Abstract
Pain is associated with stimulation of some behaviors (e.g. withdrawal reflexes) but depression of many other behaviors (e.g. feeding, locomotion, positively reinforced operant behavior). Drugs that block reuptake of serotonin, norepinephrine and/or dopamine are widely used to treat depression, and they have also emerged as useful drugs for treatment of pain. This study compared effects of selective and mixed-action inhibitors of serotonin, norepinephrine and/or dopamine reuptake in assays of acute pain-stimulated and pain-depressed behavior. Intraperitoneal injection of dilute acid served as a noxious stimulus to stimulate a writhing response or depress intracranial self-stimulation (ICSS) in Sprague-Dawley rats. Selective reuptake inhibitors of serotonin (citalopram, clomipramine) and norepinephrine (nisoxetine, nortriptyline) and a mixed-action reuptake inhibitor of serotonin and norepinephrine (milnacipran) blocked acid-stimulated writhing but failed to block acid-induced depression of ICSS. Selective dopamine reuptake inhibitors [RTI-113 (3ß-(4-chlorophenyl)tropane-2ß-carboxylic acid phenyl ester hydrochloride), bupropion] and a triple reuptake inhibitor of dopamine, serotonin and norepinephrine [RTI-112 (3ß-(3-methyl-4-chlorophenyl)tropane-2ß-carboxylic acid methyl ester hydrochloride)] blocked both acid-stimulated writhing and acid-induced depression of ICSS, although these drugs also produced an abuse-related facilitation of ICSS in the absence of the noxious stimulus. These results support further consideration of dopamine reuptake inhibitors as candidate analgesics, although abuse liability remains a concern.
Index words: analgesia, antidepressant, dopamine, intracranial self-stimulation, monoamine reuptake inhibitor
INTRODUCTION
Monoamine reuptake inhibitors are drugs that block transporters for the monoamine neurotransmitters serotonin, norepinephrine and/or dopamine 30. These drugs have a long history of use as antidepressants, and more recently, monoamine reuptake inhibitors have emerged as medications for the treatment pain 27,42,60,64,71. This latter application of monoamine reuptake inhibitors is rooted in both the neurobiology of nociception and the symptomology of pain. With regard to neurobiology, bulbospinal monoaminergic pathways have well-established roles in descending modulation of nociceptive input from primary to secondary nociceptors in the spinal dorsal horn, and serotonergic and noradrenergic pathways play primarily an inhibitory role in spinal nociceptive processing 21,59. Supraspinal monoaminergic pathways have also been implicated in preclinical measures of nociception and clinical measures of pain, and in particular, data from multiple sources suggest a role for mesocorticolimbic dopaminergic systems in the subjective experience and behavioral expression of pain 1,9,65,73. With regard to symptomology, there is high comorbidity between pain and depression 4,22,29. In particular, pain is often associated with a depression of behavior, and this pain-related depression of behavior can serve as a diagnostic indicator of pain and a target of pain treatment in both veterinary and human medicine 18,47,48. Taken together, these considerations have suggested that monoamine reuptake inhibitors with established antidepressant activity may have utility in treating pain, and especially the depression-related aspects of pain.
The objective of the present study was to systematically evaluate effects of monoamine reuptake inhibitors in complementary assays of acute pain-stimulated and pain-depressed behaviors that have been used previously to examine opioid, cannabinoid and nonsteroidal antiinflammatory drugs 35,49–51,58. Specifically, intraperitoneal injection of dilute acid was used as an acute chemical noxious stimulus in rats to stimulate a writhing response (also called a “stretching” response) and to depress intracranial self-stimulation (ICSS). Abdominal writhing is a commonly used dependent measure of nociception in assays of pain-stimulated behavior using intraperitoneal administration of dilute acid or other chemical irritants as the noxious stimulus, and antinociception is indicated by reduction in writhing 28,46,52. ICSS, by contrast, is commonly used to assess changes in motivated behavior and affect in experimental subjects 11,68, but it can also be used to evaluate effects of noxious stimuli and candidate analgesics. For example, ICSS promotes high levels of stable responding that can be depressed by intraperitoneal acid administration, and antinociception is indicated by a blockade of acid-induced depression of ICSS 48. The rationale for studying behavioral responses to an acute noxious stimulus was two-fold. First, this study was intended to serve as the first step in a broader investigation on effects of monoamine reuptake inhibitors and other drugs on behavioral depression associated with acute and chronic pain states. Second, the most salient discrepancies between preclinical and clinical research on analgesic effects of monoamine reuptake inhibitors have occurred in studies of acute pain. For example, although preclinical studies often report apparent antinociceptive effects of norepinephrine and/or serotonin reuptake inhibitors in assays of acute pain 3,8,56,62, clinical studies typically show little or no analgesic efficacy of these compounds in assays of acute experimental or clinical pain 19,20,23,69, and these monoamine reuptake inhibitors are not indicated for treatment of acute pain. Assays of acute acid-stimulated and acid-depressed behavior were intended to further elucidate this discrepancy in the literature. We hypothesized that monoamine uptake inhibitors would block acid-stimulated writhing but would be less effective in assays of acid-depressed ICSS.
METHODS
Subjects
A total of 98 male Sprague-Dawley rats (Harlan, Frederick, MD, USA) weighing 297–334 g at the time of surgery were used for the studies of lactic acid-stimulated writhing (n=46) and ICSS (n=52). Rats were housed individually and were maintained on a 12-h light/dark cycle with lights on from 6:00 a.m. to 6:00 p.m. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with National Institutes of Health guidelines on care and use of animal subjects in research, and all animal use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Intracranial Self-Stimulation (ICSS)
Surgery
All rats were implanted with a bipolar stainless steel electrode (Plastics One, Roanoke, VA, USA) using stereotaxic surgery. Each bipolar electrode consisted of a cathode (0.25 mm in diameter and covered with polyamide insulation except at the flattened tip) and an anode (0.125 mm in diameter and uninsulated). During surgery, rats were anesthetized with isoflurane gas (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ, USA). The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral from midsagittal suture, and 7.8 mm below dura). The anode was wrapped around one of three skull screws to ground the implant, and the skull screws and electrode were affixed to the skull with orthodontic resin. Rats received 5 mg/kg ketoprofen as a postoperative analgesic immediately after and 24hr after surgery, and rats recovered for at least seven days post surgery prior to commencing ICSS training.
Apparatus
ICSS experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow, and green positioned 7.6 cm directly above the response lever), a 2-W white house light, and an ICSS stimulator (Med Associates, St. Albans, VT, USA). Electrodes were connected to the stimulator via a swivel connector (Model SL2C, Plastics One, Roanoke, VA, USA). The stimulator was controlled by computer software that also controlled all programming parameters and data collection (Med Associates, St. Albans, VT, USA).
Behavioral Procedure
After initial shaping of lever-press responding, rats were trained under a continuous reinforcement schedule of brain stimulation using procedures identical to those described previously to evaluate opioids, cannabinoids and nonsteroidal anti-inflammatory drugs 35,50,51. During initial training sessions lasting 30 to 60 min, the white house light was illuminated, and responding produced electrical stimulation under a continuous schedule of reinforcement. Under this schedule, each lever press resulted in the delivery of a 0.5-s train of square-wave cathodal pulses (0.1-ms pulse duration) and illumination for 0.5-s of the colored stimulus lights over the lever. Responses during the 0.5-s stimulation period did not earn additional stimulation. Initially, the frequency of stimulation was held constant at 126 Hz, and the stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of ICSS (≥30 stimulations/min). Frequency manipulations were then introduced, and the terminal schedule consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies was presented, with a 60-s trial at each frequency. The frequency range extended from 158-56 Hz in 0.05 log increments. Each frequency trial began with a 10-s time out, during which the house light was off and responding had no scheduled consequences. During the last 5 s of this time out, 5 non-contingent stimulations were delivered once per second at the frequency available during that trial, and the lever lights were illuminated during each stimulation. This non-contingent stimulation was then followed by a 50-s “response” period, during which the house light was illuminated, and responding produced electrical stimulation under the continuous reinforcement schedule described above. Training continued with presentation of three sequential components per day, and intensity was again adjusted as necessary until rats reliably responded for at least three and no more than six trials of all components for at least two consecutive days. In general, rats were trained in groups of 10–14 for each drug. The first six rats to meet training criteria were then advanced to ICSS testing. As discussed previously 49–51,58, the remaining rats from each group were assigned to studies of acid-stimulated writhing using methods described below.
