Abstract
PURPOSE
We evaluated the validity and inter-rater reliability of encephalographer interpretation of color density spectral array (CDSA) EEG for seizure identification in critically ill children and explored predictors of accurate seizure identification.
METHODS
Conventional EEG tracings from 21 consecutive critically ill children were scored for electrographic seizures. Four two-hour long segments from each patient were converted to 8 channel CDSA displays, yielding 84 images. Eight encephalographers received CDSA training and circled elements thought to represent seizures. Images were reviewed in random order (Group A) or with information regarding seizure presence in the initial 30 minutes and with patient images in order (Group B). Sensitivity, specificity, and inter-rater reliability were calculated. Factors associated with CDSA seizure identification were assessed.
RESULTS
Seizure prevalence was 43% on conventional EEG. Specificity was significantly higher for Group A (92.3% versus 78.2%, p<0.00). Sensitivity was not significantly different between Groups A and B (64.8% versus 75%, p=0.22). Inter-rater reliability was moderate in both groups. Ten percent of images were falsely classified as containing a seizure. Seizure duration ≥2 minutes predicted identification (p<0.001).
CONCLUSIONS
CDSA may be a useful screening tool for seizure identification by encephalographers, but it does not identify all seizures and false positives occur.
Keywords: Critical Care, EEG, Pediatric, Seizure, EEG monitoring
Background
Electrographic seizures are common in critically ill children (Abend, et al. 2011, Hosain, et al. 2005, Jette, et al. 2006, Abend and Dlugos 2007, Alehan, et al. 2001, Tay, et al. 2006, Saengpattrachai, et al. 2006, Shahwan, et al., Abend, et al. 2009, Williams, et al. 2011, Greiner, et al. 2012, Kirkham, et al. 2012, Gwer, et al. 2012) and are associated with worse short-term outcome.(Greiner, Holland, Leach, et al. 2012, Kirkham, Wade, McElduff, et al. 2012, Gwer, Idro, Fegan, et al. 2012, Topjian, et al. 2013). Most electrographic seizures have no identifiable clinical correlate (Abend, Gutierrez-Colina, Topjian, et al. 2011, Jette, Claassen, Emerson, et al. 2006, Shahwan, Bailey, Shekerdemian, et al. 2010, Williams, Jarrar and Buchhalter 2011, Greiner, Holland, Leach, et al. 2012, Kirkham, Wade, McElduff, et al. 2012, Gwer, Idro, Fegan, et al. 2012, McCoy, et al. 2011) so continuous EEG monitoring (cEEG) is required for diagnosis. This has led to a rapid increase in the use of cEEG in critically ill children.(Sanchez, et al. In press.) The feasibility of monitoring multiple critically ill patients is limited by the time it takes for a trained encephalographer to review cEEG data. In addition, real time cEEG review is generally not available,(Sanchez, Carpenter, Chapman, et al. In press.) potentially resulting in delays between electrographic seizure onset and identification.
A variety of quantitative EEG tools are commercially available which may make screening cEEG more efficient for encephalographers (Scheuer and Wilson 2004), but prior surveys of neurologists indicate they are rarely used (Abend, et al. 2010). Quantitative EEG techniques utilize algorithms to separate the complex EEG signal into its components. For example, EEG power is a function of the amplitude and frequency of the EEG tracing. Often, the quantitative EEG display is compressed in time so several hours can be displayed by a single image. Color density spectral array (CDSA) EEG displays time on the x-axis, frequency on the y-axis, and power in the color dimension. Seizure evolution may involve increases in frequency and amplitude, and thus may appear on CDSA images as upward arches on the y-axis (increased frequency) in warmer colors (increased power). However, some seizures may not involve substantial increases in frequency or amplitude and thus might not be identified by CDSA. Further, some artifacts can result in increases in frequency or power and could be mistaken for seizures.
