Abstract
Mirror neurons are a class of visuomotor neurons in the monkey premotor and parietal cortices that discharge during the execution and observation of goal-directed motor acts. They are deemed to be at the basis of primates’ social abilities. In this review, the authors provide a fresh view about two still open questions about mirror neurons. The first question is their possible functional role. By reviewing recent neurophysiological data, the authors suggest that mirror neurons might represent a flexible system that encodes observed actions in terms of several behaviorally relevant features. The second question concerns the possible developmental mechanisms responsible for their initial emergence. To provide a possible answer to question, the authors review two different aspects of sensorimotor development: facial and hand movements, respectively. The authors suggest that possibly two different “mirror” systems might underlie the development of action understanding and imitative abilities in the two cases. More specifically, a possibly prewired system already present at birth but shaped by the social environment might underlie the early development of facial imitative abilities. On the contrary, an experience-dependent system might subserve perception-action couplings in the case of hand movements. The development of this latter system might be critically dependent on the observation of own movements.
Keywords: action understanding, facial imitation, mirror neurons, perception and action, sensorimotor development
The high level of complexity and sophistication of social interactions is one of the main signatures of primate behavior. Indeed, whereas for most animal species, the correct interpretation of changes in the environment is crucial for survival, for primates, also the correct interpretation of the behavior of conspecifics plays an important role in the struggle for succeeding in their complex social network. Given their direct impact on our everyday life, the neuronal and cognitive processes at the heart of the primate social skills represent a fascinating field of research in neuroscience.
About a decade ago, the general view was that action understanding was the result of a chain of rapid cognitive processes that compared previous experiences with the current visual input. In other words, action recognition and understanding were thought to be predominantly visual tasks relying on the hierarchical extraction of features of increasing complexity (e.g., Aggarwal and Cai 1999; Marr and Vaina 1982), followed by their comparison with analogous visual representations stored in the observer’s memory. This view was further corroborated by neurophysiological recordings in visual cortical areas (Bruce and others 1981; Perrett and others 1985). In a series of experiments, Perrett and co-workers showed the presence, in the superior temporal sulcus (STS in Fig. 1) of the monkey, of neurons exhibiting selective responses to human movements either performed by the experimenter in front of the monkey or presented on a computer screen (Perrett and others 1985). These neurons possessed remarkable response properties and were sensitive to many characteristics of observed movements. For example, they encoded the direction of motion of a walking person with respect to the observer (Perrett and others 1985), responded also to articulated (i.e., with arms and legs outstretched) and standing (i.e., with arms and legs close to the body) human figures presented as static snap-shots (Barraclough and others 2006), and showed a specificity for different body parts (Wachsmuth and others 1994). In addition to neurons responding to human movements, Perrett and co-workers described also a population of neurons in STS selectively responding during the observation of manipulative behaviors (i.e., hand-object interactions; Perrett and others 1989). Interestingly, for these neurons, hand movements alone miming the preferred action as well as objects alone with no hand movements elicited only a reduced discharge (Barraclough and others 2008). Even the combination of an appropriate hand movement and an object elicited a reduced neuronal discharge when the two were not physically interacting (Perrett and others 1989). Interestingly, the responses of a subset of the units selective for human actions were significantly modulated by the sound associated with that action (Barraclough and others 2005).
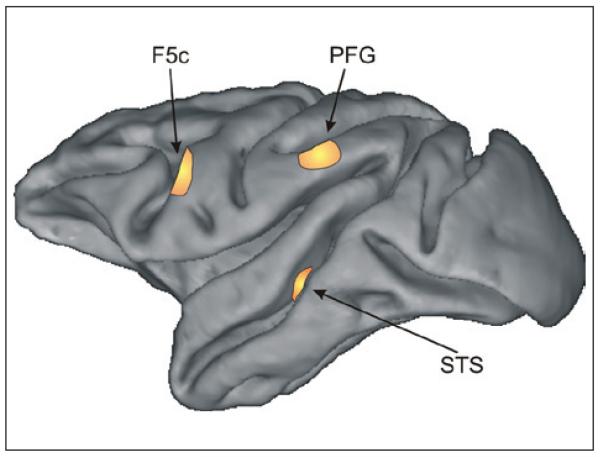
Figure 1.
Areas in the monkey brain involved in action perception. The figure shows a lateral view of the left hemisphere of a macaque brain. The three highlighted areas contain neurons that discharge during observation of bodily movements. More specifically, neurons in the superior temporal sulcus (STS) exhibit purely visual responses and do not respond during the execution of movements. Neurons responding both during the observation and execution of goal-directed movements (i.e., mirror neurons) were found in areas F5c and PFG.
Although the findings of Perrett and co-workers clearly suggest that purely visual processes strongly contribute to the analysis of complex bodily movements, the discovery of mirror neurons opened a new and interesting perspective on the issue of action perception and understanding. Mirror neurons were originally found in the convexity of the monkey premotor cortex (F5c in Fig. 1) (Gallese and others 1996; Rizzolatti and others 1996; di Pellegrino and others 1992) and later on also in the inferior parietal lobule (area PFG in Fig. 1) (Fogassi and others 2005; Gallese and others 2002; Rozzi and others 2008). They discharge both when the monkey performs a goal-directed motor act and when it observes another individual (human or monkey) performing the same or a similar action. Similar to visual neurons in the STS, hand movements alone, objects alone, or a spatially or temporally incongruent combination of the two produces a significantly lower or no degree of modulation. Figure 2 exemplifies the typical response pattern of a mirror neuron. The two panels in the right column show the responses of the same neuron while the monkey is grasping a small object (upper panel) or when the monkey is observing the experimenter grasping the same object (bottom panel). In both cases, the discharge of the neuron starts increasing shortly before the monkey’s or experimenter’s hand contacts the object (time t = 0 on the horizontal axis) and reaches its peak when the object is fully grasped.
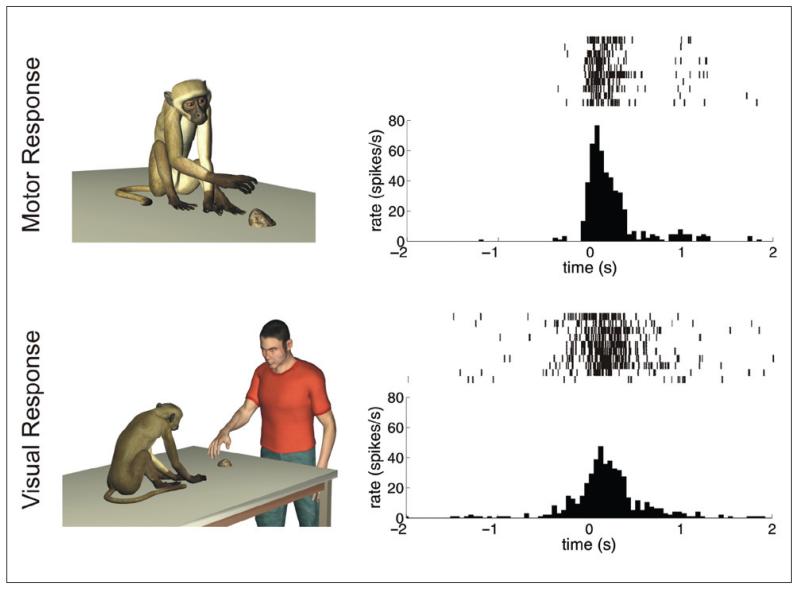
Figure 2.
Response properties of a mirror neuron. Each row in the left column schematically represents the experimental condition. The panels in the right column show the corresponding responses of the same neuron in the form of a raster plot (upper part) and peri-stimulus spike density (bottom part). (top row, right panel) Response of a neuron during active goal-directed motor acts of the monkey (e.g., grasping small objects of different shapes). (bottom row, right panel) Response of the same neuron during the observation of the same goal-directed motor acts performed by the experimenter. In the two raster plots, each vertical bar signifies the occurrence of an action potential, and different lines refer to different trials. In both figures, time t = 0 represents the moment of contact between the monkey’s (top panel) or experimenter’s (bottom panel) hand with the goal object.