Once training was completed, ICSS testing began. A test session consisted of six sequential components. The first component of each session was considered to be a “warm up” component, and data from this component were discarded. Data from the second and third components were used to calculate baseline parameters of frequency-rate curves for that session (see “Data analysis”). After the third component, rats were taken out of the ICSS chambers, administered drug and placed back into their home cages. After the designated pretreatment time elapsed, 1.8% lactic acid or its vehicle (bacteriostatic water) was administered IP in a volume of 1 ml/kg, and rats were immediately placed back into their ICSS chambers for three test components. This 30 min test session was chosen to match the session length for writhing studies (see below), and because our previous studies demonstrated that lactic acid produced sustained decrease in ICSS for up to 90 min 58. Eight different monoamine reuptake inhibitors were selected for study based on their published selectivity to function as selective serotonin reuptake inhibitors (SSRIs; citalopram 3.2–32 mg/kg and clomipramine 3.2–32 mg/kg), selective norepinephrine reuptake inhibitors (SNRIs; nisoxetine 1–10 mg/kg and nortriptyline 1–10 mg/kg), selective dopamine reuptake inhibitors (SDRIs; RTI-113 0.32–3.2 mg/kg and bupropion 3.2–32 mg/kg), a mixed-action serotonin+norepinephrine reuptake inhibitor (S+NRI; milnacipran 0.32–3.2 mg/kg), and a mixed-action inhibitor of all three monoamine (“triple reuptake inhibitor”, TRI; RTI-112 0.1–1 mg/kg) (Figure 1). Each monoamine reuptake inhibitor or its vehicle (bacteriostatic water) was administered 30 min before lactic acid or its vehicle, except for RTI-113 and RTI-112, which were administered 10 min before acid or vehicle. Doses and pretreatment times were based on previously published behavioral studies in rats 7,8,15,31,54–57,75. Each drug was tested in a group of 5–6 rats, and all rats were tested with all doses. Test drug doses were delivered in a modified Latin-square order across rats, so that each week, a rat was tested with a given dose of test drug in combination with lactic acid vehicle on one test day and with 1.8% lactic acid on another test day. All experiments with one drug in a given rat were completed before testing with a second drug was initiated, and any given rat was used to test no more than two drugs. Test sessions were typically conducted on Tuesdays and Fridays, and 30-min training sessions were conducted on Mondays, Wednesdays, and Thursdays.
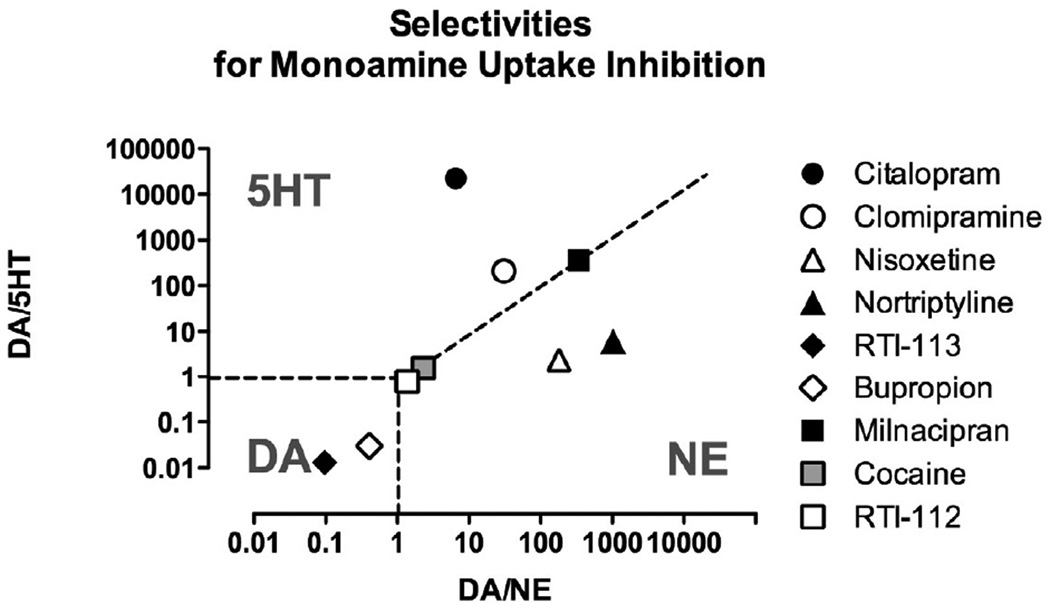
Figure 1. Functional selectivities of test compounds to inhibit reuptake of serotonin (5HT), norepinephrine (NE) or dopamine (DA).
Abscissa: Selectivity to inhibit reuptake of DA vs. NE expressed as potency to inhibit DA reuptake ÷ potency to inhibit NE reuptake. Ordinate: Selectivity to inhibit reuptake of DA vs. 5HT expressed as potency to inhibit DA reuptake ÷ potency to inhibit 5HT reuptake. Domains of selectivity for each monoamine are indicated by the abbreviation for that monoamine, and dotted lines show borders of equipotent inhibition for monoamines on either side of the border. All data are taken from published studies of monoamine reuptake in rat brain tissue as follows: citalopram, nortriptyline and bupropion 63; clomipramine 12,12; nisoxetine 12,12; RTI-113 and RTI-112 33; milnacipran 45. Data for clomipramine inhibition of dopamine reuptake and nisoxetine inhibition of serotonin uptake have not been published and were estimated from studies of transporter binding 34,34.
Repeated dosing with monoamine reuptake inhibitors is sometimes required to demonstrate efficacy in preclinical assays of antidepressant-like effects (e.g. the forced-swim test in rats) 16. Consequently, the SSRI citalopram was also tested using a dosing regimen of repeated treatment shown to be effective in the modified forced-swim test of antidepressant-like drug effects 10. Specifically, at least one week after completion of acute dosing, four rats were tested with three repeated injections of vehicle or citalopram (10 mg/kg, i.p.) at 23, 19, and 1 h before receiving acid administration and ICSS testing.
Data analysis
The primary dependent variable was reinforcement rate in stimulations per minute during each frequency trial. To normalize these data, raw reinforcement rates from each trial were converted to percent maximum control rate (%MCR), with the MCR defined as the mean of the maximal rates observed during the second and third “baseline” components for that session. Thus, %MCR values for each trial were calculated as (response rate during a frequency trial ÷ maximum control rate) × 100. For each ICSS experiment, data from the second and third baseline components were averaged to yield a baseline frequency-rate curve, and data from the three test components were averaged to yield a test frequency-rate curve. Baseline and test curves were then averaged across rats to yield mean baseline and test curves for each manipulation. For statistical analysis, results were compared by two-way analysis of variance (ANOVA), with treatment and ICSS frequency as the two factors. A significant ANOVA was followed by a Holm-Sidak post hoc test, and the criterion for significance was set at p<0.05.
To provide an additional summary of ICSS performance, the total number of stimulations obtained at all frequencies was summed for each test component and averaged across the three test components of each experimental session in each rat. Data for total stimulations per component were then expressed as a percentage of the baseline number of stimulations per component in each rat and averaged across rats. These data were also used to quantify blockade of acid-induced depression of ICSS. Specifically, “percent acid blockade” was quantified using the equation [(test-acid)/(baseline-acid)]*100, where “test” was the total number of ICSS stimulations after treatment with drug+acid, “acid” was the total number of stimulations after acid alone, and “baseline” was the total number of stimulations in the absence drug or acid. For all drugs producing greater than 50% acid blockade, linear regression in GraphPad Prism 5.0 for Macintosh (La Jolla, CA) was used to calculate an ED50 and 95% confidence limits, with ED50 defined as the effective dose producing 50% acid blockade. A value of 100% acid blockade indicated complete blockade of acid-induced depression of ICSS. Values greater than 100% acid blockade indicated facilitation of ICSS above baseline levels, and values below 0% indicated exacerbation of acid-induced depression of ICSS.
Assay of lactic acid-stimulated writhing
Behavioral procedure
Test sessions were conducted once per week using procedures described previously for opioids, cannabinoids and nonsteroidal anti-inflammatory drugs 35,50,51. Test drugs were administered IP prior to treatment with 1.8% lactic acid (IP in a volume of 1 ml/kg). Immediately after acid injection, rats were placed into acrylic test chambers (31.0cm × 20.1cm × 20.0cm) for 30 min observation periods. A writhe was operationally defined as a contraction of the abdomen followed by extension of the hind limbs, and the number of writhes during the observation period was counted. Dose effect curves were determined for citalopram (3.2–32 mg/kg), clomipramine (3.2–32 mg/kg), nisoxetine (0.32–3.2 mg/kg), nortriptyline (0.32–10 mg/kg), RTI-113 (0.32–3.2), bupropion (3.2–32 mg/kg), milnacipran (0.1–3.2 mg/kg) and RTI-112 (0.032–1 mg/kg), and each drug was tested using the same pretreatment time as used in the ICSS studies. Test drugs were delivered in a Latin-square order across rats. Each week, a rat was tested with a given drug dose in combination with 1.8% lactic acid. At the conclusion of these acute dosing studies, repeated dosing studies were conducted with citalopram (10 mg/kg) administered 23, 19, and 1 h before acid administration as in the ICSS studies above.
Data Analysis
The primary dependent variable was the number of writhes counted during each observation period in each rat. To normalize these data, raw counts were converted to percent vehicle control using the equation (drug/vehicle)*100, where “drug” was the number of writhes observed after drug + acid, and “vehicle” was the number of writhes after drug vehicle + acid. These data were then averaged across rats. For all drugs producing greater than 50% reduction in writhing, linear regression was used to calculate an ED50 and 95% confidence limits, with ED50 defined as the effective dose producing 50% control writhing.
Drugs
Lactic acid, citalopram HBr, clomipramine HCl, nisoxetine HCl, nortriptyline HCl and bupropion HCl were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Milnacipran HCl was purchased from Tocris Bioscience (Minneapolis, MN, USA). RTI-113 [3β-(4-chlorophenyl) tropane-2β-carboxylic acid phenyl ester hydrochloride] and RTI-112 [3β-(3-methyl-4-chlorophenyl) tropane-2β-carboxylic acid methyl ester hydrochloride] were synthesized at Research Triangle Institute and generously provided by Dr. Ivy Carroll. All solutions were prepared in sterile water for IP injection.
