Abstract
There is a bidirectional and complex relationship between the heart and kidneys. This interaction is physical, chemical as well as biological and is also reflected in a strong connection between renal and cardiovascular diseases. Cardiorenal syndrome type 4 (CRS type 4) is characterized by primary chronic kidney disease (CKD) leading to an impairment of cardiac function, with ventricular hypertrophy, diastolic dysfunction, and/or increased risk of adverse cardiovascular events. The incidence of CKD is increasing, and CRS type 4 is becoming a major public health problem associated with a high morbidity and mortality. In this study, we briefly review the epidemiology and pathophysiology of CRS type 4, the role of biomarkers in its early identification, and its management.
Key Words : Cardiorenal syndrome type 4, Chronic kidney disease, Biomarkers, Cardiovascular events
Introduction
As the heart and kidneys are tightly connected, primary disorders of one of these two organs often result in secondary dysfunction or injury of the other one. Such interactions play a pivotal role in the pathogenesis of a clinical entity called the cardiorenal syndrome (CRS) [1]. This term has been recently introduced in an attempt to emphasize the complex interaction between the cardiovascular and renal systems in acute and chronic diseases. CRS is classified into five different subtypes to provide a more concise approach to this condition [2] (table 1). CRS type 4, also known as chronic renocardiac syndrome, is characterized by primary chronic kidney disease (CKD; e.g. chronic glomerular disease and autosomal dominant kidney disease) leading to an impairment of cardiac function, with ventricular hypertrophy, diastolic dysfunction, and/or increased risk of adverse cardiovascular events (i.e. stroke, heart failure, and myocardial infarction). CKD is a well-known independent cardiovascular risk factor due to its role in left ventricular (LV) hypertrophy and coronary atherosclerosis pathogenesis [3]. The cardiovascular risk increases gradually with decreasing renal function [4].
Table 1.
Table 1. Definition of different types of CRS
| CRS type 1 | Acute worsening of heart function causing acute kidney injury and/or dysfunction |
| CRS type 2 | Chronic abnormalities in cardiac function leading to progressive CKD |
| CRS type 3 | Sudden worsening of renal function causing acute cardiac injury and/or dysfunction |
| CRS type 4 | Condition of primary CKD leading to a reduction in cardiac function (ventricular hypertrophy, diastolic dysfunction) and/or increased risk of cardiovascular events |
| CRS type 5 | Systemic disorders (e.g. sepsis) that concurrently induce cardiac and kidney injury and/or dysfunction |
Epidemiology and Pathophysiology of CRS Type 4
According to recent data, patients with evidence of CKD have a 10- to 20-fold increased risk for cardiac death compared to age- and sex-matched controls [1]. Even mild reductions in kidney function may result in a significant increased cardiovascular risk [5]. Evidence of elevated cardiovascular morbidity and mortality in patients with mild to moderate renal dysfunction has been reported in community-based studies [5,6,7]. All these studies documented an inverse relationship between renal function and adverse cardiovascular outcomes [1]. In the last decade, it has been demonstrated that the early stages of CKD are associated with an inflammatory state [8] which may increase the cardiovascular morbidity and mortality risk in the long term [9,10] more than the risk of progression to end-stage renal disease (ESRD) [9,11]. Patients with CKD stage 1-3 have a 25-100 times higher risk for cardiovascular events than for renal events, and it is only in CKD stage 5 that ESRD is the most likely outcome [12].
Cardiovascular risk factors such as hypertension, anemia, hyperphosphatemia, volume overload, and uremic toxins usually develop when eGFR is below 60 ml/min/1.73 m2[13], while subclinical atherosclerosis, which also affects renal circulation, starts to develop in the early stages of CKD [12]. Untreated or inadequately controlled hypertension is considered one of the most important risk factors for CKD progression [14]. Hyperphosphatemia seems to increase the cardiovascular risk in the CKD population too [15] as it plays a role in vascular calcification, which promotes arteriolosclerosis and increases vascular wall stiffness [15,16]. Indeed, serum phosphorus level has been associated with increased rates of myocardial infarction and cardiovascular death in patients with CKD stage 3-4 [15,17].