The discovery of neurons discharging during both passive observation and active execution of goal-directed motor acts had a strong impact on theoretical accounts of action perception and understanding. In particular, it was considered a decisive piece of evidence in favor of a set of theories proposing that the functional coupling between perception and action was a crucial mechanism involved in higher cognitive function (Jeannerod 1997; Liberman and Mattingly 1985; Prinz 1997). These theories suggest that the high visual sensitivity of primates for the actions of conspecifics might be due, at least partially, to a functional linkage between the motor and the visual systems. In particular, according to the direct-matching hypothesis, primates understand actions when they map their visual representations onto their correspondent internal motor representations (Rizzolatti and others 2001). In other words, action perception and understanding are not only the result of abstract processes relying only on visual representations. On the contrary, the observer’s motor system is actively involved in this process, and the specific role of mirror neurons is to represent and possibly reenact actions in terms of their motor goal. An important theoretical consequence of this proposal is that action perception and action production are not two separate cognitive processes but, on the contrary, are tightly coupled.
Several reasons render the encoding of observed actions in mirror neurons qualitatively different from that performed by purely visual areas such as the STS. First, as originally proposed by Rizzolatti and co-workers (Rizzolatti and others 1996; Rizzolatti and others 2001), the presence of motor and visual responses in the same neuron might contribute to directly link the visual analysis of observed actions to the observer’s motor repertoire. That is, the external social world would become meaningful by referring it to an internal motor knowledge. Second, the motor system seems to be suitable for extracting the goal of actions as it has the intrinsic capacity to generalize. For example, contrary to neurons in the STS, many F5 mirror neurons visually respond to more than one type of action, thus allowing them to abstract the meaning of the observed action independent of the visual details of the action. Third, the motor-related responses of mirror neurons would endow the process of encoding the actions of others with a plasticity that is directly connected to the observer’s motor experience. This allows, for example, a direct transfer of the behavioral value of own actions, learned through the interaction with the environment, to the actions of others.
Response Properties of Mirror Neurons
A large percentage of the motor neurons in area F5c have mirror properties. A subset of them shows a strict correspondence of their responses during observation and execution of actions (Gallese and others 1996). Such neurons respond when the monkey performs a specific goal-directed motor act (e.g., a precision grip) and if the monkey sees the same motor act. More often, this correspondence is defined in terms of the general goal of the motor act rather than of the exact properties of the movement, and the responses of mirror neurons often show substantial invariance with respect to the orientation, position, and kinematics of the hand executing the action. It was originally reported that F5 mirror neurons do not respond if the same action (e.g., grasping) is performed with a tool. However, more recent results showed that after extensive visual exposure of the monkey to actions executed with a tool, a subset of mirror neurons in the ventral part of area F5c started responding also to this type of visual stimuli (Ferrari and others 2005). Further studies reported additional response properties of mirror neurons. For example, it was found that some F5 mirror neurons exhibit remarkable generalization properties. Notably, they responded in a similar manner to the same action executed with different effectors (e.g., grasping with the hand and grasping with the mouth) (Ferrari and others 2003; Rizzolatti and others 2001), to the sound associated with familiar actions (Kohler and others 2002), and even to partially occluded actions that can be inferred only from their initial motion path (Umiltà and others 2001).
A question posed by the discovery of F5 mirror neurons is why the observer does not move during action observation given the activation, in her or his premotor and parietal cortices, of the same neurons that discharge also during action execution. This issue is still debated (Rizzolatti and Luppino 2001), but a possible answer has been provided by a recent and elegant study by Kraskov and co-workers (2009). In this study, the authors first identified mirror neurons that project through the pyramidal tract, likely to the spinal cord, and then investigated their responses during action production and observation. Half of these pyramidal tract mirror neurons (PTMNs) vigorously responded during action production but exhibited a complete suppression of discharge during action observation. Kraskov and co-workers speculated that the suppression of PTMNs’ responses during action observation might serve the purpose of inhibiting, likely at the spinal cord level, self-movements during action observation (Kraskov and others 2009).
In addition to area F5c, neurons with mirror properties were also found in area PF/PFG of the inferior parietal lobule (IPL) (Gallese and others 2002). More recently, it has been shown that the visual responses of a subset of IPL mirror neurons during both the observation and execution of a complex grasping act (e.g., grasping to place or grasping to eat) are modulated by the final goal of the action (placing or eating) in which the grasping was embedded (Bonini and others 2010; Fogassi and others 2005). Figure 3 shows the typical response of one of these IPL mirror neurons. The unit discharged vigorously when the monkey grasped a piece of food to eat it, but it exhibited a significantly smaller response when the same piece of food was grasped to place it into a container. Interestingly, the same selectivity was found during action observation. That is, the neuron discharged while the monkey was observing the experimenter grasping a piece of food to bring it to the mouth, whereas it remained almost silent when the experimenter grasped the same piece of food to place it into a container. Fogassi and co-workers interpreted this pattern of response as evidence that the monkey’s prediction about the final goal influenced the neuronal discharges. For this reason, it was suggested that the possible specific cognitive role of IPL mirror neurons might be that of encoding the intentions of the actor (Fogassi and others 2005). More recent investigations have shown similar response properties also in F5 mirror neurons (Bonini and others 2010). Thus, the possible differences in the functional and cognitive roles of IPL and F5 mirror neurons, respectively, remain still to be investigated in depth.
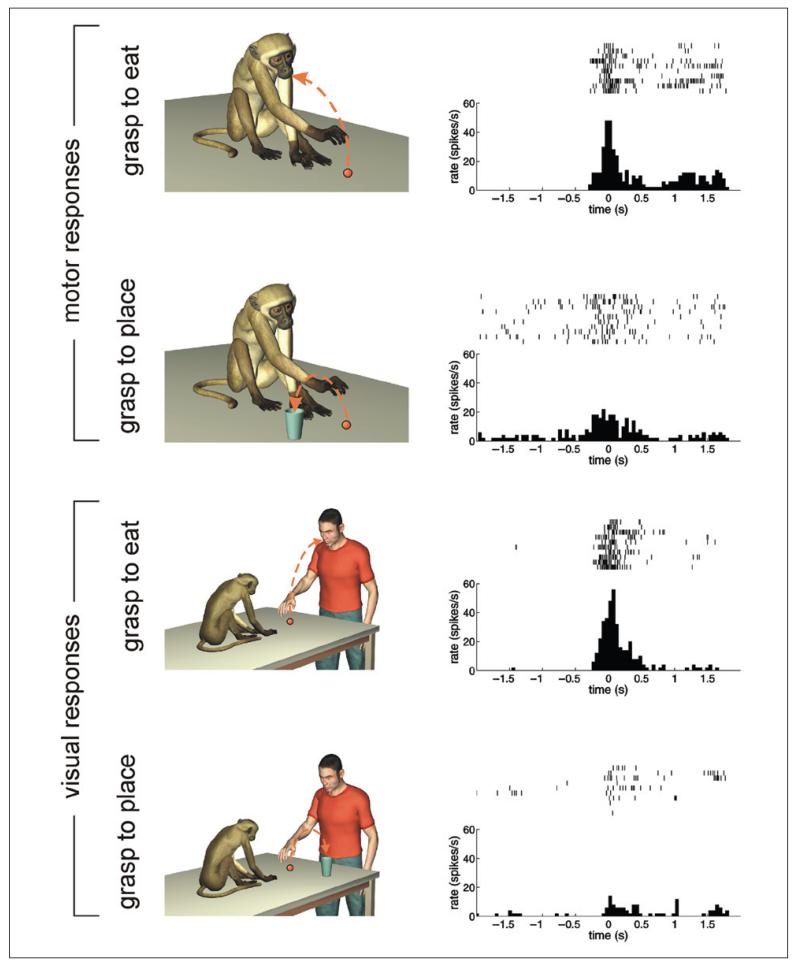
Figure 3.