RESULTS
Effects of monoamine uptake inhibitors in the assay of acid-stimulated writhing
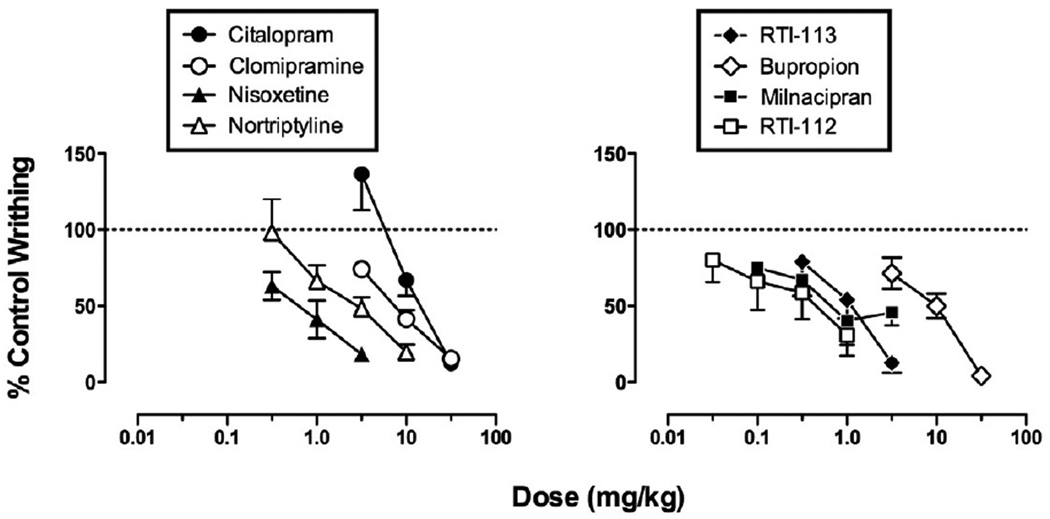
Across all 46 rats used for studies of acid-stimulated writhing, IP administration of 1.8% lactic acid (1.0 ml/kg) after drug vehicle pretreatments elicited a mean±SEM of 21.1 ± 1.4 writhes. The absolute number of control writhes elicited by acid after vehicle pretreatment in each group is reported in the legend of Figure 2. All eight monoamine uptake inhibitors produced a dose-dependent decrease in acid-stimulated writhing, and ED50 values are shown in Table 1.
Figure 2. Effects of monoamine uptake inhibitors in the assay of acid-stimulated writhing.
Abscissae: Dose in mg/kg. Ordinates: Percent control writhes. All points show mean data±SEM from 5–6 rats, and ED50 values are reported in Table 1. The mean ± SEM number of control writhes for each group were as follows: citalopram 24.7±6.8; clomipramine 17.3±3.6; nisoxetine 21.9±0.5; nortriptyline 21.4±3.5; RTI-113 22.6±4.5; bupropion 16.3±2.3; milnacipran 17.9±2.3; RTI-112 19.5±3.8.
Table 1.
ED50 values in mg/kg (95% confidence limits) for monoamine reuptake inhibitors to produce antinociception in the assays of acid-stimulated writhing or acid-induced depression of ICSS. “Inactive” indicates that a failure to produce at least 50% acid blockade in the assay of acid-depressed ICSS.
| Acid-Stimulated Writhing |
Acid-Depressed ICSS |
|
|---|---|---|
| Citalopram | 15.00 (10.74–23.50) | Inactive |
| Clomipramine | 7.77 (5.90–9.89) | Inactive |
| Nisoxetine | 0.62 (0.21–1.10) | Inactive |
| Nortriptyline | 2.55 (1.41–5.58) | Inactive |
| RTI-113 | 0.95 (0.39–2.12) | 0.20 (0.08–0.53) |
| Bupropion | 7.56 (5.04–10.52) | 1.75 (0.59–5.23) |
| Milnacipran | 1.14 (0.38–3.42) | Inactive |
| RTI-112 | 0.34 (0.09–1.28) | 0.12 (0.03–0.21) |
Effects of monoamine uptake inhibitors in the assay of acid-depressed ICSS
Acid-induced depression of ICSS
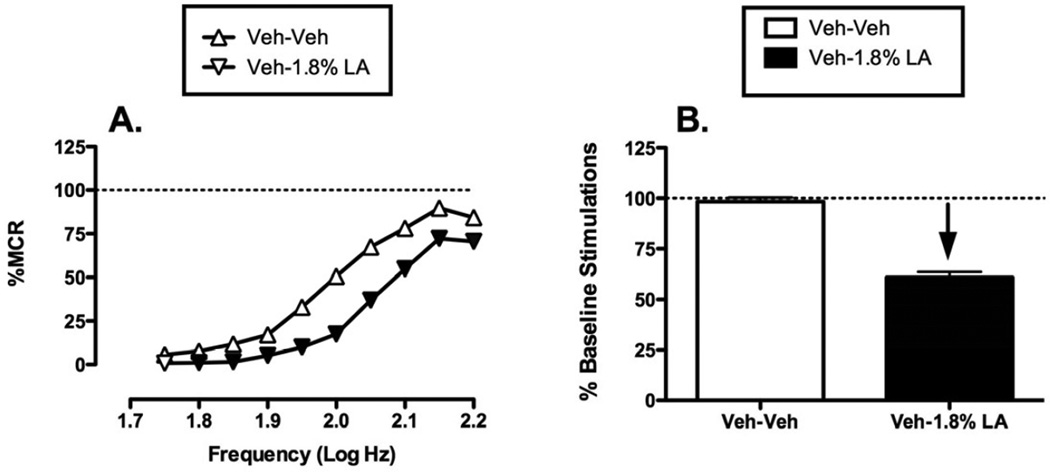
Figure 3 shows effects of the same noxious stimulus (IP injection of 1.8% lactic acid) on ICSS. During each test session, a “baseline” frequency-rate curve was determined before experimental treatments to permit determination of the Maximum Control Rate (MCR) for that session. Over the course of the entire study, the mean±SEM MCR was 54.58±0.57 stimulations per trial. Reinforcement rates during each frequency trial of a session were then expressed as a percentage of that session’s MCR, and the average frequency-rate curve for all studies with drug vehicle + acid vehicle is shown in Fig. 3. Maximum reinforcement rates were usually observed at the highest stimulation frequencies (2.15–2.2 log Hz), and responding generally decreased in a frequency-dependent manner. Administration of 1.8% lactic acid depressed ICSS, producing a rightward shift in the frequency-rate curve. Figure 3 also shows summary data for the total number of stimulations delivered across all 10 frequencies during each component. The overall mean±SEM baseline number of stimulations per component for all rats in the study was 213.58±6.26. Total ICSS after treatment with drug vehicle + acid vehicle was nearly identical to baseline predrug ICSS, but acid treatment decreased the number of stimulations per component. This acid-induced depression of ICSS provided a measure of pain-related behavioral depression, and drugs were evaluated for their ability to block this acid-induced depression of ICSS.
Figure 3. Acid-induced depression of ICSS.
Left panel (A) compares effects of pretreatment with Vehicle + Vehicle (Veh-Veh) and Vehicle + 1.8% lactic acid (Veh−1.8% LA) on full frequency-rate curves for all 52 rats used in ICSS experiments. Abscissa: Frequency of electrical brain stimulation in log Hz. Ordinate: Percent maximum control response rate (%MCR). Filled symbols indicate a significant difference from Veh-Veh (Holm-Sidak post hoc test, p<0.05). Right panel (B) shows summary data for lactic acid effects on the total number of stimulations per component. Abscissa: Pretreatment conditions. Ordinate: Percent baseline number of stimulations per component. The downward arrow indicates that lactic acid produced a significant decrease in ICSS at one or more frequencies in the full frequency-rate curve. Statistical results for two-way ANOVA of full frequency-rate curves are as follows: (A) Significant main effect of frequency [F(9,459)= 262.185, p<0.001] and treatment [F(1,51)= 202.572, p<0.001]; the interaction was also significant [F(9,459)=16.553, p<0.001].
Effects of selective serotonin reuptake inhibitors
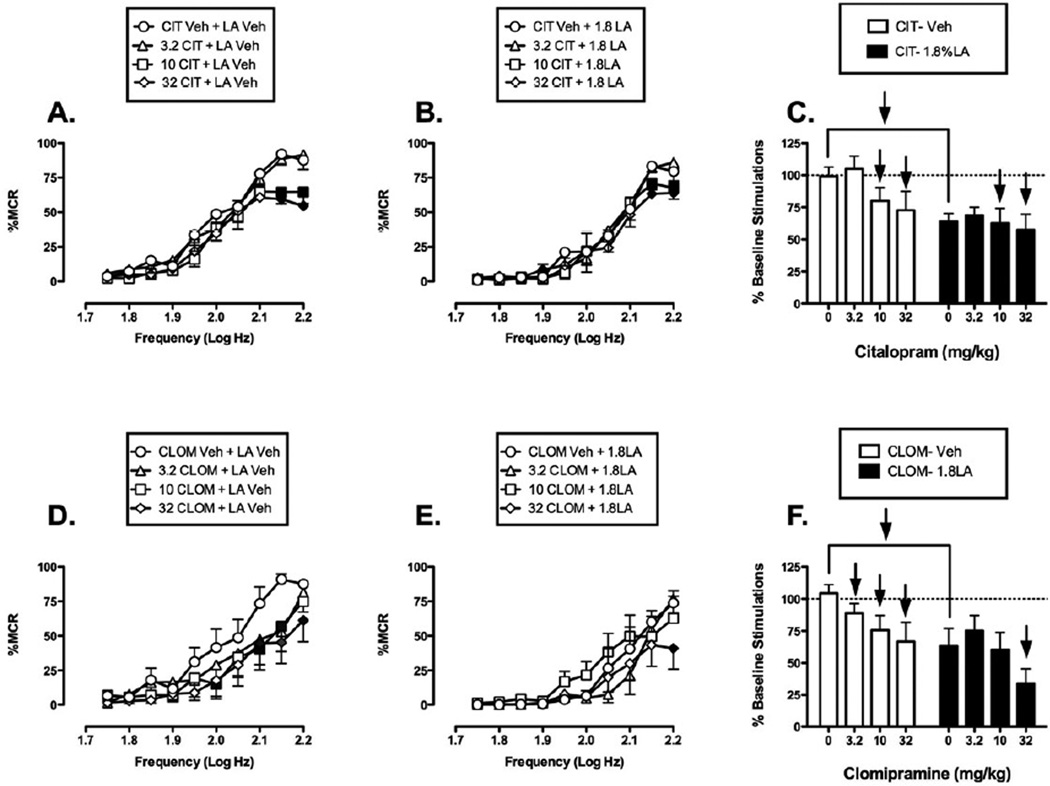
Figure 4 shows that the SSRIs citalopram and clomipramine failed to block acid-induced depression of ICSS, and consequently failed to produce antinociception in this assay. When citalopram was administered as a pretreatment to acid vehicle, citalopram produced a downward shift in the ICSS frequency-rate curve (Fig. 4A). A low dose of 3.2 mg/kg citalopram had no effect on ICSS. However, 10 and 32 mg/kg citalopram significantly decreased rates of reinforcement at the two highest frequencies (2.15 and 2.2 log Hz). When citalopram was administered as a pretreatment to 1.8% lactic acid, it exacerbated acid-induced depression of ICSS (Fig. 4B) with significant effects by 10 and 32 mg/kg citalopram at the two highest frequencies (2.15–2.2 log Hz). Overall, acute citalopram produced a depression of ICSS whether it was administered before lactic acid vehicle or 1.8% lactic acid (Fig. 4C).