While CDSA is commercially available on many EEG systems, the utility of this technique in clinical practice is uncertain. One study in which neurologists interpreted CDSA from critically ill children demonstrated an overall sensitivity for seizure identification of 83% which varied from 0–100% for individual tracings, and a low false-positive rate (Stewart, et al. 2010). A second study of quantitative EEG in critically ill children demonstrated that variability in the sensitivity across tracings may depend on modifiable factors such as user experience and display size, but also on inherent seizure characteristics like duration and spike amplitude (Akman, et al. 2011). We aimed to evaluate the accuracy and inter-rater reliability of encephalographer interpretation of CDSA EEG for electrographic seizure identification in critically ill children.
Methods
The cEEG tracings from 21 consecutive critically ill children who underwent clinically indicated cEEG in the Pediatric Intensive Care Unit at The Children's Hospital of Philadelphia were included in this study. An institutional clinical pathway suggests children with acute brain injury and encephalopathy undergo cEEG to evaluate for electrographic seizures. Informed written consent was obtained from the guardians of subjects and the study was approved by the Institutional Review Board.
Continuous EEG monitoring was performed using a Grass-Telefactor (West Warwick, RI) video-EEG system. Twenty-one gold-over-silver scalp surface electrodes were positioned according to the international 10–20 system and affixed with collodion adhesive. EEG data were acquired on a portable bedside computer networked to the hospital's EEG server. Full tracings were saved for investigational use. Each EEG tracing was reviewed by two pediatric encephalographers (NSA and DJD) to identify electrographic seizures. Electrographic seizures were defined as abnormal paroxysmal events that were different from the background, lasted longer than ten seconds (or shorter if associated with a clinical change), and had a temporal-spatial evolution in morphology, frequency, and amplitude with a plausible electrographic field. The start and end of each electrographic seizure was marked, permitting calculation of seizure duration. The number of electrodes involved at the maximal spatial extent of each seizure was assessed. Seizure morphology was categorized as epileptiform it if included spike or sharp waves or rhythmic-evolving if it included evolving rhythmic patterns but no spike or sharp waves components.
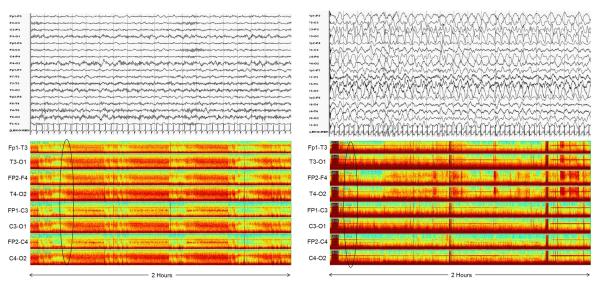
The first eight hours of each subject's conventional cEEGs were digitally converted to CDSA displays using Grass-Telefactor Twin software. The CDSA display was an eight channel, double distance, longitudinal, bipolar montage consisting of the channels Fp1-C3, C3-O1, Fp1-T3, T3-O1; Fp2-C4, C4-O2, Fp2-T4, and T4-O2. CDSA tracings displayed frequency from 0–30 Hz on the y-axis. Power was displayed on a color scale in which the colors red, orange, yellow, green, and blue represented successively lower power. Four CDSA images, each containing two hours of condensed EEG data, were obtained per subject. Thus, there were 84 total images (21 patients × 4 images per patient). Figure 1 shows an example CDSA image.
Figure 1.
Two hours of CDSA EEG is shown for eight channels using a double-distance bipolar montage. In each channel, most of the power (red) is in the low frequencies on the Y-axis consistent with diffuse delta activity. Superimposed are intermittent red arches which build from low frequency to a higher frequency. Sometimes these fade back to a low frequency and sometimes they abruptly drop back to a low frequency. These are consistent with electrographic seizures which evolve in frequency and amplitude and then either abruptly or slowly terminate. The initial three seizures are marked with black arrows.