Mirror neurons encoding the intention of the actor. The four rows show the motor (first two rows) and the visual (third and fourth rows) responses of the same neuron under different experimental conditions, which are exemplified in the left column. The first two rows show the motor responses of a neuron when the monkey grasped a piece of food to eat it (first row) or to place it into a container (second row). The third and fourth rows show the discharges of the same neuron when the monkey was observing the experimenter grasping a piece of food to bring it to the mouth (third row) or to place it into a container (fourth row). During both action execution and observation, the unit discharged selectively only when the piece of food was grasped to eat it. It did not respond when the same object was grasped to place it into a container.
All the pieces of experimental evidence reviewed so far are compatible with the view that mirror neurons integrate in their responses characteristics of observed actions that are directly related to describing them in terms of motor goals, such as the type of grip or the intention of the actor. However, two recent experiment studies suggest that mirror neurons integrate in their responses several additional pieces of information that are not directly related to the action per se.
The first study reported a new class of F5 mirror neurons, representing about 50% of all the tested mirror neurons, whose visual responses were modulated by the distance at which the observed motor acts were executed with respect to the monkey (Caggiano and others 2009). More specifically, the responses of about half of these mirror neurons were significantly modulated when the observed actions were executed in the peri-personal space of the monkey. The remaining half of the neuronal sample instead discharged more vigorously when the observed actions were executed in the extra-personal space of the monkey. Two examples of these spatially selective mirror neurons are shown in Figure 4. Unit 1 responded during the observation of a motor act only when it was executed in the peri-personal space of the monkey (represented by the yellow circle around the monkey’s body). Unit 2 exhibited a complementary response pattern. It discharged during action observation only when the action was executed in the extra-personal space of the monkey. From a functional perspective, the distance between observer and actor plays no role in understanding an observed action or representing its goal. However, it can play a decisive role in selecting possible subsequent behaviors, for example, interacting or approaching behaviors. Thus, this study suggests that the mirror neuron system might be part, together with other brain areas, of a system that contributes not only to understanding what others are doing but also in deciding the most appropriate behavioral response.
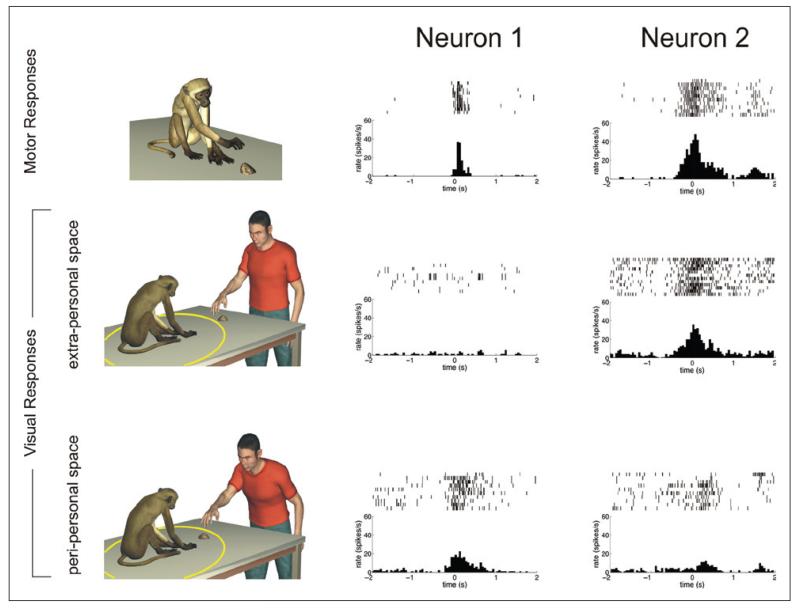
Figure 4.
Mirror neurons encoding the spatial position of an observed action. The three rows show the discharges of two neurons (neuron 1 and neuron 2) during the execution of goal-directed hand movements (top row) and during the observation of actions executed in the extra- (middle row) and peri-personal (bottom row) space of the monkey, respectively. The yellow circle represents the spatial extent of the peri-personal space of the monkey. Both neuron 1 and neuron 2 responded during the execution of goal-directed movements (top row). However, they exhibited opposite behaviors during the observation of actions performed by the experimenter in front of the monkeys. Neuron 1 discharged more strongly during the observation of an action executed in the extra-personal space of the monkey. On the contrary, neuron 2 exhibited a significantly stronger discharge when the observed action was executed in the peri-personal space of the monkey.
The second study reported that F5 mirror neurons seem to visually encode actions in a view-dependent manner (Caggiano and others 2007; Caggiano and others 2010). In this study, the visual responses of mirror neurons were studied by presenting the same actions as seen from different points of view, including also the actor’s own point of view. The discharges of mirror neurons were modulated not only by the action being observed but also by the point of view under which it was observed (e.g., a frontal or a side view). Interestingly, a consistent percentage of mirror neurons visually responded not only during the observation of the actions of another individual but also during the observation of the visual representations of the actions seen in a first-person view. This previously unreported response characteristic suggests that, similar to other neurons in premotor areas (Raos and others 2004), they could be visually activated not only by the actions of others but also by self-generated actions.
The modulations of mirror neuron discharges by the spatial position of the observed action or the point of view suggest an extension of their possible functional role in two important directions.
First, although early experimental results clearly support the view that mirror neurons integrate in their responses characteristics of observed actions that are instrumental to encode them in terms of their motor goal (Gallese and others 1996; Kohler and others 2002; Umiltà and others 2001), the two recent studies described above suggest that mirror neurons integrate in their responses several additional aspects of observed actions. The distance and point of view are two additional characteristics that are integrated in their responses, and possibly others are yet undiscovered. The advantage of this additional information integrated in the responses of mirror neurons is that of representing the actions of others in terms of features that are, for the observer, potentially relevant to plan and evaluate subsequent behaviors.
The second extension to the original proposal of the possible functional role of mirror neurons is that their visual responses might not be restricted to representing the actions of others. As discussed above, recent experimental results demonstrate the existence of a subclass of mirror neurons that discharge when an action was observed from the actor’s point of view. We believe that this finding is relevant for two important implications at ontogenetic and phylogenetic levels. The first implication is related to the possible emergence of mirror neurons during development and will be presented in the next section. The second implication concerns the possible evolution of the mirror neuron system. Philogenetically, the mirror neuron system might have evolved to map monkeys’ actions onto own motor representations possibly to provide feedback for visually directed graspings, as suggested by Arbib and co-workers (Bonaiuto and Arbib 2010; Oztop and Arbib 2002). According to this proposal, the same system was, later in evolution, “exapted,” through a generalization process, to interpret the goal-directed behaviors of others for social interaction and communication purposes. At which step, during evolution, this system possibly evolved is still completely unknown. If one looks at other primates’ cortical organization, it is clear that parieto-premotor connections were probably present already in early mammals such as tree shrews and galago (Kaas 2004). Thus, the appropriate pattern of connectivity for the possible emergence of mirror neurons was already present in common ancestors of the living primates. Further comparative experiments are needed to investigate the possible involvement of mirror neurons in the monitoring and refinement of one’s own hand manipulation behaviors within the primate lineage. If such involvement is confirmed by experimental data, it will open up an additional and unexpected perspective on their possible functional role.
A Developmental Perspective on the Mirror Neuron System
In this section, we review several pieces of behavioral evidence supporting the notion of a tight link between perception and action and examine them in relation to the broader role of the mirror neuron system proposed in the previous section. Two novel aspects differentiate the present from previous reviews. First, we focus on the developmental aspects of the putative link between perception and action and thus sort experimental results with respect to the age of the subjects. Second, in the cases where data are available in the literature, we compare behavioral and neurophysiological/brain imaging results in the two species in which a mirror neuron system has been so far reported: humans and monkeys.