Figure 4. Effects of the SSRIs citalopram (CIT, panels A-C, N=5) and clomipramine (CLOM, panels D-F, N=6) on control and acid-depressed ICSS.
Left and center panels show drug effects on full frequency-rate curves when drugs were administered as a pretreatment to vehicle (Left panels A, D) or 1.8% lactic acid (center panels B, E). Abscissae: Frequency of electrical brain stimulation in log Hz. Ordinates: Percent maximum control response rate (%MCR). Filled symbols indicate a significant difference from Veh-Veh (A, D) or Veh-LA (B, E) (Holm-Sidak post hoc test, p<0.05). Right panels (C,F) show summary data for drug effects on the total number of stimulations per component when drugs were administered as a pretreatment to vehicle (open bars) or acid (filled bars). Abscissae: Dose of drug in mg/kg. Ordinate: Percent baseline number of stimulations per component. Upward/downward arrows indicate that the drug dose produced a significant increase/decrease in ICSS at one or more frequencies in the full frequency-rate curve. Statistical results for two-way ANOVA of full frequency-rate curves are as follows: (A) Significant main effect of frequency [F(9,36)=60.71, p<0.001] and dose [F(3,12)=3.49, p=0.050], but the interaction was not significant [F(27,108)=1.57, p=0.054]. (B) Significant main effect of frequency [F(9,36)=40.84, p<0.001] but not dose [F(3,12)=0.7, p=0.573]; the interaction was significant [F(27,108)=1.64, p=0.040]. (D) Significant main effect of frequency [F(9,45)=17.94, p<.001] and dose [F(3,15)=4.33, p=0.022], but the interaction was not significant [F(27,135)=1.51, p=0.065]. (E) Significant main effect of frequency [F(9,45)=17.23, p<0.001] but not of dose [F(3,15)=1.96, p=0.164]; the interaction was significant [F(27,135)=2.04, p=0.004].
When clomipramine was administered as a pretreatment to acid vehicle, it produced a downward and rightward shift in the ICSS frequency-rate curve (Fig. 4D). All doses of clomipramine (3.2, 10, and 32 mg/kg) produced significant decrease in ICSS at the highest stimulation frequencies (2.0–2.2 log Hz). Similarly, when clomipramine was administered as a pretreatment to 1.8% lactic acid, it exacerbated acid-induced depression of ICSS. Only the highest dose of clomipramine (32 mg/kg) produced a significant decrease in ICSS rates at the highest frequency (2.2 log Hz) (Fig. 4E). Overall, acute clomipramine produced a depression of ICSS whether it was administered before lactic acid vehicle or 1.8% lactic acid (Fig. 4F).
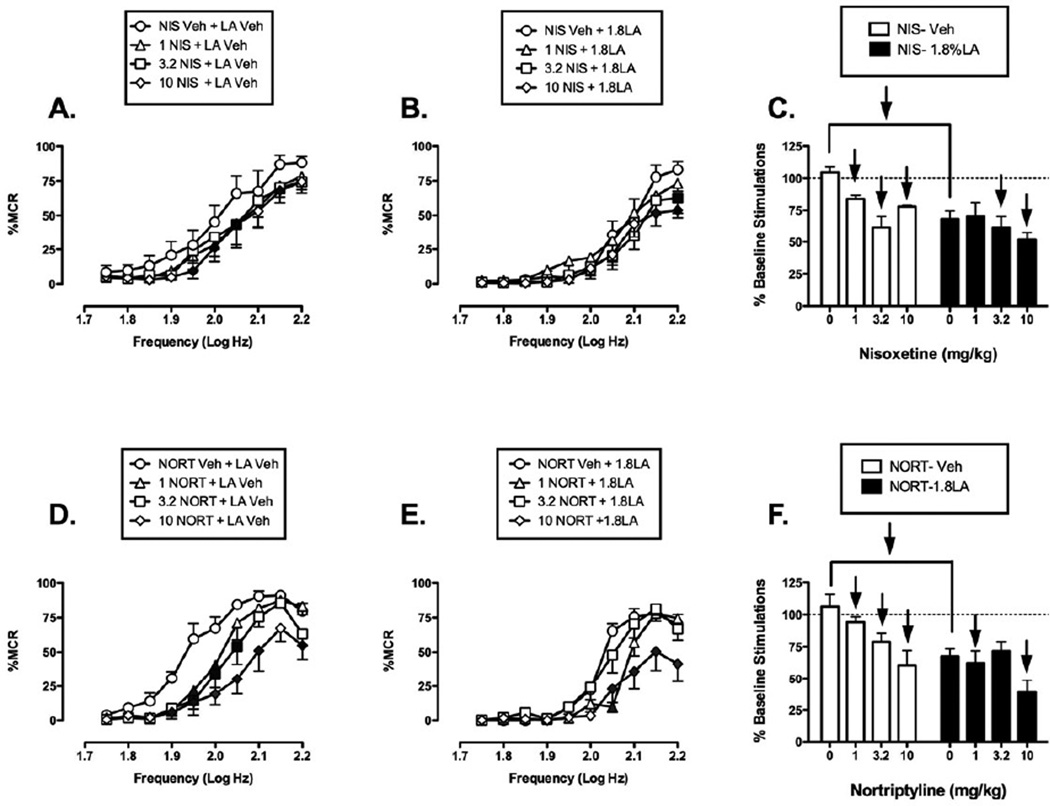
Effect of selective norepinephrine reuptake inhibitors
Figure 5 shows that nisoxetine and nortriptyline also failed to block acid-induced depression of ICSS. When nisoxetine was administered as a pretreatment to acid vehicle (Fig. 5A), it produced a downward and rightward shift of the frequency rate curve that was significant at all doses tested (1–10 mg/kg) at frequencies ranging from 1.95–2.15 log Hz. As a pretreatment to acid, nisoxetine further depressed ICSS (Figure 5B). Higher doses of 3.2 and 10 mg/kg caused significant decreases in ICSS at frequencies of 2.15–2.2 log Hz. Overall, acute nisoxetine depressed ICSS responding in the absence or presence of acid (Fig. 5C).
Figure 5. Effects of the SNRIs nisoxetine (NIS, panels A-C, N=6) and nortriptyline (NORT, panels D-F, N=6) on control and acid-depressed ICSS.
Details as in Figure 4. Statistical results for two-way ANOVA of full frequency-rate curves are as follows: (A) Significant main effect of frequency [F(9,45)=21.3, p<0.001] and dose [F(3,15)=10.18, p<0.001]; the interaction was not significant [F(27,135)=0.68, p=0.875]. (B) Significant main effects of frequency [F(9,45)=26.94, p<0.001] but not dose [F(3,15)=2.25, p=0.124]; the interaction was significant [F(27,135)=2.07, p=0.003. (D) Significant main effect of frequency [F(9,45)=51.82, p<0.001] and dose [F(3,15)=9.58, p<0.001]; the interaction was significant [F(27,135)=3.27, p<0.001]. (E) Significant main effect of frequency [F(9,45)=87.57,p<0.001] and dose [F(3,15)=5.79, p=0.008]; the interaction was significant [F(27,135)=4.48, p<0.001].
When nortriptyline was administered as a pretreatment to acid vehicle, it produced a downward and rightward shift in the ICSS frequency rate curve (Fig. 5D). All doses of nortriptyline (1, 3.2, 10 mg/kg) produced a significant decrease in ICSS at frequencies ranging from 1.9–2.2 log Hz. Similarly, when nortriptyline was administered as a pretreatment to lactic acid, it exacerbated acid-induced depression of ICSS (Fig. 4E), with significant decreases at the lowest and highest doses (1 and 10mg/kg) at a range of high frequencies (2.05–2.2 log Hz). Overall, acute nortriptyline depressed ICSS responding in the absence or presence of acid (Fig. 5F).