It has been demonstrated that inflammation enhances cardiovascular risk and mortality in hemodialysis (HD) [18] and peritoneal dialysis (PD) patients [19]. With further progression of renal parenchyma fibrosis and glomerular sclerosis, the clinical picture evolves into overt uremia. As renal function declines to the point where ‘uremia’ ensues, toxic molecules and pro-inflammatory cytokines accumulate [20,21], so the resulting hostile milieu is believed to produce heightened oxidant stress and inflammatory cytokines responsible for accelerated atherosclerosis [3]. LV hypertrophy is present in 25-40% of patients who initiate dialysis [22] and has been shown to be associated with decreased survival. Anemia and hypertension play a pivotal role in its pathogenesis [15]. Patients undergoing HD are also predisposed to develop coronary artery disease (CAD) [23]. Goodman et al. [24] suggested that CAD is common and tends to progress in young HD patients as well.
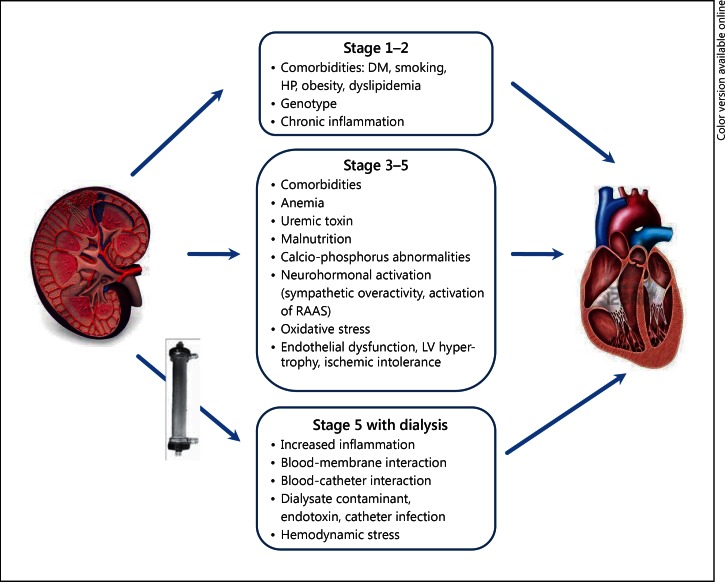
In small-to-medium-sized vessels, dyslipidemia, oxidative stress, and systemic inflammation result in endothelial dysfunction, arterial stiffness, smooth muscle cell proliferation, and consequent ischemic heart disease. Such combination of events results in a real CRS type 4 (fig. 1).
Fig. 1.
Schematic of CRS type 4 (chronic renocardiac syndrome). DM = Diabetes mellitus; HP = Hypertension; RAAS = renin angiotensin aldosterone system.
HD per se increases chronic inflammation by several mechanisms. The first one is the contact between blood and the artificial dialysis membrane, which induces complement activation, nitric oxide, and cytokine production [25,26]. The second mechanism is blood exposure to contaminants, which may be present in the dialysate and may cross the dialysis membrane by back-diffusion and back-filtration stimulating monocyte activation [27]. A third component concerns the possible presence of catheters or synthetic grafts with tunnel or exit site infection, which represents a source of infection and inflammation [28,29]. Additionally, fluid overload should also be considered an important cardiovascular risk factor responsible for extracellular volume expansion and ventricular enlargement [30,31].
The PD modality also contributes to an increased risk of cardiovascular morbidity with similar mechanisms via fluid overload, inflammation, oxidative stress, infection, malnutrition, endothelial dysfunction, and atherosclerosis [32,33].
Biomarkers for the Early Identification of CRS Type 4
Recent studies have evaluated the utility of biomarkers in the assessment of the cardiovascular risk in CKD population. Several biomarkers such as troponins, plasminogen activator inhibitor type I, homocysteine, brain natriuretic peptide (BNP), C-reactive protein, serum amyloid-A protein, ischemia-modified albumin, and advanced glycation end-products have been demonstrated to correlate with cardiovascular outcomes in CKD patients [34,35].