An important issue concerning the human and monkey mirror neuron system is how it initially emerges during development. A conclusive answer to this question can be provided only by performing neurophysiological experiments in infant monkeys. However, these types of experiments are methodologically very challenging and have not been attempted so far. Thus, the initial developmental stages of the mirror neuron system can only be indirectly inferred from behavioral results (see Box 1), brain imaging techniques, and electroencephalography (EEG) in both human and monkey infants, suggesting the presence of perception-action couplings already at early developmental stages. In this review, we focus on two different aspects of sensorimotor development: the spontaneous facial imitative abilities exhibited by human and monkey infants and the coordinated development of manual motor skills and action understanding capabilities. The choice of separately dealing with orofacial and hand movements, respectively, is motivated by two reasons. First, facial and hand mirror neurons emerge following two different but interacting developmental trajectories. Second, they pose different challenges to two fundamental aspects of the direct matching hypothesis: how observed movements are mapped onto the observer’s motor repertoire and how cells with such complex response properties such as mirror neurons can initially emerge.
Box 1. Investigating Action Perception and Understanding in Infants.
The problem that investigators face in studying the capacity to perceive and possibly understand actions at a very early stage of development is that infants can neither verbally report their perceptions nor can they be instructed to perform complex tasks. For these reasons, developmental scientists resorted to two nonverbal and implicit measures of perceptual capabilities: imitation and visual habituation. In studies of imitation capabilities, a biological movement is first presented to the infants. Their behavioral responses are then carefully scrutinized to detect possible differences with respect to baseline conditions. Differences in the rate of performance of movements are assumed to be correlated with internal processing of observed actions. This valuable experimental tool can also be used to investigate action understanding in human adults and monkeys. Visual habituation is based on the observation that infants tend to fixate visual stimuli that are novel to them (Woodward 1998). In this paradigm, infants are first habituated to a visual stimulus that can be described along two dimensions. They are then presented with two test events, each one varying along only one of the two dimensions. Longer fixation times to one of the two test events indicate which variation was surprising or unexpected for the infants and thus violates the infant’s representation of the action.
Facial Movements
Humans
In primates, the basic mechanisms for sensorimotor coordination develop in uterine life (Kurjak and others 2004). A number of studies report a surprising capacity of voluntary movements and coordinated actions in the fetus (Myowa-Yamakoshi and Takeshita 2006). Yet, at birth, the nervous system is still immature, and most of the neuronal circuitry for perceiving and acting upon the world is refined in the first months of life, possibly in a hierarchical manner (Guillery 2005). Functionally, the first systems that are refined are those associated with eye and mouth movements. Although some of these movements could be simply due to basic neonatal reflexes, others appear to involve more complex coordinated intentional movements not yet smoothly arranged. For example, already by 82 hours of life, neonates control sucking behaviors by using the same prospective principles of adult reaching behaviors (Craig and Lee 1999). This early development of oral and facial control is matched by the infants’ capacity to imitate mouth movements and facial expressions. Indeed, in a series of famous and debated experiments, Meltzoff and Moore (1977, 1983, 1989) reported that even 72-hour-old infants spontaneously reproduce facial and hand movements (for a critical review of these results, see Anisfeld 1991, 1996; Anisfeld and others 1979; Kaitz and others 1988). In their original experiment, Meltzoff and Moore (1977) presented infants between 12 and 21 days of age with three facial movements: tongue protrusion, mouth opening, and lip protrusion. They reported that in the time immediately following the presentation of each movement, the rate of performance of that movement by the infants increased with respect to the rate of the other movements (Meltzoff and Moore 1977). These results were further extended by Field and co-workers, who showed that human infants as young as 36 hours spontaneously imitate basic emotional facial expressions such as happy, sad, and surprised (Field and others 1982). These imitative abilities appear to be cross-modal. Chen and co-workers found that already by three days of age, human newborns match their mouth movements to those appropriate to producing a model sound just heard (Chen and others 2004). A puzzling result is that facial imitation in neonates starts decreasing at two months of age and is virtually absent by six months of age (Abravanel and Sigafoos 1984; Fontaine 1984).
Monkeys
The phenomenon of neonatal facial imitation is not confined to humans. For example, Myowa (1996) tested the imitative capabilities of a single chimpanzee in a longitudinal study that embraced the developmental period from 5 to 15 weeks of age. By means of a paradigm similar to that of Meltzoff and Moore, she showed that the subject of the experiment exhibited imitation capabilities between 5 and 11 weeks of life. The observation that chimpanzees can imitate facial movements was subsequently confirmed by more extended investigations (Bard 2007). More surprising is the fact that also rhesus monkeys imitate two basic facial gestures: tongue protrusion and lip smacking (Ferrari and others 2006). Furthermore, in the macaque, facial imitation capabilities in the first days of life are predictive of the development of voluntary motor skills in the first year of life (Ferrari and others 2009). Interestingly, similar to human studies, the imitative capabilities of both chimpanzee and macaques seem to be confined only to early developmental stages. More specifically, they largely disappear after day 7 in the macaque and 2 months in the chimpanzee (Bard 2007; Ferrari and others 2006; Myowa 1996).
It has been recently hypothesized that the phenomenon of neonatal facial imitation in both humans and monkeys can rely on a rudimentary mirror mechanism present at birth and capable of matching facial features with an internal motor representation (Ferrari and others 2006). However, in both humans and monkeys, neonatal imitation typically involves effectors (tongue and mouth) that the neonate cannot visually access. Thus, the main question is, how does the infant translate the observed movement into a correspondent motor program without a direct visual experience of the own face? One possible explanation might be that both face processing and the mirror neuron system, or at least the part involved in facial movements, rely on a brain network that is present already at birth and whose basic elements are probably genetically predetermined (Ferrari and others 2006; Johnson 2005; Lepage and Théoret 2007; Park and others 2009; Pascalis and Kelly 2009). In other words, part of the visual information related to facial movements appears to be matched already at birth with the corresponding motor representation. This proposal is supported by two recent developmental studies. In the first study, Sugita (2008) tested the face-processing abilities of macaque monkeys reared with no exposure to face stimuli. He found that even deprivation periods of up to two years did not interfere with the recognition and discrimination performance of face stimuli. These results strongly suggest that the basic mechanisms of face processing in the macaques, and thus also possibly in humans, are, at least partially, experience independent and possibly prewired. In a second preliminary study, Ferrari and co-workers reported that the mu rhythm was suppressed in newborn (1-7 days old) monkeys when they observed and imitated facial gestures but not during the observation of nonbiological movements (Ferrari and others 2008). The mu rhythm is an EEG oscillation with dominant frequencies in the 8 to 13 band in humans (probably in lower frequencies in 6- to 12-month-old infants), and it is considered the result of sensorimotor processes linking perception and action (Pineda 2005). Thus, the results by Ferrari and co-workers were interpreted as a signature of the activation of a mirror neuron system during the perception and later imitation of facial movements already in the first days of life.
Taken together, the experimental results reviewed in this section show that rudimentary mechanisms for perceiving simple facial movements of others, such as lip smacking or tongue protrusion, and the capacity to map them onto the observer’s motor repertoire might be prewired and already present at birth. These mechanisms might be instrumental for survival in the first period after birth because facial imitative responses might play a fundamental role in establishing and subsequently sustaining an affiliative relation with the caregiver (Ferrari and others 2006). This system can be also subjected to modification through a feedback that the infant receives from the social environment. The importance of early face-to-face exchanges in humans (Stern 1985; Trevarthen and Aitken 2001) as well as in macaques (Ferrari and others 2009) illustrates that, starting from a precocious, probably innate, social competence, the infant’s capacity to control and solicit facial gestures is critically dependent on the type of social environment feedback he or she receives (usually mainly from the mother).
Hand Movements
Contrary to facial movements, the development of reaching-grasping movements involves a long period of maturation and requires many levels of sensorimotor integration. Indeed, the muscular-skeletal apparatus is a very complex system that needs to be properly controlled in order for well-coordinated reaching and grasping movements to emerge (Bernstein 1967). It has been shown that the capacity to display skilled hand movements is highly dependent on the maturation of the corticospinal tract, which is involved in the control of arm movements (Galea and Darian-Smith 1995; Lemon 1999). In both monkeys and humans, the myelination of the corticospinal axons has a similar time course and is completed between the second and third year of life (ten Donkelaar and others 2004; Olivier and others 1997).
