Abstract
Vascular engineering seeks to design and construct functional blood vessels comprising endothelial cells and perivascular cells (PCs), with the ultimate goal of clinical translation. While endothelial behavior has been extensively investigated, PCs play an equally significant role in the development of novel regenerative strategies, providing functionality and stability to vessels. The two major classes of PCs are vascular smooth muscle cells (vSMCs) and pericytes; vSMCs can be further sub-classified as either contractile or synthetic. The inclusion of these cell types is crucial for successful regeneration of blood vessels. Furthermore, understanding distinctions between vSMCs and pericytes will enable improved therapeutics in a tissue-specific manner. Here we focus on the approaches and challenges facing the use of PCs in vascular regeneration, including their characteristics, stem cell sources, and interactions with endothelial cells. Finally, we discuss biochemical and microRNA (miR) regulators of PC behavior and engineering approaches that mimic various cues affecting PC function.
Keywords: Pericytes, Smooth muscle cells, Vascular engineering
1 Introduction
The lack of blood perfusion ultimately limits tissue function. The emerging field of vascular tissue engineering focuses on the development of technologically advanced solutions to regenerate blood vessels and restore blood perfusion for vascular disease treatment as well as in engineered tissues [1]. Incorporation of healthy vascular cells at sites of injury or into engineered constructs is a promising option toward the goal of blood vessel regeneration.
The two critical components of the vascular cellular makeup are perivascular cells (PCs) and endothelial cells (ECs). PCs – such as pericytes and vascular smooth muscle cells (vSMCs) – surround the inner endothelial lining, conferring support, and stabilization. During vessel development, both pericytes and vSMCs are recruited to stabilize newly formed vasculature. PCs wrap around blood vessels and promote vessel maturation by preventing hemorrhaging or leaking of blood vessels [2, 3]. Though they share similar functions, the two PC types localize to disparate vessels. Mature vSMCs surround and circumferentially wrap around the inner layers of larger arteries and veins including the aorta, carotid artery, and the saphenous vein. In contrast, pericytes surround smaller blood vessels or microvasculature, such as capillaries, in which a single EC makes up the inner perimeter of the blood vessel, precapillary arterioles, and postcapillary venules [4]. The greatest density of pericytes is found in microvessels of the central nervous system such as the retina and brain. In the brain, pericytes associate with the continuous endothelium that contains selective tight junctions to form and regulate the blood brain barrier [5]. Inclusion of these two cell types is crucial for successful regeneration of blood vessels. Furthermore, understanding distinctions between vSMCs and pericytes will enable improved therapeutics in a tissue-specific manner.
Blood vessel growth occurs via vasculogenesis or angiogenesis. Vasculogenesis, the formation of blood vessels de novo, transpires mainly during embryo development. In the adult, circulating progenitor stem cells (such as endothelial progenitors, hematopoietic stem cells, or stromal stem cells) have also been noted for their ability to contribute to vasculogenesis [6, 7]. Angiogenesis occurs in both embryos and adults, with vessels forming from preexisting ones [8]. In adults, most vessels are quiescent; active angiogenesis occurs in the placenta during pregnancy, in the cycling ovary, during organ growth, and during wound healing [8]. PCs exhibit important functionalities in stabilizing vessels sprouted from either of these mechanisms. Indeed, nascent vessels regress without perivascular involvement [9].
Blood vessel regeneration has extensively emphasized EC behavior and assembly; owing to their importance in vascular stability and functionality, PCs play an equally significant role in the development of novel regenerative strategies to regenerate vessels of various sizes (pericytes for capillaries and small microvasculature and vSMCs for larger vessels). In this review, we discuss vSMCs and pericytes to provide the groundwork for their use and importance in regenerative medicine pursuits: their cellular characteristics, stem cell sources, and their interactions with ECs, as well as approaches to understand their complex behavior.
2 Cellular properties of vSMCs and pericytes
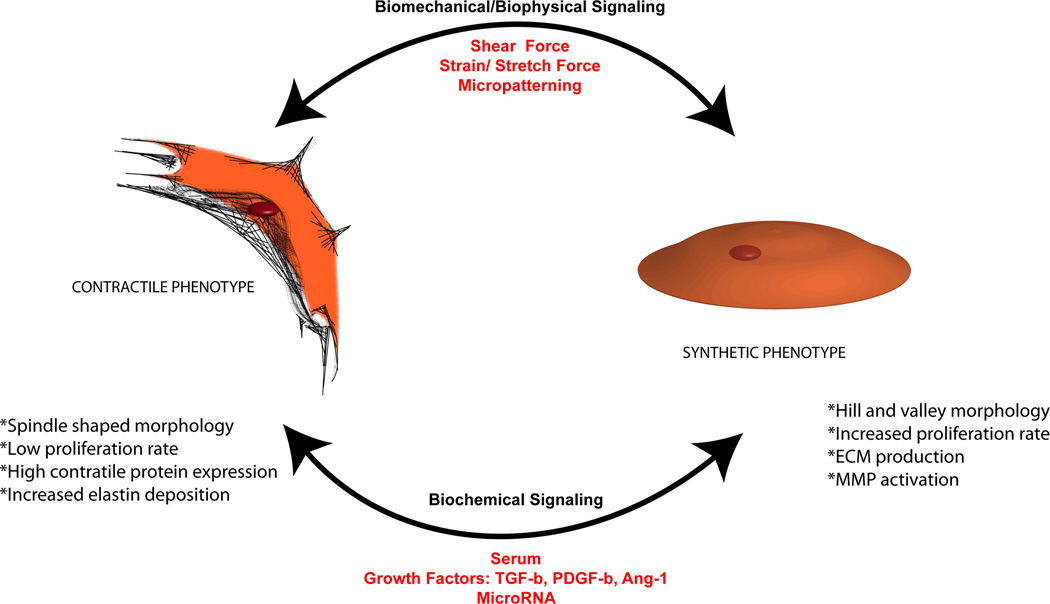
Depending on the vascular tissue engineering application needed, either vSMCs or pericytes are better suited and thus it is important to understand differences between the two types of PCs. One of the foremost distinctions between vSMCs and pericytes is their proximity to the endothelium. In larger vessels, the basement membrane and the inner elastic lamina that contains extracellular matrix (ECM) separate vSMCs from the endothelium [10]. In contrast, pericytes are typically in direct contact with ECs within the endothelial basement membrane, minimizing the diffusion distance from blood to tissue while maximizing the exchange of nutrients and oxygen (Table 1) [11, 12]. vSMCs can be further sub-classified as either contractile or synthetic (Fig. 1). During both neovascularization in the embryo [13] and vessel development, vSMCs take on a synthetic phenotype; in adult blood vessels, these cells are committed to a contractile phenotype, taking on the important task of vessel stabilization [14].
Table 1.
Comparison of cellular properties of PCs
| Vascular SMCs | Pericytes | |
|---|---|---|
| Separated from endothelium | ||
| Contractile | Synthetic | In direct contact with ECs |
| Spindle shaped morphology |
Hill and valley morphology |
Elongated stellate morphology |
| Associated with microvessels (10–100 µm diameters) or capillaries (<10 µm diameter). | ||
| Low proliferative capacity |
Increased proliferation | In capillaries: align parallel to the longitudinal axis |
| Increased contractile protein expression (SMMHC) |
Decreased contractile protein expression |
In microvessels: align circumferentially |
| Principle function: contraction |
Collagen IV, fibronectin, MMP production |
Functions: confer vessel contractility, control blood pressure |
Figure 1.
Phenotypic plasticity of vSMCs. Characteristics of the synthetic and contractile phenotypes – including morphology, proliferation, ECM and contractile protein expression, and phenotypic switch – are regulated by various biochemical and biomechanical cues.