Eight pediatric encephalographers with at least two years of EEG interpretation experience but with little or no CDSA experience reviewed a three page CDSA manual which included a brief discussion of the technique and a series of annotated example CDSA images. The study encephalographers did not have access to the underlying raw cEEG tracings. When reviewing CDSA images, encephalographers were instructed to circle events they considered to be seizures. The reviewers were divided into two groups. Group A reviewed the 84 CDSA images in random order with no information regarding the CDSA tracings. This group was intended to replicate the most basic clinical scenario, in which a neurophysiologist has no information about the EEG and had to interpret CDSA. Group B viewed the four CDSA images from each subject in consecutive order and was shown circled seizures on the initial 30 minutes of those same images. This group was intended to replicate a more likely clinical scenario in which an encephalographer might review the initial portion of cEEG and then use CDSA to target their review of subsequent cEEG data.
STATA version 11.1 (Stata Corporation, College Station, TX) was used for all statistical analyses. Descriptive statistics are presented as medians with interquartile ranges (IQR). Sensitivity and specificity for correctly identifying a seizure on each image on each image and the area under the receiver operating characteristic (ROC) curve were calculated for each group. Inter-rater reliability for classifying a CDSA image as containing a seizure or not was assessed in both groups using the parameter kappa with its 95% confidence interval (95%CI) using 1000 resamplings of the confidence interval. Moderate reliability was defined as a kappa value between 0.41 and 0.60; substantial reliability was defined as a kappa value between 0.61 and 0.80m and excellent reliability was defined as a kappa value between 0.81 and 1.00 (Landis and Koch 1977). Factors associated with CDSA seizure identification, including seizure duration, maximal spatial extent, and morphology, were evaluated using the Wilcoxon rank-sum test. Of 73 total seizures, eight occurred in the initial 30 minutes and were thus identified for group B. Thus, only the 65 seizures not shown to either group were included in the analyses of factors associated with seizure identification. A two-sided probability value of ≤0.05 was considered statistically significant.
Results
The median age of subjects was 4.4 years (IQR 0.9–6.8 years), and ten (48%) were male. Two (10%) had pre-existing epilepsy and 11 (52%) had clinically evident seizures prior to cEEG initiation. Acute encephalopathy etiologies included traumatic brain injury (4), central nervous system infection (3), metabolic/systemic etiologies (3), sepsis (3), hypoxic-ischemic encephalopathy (2), epilepsy (2), and other (4). Based on encephalographer review of conventional EEG, there were a total of 72 seizures in 9 of 21 subjects (43%).
Table 1 reports the sensitivity, specificity, positive predictive value, and negative predictive value of CDSA for identifying the presence of a seizure or not on each image. The specificities between Groups A and B (92.3% versus 78.2%) were significantly different (p<0.00). The sensitivities between Groups A and B (64.8% versus 75%) were not significantly different (p=0.22). The area under the ROC curves between Groups A and B (0.79 versus 0.77) were not significantly different (p=0.61). Ten percent of images were falsely classified as containing a seizure and among those images the mean (±standard deviation) false positive rate per hour was 1.5 ± 2 false identifications. Inter-rater reliability for classifying an image as having any seizure was moderate in both groups [Group A: kappa 0.51 (95%CI 0.35–0.66), p<0.001; Group B: kappa 0.46 (95%CI 0.33–0.59), p<0.001].
Table 1.
Sensitivity, specificity, and predictive values for seizure identification in each group.
| Group | Sensitivity (95% CI) | Specificity (95% CI) | Area under ROC Curve (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) |
|---|---|---|---|---|---|
| Group A | 64.8% (53.9%–74.7%) | 92.3% (88.3%–95.3%) | 0.79 (0.73–0.84) | 75% (63.7%–84.2%) | 88.1% (83.5%–91.8%) |
| Group B | 75% (64.6%–83.6%) | 78.2% (72.6%–83.2%) | 0.77 (0.71–0.82) | 55% (45.7%–64.1%) | 89.8% (85%–93.5%) |
CI, confidence interval; ROC Curve, receiver operating characteristic
Table 2 reports the number of seizures identified by varying numbers of raters. For example, 6 seizures (9.2%) were identified by zero raters while 14 seizures (21.5%) were identified by all eight raters. At least six raters were able to identify 55% of seizures.