Beyond the maturation of the corticospinal tract, the development and refinement of a successful control strategy for visually guided reaching movements needs also the execution of appropriate exploratory behaviors (von Hofsten 2004). These self-generated exploratory behaviors (“body babbling”; see Meltzoff and Moore 1997) serve likely both motor and cognitive purposes. The motor purpose likely consists of the integration of different functional motor circuits and their sensory consequences (e.g., gaze behavior with arm movements and proprioception). The possible cognitive purpose is the development of an internal representation of the space around the infant. The fact that this important cognitive development requires concurrent and coordinated visual and motor experience was demonstrated by Held and Bauer (1967). In their experiments, they dissociated vision and proprioception by preventing newborn macaques from seeing their own arms at birth. When tested at 35 days of age, the subjects exhibited severe impairments in visually guided reaching and grasping despite their preserved capacity to grasp under tactile guidance (Held and Bauer 1967). This result strongly suggests that the correct development of spatial maps of the peri-personal space for goal-directed hand movements entails the observation of own movements. When only proprioceptive information is available in the first stages of perinatal life, as in Held and Bauer’s experiments, severe impairments of visually guided goal-directed movements follow. Experimental data seem to suggest that also in humans, self-observation of own movement might represent an important developmental step. For example, two- to three-month-old infants spend a considerable fraction of time watching their own hands (White and others 1964). Furthermore, studies in neonates show that their spontaneous arm movements are not reflex-like and unintentional. Rather, they are purposeful and likely contribute to maintain the observed hand in their field of view (Meer and others 1995). Notably, the fact that the observation of own movements serves cognitive and not only eye-hand coordination purposes is further suggested by studies showing that congenitally blind subjects exhibit substantial differences in the multisensory representation of their peri-personal space with respect to normally sighted controls (Bremner and others 2008; Collignon and others 2009; Röder and others 2004).
In agreement with the direct-matching hypothesis, several experimental results indicate that the initial development of several perceptual abilities depends on or is triggered by the acquisition of specific motor skills. In an early study, Lederman and Klatzky (1987) suggested that manipulation behaviors can be classified based on the degree of knowledge that they afford about a manipulated object. For example, simple clutching of handheld objects tightly in the fist is appropriate for perceiving the temperature and size of an object, whereas more complex, possibly bimanual, manipulation behaviors are needed to perceive its hardness, texture, weight, or the fine details of its shape. Interestingly, by means of a meta-analysis of results in the field of human development, Bushnell and Boudreau (1993) showed that the acquisition of the different degrees of dexterous manipulation described by Lederman and Klatzky (1987) co-occurred with the acquisition of the perceptual ability to judge the corresponding properties of the manipulated object. An elegant experiment by Sommerville and co-workers (2005) clearly indicates that the improvements in motor and perceptual abilities are not merely co-occurring in time but are instead causally correlated. At three months of age, human infants are not yet able to grasp objects. Interestingly, they are also not visually sensitive to the goal structure of reaching and grasping movements performed by others. For example, they cannot distinguish between movements with different final goals and identical paths and movements with identical final goals and different paths. In their experiments, Sommerville and co-workers augmented the reaching and grasping capabilities of three-month-old infants by means of Velcro mittens that allowed them to grasp an object simply by reaching and touching it. The notable outcome of this manipulation of their motor capabilities was an improvement in their perceptual capabilities. Indeed, after motor practice with the Velcro mittens, three-month-old infants developed a visual sensitivity to the goal-directed structure of others’ actions. Further experiments showed that such causal link between motor and perceptual development is present also for more complex actions sequences (Sommerville and Woodward 2005) or actions performed with tools (Sommerville and others 2008). Furthermore, the acquisition of novel motor skills improves not only the understanding of similar motor acts but also the online prediction of their unfolding in time. For example, when observing a block-stacking task, adults make proactive eye movements similar to those they perform when executing the same task. This predictive oculomotor behavior has been considered a signature of mapping the observed actions onto the observer’s motor repertoire (Flanagan and Johansson 2003). Falck-Ytter and co-workers (2006) reasoned that if this was the case, then the development of proactive eye movements should be dependent on action development. They thus measured the eye movements of 6- and 12-month-old infants while they were observing grasping-to-place movements, which human infants master around 7 to 9 months of life (Bruner 1970). In agreement with the hypothesis that the observer’s motor repertoire plays a role in predicting the goal of observed actions, they found that 12-month-old but not 6-month-old infants performed proactive eye movements in the observation condition (Falck-Ytter and others 2006).
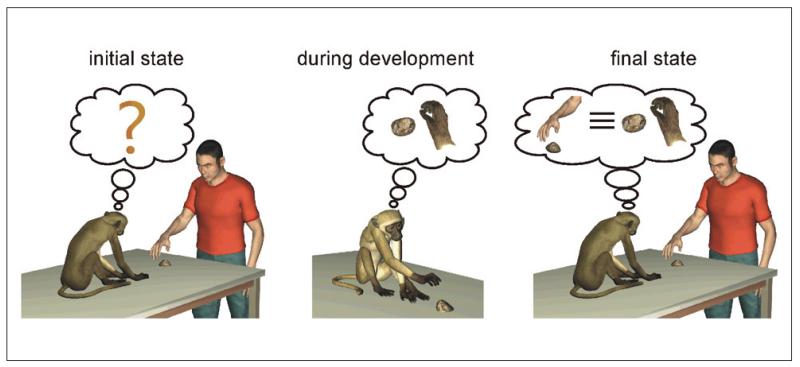
A critical point concerning hand mirror neurons is how a system with such complex response properties can initially emerge and later refine during development. The experimental evidence reviewed in this article can help us in formulating some hypotheses. One point that clearly emerges from several of the reviewed experiments is that at early stages of development, both humans and monkeys engage very often in the observation of own movements. Furthermore, as shown by Held and Bauer’s (1967) experiment, the early absence of visual feedback during active movements produces sensorimotor rather than purely motor deficits. It is thus reasonable to assume that the process of visual monitoring of own movements might play an important role also in the emergence of mirror neurons that discharge during the execution and observation of movements (Del Giudice and others 2009; Heyes 2001; Keysers and Perett 2004). The feasibility of this proposal is not only corroborated by modeling studies (Metta and others 2006; Oztop and Arbib 2002) but is also in agreement with the view-dependent responses of mirror neurons described in the previous section. In particular, if we assume that the process of associating visual with motor representations of actions in mirror neurons is mediated by Hebbian-like processes (Keysers and Perett 2004), then the result that many units are visually responding to own actions appears no longer paradoxical. On the contrary, it would become a direct consequence of the fact that the response properties of mirror neurons emerge during the observation of own movements from a subjective point of view. This process is schematically illustrated in Figure 5. At the beginning of development, infants are very likely endowed with some rudimentary capacity to code action goals, as also revealed by ultrasound investigations on fetuses and on the patterns of movement-directed behaviors described in the first weeks after birth. In this scenario, the motor system probably plays a key role in driving sensory information through the anatomical connections that are building in the developing brain. However, the visual input reaching the motor areas is probably not yet tight and committed to specific motor outputs. As a consequence, the mapping between the observed motor act and its internal motor representation, although present, is probably only broadly defined (left panel in Fig. 5). During development, the repeated synchronous coactivation of motor and visual inputs during the observation of own actions leads to the stabilization of the connectivity pattern and contributes to committing a neuron toward a specific type of visuomotor coupling (middle panel in Fig. 5). As indicated by the correlated development of motor and perceptual abilities, the visual responses to own movements are then generalized to the actions of others, which can be seen under a multiplicity of points of view (right panel in Fig. 5). Further experiments are needed to quantitatively investigate the cognitive and neuronal mechanisms underlying this generalization. Here we can offer two nonmutually exclusive speculations. The first speculation is that the process of visual generalization between own and others’ actions is driven by the observation of own hands under different points of view. The second speculation is that this process might depend on co-occurring and coordinated grasping activity (joint actions) between the infant and the adults. Indeed, during these coordinated activities, the infant would observe the hands of others under different points of view together with her or his own hand from a subjective point of view.