Both BNP and troponin T (TnT) seem to have a good predictive value for cardiovascular disease in patients with CKD [36,37]. Elevated TnT and NT-proBNP levels were found to correlate with hypervolemia and to identify a subgroup of asymptomatic ESRD patients with a 2- to 5-fold increase in cardiovascular mortality [38]. In PD patients, NT-proBNP is also a marker of LV dysfunction and cardiovascular congestion, which has been shown to be a good predictor of cardiovascular death and all-cause mortality [39]. Increased TnT levels represent a strong and independent predictor of global cardiovascular mortality in clinically stable HD patients [40,41,42] (table 2).
Table 2.
Cardiac biomarkers to evaluate the cardiovascular risk in CKD patients
| BNP and TnT | |
| – have a diagnostic power to predict cardiovascular disease in patients with CKD | |
| – have been validated as good predictors and prognostic markers in dialysis patients | |
| NT-proBNP | TnT |
| – is a marker of LV dysfunction and cardiovascular congestion | – is a useful screening tool for asymptomatic CAD in CKD |
Renal biomarkers such as cystatin C (CysC) and neutrophil gelatinase-associated lipocalin (NGAL) have been recently found to be diagnostic and prognostic markers of cardiovascular outcomes in CKD [43]. Higher levels of CysC have been demonstrated to be directly involved in the atherosclerotic process [44] and are associated with increased LV mass and its concentricity independent of renal function [45]. Increased NGAL expression has been found in atherosclerotic plaque and failing myocardium in patients with CAD and heart failure [46,47]. Its levels correlated with disease severity independent of coexisting renal injury [47,48] (table 3).
Table 3.
Table 3. Renal biomarkers to evaluate cardiovascular outcomes in CKD patients
| CysC | Directly involved in the atherosclerotic process; associated with increased LV mass and its concentricity |
| NGAL | Increased expression in atherosclerotic plaque and failing myocardium in patients with CAD and heart failure |
Management of CRS Type 4
Medical Therapies to Improve Cardiovascular Outcomes
CKD is associated with increased sympathetic activity and renin angiotensin aldosterone system (RAAS) activation which may induce chronic inflammation and oxidative stress [49]. In patients with mild to moderate renal disease, angiotensin-converting enzyme inhibitors (ACEIs) have been shown to improve cardiovascular survival independent of the severity of myocardial disease [50]. Their favorable effects on neurohormonal activation, hemodynamics, and ventricular remodelling may explain their positive effect on arrhythmic events in dialysis patients. Pun et al. [51] found that the use of ACEIs or angiotensin receptor blockers (ARBs) and beta-blockers (BBs) was significantly associated with improved survival in ESRD patients after cardiac arrest. ARBs have been shown to reduce oxidative stress and inflammation, suppress the RAAS, and decrease cardiovascular events in hypertensive patients [52,53]. There is a paucity of data regarding the use of ARBs in the prevention of cardiac mortality in dialysis patients. A small randomized trial of candesartan in dialysis patients demonstrated a reduction in cardiovascular events and fatal arrhythmias, though the significance of the latter finding was limited by the small number of events [54]. Another small trial by Suzuki et al. [55] proved that treatment with an ARB may effectively reduce fatal and nonfatal cardiovascular events in dialysis patients. Larger trials are needed before any firm conclusion on the use of ARBs for preventing cardiovascular events and sudden death in dialysis patients can be drawn. BBs have been demonstrated to reduce the risk of cardiovascular death in patients after myocardial infarction and heart failure. In the Bezafibrate Infarction Prevention study, the authors found that BBs reduce the cardiac risk in CAD patients with or without CKD [56]. Cice et al. [57] randomized 114 dialysis patients to receive carvedilol or placebo and demonstrated a significant reduction in cardiovascular mortality and a trend towards a reduction in the occurrence of sudden death. Some studies suggested that HD patients treated with BBs had lower overall and cardiovascular mortality [58,59].
Up to now, there is no final clinical recommendation regarding the best mode of coronary intervention in CRS type 4 patients.
Dialytic Strategies to Improve Cardiovascular Outcomes
Advances in dialysis technology may improve hemodynamic stability, reduce oxidative and inflammatory stress and produce more efficient removal of low and middle toxins leading to the concept of ‘cardioprotective dialysis’. The application of new technologies involves the use of new biomaterials designed to ameliorate inflammatory responses and enhance membrane performance as well as the use of machines whose functions are well integrated in terms of safety, quality of therapy, performance and monitoring.