Table 2.
Number of raters identifying seizures.
| Number of Raters who Identified Seizure | N (%) of Seizures (Total N=65) |
|---|---|
| 0 | 6 (9.2%) |
| 1 | 10 (15.4%) |
| 2 | 3 (4.6%) |
| 3 | 4 (6.2%) |
| 4 | 4 (6.2%) |
| 5 | 2 (3.1%) |
| 6 | 6 (9.2%) |
| 7 | 16 (24.6%) |
| 8 | 14 (21.5%) |
Table 3 reports the association between seizure characteristics and encephalographer CDSA seizure identification. Seizure duration ≥2 minutes predicted seizure identification (p=0.001). The spatial extent and morphology of seizures did not predict seizure identification (p=NS). We reviewed the 10 CDSA images which were the source of the most false positives and the corresponding EEG. The most common encephalographic changes underlying the false positives were artifact related to artifact (electrode dysfunction or movement) in 6 images, fluctuations in burst suppression in 2 images, background frequency fluctuations (periodic diffuse beta activity) in 1 image, and state changes in 1 image. Figure 2 shows an example of a CDSA image with a false positive and a CDSA example with a false negative.
Table 3.
Electrographic seizure characteristics associated with larger number of raters identifying them in CDSA (N=65).
| Seizure Characteristic | Number of Seizures | Median Number of Raters (IQR) | Wilcoxon Rank Sum |
|---|---|---|---|
|
| |||
| Duration | p<0.001 | ||
| ≥2 Minutes | 38 | 7 (6, 8) | |
| <2 Minutes | 27 | 1 (1, 4) | |
|
| |||
| Spatial Extent | p=0.19 | ||
| ≥5 Electrodes | 21 | 4 (3, 6) | |
| ≤4 Electrodes | 44 | 7 (1, 7.5) | |
|
| |||
| Morphology | p=0.12 | ||
| Epileptiform | 42 | 6 (1, 7) | |
| Rhythmic | 23 | 5 (3, 8) | |
IQR, Interquartile Range
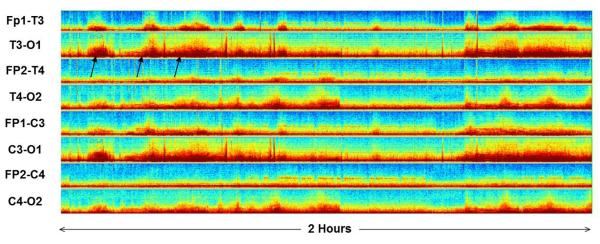
Figure 2.
Example of false negative and positive CDSA images.
Left: False positive. An increase in power is seen periodically at beta frequencies (black oval). These periods corresponded to periods with diffuse beta activity and not electrographic seizures.
Right: False negative. A very slight increase in power is seen at theta-alpha frequencies. The electrographic seizure (black oval) slowly evolves and only reaches about 2–3 Hz. Therefore the seizure does not stand out well form the background on CDSA.
Discussion
When interpreted by encephalographers, CDSA may be a useful screening tool for electrographic seizure identification, although it does not identify all seizures and has only moderate inter-rater reliability. False positives occur indicating that seizure confirmation by conventional EEG review is still needed.
Sensitivity (65% for Group A and 75% for Group B) and negative predictive values (88% for Group A and 90% for Group B) were not 100%, indicating that some electrographic seizures identified on conventional cEEG would not be identified using CDSA. The finding that some seizures are not identified by CDSA is highlighted by the finding that only about half of seizures were identified by six or more raters. While imperfect, CDSA permits identification of many seizures, is easy to implement, and is commercially available. Further, a higher sensitivity of 83% was reported by a study with more extensive CDSA training (Stewart, Otsubo, Ochi, et al. 2010). However, since many seizures are not identified, conventional cEEG review is likely still required. With further development, coordinated care models may be able to blend the accuracy of conventional EEG with the efficiency of CDSA to make cEEG monitoring feasible in large numbers of at-risk critically ill children despite limited encephalographer resources.