Figure 5.
Exemplification of the proposed mechanism for the initial emergence of mirror neurons. At the beginning of development, there is in the infant (human or monkey) no clear mapping between the observed motor act and its internal motor representation (left panel). During development, the repeated synchronous coactivation of motor and visual representations during observation of own movements leads to the emergence of neurons that exhibit a visuomotor coupling between a specific action and its visual representation from an egocentric point of view (middle panel). These visual representations are then generalized to the actions of others, which can be seen under a multiplicity of points of view (right panel).
Differently from hand mirror neurons, mouth mirror neurons are probably subjected to a different developmental trajectory. This hypothesis is supported not only by the developmental and behavioral data described so far but also by functional and anatomical data suggesting that mouth and hand mirror neurons, although interconnected, could be constructed by exploiting different brain networks. In humans, the observation of mouth actions activated a more lateral sector of the premotor cortex than when subjects observed hand actions (Buccino and others 2001). This observation is confirmed by neurophysiological studies in monkeys showing that hand and mouth mirror neurons are found in two different sectors of F5. More precisely, the medial part of F5 contains only hand mirror neurons, whereas the most lateral sector of the F5 convexity contains also mouth mirror neurons (Ferrari and others 2003). Monkey anatomical studies might help in clarifying whether the functional difference between the medial and lateral sectors of area F5 might be due to distinct patterns of connectivity with cortical and subcortical areas. In particular, in comparison with its medial sector, the lateral sector of F5 has significant connections with the facial nucleus (Morecraft and others 2001), the insular and anterior cingulate cortices (Barbas and Pandya 1987; Matelli and others 1986), and the amygdala. Taken together, these anatomical findings suggest that mouth mirror neurons could be involved in anatomo-functional circuits that, differently from hand mirror neurons, are linked to visceromotor sensations and emotional processing related to both ingestive behaviors and face processing. Further anatomo-functional experiments are needed to test this hypothesis.
Conclusions
Mirror neurons are units in the monkey frontal and parietal cortices that discharge during both passive observation and active execution of goal-directed motor acts. In this article, we tackled two still debated questions connected with their intriguing response properties: what is their possible functional role and how can they initially emerge during development, respectively.
To provide a possible answer to the first question, we reviewed current experimental results about the response properties of mirror neurons in the monkey. The picture that emerged is that it seems that there exist many classes of mirror neurons, with each class influenced by a different aspect of an observed action (e.g., type of the observed motor act, distance of the observer from the observed action, point of view from which the action is observed). This observation motivated an extension to the current proposal about their possible functional role. More specifically, we proposed that mirror neurons not only encode the goal of an observed motor act but might represent a flexible system that integrates several of its behaviorally relevant characteristics. Such an encoding mechanism has, among others, two important characteristics. First, as originally proposed by Rizzolatti and co-workers, the projection of an observed action onto the observer’s motor repertoire gives an individual an immediate representation of it “from the inside” and an unambiguous understanding of its meaning (Rizzolatti and others 1996; Rizzolatti and others 2001; Rizzolatti and Sinigaglia 2010). Second, the proposed integration is experience dependent and thus also possibly subject dependent. Indeed, the subclasses of mirror neurons integrating in their responses different characteristics of observed actions are likely better specified through sensorimotor learning. This translates to the fact that the encoding of actions at the level of the single subject will depend on her or his sensorimotor history (Calvo-Merino and others 2006; Casile and Giese 2006; Reithler and others 2007). Thus, two observers can and likely will have internal representations of the same observed action at different levels of detail.
In the second part of the review, we speculated about possible developmental mechanisms that can account for the initial emergence of units with such complex motor and visual responses, such as those exhibited by mirror neurons. For this purpose, we reviewed and compared in humans and monkeys two different aspects of sensorimotor development: facial and hand movements. We proposed that possibly two different “mirror” systems might underlie the development of cognitive and imitative abilities in the two cases. More specifically, a possibly prewired system already present at birth might underlie the early development of facial imitative abilities in newborns. This system is, however, subjected to modification through social experience. In fact, it is likely that social factors provide an important feedback to the infant about the meaning of her or his own facial movements. The caregiver, who in primates’ early life is typically the mother, through co-occurrence (i.e., imitation and body synchronization) and turn-taking behaviors might actively shape and nurture the infant capacity to match the own gestures with that of others (see also Del Giudice and others 2009; Trevarthen and Aitken 2001). On the contrary, a different experience-dependent system might subserve perceptionaction couplings in the case of hand movements. In this latter case, from a review of the literature, it emerged that, during infancy, (1) the development of specific motor abilities is causally connected to the development of the correspondent perceptual abilities starting from an internal representation of goals and from broadly defined sensorimotor mapping, and (2) observation of own movements represents an important developmental step. Motivated by these pieces of evidence, we proposed that the mirror neuron system, putatively at the basis of perception-action couplings, might initially emerge and be refined following a process of Hebbian learning during the observation of own movements. This idea in itself is not new and was already suggested by other researchers (Del Giudice and others 2009; Heyes 2010; Oztop and Arbib 2002). However, the neurophysiological results reviewed here provide evidence for the conceptual and simulation models presented in the literature. In particular, the intriguing finding that a subclass of mirror neurons responds to the visual representations of own actions (Caggiano and others 2007; Caggiano and others 2010) is compatible with a Hebbian account of their initial emergence.
In our opinion, the discovery of mirror neurons represents one of the most exciting developments of modern cognitive neuroscience. Despite almost two decades of intense studies, two important questions remain still largely unanswered: what is their possible functional and cognitive role and if and how do their response properties emerge as a consequence of an associative learning process. Although in the present review, we speculated about possible answers to these questions, only sound and carefully conducted experiments can provide a final answer. Yet, the fact that such important points are still open renders mirror neurons and, more generally, perceptionaction couplings a very interesting and flourishing field of research.
Acknowledgment
We thank Luca Bonini for many stimulating discussions and for valuable help in preparing Figure 3.