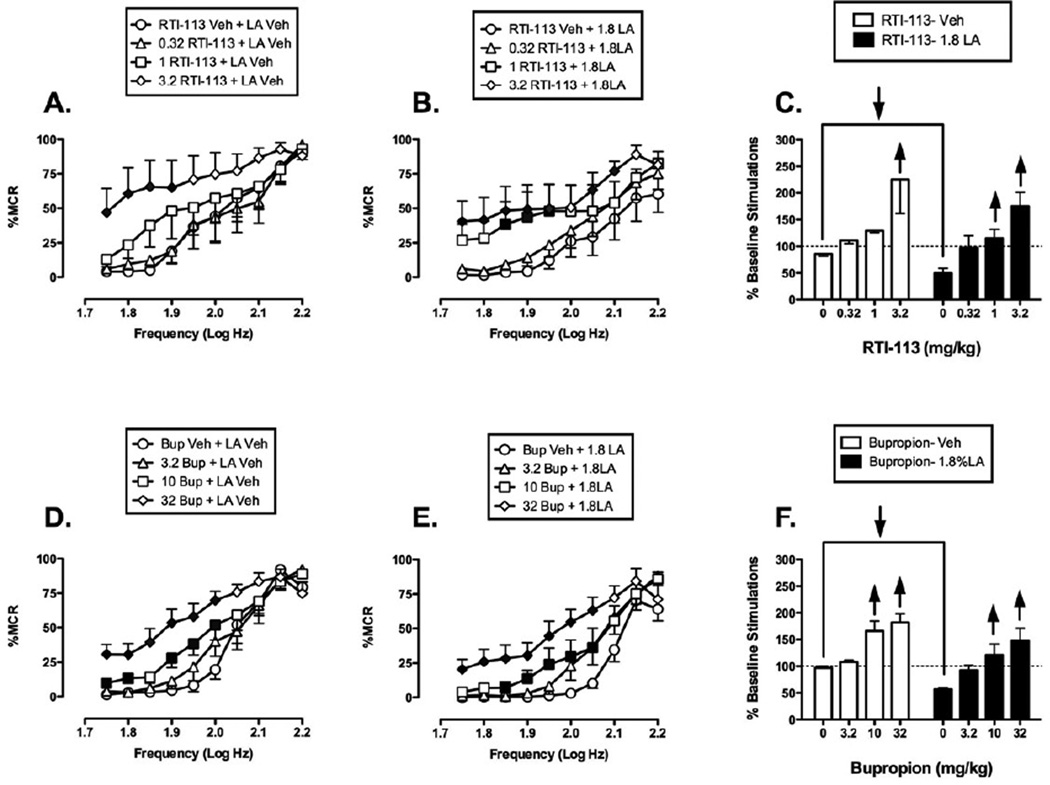
Effect of selective dopamine reuptake inhibitors
Figure 6 shows that, in contrast to the SSRIs and SNRIs, the SDRIs RTI-113 and bupropion dose-dependently and completely blocked acid-induced depression of ICSS at or near doses that also facilitated control ICSS in the absence of the noxious acid stimulus. When administered as a pretreatment to acid vehicle, RTI-113 produced a dose-dependent leftward and upward shift in the ICSS frequency-rate curve, and the highest dose of 3.2 mg/kg RTI-113 significantly facilitated ICSS at frequencies of 1.75–1.9 log Hz (Fig. 6A). Similarly, when administered as a pretreatment to 1.8% lactic acid, RTI-113 increased ICSS responding and ameliorated acid-induced depression of ICSS (Fig. 6B). Significant increases in ICSS responding were observed after pretreatment with 1 and 3.2 mg/kg RTI-113 at a broad range of frequencies ranging from 1.75–2.1 log Hz. Overall, acute RTI-113 produced non-selective facilitation of ICSS in the absence or presence of acid (Fig. 6C).
Figure 6. Effects of the SDRIs RTI-113 (panels A-C, N=6) and bupropion (Bup, panels D-F, N=8) on control and acid-depressed ICSS.
Details as in Figure 4. Statistical results for two-way ANOVA of full frequency-rate curves are as follows: (A) Significant main effect of frequency [F(9,45)=14.35, p<0.001] and dose [F(3,15)=5.27, p=0.011]; the interaction was significant [F(27,135)=2.27, p=0.001]. (B) Significant main effects of frequency [F(9,45)=16.99, p<0.001] and dose [F(3,15)=10.42, p<0.001]; the interaction was not significant [F(27,135)=0.69, p=0.0871]. (D) Significant main effect of frequency [F(9,63)=47.70, p<0.001] and dose [F(3,21)=23.04, p<0.001]; the interaction was significant [F(27,189)=4.9, p<0.001]. (E) Significant main effect of frequency [F(9,63)=54.97,p<0.001] and dose [F(3,21)=11.12, p<0.001]; the interaction was significant [F(27,189)=2.63, p<0.001].
Pretreatment with bupropion also non-selectively increased ICSS responding in the absence (Fig. 6D) or presence of acid (Fig. 6E). High doses of 10 and 32 mg/kg bupropion significantly increased rates of reinforcement under both conditions across a broad range of frequencies from 1.75–2.05 log Hz, and these effects of bupropion are summarized in Fig. 6F.
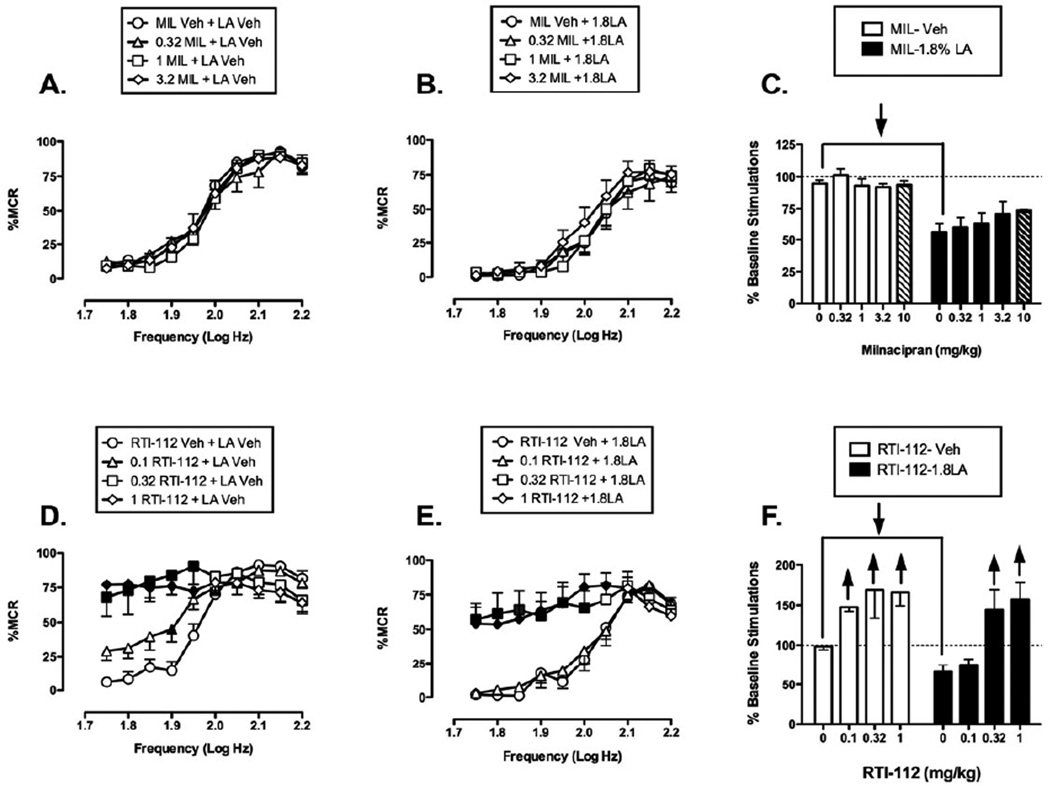
Effect of mixed-action monoamine reuptake inhibitors
Figure 7 shows the effects of the S+NRI milnacipran and the TRI RTI-112 on control and acid-depressed ICSS. Pretreatment with 0.32–3.2 mg/kg milnacipran did not significantly alter either control ICSS (Fig. 7A) or acid-depressed ICSS (Fig. 7B), and these data are summarized in Fig. 7C. A higher dose of 10 mg/kg was tested in 2 rats, but this higher dose also had little effect on ICSS in the absence or presence of the noxious stimulus. Overall, there was a trend for milnacipran to attenuate acid-induced depression of ICSS without affecting control ICSS, but this trend did not achieve statistical significance despite the use of a relatively large group of rats (N=9) and the evaluation of milnacipran doses that significantly reduced acid-stimulated writhing.
Figure 7. Effects of the S+NRI milnacipran (MIL, panels A-C, N=9) and the TRI RTI-112 (panels D-F, N=6) on control and acid-depressed ICSS.
Hatched bars in panel C show effects of the highest milnacipran dose in a subset of 2 rats. All other details as in Figure 4. Statistical results for two-way ANOVA of full frequency-rate curves are as follows: (A) Significant main effect of frequency [F(9,72)=73.701, p<0.001], but not of dose [F(3,24)=0.314, p=0.815]; the interaction was not significant [F(27,216)=0.785, p=0.769]. (B) Significant main effect of frequency [F(9,72)=53.313, p<0.001] but not of dose [F(3,24)=0.743, p=0.537]; the interaction was not significant [F(27,216)=0.864 p=0.663] (D) Significant main effect of frequency [F(9,45)=27.022, p<0.001] and dose [F(3,15)=5.403, p=0.010]; the interaction was significant [F(27,135)=11.075, p<0.001]. (E) Significant main effect of frequency [F(9,45)=36.328, p<0.001] and dose [F(3,15)=12.033, p<0.001]; the interaction was significant [F(27,135)=7.585, p<0.001].