The principal function of contractile vSMCs is, as its name suggests, contraction. At a cellular level, the contractile vSMC phenotype is characterized by a spindle-shaped morphology; cell quiescence, marked by the cell’s low proliferative capacity; and increased contractile protein expression [15]. When surrounding vessels, contractile vSMCs replicate at the low frequency of 0.047% per day [16]. Cyclic AMP synthesis and signaling have been suggested to promote vSMC quiescence [14], while growth factors, as well as fetal calf serum, drive their proliferative capacity. Healthy blood vessels in the body contain vSMCs that exhibit the contractile phenotype. Thus, in the context of vascular engineering, the contractile phenotype is desired to generate stabilized and sturdy vasculature (Fig. 2). The most mature marker that demarcates a contractile vSMC is smooth muscle myosin heavy chain (SMMHC), a protein that powers contraction. Two isoforms of SMMHC (SM-1 and SM-2) are distinct markers for vSMCs. Fetal tissue in both rabbits and humans contain the SM-1 isoform, while the SM-1 and SM-2 isoforms exist in adult vSMCs [17]. Elastin, a non-basement membrane ECM protein that provides blood vessels with elasticity and resilience, has been shown to promote the contractile phenotype of vSMCs [18, 19]. Constituting 28–32% of major vascular vessels, elastin has been shown to induce actin stress fiber organization, regulate migration, and inhibit proliferation in a study using mouse vSMCs lacking elastin (Eln−/−) [19, 20]. A 3-D topography has been suggested to promote elastin production. Lin et al. (2011) showed that vSMCs cultured in 3-D scaffolds produced more elastin than vSMCs cultured in coverslips [21]. In healthy vessels, contractile vSMCs wrap circumferentially rather than longitudinally around blood vessels. The wrapping improves the mechanical properties of the vessel by providing circumferential force [10]. This orientation also helps to manage vasoactivity. Fibroblast growth factor 9 has been suggested to induce wrapping of vSMC around endothelial tubes, supporting their continuous stabilization [22].
Figure 2.
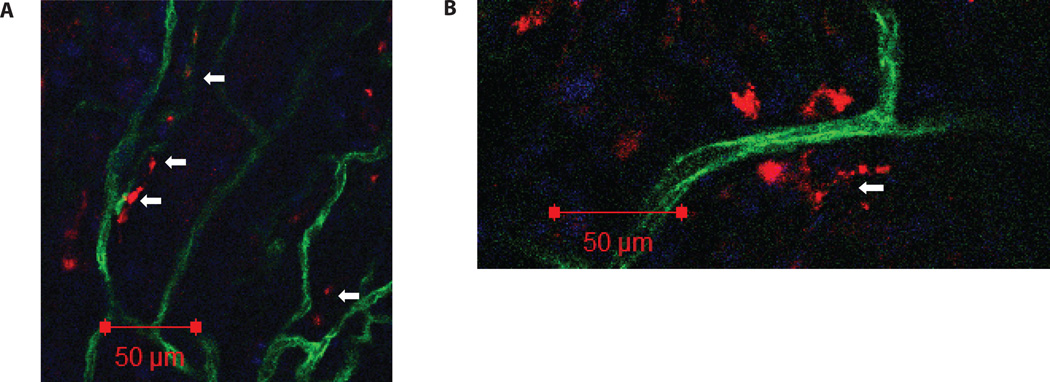
Functionality of hPSC derivatives. Synthetic-vSMCs and contractile-vSMCs derived from hPSCs, transplanted subcutaneously, were shown to: (A) migrate to the host vasculature and locate in the outer layers of the mouse blood vessels that penetrated into the Matrigel plug. (B) On some occasions, the human contractile vSMCs were found to wrap the smaller mouse vasculature circumferentially. Human cells in red, mouse vasculature in green, and nuclei in blue. Some human cells are indicated with white arrows. For details about method please refer to ref. [75].
The synthetic phenotype is prevalent in injured or diseased vessels or during vessel remodeling. For instance, vSMCs found in diseased atherosclerotic plaques are mostly synthetic, exhibiting altered characteristics such as increased golgi and rough endoplasmic reticulum, decreased myofilaments, and altered lipid metabolism [23]. The synthetic vSMC phenotype is characterized by an in vitro “hill and valley” morphology, increased cell proliferation, and decreased contractile protein expression [15]. Synthetic vSMCs produce ECM proteins, such as fibronectin and collagen, as well as matrix metalloproteinases (MMPs) to aid in migration [24]. After vascular injury, vSMCs proliferate and migrate from the tunica media to the intimal layer of vessels causing intimal thickening [25]. Additionally, vSMCs found in aortic vessels that had experience aneurysms were found to experience a phenotypic change such as increased MMP2 and MMP9 [26]. After arterial injury of MMP9−/− mice, vSMCs harvested from the artery demonstrated significantly reduced proliferation and migration [27].
Typically, pericytes exhibit an elongated, stellate morphology and are associated with microvessels (10–100 µm diameters) or capillaries (<10 µm diameter). However, depending on their location, they can change their shape, size, distribution, attachments, and density [12]. For example, capillary pericytes align parallel to the longitudinal axis of the vessel; in microvessels, pericytes align circumferentially [28]. Pericyte coverage of the abluminal vessel ranges from 10 to 50% as a result of the various morphologies of pericytes, as well as the differences in the ratio of pericytes to ECs based on tissue type [29]. Functionally, pericytes confer vessel contractility as well as blood pressure control [30]. The contractility of pericytes has been reported to be regulated by Rho-GTPase-dependent signaling [31]. Bovine pericytes expressing the constitutively active GTP-bound Rho GTPase display a hypercontractile phenotype that includes numerous actin-enriched projections, as well as generation of a sufficiently large contractile force to deform silicon substrates [31]. In contrast, pericytes with Rho GTPase irreversibly locked in a GDP-bound state are polygonally shaped and exhibit diminished contraction [31]. In the body, vessel injury prompts pericytes to switch from encircling vessels to migrating from their vascular locations [32, 33]. In addition, pericytes experience phenotypic changes during inflammation of vessels due to injury. In response to inflammation, inflammatory cells transverse the endothelium and the pericyte layer, resulting in the disruption of pericyte association with the basement membrane [34] as well as a pericyte phenotypic change to a relaxed state [35], likely due to a reduction of RhoA, which has been reported to induce a relaxed phenotype marked by loss of focal adhesion, stress fibers, and increased migration [35].
3 Perivascular marker expression profiles
A useful tool to isolate and distinguish vSMCs and pericytes in tissue engineering and regeneration employs marker expression profiles; because no single marker identifies either cell type, an array of markers is necessary and commonly utilized.
Contractile vSMC markers can be categorized according to their expression during development. Alpha smooth muscle actin (αSMA) is expressed early in development, whereas transgelin (SM22α) is an early intermediate marker [15]. Basic calponin, caldesmon heavy chain, and smoothelin are expressed intermediately during development. SMMHC is expressed late during development; therefore, it functions as a mature vSMC marker [15]. Synthetic vSMCs are identified by caldesmon light chain, vimentin, non-smooth muscle myosin heavy chain B (SMemb), tropomyosin 4, and cellular retinol binding protein 1 [15].