Several studies have shown that dialysis treatment does not significantly reduce levels of inflammatory markers [60,61]. It is intuitive that HD or PD which lead to negligible improvement in inflammatory biomarkers or worsening levels over time, are unlikely to have a significant impact on cardiovascular outcomes. A Cochrane meta-analysis on biocompatible membranes revealed a reduction in the beta-2-microglobulin (β2M) level, an increase in albumin concentration, and an improvement of Kt/V, although mortality was not affected [62]. House et al. [63] did not find any benefit of high-flux polysulfone membranes compared with low-flux membranes in terms of lipids and homocysteine levels in a controlled trial. In contrast, Chauveau et al. [64], in an observational study, have shown that high-flux membranes were associated with improved 2-year survival. Different studies have reported that ‘hemofiltration’ or ‘hemodiafiltration’ treatment was associated with better blood pressure control, lower incidence of intradialytic hypotension or arrhythmia, better β2M and phosphate clearance, reduced inflammation and oxidative stress as well as reduced hospitalization rate [65,66,67]. Importantly, improvements in patient monitoring during HD may improve safety and tolerability. Blood volume control using biofeedback systems has been developed to modulate blood volume and plasma refilling by adjusting the ultrafiltration rate and conductivity.
PD might circumvent the hemodynamic instability of frequent and rapid ultrafiltration associated with conventional HD. However, some data suggest that PD is associated with a lower mortality than HD in the first 1-2 years; afterwards, the mortality may be higher on PD than HD. Conversely, other registry data do not support this finding. Further long-term studies assessing surrogate and hard endpoints of cardiovascular outcomes in PD are required [33].
Conclusion
In conclusion, it is clear that CKD patients face an unrelenting assault on the cardiovascular system from a variety of fronts. It is likely that many factors will be discovered and woven into an already complex fabric of metabolic, inflammatory, infectious, immune, and genetic factors. For instance, the roles of apoptosis, altered immune homeostasis, and latent infection on cardiovascular function and pathology in uremia remain to be completely explored. A better understanding of this pathophysiological mechanism may provide a potential target for intervention. In uremic and dialyzed patients, an altered monocyte function and a decreased monocyte HLA-DR expression may represent other interesting aspects to explore and to evaluate immuno-mediated organ cross talk and heart-kidney interactions.
Disclosure Statement
The authors have no conflicts of interest to declare. This study is not under consideration for publication elsewhere in a similar form, in any language.
References
- 1.Ronco C, Chionh CY, Haapio M, Anavekar NS, House A, Bellomo R. The cardiorenal syndrome. Blood Purif. 2009;27:114–126. doi: 10.1159/000167018. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, House AA, Haapio M. Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intensive Care Med. 2008;34:957–962. doi: 10.1007/s00134-008-1017-8. [DOI] [PubMed] [Google Scholar]
- 4.Ronco C, House AA, Haapio M. Cardiorenal and renocardiac syndromes: the need for a comprehensive classification and consensus. Nat Clin Pract Nephrol. 2008;4:310–311. doi: 10.1038/ncpneph0803. [DOI] [PubMed] [Google Scholar]
- 5.Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Coronado BE, Greene T, Wang SR, Kusek JW, Beck GJ, Levey AS. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002;57:327–335. doi: 10.5414/cnp57327. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P, Barany P, Heimburger O, Pecoits-Filho R, Lindholm B. Mortality, malnutrition, and atherosclerosis in ESRD: what is the role of interleukin-6? Kidney Int Suppl. 2002;80:103–108. doi: 10.1046/j.1523-1755.61.s80.19.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 10.Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601–1608. doi: 10.1016/S0140-6736(00)04728-0. [DOI] [PubMed] [Google Scholar]