Financial Disclosure/Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by the Deutsche Forschungsgemeinschaft (grant SFB 550-C10), the Hermann and Lilly Schilling Foundation, the National Institute of Health (grant 1-P01-HD064653-01), and the Ministero Italiano per l’Università e la Ricerca (grants 2004057380 and 2006052343).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- Abravanel E, Sigafoos AD. Exploring the presence of mitation during early infancy. Child Dev. 1984;55(2):381–92. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/6723442. [PubMed] [Google Scholar]
- Aggarwal JK, Cai Q. Human motion analysis: a review. Comput Vision Image Understanding. 1999;73:428–40. [Google Scholar]
- Anisfeld M. Neonatal imitation. Dev Rev. 1991;11:60–97. [Google Scholar]
- Anisfeld M. Only tongue protrusion modeling is matched by neonates. Dev Rev 16. 1996;2:149–61. [Google Scholar]
- Anisfeld M, Masters JC, Jacobson SW, Kagan J, Meltzoff AN, Moore MK. Interpreting “imitative” responses in early infancy. Science. 1979;205:214–9. doi: 10.1126/science.451593. Retrieved from: http://images2.wikia.nocookie.net/imitation/images/9/9f/Anisfeld_et_al_1979.pdf. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256(2):211–28. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Bard KA. Neonatal imitation in chimpanzees (Pan troglodytes) tested with two paradigms. Anim Cogn. 2007;10(2):233–42. doi: 10.1007/s10071-006-0062-3. [DOI] [PubMed] [Google Scholar]
- Barraclough NE, Keith RH, Xiao D, Oram MW, Perrett DI. Visual adaptation to goal-directed hand actions. J Cogn Neurosci. 2008;21(9):1805–19. doi: 10.1162/jocn.2008.21145. [DOI] [PubMed] [Google Scholar]
- Barraclough NE, Xiao D, Baker CI, Oram MW, Perrett DI. Integration of visual and auditory information by superior temporal sulcus neurons responsive to the sight of actions. J Cogn Neurosci. 2005;17(3):377–91. doi: 10.1162/0898929053279586. [DOI] [PubMed] [Google Scholar]
- Barraclough NE, Xiao D, Oram MW, Perrett DI. The sensitivity of primate STS neurons to walking sequences and to the degree of articulation in static images. Prog Brain Res. 2006;154:135–48. doi: 10.1016/S0079-6123(06)54007-5. [DOI] [PubMed] [Google Scholar]
- Bernstein N. The co-ordination and regulation of movements. Pergamon; London: 1967. [Google Scholar]
- Bonaiuto J, Arbib MA. Extending the mirror neuron system model, II: what did I just do? A new role for mirror neurons. Biol Cybern. 2010;102(4):341–59. doi: 10.1007/s00422-010-0371-0. [DOI] [PubMed] [Google Scholar]
- Bonini L, Rozzi S, Serventi F, Simone L, Ferrari PF, Fogassi L. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb Cortex. 2010;20(6):1372–85. doi: 10.1093/cercor/bhp200. [DOI] [PubMed] [Google Scholar]
- Bremner AJ, Mareschal D, Lloyd-Fox S, Spence C. Spatial localization of touch in the first year of life: early influence of a visual spatial code and the development of remapping across changes in limb position. J Exp Psychol Gen. 2008;137(1):149–62. doi: 10.1037/0096-3445.137.1.149. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 1981;46:369–84. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- Bruner JS. In: Mechanisms of motor skill development. Connolly KJ, editor. Academic Press; London: 1970. pp. 63–91. [No title] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13(2):400–4. Retrieved from: http://www.ingentaconnect.com/content/bsc/ejn/2001/00000013/00000002/art00020. [PubMed] [Google Scholar]
- Bushnell EW, Boudreau JP. Motor development and the mind: the potential role of motor abilities as a determinant of aspects of perceptual development. Child Dev. 1993;64(4):1005–21. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/8404253. [PubMed] [Google Scholar]
- Caggiano V, Fogassi L, Giese MA, Rizzolatti G, Thier P, Casile A. Neurons in monkey pre-motor cortex (area F5) responding to filmed actions. Perception. 2007;39:73–4. [Google Scholar]
- Caggiano V, Fogassi L, Rizzolatti G, Thier P, Casile A. Mirror neurons differentially encode the peripersonal and extrapersonal space of monkeys. Science. 2009;324(5925):403–6. doi: 10.1126/science.1166818. [DOI] [PubMed] [Google Scholar]
- Caggiano V, Fogassi L, Rizzolatti G, Thier P, Giese MA, Casile A. View-based encoding of actions in mirror neurons in area F5 in macaque premotor cortex. Curr Biol. 2011 doi: 10.1016/j.cub.2010.12.022. Forthcoming. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol. 2006;16(19):1905–10. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Casile A, Giese MA. Nonvisual motor training influences biological motion perception. Curr Biol. 2006;16(1):69–74. doi: 10.1016/j.cub.2005.10.071. [DOI] [PubMed] [Google Scholar]
- Chen X, Striano T, Rakoczy H. Auditory-oral matching behavior in newborns. Dev Sci. 2004;7(1):42–7. doi: 10.1111/j.1467-7687.2004.00321.x. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/15323117. [DOI] [PubMed] [Google Scholar]
- Collignon O, Charbonneau G, Lassonde M, Lepore F. Early visual deprivation alters multisensory processing in peripersonal space. Neuropsychologia. 2009;47(14):3236–43. doi: 10.1016/j.neuropsychologia.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Craig CM, Lee DN. Neonatal control of nutritive sucking pressure: evidence for an intrinsic tau-guide. Exp Brain Res. 1999;124(3):371–82. doi: 10.1007/s002210050634. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/9989443. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Manera V, Keysers C. Programmed to learn? The ontogeny of mirror neurons. Dev Sci. 2009;12(2):350–63. doi: 10.1111/j.1467-7687.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91(1):176–80. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter T, Gredebäck G, von Hofsten C. Infants predict other people’s action goals. Nat Neurosci. 2006;9(7):878–9. doi: 10.1038/nn1729. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur J Neurosci. 2003;17(8):1703–14. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Paukner A, Ruggiero A, Darcey L, Unbehagen S, Suomi SJ. Interindividual differences in neonatal imitation and the development of action chains in rhesus macaques. Child Dev. 2009;80(4):1057–68. doi: 10.1111/j.1467-8624.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J Cogn Neurosci. 2005;17(2):212–26. doi: 10.1162/0898929053124910. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Vanderwert R, Herman K, Paukner A, Fox NA, Suomi SJ. 2008 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2008. EEG activity in response to facial gestures in 1-7 days old infant rhesus macaques. [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi S. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4(9):1501–8. doi: 10.1371/journal.pbio.0040302. Retrieved from: http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field TM, Woodson R, Greenberg R, Cohen D. Discrimination and imitation of facial expressions by neonates. Science. 1982;218:179–81. doi: 10.1126/science.7123230. Retrieved from: http://books.google.com/books?hl=en&lr=&id=VcryqelKfncC&oi=fnd&pg=PA119&dq=discrimination+and+imitation+of+facial+expressions+by+neonates&ots=5tuQAF-y4v&sig=jOhdN3EvUw8V-XnMzsXBOQdyIOZE. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Johansson RS. Action plans used in action observation. Nature. 2003;424(6950):769–71. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–7. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fontaine R. Imitative skills between birth and six months. Infant Behav Dev. 1984;7(1977):323–33. Retrieved from: http://doi.apa.org/?uid=1985-09178-001. [Google Scholar]
- Galea MP, Darian-Smith I. Postnatal maturation of the direct corticospinal projections in the macaque monkey. Cereb Cortex. 1995;5(6):518–40. doi: 10.1093/cercor/5.6.518. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/8590825. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(2):593. doi: 10.1093/brain/119.2.593. Retrieved from: http://brain.oxfordjournals.org/cgi/content/abstract/119/2/593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action representation and the inferior parietal lobule. In: Prinz W, Hommel B, editors. Common mechanisms in perception and action: attention and performance. XIX. Oxford University Press; Oxford, UK: 2002. pp. 247–66. [Google Scholar]
- Guillery RW. Is postnatal neocortical maturation hierarchical? Trends Neurosci. 2005;28(10):512–7. doi: 10.1016/j.tins.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Held R, Bauer JJ. Visually guided reaching in infant monkeys after restricted rearing. Science. 1967;155:718–20. doi: 10.1126/science.155.3763.718. [DOI] [PubMed] [Google Scholar]
- Heyes C. Causes and consequences of imitation. Trends Cogn Sci. 2001;5(6):253–61. doi: 10.1016/s1364-6613(00)01661-2. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/11390296. [DOI] [PubMed] [Google Scholar]
- Heyes C. Where do mirror neurons come from? Neurosci Biobehav Rev. 2010;34(4):575–83. doi: 10.1016/j.neubiorev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The cognitive neuroscience of action. Blackwell; Malden, MA: 1997. [Google Scholar]