The most common identification for pericytes is a CD146+PDGFRβ+CD34−CD31− population. However, a variety of other markers are present on pericytes depending on their location in the body. The regulator G-protein signaling 5 (RGS5) designates an “activated” pericyte, present in vascular remodeling and neovascularization [36]. The 3G5 antigen is widely accepted as a ubiquitous pericyte marker of the microvasculature [30] though also expressed by other cell types. The transmembrane chondroitin sulfate proteoglycan neuron-glial 2 (NG2 or cspg4) and αSMA help to distinguish pericytes in various types of vessels [30]. Namely, pericytes of the capillaries are NG2+αSMA−, of the venules are NG2−αSMA+, and of the arterioles are NG2+αSMA+. When cultured, pericytes are positive for NG2, αSMA, CD44, CD146, platelet derived growth factor β (PDGFRβ), and nestin and negative for CD56, CD34, CD31, and von Willebrand factor. Finally, pericytes also have mesenchymal stem cell (MSC) features [37, 38] and express MSC markers, such as CD44, CD73, CD90, and CD105.
4 Perivascular stem cell sources
Because PCs derived via biopsies may be diseased or have low proliferative capacity, it is imperative to have a source of PCs for tissue regeneration endeavors that is amenable to clinical translation. To this end, stem cell sources have been used to derive PCs, either for developmental studies or use in tissue-engineered constructs.
Adult stem cells are undifferentiated cells found in various tissues of the human body that can differentiate into specialized cell types of that tissue [39]. Specifically, MSCs serve as a cell source for the derivation of pericytes and vSMCs. These cells are advantageous for vascular engineering applications because they lack major histocompatibility complex II molecules, which incite an immune response [40]. Thus, allogeneic MSCs have the potential for transplantation with limited risk for immune rejection, though it is uncertain whether cells differentiated from MSCs are immunogenic. More detailed information about the relationship between PCs and MSCs can be found in refs. [40–42].
Pluripotent stem cells (PSCs), including human embryonic stem cells (hESCs) and induced PSCs (hiPSCs), can differentiate into all cell types of the body, including pericytes and vSMCs [43, 44]. ESCs are derived from a developing embryo, while iPSCs are generated by reprogramming somatic or adult progenitor cells. Both have an unlimited ability to self-renew, making them easy to expand for therapeutic use [45, 46]. Collagen IV [47], retinoic acid [48–50], and the growth factors PDGF-BB [50–52] and transforming growth factor β1 (TGF-β1) [51] have been implicated in the derivation of vSMCs. We have previously demonstrated the derivation of smooth muscle-like cells (SMLCs) from hESCs in a 2-D, feeder-free approach [51]. After supplementation with PDGF-BB and TGF-β1, SMLCs resembled mature vSMCs with respect to marker expression (Fig. 3) as well ECM deposition and contractility. vSMCs have also been derived from hiPSCs from skin fibroblasts [53] and aortic SMCs [54]. Recently, Bajpai et al. (2012) differentiated hiPSCs toward contractile vSMCs through an intermediate population of clonogenic and multipotent intermediates (MSCs). The hiPSC-derived MSC intermediates were able to differentiate into osteogenic, chondrogenic, or adipogenic lineages [55]. Recently, Tang et al. have questioned the existence of the synthetic phenotype with the discovery of a new source of multipotent vascular stem cells present in the vessel walls [56]. From lineage tracking of SMMHC, they concluded that remodeling of blood vessels is not a result of the phenotypic switching of contractile vSMCs to a synthetic phenotype but rather the differentiation of MSCs to proliferative SMCs.
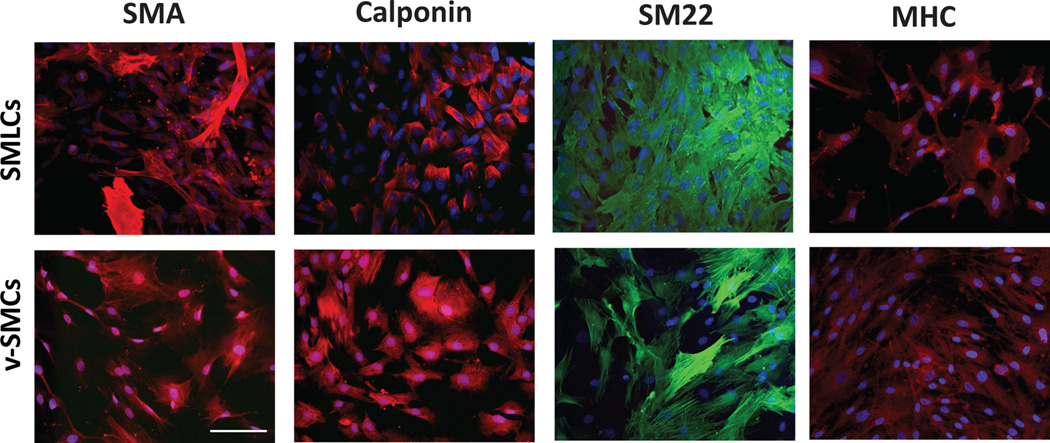
Figure 3.
Comparison of hESC-derived SMLCs and control vSMCs. Comparative immunofluorescence analysis demonstrates the expression of specific SMC markers such as SMA, calponin, SM22, and SM-MHC. Scale bar is 100 µm. Reproduced with kind permission from Springer Science and Business Media [51].
The utilization of PSCs as a source of pericytes has also been demonstrated, though much less studied than vSMC differentiation. In a recent study by Dar et al., human PSCs spontaneously differentiated via embryoid body formation were found to express a CD105+CD31− sub-population [57]. Following further culture of this sub-population, derived pericytes were found to express pericyte-specific markers, assemble with ECs to form a vascular network in vitro and in vivo, and exhibit mesenchymal differentiation potential. Flk1 has been noted as a progenitor marker of pericytes in mouse ESCs [58]. Flk1+ cells isolated from differentiating mouse ESCs were observed to differentiate into functional pericytes which could support EC tubes. De novo differentiation of SMA+ pericytes in this system occurred even in the absence of exogenous growth factors in serum-free conditions. The differentiation of hPSCs toward either PC type has been comprehensively reviewed in ref. [43].
Interestingly, many studies have suggested that pericytes themselves are multipotent vascular stem cells that migrate from their vascular niches to sites of injured tissues with the purpose of repairing these tissues [59]. Pericytes have been shown to produce progeny similar to multilineage mesodermal progenitor cells [38], including, but not limited to, adipocytes, osteoblasts, chondrocytes, and odontoblasts [60].
5 Interactions between perivascular cells and ECs
PCs are essential to prevent regression of assembled endothelial tubes, both in vivo and in vitro. To engineer a tissue for transplantation, its vasculature must be supported by PC types to facilitate and ensure its longevity and durability. Directing the complex process of vessel formation [12, 61], interactions between ECs and PCs have been examined in various in vitro and in vivo systems. In this section, we specifically focus on insights gained from in vitro model systems developed to study the heterocellular crosstalk of the vasculature.
vSMCs support the EC infrastructure [62, 63]. It has been demonstrated that co-cultures of ECs and SMCs alter expression of angiogenic factors VEGF, PDGF-AA, PDGF-BB, and TGF-β compared to monocultures [64]. SMLCs derived from hESCs have been shown to support cord-like structures of endothelial progenitors in a Matrigel system [51]. After 48 h, cord structures created by endothelial progenitor alone collapsed, whereas those co-cultured with derived SMLCs were stabilized and created tubes with thicknesses between 20 and 30 µm (Fig. 4). Co-cultures of ECs with a PC source (10T1/2, a multipotent mesenchymal cell) demonstrated that VEGF expression was dependent on heterocellular contacts. Inhibiting VEGF yielded a drastic increase in EC apoptosis. Thus EC survival seems dependent on perivascular-derived VEGF [65]. Another co-culture model was developed to mimic the arterial vessel wall [66]. Human umbilical artery SMCs were induced toward a contractile phenotype (via serum deprivation) and cultured with ECs, separated by a collagen gel layer. Under static conditions, αSMA expression dramatically decreased, suggesting reversion to a synthetic phenotype, whereas under the influence of shear stress, αSMA was maintained as in the contractile phenotype.