- 11.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hallan SI, Stevens P. Screening for chronic kidney disease: which strategy? J Nephrol. 2010;23:147–155. [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O'Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT., Jr Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35:329–344. doi: 10.1016/j.pop.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchison JA. Vascular calcification in dialysis patients. Prilozi. 2007;28:215–224. [PubMed] [Google Scholar]
- 17.Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: the DALLAS Heart Study. J Am Soc Nephrol. 2005;16:507–513. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 19.Noh H, Lee SW, Kang SW, Shin SK, Choi KH, Lee HY, Han DS. Serum C-reactive protein: a predictor of mortality in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1998;18:387–394. [PubMed] [Google Scholar]
- 20.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 21.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–1325. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 22.Middleton JP, Pun PH. Hypertension, chronic kidney disease, and the development of cardiovascular risk: a joint primacy. Kidney Int. 2010;77:753–755. doi: 10.1038/ki.2010.19. [DOI] [PubMed] [Google Scholar]
- 23.Navab KD, Elboudwarej O, Gharif M, Yu J, Hama SY, Safarpour S, Hough GP, Vakili L, Reddy ST, Navab M, Vaziri ND. Chronic inflammatory disorders and accelerated atherosclerosis: Chronic kidney disease. Curr Pharm Des. 2011;17:17–20. doi: 10.2174/138161211795049787. [DOI] [PubMed] [Google Scholar]
- 24.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 25.Hakim RM. Clinical implications of hemodialysis membrane biocompatibility. Kidney Int. 1993;44:484–494. doi: 10.1038/ki.1993.272. [DOI] [PubMed] [Google Scholar]
- 26.Amore A, Bonaudo R, Ghigo D, Arese M, Costamagna C, Cirina P, Gianoglio B, Perugini L, Coppo R. Enhanced production of nitric oxide by blood-dialysis membrane interaction. J Am Soc Nephrol. 1995;6:1278–1283. doi: 10.1681/ASN.V641278. [DOI] [PubMed] [Google Scholar]
- 27.Tielemans C, Husson C, Schurmans T, Gastaldello K, Madhoun P, Delville JP, Marchant A, Goldman M, Vanherweghem JL. Effects of ultrapure and non-sterile dialysate on the inflammatory response during in vitro hemodialysis. Kidney Int. 1996;49:236–243. doi: 10.1038/ki.1996.33. [DOI] [PubMed] [Google Scholar]
- 28.Ashby DR, Power A, Singh S, Choi P, Taube DH, Duncan ND, Cairns TD. Bacteremia associated with tunneled hemodialysis catheters: outcome after attempted salvage. Clin J Am Soc Nephrol. 2009;4:1601–1605. doi: 10.2215/CJN.01840309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein SL, Ikizler TA, Zappitelli M, Silverstein DM, Ayus JC. Non-infected hemodialysis catheters are associated with increased inflammation compared to arteriovenous fistulas. Kidney Int. 2009;76:1063–1069. doi: 10.1038/ki.2009.303. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal R, Weir MR. Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1255–1260. doi: 10.2215/CJN.01760210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stegmayr BG. Ultrafiltration and dry weight – what are the cardiovascular effects? Artif Organs. 2003;27:227–229. doi: 10.1046/j.1525-1594.2003.07205.x. [DOI] [PubMed] [Google Scholar]
- 32.Chung SH, Heimburger O, Stenvinkel P, Wang T, Lindholm B. Influence of peritoneal transport rate, inflammation, and fluid removal on nutritional status and clinical outcome in prevalent peritoneal dialysis patients. Perit Dial Int. 2003;23:174–183. [PubMed] [Google Scholar]
- 33.Fassett RG, Driver R, Healy H, Coombes JS. Cardiovascular disease in peritoneal dialysis patients. Panminerva Med. 2009;51:151–161. [PubMed] [Google Scholar]