Although specificity (92% in Group A and 78% in Group B) and positive predictive values (75% for Group A and 55% for Group B) are high, they were not 100%, indicating that when CDSA is interpreted as containing seizures, the patient may not actually be experiencing seizures. This misclassification could lead some patients to receive anticonvulsants unnecessarily. If an event on CDSA is considered to represent a seizure, confirmation by review of conventional EEG may be required. Fortunately, only 10% of the two-hour images were falsely classified as containing a seizure, indicating that conventional EEG “proof-reading” might not place a substantial burden on encephalography resources. Our false positive seizure identification rate was 1.5 identifications per hour, but this rate might be lower in clinical use since if a CDSA pattern was determined to be non-ictal initially, then similar future patterns might not be falsely identified as seizures.
Group A viewed the CDSA images in random order with no information provided to mimic an encephalographer who came on shift and must view the CDSA without prior information. Group B was given information regarding full array EEG interpretation for the first 30 minutes of CDSA and reviewed each patient's images in order. This was intended to mimic a more realistic clinical scenario in which the initial portion of EEG was reviewed by an encephalographer and each CDSA image could be interpreted in the context of conventional EEG data and prior CDSA images. Specificity was significantly higher in Group A than in Group B, indicating fewer false positives in Group A than in Group B. Since encephalographers in Group B were provided with some information about seizures in the initial 30 minutes, they may have had a heightened awareness of the presence of seizures leading them to identify more CDSA changes as possible seizures. Although not statistically different, sensitivity was higher for Group B than Group A, suggesting there was a trend toward higher seizure identification when some information about the initial 30 minutes was available. Importantly, our study contained fewer CDSA images with seizures than without seizures, resulting in less power to identify significant differences in sensitivity than specificity. A larger study in which more images contained seizures might have shown a significant difference in sensitivity. Overall, the comparison of Groups A and B indicates that providing some information about seizures in the first 30 minutes may yield more false positives but also more true positives. Achieving a higher sensitivity (more true positives) with a lower specificity (more false positives) may be acceptable in clinical practice since an encephalographer can rapidly review the conventional EEG to determine whether a CDSA change represents a seizure and can learn to dismiss subsequent CDSA patterns that do not represent seizures.
Inter-rater reliability was only moderate (kappa 0.46 and 0.51). This is lower than reported by a prior study of CDSA in critically ill children (Stewart, Otsubo, Ochi, et al. 2010). In the prior study, 27 EEG tracings lasting a mean of 18 hours were converted to displays containing CDSA and amplitude integrated EEG for eight channels (double-distance longitudinal bipolar montage) with eight hours on a screen. Seventeen of the 27 tracings contained 553 seizures. Three neurophysiologists received quantitative EEG interpretation training and then identified events suspected to be seizures. There was substantial (kappa 0.78) inter-rater reliability among neurophysiologists reviewing CDSA (Stewart, Otsubo, Ochi, et al. 2010). The prior study involved three neurophysiologists while our study involved four in each group, and this may have impacted inter-rater reliability. Additionally, our study used a brief written document for CDSA training while the prior study used a two-hour hands-on training session that included review of CDSA with simultaneous access to the underlying raw EEG. This discrepancy suggests that a more detailed and interactive training approach may yield improved inter-rater reliability.