Figure 4.
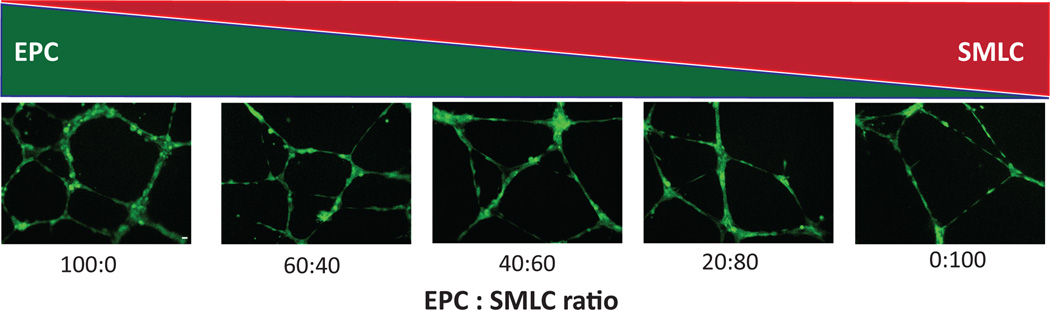
Network stabilization by SMLCs. hESC-derived SMLCs contribute to formation and stabilization of endothelial progenitor cell (EPC) networks. Fluorescent microscopy images of viable cord like structures formed on Matrigel following seeding with ratios of 100:0, 60:40, 40:60, 20:80, and 0:100 (EPCs:SMLCs). Reproduced with kind permission from Springer Science and Business Media [51].
Co-cultures of ECs and pericytes or SMCs revealed that PCs inhibit EC proliferation; however, co-culture with fibroblast, epithelial, or 3T3 cells actually stimulated EC growth [67]. Though they limit proliferation, PCs seemingly do not impair endothelial ability to form tube structures, to which they are recruited and stabilize. By using defined conditions, the growth factors and cytokines necessary for vascular morphogenesis and pericyte stabilization have been realized; VEGF, fibroblast growth factor-2, stem cell factor, interleukin-3, and stromal-derived factor-1 are necessary for ECs to form vascular guidance channels to which pericytes home. Pericyte recruitment, triggered by EC-derived PDGF and epidermal growth factor [68], is necessary for the formation of stabilized vessel structures as well as ECM deposition [61]. Overall, these systems have led to vital insights into the importance of the presence of PCs to recapitulate physiological processes.
6 Biomolecular regulators of PC behavior
Biomolecular understanding and manipulation of cells is another useful tool that has been utilized to study the complex behavior and multifaceted functionalities of PCs and their use in regeneration. Serum, growth factors, and microRNA (miR) have been the mostly widely studied regulators of perivascular properties.
6.1 Biochemical signaling: Serum
For vSMCs, serum deprivation is known to induce a more contractile phenotype. After serum starvation, human umbilical arterial vSMCs adopt an elongated spindle shape, re-acquire contractility, and exhibit elevated SMA, SMMHC, calponin, and SM22α protein expression [69]. Similarly, after serum deprivation of cloned vSMCs from the human thoracic artery, random migration, ECM production, and proliferation decreased, and the vSMCs adopted an elongated spindle-shaped morphology. SMMHC was also shown to be upregulated after serum starvation [70].
The molecules that regulate the change to a contractile phenotype include serum response factor (SRF), myocardin, extracellular signal-regulated kinase 1/2 (ERK-1/2), E twenty-six (ETS)-like transcription factor 1 (ELK-1), and Kruppel-like factor 4 (KLF4). Studies have shown that almost all vSMC genes depend on motifs such as CC(AT)6GG, called CArG elements, found in the vSMC marker gene promoter on intronic sequences [14, 71]. CArG elements serve as binding sites for SRF [71]. The loss of SRF transactivation by suppression using RNAi induced synthetic phenotype characteristics, including decreased SMA expression, increased proliferation, and increased migration [72]. Myocardin, a muscle-specific SRF coactivator, forms a ternary complex with the bound SRF to activate SMC gene expression [71, 73]. Myocardin overexpression during embryoid body differentiation increased carbachol-induced contraction, as well as the number of cells positive for the contractile proteins SMA and SMMHC [74].Contrastingly, the presence of serum induces a change to a more synthetic phenotype [75]. ERK1/2 has been shown to phosphorylate ELK-1. After phosphorylation, ELK-1 binds to SRF binding sites in CArG elements [76, 77]. The binding displaces myocardin and therefore represses contractile vSMC protein expression [77]. KLF4 represses the expression of multiple vSMC genes by both downregulating myocardin as well as preventing SRF/myocardin complexes from associating with vSMC gene promoters [78]. Additionally, proteasomal degradation of myocardin by urokinase-type plasminogen activator has been reported to lead to a synthetic phenotype [79].
The effect of serum has not been correlated with pericyte properties.
6.2 Biochemical signaling: Growth factors
6.2.1 Contractile vSMC phenotype
TGF-β1 and its receptor interactions play an important role in vessel formation. TGF-β1 bind to type I receptors, which form heterodimers with type II receptors, ultimately leading to the activation of SMAD transcription factors [80]. TGF-β1 is commonly reported to differentiate vascular progenitor cells into pericytes and vSMCs [49–51, 81]. Disruption of TGF-β in ECs reduces TGF-β1 availability and thus the ability to promote recruitment and differentiation of vSMCs [82]. TGF-β1 has also been found to increase contractile protein expression in vSMCs [83]. Sieczkiewicz et al. (2003) have shown that TGF-β1 reduces retinal pericyte proliferation and increases contractile protein expression.
6.2.2 Synthetic vSMC phenotype
PDGF-B secreted by ECs promotes the recruitment of pericytes and vSMCs during vessel growth and remodeling through PDGF-B/PDGFR-β interactions. PDGF-B has been reported to repress the contractile phenotype, therefore making it a negative regulator of vSMC gene expression. After 24 h exposure to PDGF-BB, messenger RNA (mRNA) expression of SMMHC decreased by 80% in rat vSMCs [84]. PDGF-BB treatment repressed SMA, SMMHC, and SM22α promoters of rat vSMCs [85]. Reportedly, activation of KLF4 is required for the PDGF-BB-induced effect of repressing the vSMC contractile phenotype [86].
6.2.3 Pericytes
PDGF is the most widely appreciated regulator of pericyte behavior. Endothelial-derived PDGF-BB has been demonstrated to promote pericyte recruitment and stabilization of nascent endothelial tubes [68]. Mouse embryos with PDGF-B deficiencies were found to lack pericytes; this resulted in the formation of tortuous capillaries, many of which ruptured at late gestation because they had formed microaneurysms [3]. The ECs of these pericyte-lacking sprouting capillaries were incapable of attracting PDGFR-β+ pericyte progenitor cells, which contributed to the deformation of the capillaries [3]. Similarly, Benjamin et al. (1998) found that adding exogenous PDGF-BB during vessel remodeling disrupted endogenous cues, impeding endothelial-pericyte interactions [87]. Proliferation of PDGFR-β pericytes and vSMC progenitors was observed at endothelial PDGF-B expression sites [88].
Other angiogenic growth factors have also been implicated in regulating pericyte activity. Though widely known for its mitogenic effects on ECs, exogenous VEGF has been demonstrated to stimulate pericyte migration in a dose-dependent manner, purporting it as a mitogen for pericytes as well [89]. TGF-β1 regulates the contractile phenotype, induces SMA expression, and limits proliferation of pericytes [90]. TNF-a and IL-1β have been reported to induce a relaxed phenotype in pericytes yielding increased microvascular permeability in co-culture with microvascular ECs [91].