- 34.Mutluay R, Konca C, Erten Y, Pasaoglu H, Deger SM, Agirgun C, Derici U, Arinsoy T, Sindel S. Predictive markers of asymptomatic atherosclerosis in end-stage renal disease patients. Ren Fail. 2010;32:448–454. doi: 10.3109/08860221003658258. [DOI] [PubMed] [Google Scholar]
- 35.Arikan H, Koc M, Tuglular S, Ozener C, Akoglu E. Elevated plasma levels of PAI-1 predict cardiovascular events and cardiovascular mortality in prevalent peritoneal dialysis patients. Ren Fail. 2009;31:438–445. doi: 10.1080/08860220902963772. [DOI] [PubMed] [Google Scholar]
- 36.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 37.DeFilippi CR, Fink JC, Nass CM, Chen H, Christenson R. N-terminal pro-B-type natriuretic peptide for predicting coronary disease and left ventricular hypertrophy in asymptomatic CKD not requiring dialysis. Am J Kidney Dis. 2005;46:35–44. doi: 10.1053/j.ajkd.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Codognotto M, Piccoli A, Zaninotto M, Mion MM, Ruzza L, Barchita A, Naso A, Plebani M. Effect of a dialysis session on the prognostic values of NT-proBNP, troponins, endothelial damage and inflammation biomarkers. J Nephrol. 2010;23:465–471. [PubMed] [Google Scholar]
- 39.Wang AY, Lam CW, Yu CM, Wang M, Chan IH, Zhang Y, Lui SF, Sanderson JE. N-terminal pro-brain natriuretic peptide: an independent risk predictor of cardiovascular congestion, mortality, and adverse cardiovascular outcomes in chronic peritoneal dialysis patients. J Am Soc Nephrol. 2007;18:321–330. doi: 10.1681/ASN.2005121299. [DOI] [PubMed] [Google Scholar]
- 40.Ooi DS, Zimmerman D, Graham J, Wells GA. Cardiac troponin T predicts long-term outcomes in hemodialysis patients. Clin Chem. 2001;47:412–417. [PubMed] [Google Scholar]
- 41.Dierkes J, Domrose U, Westphal S, Ambrosch A, Bosselmann HP, Neumann KH, Luley C. Cardiac troponin T predicts mortality in patients with end-stage renal disease. Circulation. 2000;102:1964–1969. doi: 10.1161/01.cir.102.16.1964. [DOI] [PubMed] [Google Scholar]
- 42.Mallamaci F, Zoccali C, Parlongo S, Tripepi G, Benedetto FA, Cutrupi S, Bonanno G, Fatuzzo P, Rapisarda F, Seminara G, Stancanelli B, Bellanuova I, Cataliotti A, Malatino LS. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2002;40:68–75. doi: 10.1053/ajkd.2002.33914. [DOI] [PubMed] [Google Scholar]
- 43.Iwanaga Y, Miyazaki S. Heart failure, chronic kidney disease, and biomarkers – an integrated viewpoint. Circ J. 2010;74:1274–1282. doi: 10.1253/circj.cj-10-0444. [DOI] [PubMed] [Google Scholar]
- 44.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel PC, Ayers CR, Murphy SA, Peshock R, Khera A, de Lemos JA, Balko JA, Gupta S, Mammen PP, Drazner MH, Markham DW. Association of cystatin C with left ventricular structure and function: the DALLAS Heart Study. Circ Heart Fail. 2009;2:98–104. doi: 10.1161/CIRCHEARTFAILURE.108.807271. [DOI] [PubMed] [Google Scholar]
- 46.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 47.Yndestad A, Landro L, Ueland T, Dahl CP, Flo TH, Vinge LE, Espevik T, Froland SS, Husberg C, Christensen G, Dickstein K, Kjekshus J, Oie E, Gullestad L, Aukrust P. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229–1236. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 48.Zografos T, Haliassos A, Korovesis S, Giazitzoglou E, Voridis E, Katritsis D. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am J Cardiol. 2009;104:917–920. doi: 10.1016/j.amjcard.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Koomans HA, Blankestijn PJ, Joles JA. Sympathetic hyperactivity in chronic renal failure: a wake-up call. J Am Soc Nephrol. 2004;15:524–537. doi: 10.1097/01.asn.0000113320.57127.b9. [DOI] [PubMed] [Google Scholar]
- 50.Schmieder RE, Delles C, Mimran A, Fauvel JP, Ruilope LM. Impact of telmisartan versus ramipril on renal endothelial function in patients with hypertension and type 2 diabetes. Diabetes Care. 2007;30:1351–1356. doi: 10.2337/dc06-1551. [DOI] [PubMed] [Google Scholar]