Some seizures types may be more easily identified using CDSA. Our data indicated that CDSA identification was better for seizures ≥2 minutes in duration. This is consistent with a prior study in which the sensitivity for seizure identification on individual tracings ranged from 0–100%, with seizures more likely to be missed if they were low voltage (<75 microvolts), short duration (<1 min), or remained focal (Stewart, Otsubo, Ochi, et al. 2010). In a study of two types of quantitative EEG, envelope trend and CDSA, performed on EEG tracings from critically ill children, some modifiable factors influenced seizure identification including interpreter experience, display size, and quantitative EEG method. However, some non-modifiable factors inherent to the EEG pattern also impacted identification, including the maximum spike amplitude, seizure duration, seizure frequency, and seizure duration (Akman, Micic, Thompson, et al. 2011). Together, these studies indicate that further optimization of quantitative EEG displays may improve accuracy, but that characteristics of an individual's seizures are also important and may preclude development of a quantitative EEG tool that is perfect for all patients. Since CDSA is based on amplitude and frequency characteristics compressed over time, it is not surprising that seizure morphology did not impact seizure identification. Seizure spatial extent did not impact seizure identification, but we were displaying multiple CDSA channels. If we had used a more limited montage, seizure spatial extent may have impacted identification.
Practically, it is possible that not every seizure needs to be identified and a sensitivity of 100% may not be critical. For example, one study found that electrographic status epilepticus was associated with higher mortality and worse short-term outcome among survivors, while electrographic seizures that did not meet criteria for status epilepticus were not associated with increased mortality or worse short-term outcome (Topjian, Gutierrez-Colina, Sanchez, et al. 2013). Larger studies involving longer-term outcome in children with electrographic seizures are needed, but this initial data suggests that techniques which identify longer recurrent seizures may be useful even if they fail to identify rare brief seizures.
There have been more studies of the utility of quantitative EEG techniques in neonates than in children. Amplitude integrated EEG (aEEG) is available in many neonatal ICUs, (Glass, et al. 2012) and studies have demonstrated that about one-quarter to three-quarter of seizures can be identified using aEEG (Rennie, et al. 2004, Shah, et al. 2008, Shellhaas, et al. 2007). Both user experience and characteristics of the seizure impact seizure identification. Trends beyond aEEG have also been investigated in neonates. Envelope trend with a 6-channel display has been shown to have a sensitivity of 88% for long discrete seizures but only 40% for brief seizures and 20% for slowly evolving seizures (Abend, et al. 2008).
This study has several limitations. First, in clinical practice CDSA users would likely have access to more patient information and would be able to access underlying conventional EEG when needed. Checking whether the initial several CDSA patterns represented seizures using conventional EEG may have precluded falsely identifying later CDSA patterns as seizures, and thus may have improved specificity and reduced the false positive rate. Thus, our reported sensitivity and specificity may be lower than could be achieved in clinical practice. The facts that sensitivity and specificity were reasonable and that a more intensive training program might yield higher values (Stewart, Otsubo, Ochi, et al. 2010) suggest that with further development, CDSA could be one useful component of ICU EEG monitoring. Second, only CDSA was used. Studies that included both aEEG and CDSA have reported that significantly more seizures were missed with CDSA (21%) than amplitude integrated EEG (14%) (Stewart, Otsubo, Ochi, et al. 2010). Prior studies have also indicated that using multiple quantitative EEG displays together improves accuracy (Akman, Micic, Thompson, et al. 2011). Third, we displayed CDSA data from eight channels expecting this extensive coverage would improve identification of focal seizures. Averaging channels, for example, to create right and left hemispheric displays, might preclude identification of very focal seizures but might also reduce identification of single electrode artifact as seizure. Further study is needed to determine whether accuracy is improved by permitting access to underlying conventional EEG, averaging channels together, displaying multiple quantitative EEG panels in combination, and providing more intensive training. Finally, while two pediatric encephalographers reviewed the conventional EEG tracings to ensure seizure identification consensus and this was taken as the gold standard for CDSA comparison, studies have demonstrated imperfect inter-rater reliability for seizure identification even using conventional EEG (Abend, et al. 2011, Ronner, et al. 2009) which is only partially improved by group discussion and implementation of interpretation rules (Azuma, et al. 2003).
These data indicate that CDSA may be a useful for screening tool for encephalographers aiming to identify seizures in critically ill children and that providing information about electrographic seizures in the first 30 minutes of conventional EEG recording may enhance the encephalographers' ability to identify seizures. However, some seizures are not identified and some CDSA patterns are falsely identified as seizures, indicating that review of conventional EEG is still needed. Further study is needed to optimize CDSA training and display parameters, and also to determine whether programs combining bedside caregiver CDSA interpretation with encephalographer confirmation using conventional EEG can lead to more accurate, rapid, and efficient seizure identification.