6.2.4 Common
Pericytes and vSMCs also express angiopoietin-1 (Ang-1) on their surfaces, and ECs express Tie-2 receptor [11]. The tight endothelial-pericyte interactions are a result of the binding of Ang-1 and Tie-2 [11]. Angiopoietin-2 (Ang-2), an antagonist on Ang-1 [92], is mainly expressed in ECs and vSMCs. Ang-1 and Ang-2 both bind to Tie-2, but only Ang-1 phosphorylates Tie-2. Ang-2 is thought to destabilize blood vessels by dissociating vSMCs and ECs. Ang-2 was found to inhibit EC-induced vSMC migration [93]. Direct injection of Ang-2 into the eyes or rats caused a dose-dependent loss of pericytes after 7 days [94].
6.3 MicroRNA
Recently, miR has been implicated in controlling the phenotype of vSMCs. miRs are single-stranded non-coding RNAs that affect various cellular outcomes by altering mRNA function [95]. miR plays different roles in vascular development of healthy compared to diseased vessels by altering gene function and therefore may serve as a potential therapeutic resource. For instance, both miR-143 and miR-145 cooperatively target KLF4, myocardin, and ELK-1 in order to promote differentiation from mouse ESCs and to repress proliferation as vSMCs mature [96]. Boucher et al. (2011) found that the activation of Notch signaling by Jagged-1 (Jag-1) in vSMCs resulted a contractile phenotype. Additionally, they reported that Jag-1/Notch signaling requires both miR-143 and miR-145 to promote the vSMC contractile phenotype [97]. The contractile vSMC markers SMA, calponin, and SMMHC were found to be upregulated by the precursor to miR-145 (or pre-miR-145) [98]. Additionally, miR-195 reduced the proliferation and migration of vSMCs, indicating a contractile phenotype [99]. Similarly, miR-133a reportedly induced a contractile vSMC phenotype by inhibiting the transcription factor, specifity protein 1 (Sp-1) [100].
TGF-β and bone morophogenic protein (BMP) [101] signaling and subsequent SMAD protein binding was reported to induce a contractile phenotype via increased miR-21 and downregulation of programmed cell death 4 (PCDC4) [102].
However, unlike growth factor regulation of miR-21, hypoxia-induced upregulation of miR-21 has been reported to induce increased proliferation and migration, characteristic of the synthetic phenotype [103, 104]. This illustrates that the same miR may have different responses under different conditions. Increased proliferation of vSMCs was also observed with the increased expression of miR-221 and miR-222 by targeting p27 and p57, which are negative regulators of vSMC proliferation [105]. Knock-down of miR-221 and miR-222 reduced neointimal lesion formation caused by proliferating vSMCs [105]. miR-145 has also been found to be expressed in NG2+ pericytes via in situ hybridization on tissue sections [106]. In knockout mice with reduced pericyte-investment in microvessels, expression of miR-145 was reduced in harvested microvessels, whereas other miRNAs were not affected. Furthermore, altered levels of miR-145 diminished migration in vitro, suggesting its relevance to pericyte behavior.
7 Engineering approaches to study PC behavior
Technological advancements in recent years have enabled researchers to more accurately mimic the perivascular environment. In this section, we discuss the insights gained from the recreation of physiologically relevant biomechanical forces, micropatterned topographies, and substrate stiffness in engineered niches.
7.1 Biomechanical forces
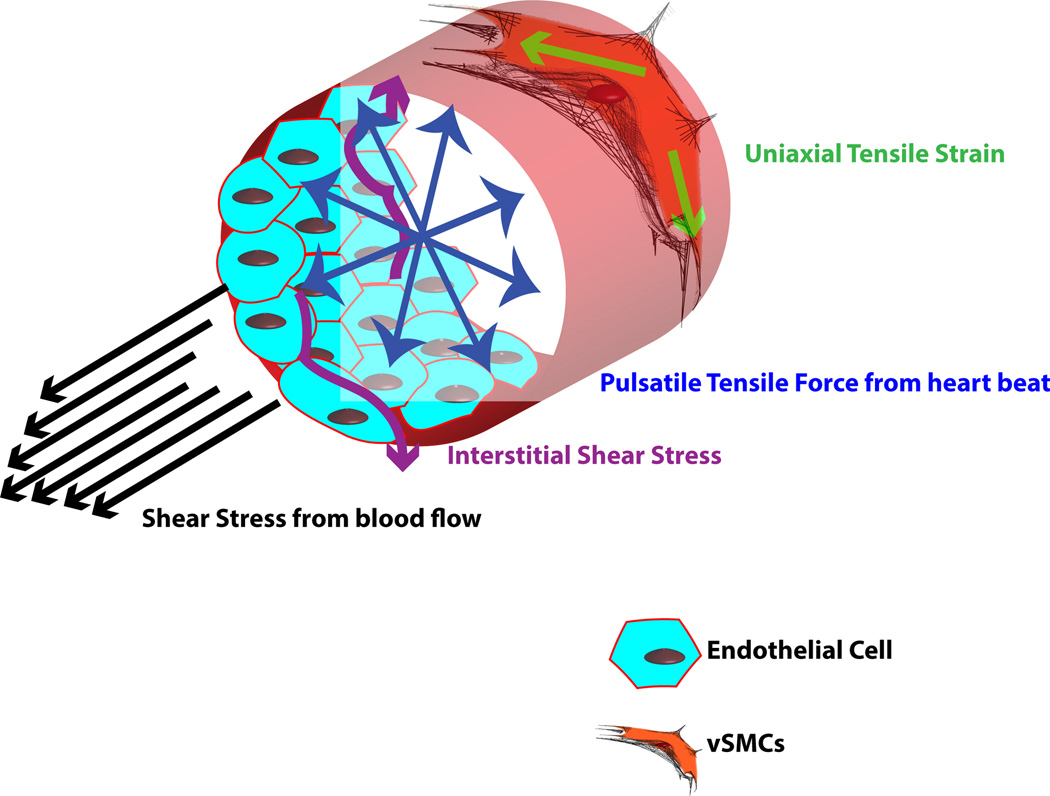
Pre-conditioning tissue-engineered constructs using mechanical forces may translate to improved cell adaptability when exposed to similar biomechanical forces once implanted in the body. Pericytes and vSMCs experience two major biomechanical forces: shear force generated from interstitial blood flow and uniaxial cyclic strain force generated from heart beats (Fig. 5). In healthy vessels, pericytes and vSMCs are not in direct contact with the blood flow, unlike ECs. However, they experience low transverse shear stress resulting from differences in blood vessel pressure and tissue pressure [107]. Studies have approximated this interstitial shear stress at an average 1 dyn/cm2 [108]. Exposure of aortic vSMCs to a shear stress of 0.05 dyn/cm2 resulted in enhanced MMP-dependent vSMC motility [109] indicative of a synthetic phenotype. In contrast, vSMCs close to the internal elastic lamina may experience approximately 100 times higher shear than those away from the internal elastic lamina [110]. Additionally, after injury to the endothelium, vSMCs may endure higher shear resulting from exposure to blood flow [107]. In response to higher shear flow rates, canine vSMCs [111] and ovine vSMCs [112] aligned perpendicularly when a shear fluid force of up to 20 dyn/cm2 was applied for between 48 and 96 h. Proliferation of human vSMCs increased after exposure to shear stress of between 5 and 25 dyn/cm2 for 24 h [113]. Vascular vSMCs also have been reported to take on a more contractile phenotype after shear stress [112].
Figure 5.