- 51.Pun PH, Lehrich RW, Smith SR, Middleton JP. Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin J Am Soc Nephrol. 2007;2:491–500. doi: 10.2215/CJN.02360706. [DOI] [PubMed] [Google Scholar]
- 52.Dohi Y, Ohashi M, Sugiyama M, Takase H, Sato K, Ueda R. Candesartan reduces oxidative stress and inflammation in patients with essential hypertension. Hypertens Res. 2003;26:691–697. doi: 10.1291/hypres.26.691. [DOI] [PubMed] [Google Scholar]
- 53.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi A, Takase H, Toriyama T, Sugiura T, Kurita Y, Ueda R, Dohi Y. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis – a randomized study. Nephrol Dial Transplant. 2006;21:2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki H, Kanno Y, Sugahara S, Ikeda N, Shoda J, Takenaka T, Inoue T, Araki R. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52:501–506. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 56.Chonchol M, Benderly M, Goldbourt U. Beta-blockers for coronary heart disease in chronic kidney disease. Nephrol Dial Transplant. 2008;23:2274–2279. doi: 10.1093/ndt/gfm950. [DOI] [PubMed] [Google Scholar]
- 57.Cice G, Ferrara L, D'Andrea A, D'Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabro R. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 58.Koch M, Thomas B, Tschope W, Ritz E. Survival and predictors of death in dialysed diabetic patients. Diabetologia. 1993;36:1113–1117. doi: 10.1007/BF02374508. [DOI] [PubMed] [Google Scholar]
- 59.Ritz E, Dikow R, Adamzcak M, Zeier M. Congestive heart failure due to systolic dysfunction: The Cinderella of cardiovascular management in dialysis patients. Semin Dial. 2002;15:135–140. doi: 10.1046/j.1525-139x.2002.00044.x. [DOI] [PubMed] [Google Scholar]
- 60.Borazan A, Ustun H, Ustundag Y, Aydemir S, Bayraktaroglu T, Sert M, Yilmaz A. The effects of peritoneal dialysis and hemodialysis on serum tumor necrosis factor-alpha, interleukin-6, interleukin-10 and C-reactive-protein levels. Mediators Inflamm. 2004;13:201–204. doi: 10.1080/09511920410001713493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int. 2004;65:2371–2379. doi: 10.1111/j.1523-1755.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 62.Macleod AM, Campbell M, Cody JD, Daly C, Donaldson C, Grant A, Khan I, Rabindranath KS, Vale L, Wallace S. Cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD003234.pub2. CD003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.House AA, Wells GA, Donnelly JG, Nadler SP, Hebert PC. Randomized trial of high-flux vs low-flux haemodialysis: effects on homocysteine and lipids. Nephrol Dial Transplant. 2000;15:1029–1034. doi: 10.1093/ndt/15.7.1029. [DOI] [PubMed] [Google Scholar]
- 64.Chauveau P, Nguyen H, Combe C, Chene G, Azar R, Cano N, Canaud B, Fouque D, Laville M, Leverve X, Roth H, Aparicio M. Dialyzer membrane permeability and survival in hemodialysis patients. Am J Kidney Dis. 2005;45:565–571. doi: 10.1053/j.ajkd.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Gerdemann A, Wagner Z, Solf A, Bahner U, Heidland A, Vienken J, Schinzel R. Plasma levels of advanced glycation end products during haemodialysis, haemodiafiltration and haemofiltration: potential importance of dialysate quality. Nephrol Dial Transplant. 2002;17:1045–1049. doi: 10.1093/ndt/17.6.1045. [DOI] [PubMed] [Google Scholar]
- 66.Lin CL, Yang CW, Chiang CC, Chang CT, Huang CC. Long-term on-line hemodiafiltration reduces predialysis beta-2-microglobulin levels in chronic hemodialysis patients. Blood Purif. 2001;19:301–307. doi: 10.1159/000046958. [DOI] [PubMed] [Google Scholar]
- 67.Canaud B, Morena M, Leray-Moragues H, Chalabi L, Cristol JP. Overview of clinical studies in hemodiafiltration: what do we need now? Hemodial Int. 2006;10((suppl 1)):S5–S12. doi: 10.1111/j.1542-4758.2006.01183.x. [DOI] [PubMed] [Google Scholar]