Effects of the TRI RTI-112 were similar to effects of the SDRIs discussed above. When RTI-112 was administered as a pretreatment to acid vehicle, it produced a dose-dependent leftward and upward shift in the ICSS frequency-rate curve (Fig. 7D). Significant increases in ICSS responding were observed after pretreatment with all doses of RTI-112 at lower frequencies (1.75–1.95 log Hz). When RTI-112 was administered as a pretreatment to lactic acid, significant leftward shifts in the frequency-rate curve were seen only with 0.32 and 1 mg/kg RTI-112 at the lower range of frequencies (1.75–2.05 log Hz) (Fig. 7E). Overall, acute RTI-112 produced non-selective facilitation of ICSS in the absence or presence of acid (Fig. 7F).
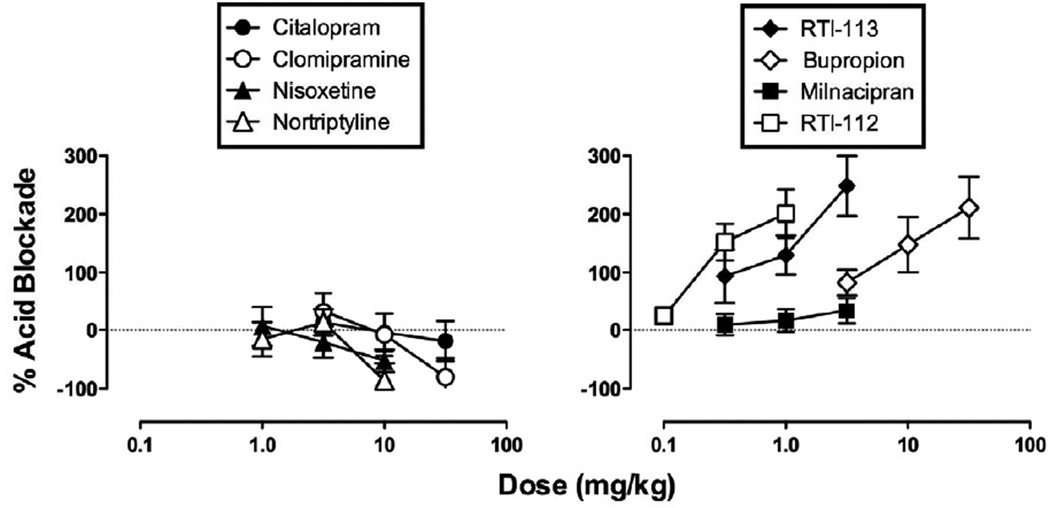
Summary of monoamine releaser effects on acid-induced depression of ICSS
Figure 8 shows effects of all eight monoamine releasers expressed as “percent acid blockade” in the assay of acid-induced depression of ICSS. The SSRIs, SNRIs and the S+NRI milnacipran all failed to produce greater than 50% acid blockade, and ED50 values could not be calculated (Table 1). The SDRIs and the TRI RTI-112 produced dose-dependent increases in % acid blockade to values well above 100%, indicating facilitation of ICSS above baseline levels. Nonetheless, ED50s could be calculated for these drugs, and these ED50 values are reported in Table 1 for comparison with ED50 values in the assay of acid-stimulated writhing. There was a trend for the dopaminergic drugs to be more potent in the assay of acid-depressed ICSS than in the assay of acid-stimulated writhing; however, ED50 values across assays were not considered to be statistically significant because 95% confidence limits overlapped.
Figure 8. Effects of monoamine uptake inhibitors in the assay of acid-depressed ICSS expressed as % acid blockade.
Abscissae: Dose in mg/kg. Ordinates: Percent blockade of acid-induced depression of ICSS, calculated as described in Methods. All points show mean data±SEM from 5–6 rats, and ED50 values are reported in Table 1.
Effects of repeated citalopram
Citalopram was re-tested using a repeated-dosing regimen shown previously to produce antidepressant effects in a forced-swim test in rats 10. As with acute administration, repeated citalopram (10 mg/kg × 3 doses) significantly decreased acid-stimulated writhing from a mean±SEM of 13.17 ± 2.40 writhes after treatment with citalopram vehicle to 5.67±2.09 writhes after repeated citalopram (t(5)=3.16, p=0.025). However, when repeated citalopram was administered as a pretreatment to lactic acid in the ICSS procedure, it failed to block acid-induced depression of ICSS. Two-way repeated measures ANOVA between effects of repeated vehicle or repeated citalopram + 1.8% lactic acid on full frequency-rate ICSS curves revealed a significant effect of frequency [F(9,27)=32.33, p<0.001], but no significant effects of citalopram treatment [F(1,3)=8.56 p=0.06] and no interaction [F(9,27)=1.86, p=0.10], and the mean±SEM % baseline stimulations after repeated vehicle or repeated citalopram + acid were 69.80±2.79 and 62.74±5.05 respectively. Thus, as with acute citalopram, repeated citalopram produced antinociception in the assay of acid-stimulated writhing but not in the assay of acid-depressed ICSS.
DISCUSSION
This study investigated effects of selective and mixed-action monoamine reuptake inhibitors in preclinical assays of acute pain-stimulated and pain-depressed behavior in rats. There were three main findings. First, in agreement with many previous studies of pain-stimulated behaviors, all monoamine reuptake inhibitors significantly reduced acid-stimulated writhing. Second, the selective serotonin and norepinephrine reuptake inhibitors and the mixed-action serotonin+norepinephrine reuptake inhibitor milnacipran failed to block acid-induced depression of ICSS up to doses that were effective in the writhing assay and that (except for milnacipran) decreased ICSS in the absence of the noxious stimulus. Finally, in contrast to the serotonergic and noradrenergic compounds, the selective dopamine and triple reuptake inhibitors did block acid-induced depression of ICSS. These compounds also facilitated control ICSS in the absence of the noxious stimulus, which is consistent with abuse liability of dopamine reuptake inhibitors 61 and suggests that efficacy to block acid-induced depression of ICSS may reflect nonselective behavioral stimulation rather than reduced nociception. However, the antinociceptive efficacy of selective and mixed-action dopamine reuptake inhibitors is consistent with previous results with cocaine in this assay 50, with analgesic efficacy of dopamine reuptake inhibitors and releasers in assays of acute pain in humans 70,72,74, and with a role for dopamine in modulating affective dimensions of pain 73. These results support further consideration of selective or mixed-action dopamine reuptake inhibitors as analgesics, especially under circumstances where pain relief without sedation may be especially desirable.
Effects of monoamine reuptake inhibitors on acid-stimulated writhing
The effects of monoamine reuptake inhibitors in the present assay of acid-stimulated writhing are consistent with effects of these compounds in previous studies of acute pain-stimulated behaviors elicited by chemical noxious stimuli such as writhing elicited by intraperitoneal administration of chemical irritants 2,3,38,62 or paw flinching elicited by intraplantar administration of formalin 8,44,62,75. Monoamine reuptake inhibitors also produced apparent antinociception in some assays of acute thermal nociception, although these effects were less reliable 8,24,62. For example, both clomipramine and nortriptyline produced dose dependent antinociception in assays of acetic acid-induced writhing and hot plate thermal nociception in mice, although both drugs were less potent and less efficacious in the thermal assay 62. Moreover, these preclinical measures of acute pain-stimulated behavior are often more responsive to selective or mixed-action norepinephrine or serotonin reuptake inhibitors than to drugs like cocaine or bupropion with prominent dopaminergic effects 17,50,56. However, this preclinical efficacy of norepinephrine/serotonin reuptake inhibitors translates poorly to efficacy in assays of acute experimental or clinical pain in humans 19,20,23,69.
Effects of monoamine reuptake inhibitors on acid-induced depression of ICSS
These are the first studies to examine effects of monoamine reuptake inhibitors in an assay of pain-depressed behavior. In agreement with previous results, intraperitoneal administration of dilute acid depressed ICSS 49–51,58, and drugs were evaluated for their ability to block acid-induced depression of ICSS. Only the reuptake inhibitors with prominent dopaminergic effects (RTI-112, RTI-113, bupropion) were effective under these conditions. These compounds also facilitated control ICSS in the absence of the noxious stimulus, and even in the presence of acid, they increased ICSS well above baseline levels. These effects distinguish dopamine reuptake inhibitors from prominent clinical analgesics such as mu opioid agonists and nonsteroidal anti-inflammatory drugs that block acid-induced depression of ICSS while having little or no effect on control ICSS 35,50,58. Taken together, these findings suggest that apparent antinociception by dopamine reuptake inhibitors may have reflected nonselective stimulation of behavior rather than a selective blockade of sensory sensitivity to the noxious stimulus. Nonetheless, three points warrant mention. First, all three compounds decreased rather than increased acid-stimulated writhing, so these compounds were effective in decreasing a pain-stimulated behavior as well as increasing a pain-depressed behavior. Second, these preclinical findings may be related to clinical reports of analgesia produced by dopamine reuptake inhibitors and releasers under conditions of acute pain 70,72,74. For example, intranasal cocaine reduced pain scores in a human model of ischemic tourniquet pain 70, and reduced pain scores in human assays of acute pain were also produced by nonpharmacological manipulations that activated brain reward regions populated by dopaminergic neurons 67,76. Lastly, these results extend a literature suggesting an inverse relationship between supraspinal dopaminergic activity and some components of pain 32,73.
