 2000;93:237–244. doi: 10.1093/qjmed/93.4.237. [DOI] [PubMed] [Google Scholar]
- 12.Ashkenazi A, Zaizov R, Matoth Y. Effect of splenectomy on destructive bone changes in children with chronic (Type I) Gaucher disease. Eur J Pediatr. 1986;145:138–141. doi: 10.1007/BF00441877. [DOI] [PubMed] [Google Scholar]
- 13.Fleshner PR, Aufses AH, Jr., Grabowski GA, Elias R. A 27-year experience with splenectomy for Gaucher's disease. Am J Surg. 1991;161:69–75. doi: 10.1016/0002-9610(91)90363-i. [DOI] [PubMed] [Google Scholar]
- 14.Mistry PK, Sirrs S, Chan A, et al. Pulmonary hypertension in type 1 Gaucher's disease: Genetic and epigenetic determinants of phenotype and response to therapy. Mol Genet Metab. 2002;77:91–98. doi: 10.1016/s1096-7192(02)00122-1. [DOI] [PubMed] [Google Scholar]
- 15.Baron Duffy ML. Trends in median age at death: New Jersey, 1970–2000. [July 13, 2007];Topics in Health Statistics. 2004 Available from: http://www.state.nj.us/health/chs.
- 16.Minino AM, Heron MP, Smith BL. Deaths: Preliminary data for 2004. National Vital Statistics Reports. 2006;54:1–50. [PubMed] [Google Scholar]
- 17.Ginsberg H, Grabowski GA, Gibson JC, et al. Reduced plasma concentrations of total, low density lipoprotein and high density lipoprotein cholesterol in patients with Gaucher type I disease. Clin Genet. 1984;26:109–116. doi: 10.1111/j.1399-0004.1984.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 18.Corssmit EPM, Hollak CEM, Endert E, et al. Increased basal glucose production in type 1 Gaucher's disease. J Clin Endocrinol Metab. 1995;80:2653–2657. doi: 10.1210/jcem.80.9.7673407. [DOI] [PubMed] [Google Scholar]
- 19.Langeveld M, Ghauharali KJM, Sauerwein HP, et al. Gaucher disease type 1, a glycosphingolipid storage disorder, is associated with insulin resistance. J Clin Endocrinol Metab. 2008;93:845–851. doi: 10.1210/jc.2007-1702. [DOI] [PubMed] [Google Scholar]
- 20.Langeveld M, Scheij S, Dubbelhuis P, et al. Very low serum adiponectin levels in patients with Gaucher disease type 1 without overt hyperglycemia. Metabolism. 2007;56:314–319. doi: 10.1016/j.metabol.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Allen MJ, Myer BJ, Khokher AM, et al. Pro-inflammatory cytokines and the pathogenesis of Gaucher's disease: increased release of interleukin-6 and interleukin-10. Q J Med. 1997;90:19–25. doi: 10.1093/qjmed/90.1.19. [DOI] [PubMed] [Google Scholar]
- 22.Palmore JA, Gardner RW. Measuring Mortality, Fertility and Natural Increase—A Self-Teaching Guide to Elementary Measures. East-West Center; Honolulu: 1996. [Google Scholar]
- 23.Rothman K. Epidemiology: An Introduction. Oxford University Press; New York: 2002. [Google Scholar]
- 24. [July 13, 2007];World Population Prospects, 2002, UN. Available from : http://www.un.org/esa/population/publications/wpp2002/WPP2002-HIGHLIGHTSrev1.PDF.