- Johnson MH. Subcortical face processing. Nat Rev Neurosci. 2005;6(10):766–74. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Record A. 2004;281(1):1148–56. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- Kaitz M, Meschulach-Sarfaty O, Auerbach J, Eidelman A. A reexamination of newborns’ ability to imitate facial expressions. Dev Psychol. 1988;24(1):3–7. [Google Scholar]
- Keysers C, Perett DI. Demystifying social cognition: a Hebbian perspective. Trends Cogn Sci. 2004;8(11):501–7. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umiltà MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 2002;297(5582):846–8. doi: 10.1126/science.1070311. Retrieved from: http://www.sciencemag.org/cgi/content/abstract/297/5582/846. [DOI] [PubMed] [Google Scholar]
- Kraskov A, Dancause N, Quallo MM, Shepherd S, Lemon RN. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron. 2009;64(6):922–30. doi: 10.1016/j.neuron.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjak A, Stanojevic M, Andonotopo W, Salihagic-Kadic A, Carrera JM, Azumendi G. Behavioral pattern continuity from prenatal to postnatal life: a study by four-dimensional (4D) ultrasonography. J Perinat Med. 2004;32(4):346–53. doi: 10.1515/JPM.2004.065. [DOI] [PubMed] [Google Scholar]
- Lederman SJ, Klatzky RL. Hand movements: a window into haptic object recognition. Cogn Psychol. 1987;19(3):342–68. doi: 10.1016/0010-0285(87)90008-9. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/3608405. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Neural control of dexterity: what has been achieved? Exp Brain Res. 1999;128(1-2):6–12. doi: 10.1007/s002210050811. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/10473734. [DOI] [PubMed] [Google Scholar]
- Lepage J-F, Théoret H. The mirror neuron system: grasping others’ actions from birth? Dev Sci. 2007;10(5):513–23. doi: 10.1111/j.1467-7687.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21(1):1–36. doi: 10.1016/0010-0277(85)90021-6. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/4075760. [DOI] [PubMed] [Google Scholar]
- Marr D, Vaina L. Representation and recognition of the movement of shapes. Proc R Soc London B. 1982;214:501–24. doi: 10.1098/rspb.1982.0024. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251(3):281–98. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Meer ALVD, Weel FRVD, Lee DN. The functional significance of arm movements in neonates. Science. 1995;267:693–95. doi: 10.1126/science.7839147. Retrieved from: http://www.sciencemag.org/cgi/content/abstract/sci;267/5198/693. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198(4312):75–8. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Newborn infants imitate adult facial gestures. Child Dev. 1983;54(3):702–9. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/6851717. [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation in newborn infants: exploring the range of gestures imitated and the underlying mechanisms. Dev Psychol. 1989;25(6):954–62. doi: 10.1037/0012-1649.25.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Explaining facial imitation: a theoretical model. Early Dev Parenting. 1997;6:179–92. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metta G, Sandini G, Natale L, Craighero L, Fadiga L. Understanding mirror neurons. Interact Stud. 2006;7(2):197–232. [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124(Pt 1):176–208. doi: 10.1093/brain/124.1.176. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/11133797. [DOI] [PubMed] [Google Scholar]
- Myowa M. Imitation of facial gestures by an infant chimpanzee. Primates. 1996;37(2):207–13. Retrieved from: http://www.springerlink.com/index/53363132M815M643.pdf. [Google Scholar]
- Myowa-Yamakoshi M, Takeshita H. Do human fetuses anticipate self-oriented actions? A study by four-dimensional (4d) ultrasonography. Infancy. 2006;10(3):289–301. [Google Scholar]
- Olivier E, Edgley SA, Armand J, Lemon RN. An electrophysiological study of the postnatal development of the corticospinal system in the macaque monkey. J Neurosci. 1997;17(1):267–76. doi: 10.1523/JNEUROSCI.17-01-00267.1997. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/8987754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztop E, Arbib MA. Schema design and implementation of the grasp-related mirror neuron system. Biol Cybern. 2002;87(2):116–40. doi: 10.1007/s00422-002-0318-1. Retrieved from: http://www.springerlink.com/index/93AVY2V9J4M4QYF6.pdf. [DOI] [PubMed] [Google Scholar]
- Park J, Newman LI, Polk TA. Face processing: the inter-play of nature and nurture. Neuroscientist. 2009;15(5):445–9. doi: 10.1177/1073858409337742. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Kelly DJ. The origins of face processing in humans: phylogeny and ontogeny. Perspect Psychol Sci. 2009;4(2):200–9. doi: 10.1111/j.1745-6924.2009.01119.x. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Harries MH, Bevan R, Thomas S, Benson PJ, Mistlin AJ. Frameworks of analysis for the neural representation of animate objects and actions. J Exp Biol. 1989;146(1):87–113. doi: 10.1242/jeb.146.1.87. Retrieved from: http://jeb.biologists.org/cgi/content/abstract/146/1/87. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Mistlin AJ, Chitty AJ, Head AS, Potter DD. Visual analysis of body movements by neurones in the temporal cortex of the macaque monkey: a preliminary report. Behav Brain Res. 1985;16(2-3):153–70. doi: 10.1016/0166-4328(85)90089-0. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/4041214. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res Rev. 2005;50(1):57–68. doi: 10.1016/j.brainresrev.2005.04.005. Retrieved from: http://linkinghub.elsevier.com/retrieve/pii/S0165017305000573. [DOI] [PubMed] [Google Scholar]
- Prinz W. Perception and action planning. Eur J Cogn Psychol. 1997;9(2):129–54. [Google Scholar]
- Raos V, Umiltá MA, Gallese V, Fogassi L. Functional properties of grasping-related neurons in the dorsal premotor area F2 of the macaque monkey. J Neurophysiol. 2004;92(4):1990–2002. doi: 10.1152/jn.00154.2004. [DOI] [PubMed] [Google Scholar]
- Reithler J, van Mier HI, Peters JC, Goebel R. Nonvisual motor learning influences abstract action observation. Curr Biol. 2007;17(14):1201–7. doi: 10.1016/j.cub.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V. Premotor cortex and the recognition of motor actions. Cogn Brain Res. 1996;3:131–41. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2(9):661–70. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinter-pretations. Nat Rev Neurosci. 2010;11(4):264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci. 2008;28:1569–88. doi: 10.1111/j.1460-9568.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- Röder B, Rösler F, Spence C. Early vision impairs tactile perception in the blind. Curr Biol. 2004;14(2):121–4. [PubMed] [Google Scholar]
- Sommerville JA, Hildebrand EA, Crane CC. Experience matters: the impact of doing versus watching on infants’ subsequent perception of tool-use events. Dev Psychol. 2008;44(5):1249–56. doi: 10.1037/a0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward A. Pulling out the intentional structure of action: the relation between action processing and action production. Cognition. 2005;95:1–30. doi: 10.1016/j.cognition.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward A, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96(1):B1–11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. The interpersonal world of the infant: a view from psychoanalysis and developmental psychology. Basic Books; New York: 1985. [Google Scholar]
- Sugita Y. Face perception in monkeys reared with no exposure to faces. Proc Natl Acad Sci U S A. 2008;105(1):394–8. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Donkelaar HJ, Lammens M, Wesseling P, Hori A, Keyser A, Rotteveel J. Development and malformations of the human pyramidal tract. J Neurol. 2004;251(12):1429–42. doi: 10.1007/s00415-004-0653-3. [DOI] [PubMed] [Google Scholar]
- Trevarthen C, Aitken KJ. Infant intersubjectivity: research, theory, and clinical applications. J Child Psychol Psychiatry Allied Disciplines. 2001;42(1):3–48. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/11205623. [PubMed] [Google Scholar]
- Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C. I know what you are doing: a neurophysiological study. Neuron. 2001;31(1):155–65. doi: 10.1016/s0896-6273(01)00337-3. Retrieved from: http://linkinghub.elsevier.com/retrieve/pii/S0896627301003373. [DOI] [PubMed] [Google Scholar]
- von Hofsten C. An action perspective on motor development. Trends Cogn Sci. 2004;8(6):266–72. doi: 10.1016/j.tics.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E, Oram MW, Perrett DI. Recognition of objects and their component parts: responses of single units in the temporal cortex of the macaque. Cereb Cortex. 1994;5:509–22. doi: 10.1093/cercor/4.5.509. [DOI] [PubMed] [Google Scholar]
- White B, Castle P, Held R. Observations on the development of visually-directed reaching. Child Dev. 1964;35:349–64. doi: 10.1111/j.1467-8624.1964.tb05944.x. Retrieved from: http://www.jstor.org/stable/1126701. [DOI] [PubMed] [Google Scholar]
- Woodward A. Infants selectively encode the goal object of an actor’s reach. Cognition. 1998;69(1):1–34. doi: 10.1016/s0010-0277(98)00058-4. [DOI] [PubMed] [Google Scholar]