A clear limiting factor to the use of dopamine reuptake inhibitors for treatment of pain is their high abuse liability, and in the present study, the ability of these drugs to facilitate control ICSS in the absence of the noxious stimulus provides one source of evidence for that abuse liability 68. However, triple reuptake inhibitors with reduced proportions of dopaminergic activity may provide an approach to retain analgesic efficacy with reduced abuse liability. For example, bicifadine has been classified as a triple reuptake inhibitor, although its potency to inhibit dopamine reuptake is approximately 10-fold lower than its potency to inhibit serotonin or norepinephrine reuptake 6. The effects of bicifadine on pain-depressed behavior are unknown, but it produced antinociception in a range of other preclinical and clinical assays 6, and preclinical evidence suggests that it has weaker reinforcing efficacy than reuptake inhibitors such as cocaine that have more prominent dopaminergic components 53. A related limitation to the use of dopamine reuptake inhibitors is their ability to stimulate behavior in a manner that may aggravate injury and impede healing. However, analgesia coupled with some behavioral stimulation may be advantageous in some situations. For example, physical therapy can play a key role in rehabilitation and pain reduction, and analgesic stimulants may warrant consideration as tools to facilitate patient participation in rehabilitative activities 66. Overall, the present results support further consideration of selective or mixed-action “triple” dopamine reuptake inhibitors as candidate analgesics.
In contrast to the apparent antinociceptive effects of the selective dopamine and triple reuptake inhibitors, the selective serotonin and norepinephrine reuptake inhibitors failed to produce antinociception in the assay of acid-depressed ICSS. Rather, at doses that decreased acid-stimulated writhing, these drugs also generally decreased control ICSS and exacerbated acid-induced depression of ICSS, suggesting that they produced a profile of general behavioral depression. These behavioral depressant effects of serotonin and norepinephrine reuptake inhibitors are similar to results reported previously on ICSS in the absence of noxious stimulation 25,36,39, and the lack of antinociception is consistent with a lack of analgesic effects in human assays of acute pain 19,20,23,69.
Although effects of milnacipran did not attain statistical significance in the assay of acid-depressed ICSS, it did not depress control ICSS, and it trended toward an attenuation of acid-induced depression of ICSS. The reason for this distinct profile will require further study. In the present study, milnacipran was notable for the small magnitude of its effects in all three assays, although it was tested in a range of doses shown to be effective previously in other assays in rats 5,7. For example, milnacipran significantly decreased acid-stimulated writhing, but its effects appeared to plateau at a dose of 1.0 mg/kg, and a higher dose of 3.2 mg/kg did not produce a larger effect. Milnacipran was also reported to produce weaker effects than other monoamine reuptake inhibitors in an assay of acid-stimulated writhing in mice 2, and weaker relief of fibromyalgia pain than amitriptyline or duloxetine in humans 26. Nonetheless, the ability of milnacipran to attenuate acid-stimulated writhing without affecting control ICSS and while trending to attenuate acid-induced depression of ICSS suggests that mixed serotonin+norepinephrine reuptake inhibitors may have dissociable effects from selective serotonin or selective norepinephrine reuptake inhibitors on pain-depressed behaviors. Any attenuation by milnacipran of acid-induced depression of ICSS is unlikely to reflect a dopaminergic contribution, because milnacipran displays more than 100-fold selectivity to block reuptake of serotonin and norepinephrine than dopamine (see figure 1) 45.
Dissociable drug effects in assays of pain-stimulated vs. pain-depressed behavior
These results add to a growing body of evidence to suggest that drugs can produce dissociable evidence for antinociception in assays of pain-stimulated vs. pain-depressed behavior. Historically, preclinical research on candidate analgesics has relied almost exclusively on assays of pain-stimulated behavior; however, a growing literature describes efforts to develop novel assays of pain-depressed behavior to model clinically relevant aspects of pain that include functional impairment and depression of behavior and mood 14,40,41,48. These new approaches have the potential to complement traditional procedures and improve translational validity of preclinical research on pain and analgesia.
Perspective.
Monoamine reuptake inhibitors are used to treat depression and some forms of pain. This study examined effects of monoamine reuptake inhibitors in a preclinical assay of pain-related behavioral depression. The results support further consideration of dopamine reuptake inhibitors as candidate analgesics under selected circumstances, although abuse liability remains a concern.
Acknowledgments
This work was supported by R01-NS070715 (SSN) and R01-DA05477 (FIC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None of the authors have professional or financial relationships that could result in conflicts of interest related to work described in this manuscript.
REFERENCES
- 1.Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 1999;65:2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- 2.Aoki M, Tsuji M, Takeda H, Harada Y, Nohara J, Matsumiya T, Chiba H. Antidepressants enhance the antinociceptive effects of carbamazepine in the acetic acid-induced writhing test in mice. Eur J Pharmacol. 2006;550:78–83. doi: 10.1016/j.ejphar.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 3.Ardid D, Marty H, Fialip J, Privat AM, Eschalier A, Lavarenne J. Comparative effects of different uptake inhibitor antidepressants in two pain tests in mice. Fundam Clin Pharmacol. 1992;6:75–82. doi: 10.1111/j.1472-8206.1992.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 4.Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70:890–897. doi: 10.1097/PSY.0b013e318185c510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardin L, Gregoire S, Aliaga M, Malfetes N, Vitton O, Ladure P, Newman-Tancredi A, Depoortere R. Comparison of milnacipran, duloxetine and pregabalin in the formalin pain test and in a model of stress-induced ultrasonic vocalizations in rats. Neurosci Res. 2010;66:135–140. doi: 10.1016/j.neures.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Basile AS, Janowsky A, Golembiowska K, Kowalska M, Tam E, Benveniste M, Popik P, Nikiforuk A, Krawczyk M, Nowak G, Krieter PA, Lippa AS, Skolnick P, Koustova E. Characterization of the antinociceptive actions of bicifadine in models of acute, persistent, and chronic pain. J Pharmacol Exp Ther. 2007;321:1208–1225. doi: 10.1124/jpet.106.116483. [DOI] [PubMed] [Google Scholar]
- 7.Berrocoso E, Mico JA, Vitton O, Ladure P, Newman-Tancredi A, Depoortere R, Bardin L. Evaluation of milnacipran, in comparison with amitriptyline, on cold and mechanical allodynia in a rat model of neuropathic pain. Eur J Pharmacol. 2011;655:46–51. doi: 10.1016/j.ejphar.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 2005;48:252–263. doi: 10.1016/j.neuropharm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Borsook D, Becerra L, Carlezon WA, Jr, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain. 2007;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 11.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 12.Cheetham SC, Viggers JA, Butler SA, Prow MR, Heal DJ. [3H]nisoxetine--a radioligand for noradrenaline reuptake sites: correlation with inhibition of [3H]noradrenaline uptake and effect of DSP-4 lesioning and antidepressant treatments. Neuropharmacology. 1996;35:63–70. doi: 10.1016/0028-3908(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Cheetham SC, Viggers JA, Slater NA, Heal DJ, Buckett WR. [3H]paroxetine binding in rat frontal cortex strongly correlates with [3H]5-HT uptake: effect of administration of various antidepressant treatments. Neuropharmacology. 1993;32:737–743. doi: 10.1016/0028-3908(93)90181-2. [DOI] [PubMed] [Google Scholar]
- 14.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook CD, Carroll IF, Beardsley PM. Cocaine-like discriminative stimulus effects of novel cocaine and 3-phenyltropane analogs in the rat. Psychopharmacology (Berl) 2001;159:58–63. doi: 10.1007/s002130100891. [DOI] [PubMed] [Google Scholar]
- 16.Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–112. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- 17.Duarte ID, Nakamura M, Ferreira SH. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res. 1988;21:341–343. [PubMed] [Google Scholar]
- 18.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Enggaard TP, Klitgaard NA, Gram LF, Arendt-Nielsen L, Sindrup SH. Specific effect of venlafaxine on single and repetitive experimental painful stimuli in humans. Clin Pharmacol Ther. 2001;69:245–251. doi: 10.1067/mcp.2001.114873. [DOI] [PubMed] [Google Scholar]
- 20.Enggaard TP, Poulsen L, Arendt-Nielsen L, Hansen SH, Bjornsdottir I, Gram LF, Sindrup SH. The analgesic effect of codeine as compared to imipramine in different human experimental pain models. Pain. 2001;92:277–282. doi: 10.1016/s0304-3959(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 21.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon LR, Koltzenburg M, editors. Textbook of Pain. London: Elsevier; 2006. pp. 125–142. [Google Scholar]