Biomechanical forces affecting vSMCs. Various forces in the blood vessel act on the vSMCs, including shear stress, interstitial shear stress, pulsatile tensile force, and uniaxial tensile strain.
Because PCs circumferentially predominantly surround vessel walls, they experience a predominantly uniaxial tensile force or strain. As the heart beats, it generates a pulsatile pressure resulting in an outward tension experienced by the vessel [114]. Since the heart beats rhythmically, the tension felt by the cells is also cyclic [115].
A number of researchers have mimicked these mechanical cues by applying strain to cultured vSMCs on 2-D surfaces. The expression of contractile vSMC proteins SMA, calponin, and SM22a were also shown to increase after cyclic strain [116, 117]. vSMCs align perpendicular to the direction of uniaxial cyclic strain [118]. Uniaxial cyclic strain also regulates the expression of vSMC markers in MSCs by transiently increasing SMA and SM22a after 1 day [115]. After the return of these proteins to basal levels, the cells align perpendicularly to the direction of strain. Studies have shown that 2-D uniaxial cyclic strain affects vSMC ECM deposition, marker expression, and alignment. Expression of ECM proteins, such as collagen I, collagen IV, and fibronectin increased under uniaxial cyclic strain [119, 120]. In contrast, equiaxial strain downregulates SMA and SM22a in MSCs.
To achieve 3-D strain, pericytes and vSMCs are either embedded in a 3-D matrix that is mechanically strained or the cells themselves are strained using pulsatile perfusion bioreactors. In these systems, vSMCs align parallel to the direction of strain [121, 122]. After 5 weeks of cyclic distension of adult SMCs embedded in collagen gels, the cells were aligned parallel to the direction of strain and elastin deposition was observed [121]. After 8 weeks of 3-D strain generated by pulsatile flow through a poly(lactide-co-caprolactone) scaffold seeded with rabbit aortic vSMCs, collagen production, proliferation, and SMA expression were enhanced [122]. Wang et al. (2010) subjected vSMCs derived from human adipose stem cells and seeded on polyglycolic acid (PGA) unwoven mesh scaffolds to 8 weeks of pulsatile shear that was gradually increased from 75 to 150 mmHg [123]. Pulsatile stimulation increased SMA, calponin, and collagen expression [123]. The 3-D strain resulting from the pulsatile stimulation of vSMCs has also brought about an increase in mechanical strength (tensile strength, suture strength, elastic modulus, and burst pressure), oriented vSMCs, and organized collagen fibers compared to the static controls [123, 124].
7.2 Micropatterned surfaces
By controlling cell shape, micropatterned surfaces have also been studied in vSMC phenotype control. Micropatterning is a robust method of presenting micro- and nanometer scale topological features in distinct spatial patterns that serve as instructive cues and allow the control of various cell responses. Thakar et al. (2009) seeded human aortic vSMCs on non-patterned and micropatterned fibronectin-inked poly(dimethylsiloxane) (PDMS) microgrooves and microstamps fabricated using soft lithography for 24 h and then applied cyclic uniaxial strain (1 Hz, 5% elongation) for an additional 24 h. When compared to non-patterned vSMCs, cells restricted to micropatterned PDMS microgrooves and microstamps exhibited contractile phenotype characteristics, such as a decreased proliferation rate and a more elongated morphology [125]. Additionally, after application of cyclic strain, vSMCs seeded on micropatterned surfaces aligned in the direction parallel to microgrooves, while vSMCs seeded on non-patterned surfaces aligned perpendicularly to the direction of stretch [125]. Thakar et al. (2003) also studied bovine vSMCs on collagen striped micropatterns. When grown on these 20–50 µm wide stripes, bovine vSMCs exhibited lower proliferation rates [126]. Williams et al. (2011) used a comb polymer to act as protein/cell resistant barriers in order to micropattern tissue culture polystyrene surfaces. vSMCs attached to the non-printed regions of 10 µm-wide lanes of tissue culture polystyrene interspersed between 100 µm-wide microcontact printed comb polymer patterns [127]. Micropatterning using the comb polymer was found to upregulate SMMHC as effectively as TGF-β at low cell passages [127].
7.3 Substrate stiffness
The mechanical microenvironment of cells has been shown to affect cell function and phenotype. In the body, vSMCs are located in specific niches consisting of different ECM proteins that provide the cells with an appropriate mechanical environment to function properly. Altering substrate stiffness is a popular approach to emulate different mechanical microenvironments, in contrast to genetic modulation, that control the vSMC phenotype [128, 129]. Therefore, a suitable substrate stiffness should be considered when designing implantable vessel constructs in order to ensure proper vSMC function. For instance, rat aortic vSMCs cultured on softer (Young’s modulus of approximately 6 MPa) biocompatible polyelectrolyte multilayer (PEMU) surfaces adopted a synthetic phenotype, while vSMCs cultured on more rigid (Young’s modulus of about 8 GPa) PEMU surfaces adopted a contractile phenotype [129]. Brown et al. reported an increase in bovine vSMC proliferation and cell spreading after increasing the stiffness of polyacrylamide (PAAm) gels having RGD peptides with elastic moduli of 19 kPa to elastic moduli of 84 kPa. Human umbilical artery vSMCs encapsulated in methacrylated and lysine functionalized dextran hydrogels had high vSMC proliferation and rapid cell spreading in softer gels (shear storage moduli ranging from 898 to 3124 Pa) [130].
8 Summary and future directions
PCs play a pivotal role in the endurance and function of blood vessels, making them necessary for engineering the vasculature. Current difficulties facing the inclusion of PCs include their cryptic marker expression profiles, which limits efficient isolation and derivation techniques, misalignment of cells, and the ability of vSMCs to assume either a synthetic or contractile phenotype. We need to better understand and control the fate decision toward either the synthetic or contractile phenotype to optimize and engineer blood vessels. Recent work in our lab has demonstrated how hPSCs can be guided toward the synthetic or contractile phenotypes with characteristic functionalities in vitro and in vivo [75]. Further investigation of the biochemical cues and the molecular pathways activated is needed to understand the switch between the two phenotypes. Research has also suggested that miR is involved in the genetic regulation of growth factors and transcription factors involved in phenotypic switching [131].
With advancing technologies, opportunities to harness perivascular potential for regenerative medicine are envisioned to dramatically increase. Development of improved biomaterials that promote perivascular maturation and facilitate incorporation with EC tubes are needed to promote perivascular function in tissue constructs. For instance, Parizek et al. (2009) observed an improvement in the adhesion, maturation, and growth of vSMCs seeded on high-density polyethylene foils modified by an argon (Ar+) plasma discharge and grafted with biomolecules [132]. Development of bioreactors that apply physiologically relevant mechanical forces to tissue-engineered blood vessels could enhance the functionality of vessels after transplantation. Similarly, ease of imaging and quantification of parameters in tissue constructs would become possible with the development of microscale systems where microvascular chambers maintained at 37°C are pressurized and placed on a microscope stage [133]. Ultimately, approaches balancing biochemical, biomechanical, and biophysical cues could yield optimal perivascular function in engineered blood vessels.
Acknowledgments
This work was supported by the National Institutes of Health grant R01HL107938. M. W. is an IGERT trainee. S. K. is sponsored by the National Institutes of Health (NIH; grant F31HL112644).
((Funded by:
-
▪
National Institutes of Health grant R01HL107938
-
▪
National Institutes of Health (NIH; grant F31HL112644)))
Abbreviations
- EC
endothelial cell
- ECM
extracellular matrix
- hESC
human embryonic stem cell
- hiPSC
human induced pluripotent stem cell
- KLF4
Kruppel-like factor 4
- miR
microRNA
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- NG2
neuron-glial 2
- PC
perivascular cell
- PDGFRβ
platelet derived growth factor β
- PSC
pluripotent stem cell
- αSMA
alpha smooth muscle actin
- SMLC
smooth muscle like cell
- SMMHC
smooth muscle myosin heavy chain
- SRF
serum response factor
- vSMC
vascular smooth muscle cell
Footnotes
The authors declare no conflict of interest.