Acknowledgments
Funding: Lauren Beslow is funded by K12 NS049453, T32 NS007413, and L. Morton Morley Funds of The Philadelphia Foundation. Alexis Topjian is funded by K12 HD047349 Nicholas Abend is funded by K23 NS076550
Footnotes
Financial Disclosures None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol. 2005;32:162–165. doi: 10.1016/j.pediatrneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007;37:165–170. doi: 10.1016/j.pediatrneurol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Alehan FK, Morton LD, Pellock JM. Utility of electroencephalography in the pediatric emergency department. J Child Neurol. 2001;16:484–487. doi: 10.1177/088307380101600704. [DOI] [PubMed] [Google Scholar]
- Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia. 2006;47:1504–1509. doi: 10.1111/j.1528-1167.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47:1510–1518. doi: 10.1111/j.1528-1167.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia. 2010;51:1198–1204. doi: 10.1111/j.1528-1167.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011;52:1130–1136. doi: 10.1111/j.1528-1167.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012;129:e748–755. doi: 10.1542/peds.2011-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012;38:853–862. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwer S, Idro R, Fegan G, et al. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child. 2012;97:343–349. doi: 10.1136/archdischild-2011-300935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Critical Care Medicine. 2013;31:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011;52:1973–1978. doi: 10.1111/j.1528-1167.2011.03291.x. [DOI] [PubMed] [Google Scholar]
- Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG Monitoring: Current Resources and Practice in the United States and Canada. Journal of Clinical Neurophysiology. doi: 10.1097/WNP.0b013e31827eda27. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer ML, Wilson SB. Data analysis for continuous EEG monitoring in the ICU: seeing the forest and the trees. J Clin Neurophysiol. 2004;21:353–378. [PubMed] [Google Scholar]
- Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010;12:382–389. doi: 10.1007/s12028-010-9337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CP, Otsubo H, Ochi A, Sharma R, Hutchison JS, Hahn CD. Seizure identification in the ICU using quantitative EEG displays. Neurology. 2010;75:1501–1508. doi: 10.1212/WNL.0b013e3181f9619e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akman CI, Micic V, Thompson A, Riviello JJ., Jr Seizure detection using digital trend analysis: Factors affecting utility. Epilepsy Res. 2011;93:66–72. doi: 10.1016/j.eplepsyres.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46:111–115. doi: 10.1016/j.pediatrneurol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie JM, Chorley G, Boylan GB, Pressler R, Nguyen Y, Hooper R. Non-expert use of the cerebral function monitor for neonatal seizure detection. Arch Dis Child Fetal Neonatal Ed. 2004;89:F37–40. doi: 10.1136/fn.89.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DK, Mackay MT, Lavery S, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–1154. doi: 10.1542/peds.2007-1839. [DOI] [PubMed] [Google Scholar]
- Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–777. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]
- Abend NS, Dlugos D, Herman S. Neonatal seizure detection using multichannel display of envelope trend. Epilepsia. 2008;49:349–352. doi: 10.1111/j.1528-1167.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- Abend NS, Gutierrez-Colina AM, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. Journal of Clinical Neurophysiology. 2011;28:15–19. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronner HE, Ponten SC, Stam CJ, Uitdehaag BM. Inter-observer variability of the EEG diagnosis of seizures in comatose patients. Seizure. 2009;18:257–263. doi: 10.1016/j.seizure.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Azuma H, Hori S, Nakanishi M, Fujimoto S, Ichikawa N, Furukawa TA. An intervention to improve the interrater reliability of clinical EEG interpretations. Psychiatry Clin Neurosci. 2003;57:485–489. doi: 10.1046/j.1440-1819.2003.01152.x. [DOI] [PubMed] [Google Scholar]