- 22.Goldenberg DL. Pain/Depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am J Med. 2010;123:675–682. doi: 10.1016/j.amjmed.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Gordon NC, Heller PH, Gear RW, Levine JD. Interactions between fluoxetine and opiate analgesia for postoperative dental pain. Pain. 1994;58:85–88. doi: 10.1016/0304-3959(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 24.Hall FS, Schwarzbaum JM, Perona MT, Templin JS, Caron MG, Lesch KP, Murphy DL, Uhl GR. A greater role for the norepinephrine transporter than the serotonin transporter in murine nociception. Neuroscience. 2011;175:315–327. doi: 10.1016/j.neuroscience.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall FS, Stellar JR, Kelley AE. Acute and chronic desipramine treatment effects on rewarding electrical stimulation of the lateral hypothalamus. Pharmacol Biochem Behav. 1990;37:277–281. doi: 10.1016/0091-3057(90)90334-e. [DOI] [PubMed] [Google Scholar]
- 26.Hauser W, Petzke F, Uceyler N, Sommer C. Rheumatology. Vol. 50. Oxford: 2010. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis; pp. 532–543. [DOI] [PubMed] [Google Scholar]
- 27.Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs. 2012;26:297–307. doi: 10.2165/11598970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Hendershot LC, Forsaith J. Antagonism of the frequency of phenylquinone-induced writhing in the mouse by weak analgesics and nonanalgesics. J Pharmacol Exp Ther. 1959;125:237–240. [PubMed] [Google Scholar]
- 29.Institute oM. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 30.Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82–88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311:576–584. doi: 10.1124/jpet.104.070656. [DOI] [PubMed] [Google Scholar]
- 32.Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain. 2012;153:744–754. doi: 10.1016/j.pain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhar MJ, McGirr KM, Hunter RG, Lambert PD, Garrett BE, Carroll FI. Studies of selected phenyltropanes at monoamine transporters. Drug Alcohol Depend. 1999;56:9–15. doi: 10.1016/s0376-8716(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 34.Kula NS, Baldessarini RJ, Tarazi FI, Fisser R, Wang S, Trometer J, Neumeyer JL. [3H]beta-CIT: a radioligand for dopamine transporters in rat brain tissue. Eur J Pharmacol. 1999;385:291–294. doi: 10.1016/s0014-2999(99)00695-0. [DOI] [PubMed] [Google Scholar]
- 35.Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated vs. pain-depressed behavior in rats. J Pharmacol Exp Ther Epub ahead of print. 2012 Aug 14; doi: 10.1124/jpet.112.197780. pmid:22892341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K, Kornetsky C. Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav. 1998;60:539–544. doi: 10.1016/s0091-3057(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 37.Letchworth SR, Smith HR, Porrino LJ, Bennett BA, Davies HM, Sexton T, Childers SR. Characterization of a tropane radioligand, [(3)H]2beta-propanoyl-3beta-(4-tolyl) tropane ([(3)H]PTT), for dopamine transport sites in rat brain. J Pharmacol Exp Ther. 2000;293:686–696. [PubMed] [Google Scholar]
- 38.Leventhal L, Smith V, Hornby G, Andree TH, Brandt MR, Rogers KE. Differential and synergistic effects of selective norepinephrine and serotonin reuptake inhibitors in rodent models of pain. J Pharmacol Exp Ther. 2007;320:1178–1185. doi: 10.1124/jpet.106.109728. [DOI] [PubMed] [Google Scholar]
- 39.Marston HM, Martin FD, Papp M, Gold L, Wong EH, Shahid M. Attenuation of chronic mild stress-induced ‘anhedonia’ by asenapine is not associated with a ‘hedonic’ profile in intracranial self-stimulation. J Psychopharmacol. 2010;25:1388–1398. doi: 10.1177/0269881110376684. [DOI] [PubMed] [Google Scholar]
- 40.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 41.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 42.Mico JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27:348–354. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Millan MJ, Gobert A, Lejeune F, Newman-Tancredi A, Rivet JM, Auclair A, Peglion JL. S33005, a novel ligand at both serotonin and norepinephrine transporters: I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. J Pharmacol Exp Ther. 2001;298:565–580. [PubMed] [Google Scholar]
- 44.Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol. 2004;19(Suppl 1):S15–19. doi: 10.1002/hup.620. [DOI] [PubMed] [Google Scholar]
- 45.Mochizuki D, Tsujita R, Yamada S, Kawasaki K, Otsuka Y, Hashimoto S, Hattori T, Kitamura Y, Miki N. Neurochemical and behavioural characterization of milnacipran, a serotonin and noradrenaline reuptake inhibitor in rats. Psychopharmacology (Berl) 2002;162:323–332. doi: 10.1007/s00213-002-1111-5. [DOI] [PubMed] [Google Scholar]
- 46.Mogil JS, Wilson SG, Wan Y. Assessing nociception in murine subjects. In: Kruger L, editor. Methods in Pain Research. Boca Raton, FL: CRC Press; 2001. pp. 11–39. [Google Scholar]
- 47.National RC. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington DC: 2003. [PubMed] [Google Scholar]
- 48.Negus SS, Bilsky EJ, Pereira Do Carmo G, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. In: Szallasi A, editor. Methods in Molecular Biology: Analgesia. New York: Humana Press; 2010. pp. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther. 2012;340:501–509. doi: 10.1124/jpet.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Negus SS, Rosenberg MB, Altarifi AA, O’Connell RH, Folk JE, Rice KC. Effects of the delta opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain. 2012;13:317–327. doi: 10.1016/j.jpain.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical Assessment of Candidate Analgesic Drugs: Recent Advances and Future Challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 53.Nicholson KL, Balster RL, Golembiowska K, Kowalska M, Tizzano JP, Skolnick P, Basile AS. Preclinical evaluation of the abuse potential of the analgesic bicifadine. J Pharmacol Exp Ther. 2009;330:236–248. doi: 10.1124/jpet.109.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otsuka N, Kiuchi Y, Yokogawa F, Masuda Y, Oguchi K, Hosoyamada A. Antinociceptive efficacy of antidepressants: assessment of five antidepressants and four monoamine receptors in rats. J Anesth. 2001;15:154–158. doi: 10.1007/s005400170018. [DOI] [PubMed] [Google Scholar]
- 55.Paul IA, Duncan GE, Kuhn C, Mueller RA, Hong JS, Breese GR. Neural adaptation in imipramine-treated rats processed in forced swim test: assessment of time course, handling, rat strain and amine uptake. J Pharmacol Exp Ther. 1990;252:997–1005. [PubMed] [Google Scholar]
- 56.Pedersen LH, Nielsen AN, Blackburn-Munro G. Anti-nociception is selectively enhanced by parallel inhibition of multiple subtypes of monoamine transporters in rat models of persistent and neuropathic pain. Psychopharmacology (Berl) 2005;182:551–561. doi: 10.1007/s00213-005-0120-6. [DOI] [PubMed] [Google Scholar]
- 57.Pelissier T, Hernandez A, Mestre C, Eschalier A, Laurido C, Paeile C, Alvarez P, Soto-Moyano R. Antinociceptive effect of clomipramine in monoarthritic rats as revealed by the paw pressure test and the C-fiber-evoked reflex. Eur J Pharmacol. 2001;416:51–57. doi: 10.1016/s0014-2999(01)00848-2. [DOI] [PubMed] [Google Scholar]
- 58.Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 60.Richards BL, Whittle SL, Buchbinder R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2011:CD008920. doi: 10.1002/14651858.CD008920.pub2. [DOI] [PubMed] [Google Scholar]
- 61.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 62.Rojas-Corrales MO, Casas J, Moreno-Brea MR, Gibert-Rahola J, Mico JA. Antinociceptive effects of tricyclic antidepressants and their noradrenergic metabolites. Eur Neuropsychopharmacol. 2003;13:355–363. doi: 10.1016/s0924-977x(03)00017-8. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol. 1999;19:467–489. doi: 10.1023/a:1006986824213. [DOI] [PubMed] [Google Scholar]
- 64.Sawynok J, Esser MJ, Reid AR. Antidepressants as analgesics: an overview of central and peripheral mechanisms of action. J Psychiatry Neurosci. 2001;26:21–29. [PMC free article] [PubMed] [Google Scholar]
- 65.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 66.Veizi IE, Chelimsky TC, Janata JW. Chronic regional pain syndrome: what specialized rehabilitation services do patients require? Curr Pain Headache Rep. 2012;16:139–146. doi: 10.1007/s11916-012-0253-3. [DOI] [PubMed] [Google Scholar]
- 67.Villemure C, Laferriere AC, Bushnell MC. The ventral striatum is implicated in the analgesic effect of mood changes. Pain Res Manag. 2012;17:69–74. doi: 10.1155/2012/371362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vlachou S, Markou A. Intracranial self-stimulation, Neuromethods. In: Olmstead MC, editor. Animal Models of Drug Addiction. Vol. 53. New York: Humana Press; 2011. pp. 3–56. [Google Scholar]
- 69.Wallace MS, Barger D, Schulteis G. The effect of chronic oral desipramine on capsaicin-induced allodynia and hyperalgesia: a double-blinded, placebo-controlled, crossover study. Anesth Analg. 2002;95:973–978. doi: 10.1097/00000539-200210000-00034. table of contents. [DOI] [PubMed] [Google Scholar]
- 70.Wang RI, Johnson RP, Lee JC, Waite EM. The oral analgesic efficacy of bicifadine hydrochloride in postoperative pain. J Clin Pharmacol. 1982;22:160–164. doi: 10.1002/j.1552-4604.1982.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 71.Watson CPN, Chipman ML, Monks RC. Antidepressant analgesics: a systematic review and comparative study. In: McMahon LR, Koltzenburg M, editors. Textbook of Pain. London: Elsevier; 2006. pp. 481–497. [Google Scholar]
- 72.Webb SS, Smith GM, Evans WO, Webb NC. Toward the development of a potent, nonsedating, oral analgesic. Psychopharmacology (Berl) 1978;60:25–28. doi: 10.1007/BF00429174. [DOI] [PubMed] [Google Scholar]
- 73.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8:781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 74.Yang JC, Clark WC, Dooley JC, Mignogna FV. Effect of intranasal cocaine on experimental pain in man. Anesth Analg. 1982;61:358–361. [PubMed] [Google Scholar]
- 75.Yokogawa F, Kiuchi Y, Ishikawa Y, Otsuka N, Masuda Y, Oguchi K, Hosoyamada A. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95:163–168. doi: 10.1097/00000539-200207000-00029. table of contents. [DOI] [PubMed] [Google Scholar]
- 76.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS One. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]