References
- 1.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mancini ML, Terzic A, Conley BA, Oxburgh LH, et al. Endoglin plays distinct roles in vascular smooth muscle cell recruitment and regulation of arteriovenous identity during angiogenesis. Dev. Dynam. 2009;238:2479–2493. doi: 10.1002/dvdy.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 4.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Science; 2002. [Google Scholar]
- 5.Armulik A, Genove G, Mae M, Nisancioglu MH, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 6.Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res. Part C: Embryo Today: Rev. 2003;69:73–82. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- 7.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow–derived cells. Blood. 2005;105:1068–1077. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 8.Fischer C, Schneider M, Carmeliet P. Principles and Therapeutic Implications of Angiogenesis, Vasculogenesis and Arteriogenesis. In: Moncada S, Higgs A, editors. The Vascular Endothelium II. Berlin Heidelberg: Springer; 2006. pp. 157–212. [DOI] [PubMed] [Google Scholar]
- 9.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovas. Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 10.Clifford PS. Local control of blood flow. Adv. Physiol. Educ. 2011;35:5–15. doi: 10.1152/advan.00074.2010. [DOI] [PubMed] [Google Scholar]
- 11.Eble JA, Niland S. The extracellular matrix of blood vessels. Curr. Pharm. Des. 2009;15:1385–1400. doi: 10.2174/138161209787846757. 1316. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 13.Ball SG, Shuttleworth CA, Kielty CM. Platelet-derived growth factor receptors regulate mesenchymal stem cell fate: Implications for neovascularization. Exp. Opin. Biol. Ther. 2010;10:57–71. doi: 10.1517/14712590903379510. [DOI] [PubMed] [Google Scholar]
- 14.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 15.Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: Implications for vascular tissue engineering. Tissue Eng., Part B. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lombardi DM, Reidy MA, Schwartz SM. Methodologic considerations important in the accurate quantitation of aortic smooth muscle cell replication in the normal rat. Am. J. Pathol. 1991;138:441–446. [PMC free article] [PubMed] [Google Scholar]
- 17.Aikawa M, Sivam PN, Kuro-o M, Kimura K, et al. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ. Res. 1993;73:1000–1012. doi: 10.1161/01.res.73.6.1000. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Yamamoto K, Noumura T. Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp. Cell Res. 1993;204:121–129. doi: 10.1006/excr.1993.1016. [DOI] [PubMed] [Google Scholar]
- 19.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 20.Mithieux SM, Weiss AS, Elastin . In: Advances in Protein Chemistry. David ADP, John MS, editors. Academic Press; 2005. pp. 437–461. [DOI] [PubMed] [Google Scholar]
- 21.Lin S, Sandig M, Mequanint K. Three-dimensional topography of synthetic scaffolds induces elastin synthesis by human coronary artery smooth muscle cells. Tissue Eng., Part A. 2011;17:1561–1571. doi: 10.1089/ten.TEA.2010.0593. [DOI] [PubMed] [Google Scholar]
- 22.Frontini MJ, Nong Z, Gros R, Drangova M, et al. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat. Biotechnol. 2011;29:421–427. doi: 10.1038/nbt.1845. [DOI] [PubMed] [Google Scholar]
- 23.Frink RJ, Foundation HR. Inflammatory Atherosclerosis: Characteristics of the Injurious Agent. Heart Research Foundation; 2002. [Google Scholar]
- 24.Cecchettini A, Rocchiccioli S, Boccardi C, Citti L. Chapter two – Vascular smooth-muscle-cell activation: Proteomics point of view. In: Kwang WJ, editor. International Review of Cell and Molecular Biology. Academic Press; 2011. pp. 43–99. [DOI] [PubMed] [Google Scholar]
- 25.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 26.Ailawadi G, Moehle CW, Pei H, Walton SP, et al. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2009;138:1392–1399. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ. Res. 2002;91:845–851. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 28.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 29.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 30.Crisan M, Corselli M, Chen WC, Péault B. Perivascular cells for regenerative medicine. J. Cell. Mol. Med. 2012;16:2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutcher ME, Kolyada AY, Surks HK, Herman IM. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am. J. Pathol. 2007;171:693–701. doi: 10.2353/ajpath.2007.070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonul E, Duz B, Kahraman S, Kayali H, et al. Early pericyte response to brain hypoxia in cats: An ultrastructural study. Microvasc. Res. 2002;64:116–119. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 33.Dore-Duffy P, Owen C, Balabanov R, Murphy S, et al. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc. Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- 34.Voisin M-B, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arteriosclerosis, Thrombosis, Vasc. Biol. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Cao C, Chen Z, Bankaitis V, et al. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS One. 2012;7:45499. doi: 10.1371/journal.pone.0045499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger M, Bergers G, Arnold B, Hämmerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094–1101. doi: 10.1182/blood-2004-06-2315. [DOI] [PubMed] [Google Scholar]
- 37.Caplan A. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Crisan M, Yap S, Casteilla L, Chen C-W, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Körbling M, Estrov Z. Adult stem cells for tissue repair – A new therapeutic concept? New Engl. J. Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 40.Caplan AI. Why are MSCs therapeutic? New data: New insight. . J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang NF, Li S. Mesenchymal stem cells for vascular regeneration. Regen. Med. 2008;3:877–892. doi: 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng J, Mantesso A, Sharpe PT. Perivascular cells as mesenchymal stem cells. Exp. Opin. Biol. Ther. 2010;10:1441–1451. doi: 10.1517/14712598.2010.517191. [DOI] [PubMed] [Google Scholar]
- 43.Dar A, Itskovitz-Eldor J. Therapeutic potential of perivascular cells from human pluripotent stem cells. J. Tissue Eng. Regen. Med. 2013 doi: 10.1002/term.1698. [DOI] [PubMed] [Google Scholar]
- 44.Kusuma S, Gerecht S. Comparison of induced pluripotent stem cell and embryonic stem cell differentiation toward vascular lineages. Regen. Med. 2009;4:805. [Google Scholar]
- 45.Takahashi K, Tanabe K, Ohnuki M, Narita M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 47.Xiao Q, Zeng L, Zhang Z, Hu Y, Xu Q. Stem cell-derived Sca-1+ progenitors differentiate into smooth muscle cells, which is mediated by collagen IV-integrin α1/β1/αv and PDGF receptor pathways. Am. J. Physiol–Cell Physiol. 2007;292:C342–C352. doi: 10.1152/ajpcell.00341.2006. [DOI] [PubMed] [Google Scholar]
- 48.Huang H, Zhao X, Chen L, Xu C, et al. Differentiation of human embryonic stem cells into smooth muscle cells in adherent monolayer culture. Biochem. Biophys. Res. Commun. 2006;351:321–327. doi: 10.1016/j.bbrc.2006.09.171. [DOI] [PubMed] [Google Scholar]
- 49.Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK. Transforming growth factor-β1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am. J. Physiol, – Cell Physiol. 2004;287:C1560–C1568. doi: 10.1152/ajpcell.00221.2004. [DOI] [PubMed] [Google Scholar]
- 50.Vazão H, Neves RPd, Grãos M, Ferreira L. Towards the maturation and characterization of smooth muscle cells derived from human embryonic stem cells. PLoS One. 2011;6:e17771. doi: 10.1371/journal.pone.0017771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vo E, Hanjaya-Putra D, Zha Y, Kusuma S, Gerecht S. Smooth-muscle-like cells derived from human embryonic stem cells support and augment cord-like structures in vitro. Stem Cell Rev. Rep. 2010;6:237–247. doi: 10.1007/s12015-010-9144-3. [DOI] [PubMed] [Google Scholar]
- 52.Oyamada N, Itoh H, Sone M, Yamahara K, et al. Transplantation of vascular cells derived from human embryonic stem cells contributes to vascular regeneration after stroke in mice. J. Trans. Med. 2008;6:54. doi: 10.1186/1479-5876-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taura D, Sone M, Homma K, Oyamada N, et al. Induction isolation of vascular cells from human induced pluripotent stem cells – Brief report. Arteriosclerosis Thrombosis Vasc Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- 54.Lee T-H, Song S-H, Kim KL, Yi J-Y, et al. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ. Res. 2010;106:120–128. doi: 10.1161/CIRCRESAHA.109.207902. [DOI] [PubMed] [Google Scholar]
- 55.Bajpai VK, Mistriotis P, Loh Y-H, Daley GQ, Andreadis ST. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc Res. 2012 doi: 10.1093/cvr/cvs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang Z, Wang A, Yuan F, Yan Z, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat. Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dar A, Domev H, Ben-Yosef O, Tzukerman M, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb/clinical perspective. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita J, Itoh H, Hirashima M, Ogawa M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 59.Crisan M, Chen C-W, Corselli M, Andriolo G, et al. Perivascular multipotent progenitor cells in human organs. Ann. N. Y. Acad. Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 60.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 61.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harvey K, Welch Z, Sliva D, Siddiqui R. Role of Rho kinase in sphingosine 1-phosphate-mediated endothelial and smooth muscle cell migration and differentiation. Mol. Cell. Biochem. 2010;342:7–19. doi: 10.1007/s11010-010-0461-2. [DOI] [PubMed] [Google Scholar]
- 63.Moon JJ, Saik JE, Poché RA, Leslie-Barbick JE, et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31:3840–3847. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heydarkhan-Hagvall S, Helenius G, Johansson BR, Li JY, et al. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J. Cell. Biochem. 2003;89:1250–1259. doi: 10.1002/jcb.10583. [DOI] [PubMed] [Google Scholar]
- 65.Darland DC, Massingham LJ, Smith SR, Piek E, et al. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev. Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Sakamoto N, Kiuchi T, Sato M. Development of an endothelial-smooth muscle cell coculture model using phenotype-controlled smooth muscle cells. Ann. Biomed. Eng. 2011;39:2750–2758. doi: 10.1007/s10439-011-0372-8. [DOI] [PubMed] [Google Scholar]
- 67.Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J. Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han M, Wen J-K, Zheng B, Cheng Y, Zhang C. Serum deprivation results in redifferentiation of human umbilical vascular smooth muscle cells. Am. J. Physiol-Cell Physiol. 2006;291:C50–C58. doi: 10.1152/ajpcell.00524.2005. [DOI] [PubMed] [Google Scholar]
- 70.Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by β1-integrin signaling pathways. Hypertension. 2003;41:903–911. doi: 10.1161/01.HYP.0000062882.42265.88. [DOI] [PubMed] [Google Scholar]
- 71.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J. Clin. Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaplan-Albuquerque N, Van Putten V, Weiser-Evans MC, Nemenoff RA. Depletion of serum response factor by RNA interference mimics the mitogenic effects of platelet derived growth factor-BB in vascular smooth muscle cells. Circ. Res. 2005;97:427–433. doi: 10.1161/01.RES.0000179776.40216.a9. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida T, Sinha S, Dandré F, Wamhoff BR, et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ. Res. 2003;92:856–864. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 74.Raphel L, Talasila A, Cheung C, Sinha S. Myocardin overexpression is sufficient for promoting the development of a mature smooth muscle cell-like phenotype from human embryonic stem cells. PLoS One. 2012;7:e44052. doi: 10.1371/journal.pone.0044052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wanjare M, Kuo F, Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 2012 doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee S-M, Vasishtha M, Prywes R. Activation and repression of cellular immediate early genes by serum response factor cofactors. J. Biol. Chem. 2010;285:22036–22049. doi: 10.1074/jbc.M110.108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z, Wang D-Z, Hockemeyer D, McAnally J, et al. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Sinha S, McDonald OG, Shang Y, et al. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J. Biol. Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 79.Kiyan Y, Limbourg A, Kiyan R, Tkachuk S, et al. Urokinase receptor associates with myocardin to control vascular smooth muscle cells phenotype in vascular disease. Arteriosclerosis, Thrombosis, Vasc. Biol. 2012;32:110–122. doi: 10.1161/ATVBAHA.111.234369. [DOI] [PubMed] [Google Scholar]
- 80.Massagué J. TGF-β signal transduction. Ann. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 81.Klein D, Weißhardt P, Kleff V, Jastrow H, et al. Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS One. 2011;6:e20540. doi: 10.1371/journal.pone.0020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carvalho RLC, Jonker L, Goumans M-J, Larsson J, et al. Defective paracrine signalling by TGFβ in yolk sac vasculature of endoglin mutant mice: A paradigm for hereditary haemorrhagic telangiectasia. Development. 2004;131:6237–6247. doi: 10.1242/dev.01529. [DOI] [PubMed] [Google Scholar]
- 83.Deaton RA, Su C, Valencia TG, Grant SR. Transforming growth factor-β1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J. Biol. Chem. 2005;280:31172–31181. doi: 10.1074/jbc.M504774200. [DOI] [PubMed] [Google Scholar]
- 84.Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ. Res. 1992;71:1525–1532. doi: 10.1161/01.res.71.6.1525. [DOI] [PubMed] [Google Scholar]
- 85.Dandré F, Owens GK. Platelet-derived growth factor-BB and Ets-1 transcription factor negatively regulate transcription of multiple smooth muscle cell differentiation marker genes. Am. J. Physiol. – Heart Circ. Physiol. 2004;286:H2042–H2051. doi: 10.1152/ajpheart.00625.2003. [DOI] [PubMed] [Google Scholar]
- 86.Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am. J. Physiol. – Heart Circ. Physiol. 2009;296:H1027–H1037. doi: 10.1152/ajpheart.01230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 88.Hellstrom M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 89.Yamagishi SI, Yonekura H, Yamamoto Y, Fujimori H, et al. Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Lab. Invest. 1999;79:501–509. [PubMed] [Google Scholar]
- 90.Sieczkiewicz GJ, Herman IM. TGF-β1 signaling controls retinal pericyte contractile protein expression. Microvasc. Res. 2003;66:190–196. doi: 10.1016/s0026-2862(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 91.Kerkar S, Williams M, Blocksom JM, Wilson RF, et al. TNF-α and IL-1β increase pericyte/endothelial cell co-culture permeability. J. Surg. Res. 2006;132:40–45. doi: 10.1016/j.jss.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 92.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi H, DeBusk LM, Babichev YO, Dumont DJ, Lin PC. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood. 2006;108:1260–1266. doi: 10.1182/blood-2005-09-012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hammes H-P, Lin J, Wagner P, Feng Y, et al. Angiopoietin-2 causes pericyte dropout in the normal retina. Diabetes. 2004;53:1104–1110. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- 95.Daubman S. MicroRNAs in angiogenesis and vascular smooth muscle cell function. Circ. Res. 2010;106:423–425. doi: 10.1161/RES.0b013e3181d61a0d. [DOI] [PubMed] [Google Scholar]
- 96.Cordes KR, Sheehy NT, White MP, Berry EC, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boucher JM, Peterson SM, Urs S, Zhang C, Liaw L. The miR-143/145 cluster is a novel transcriptional target of jagged-1/notch signaling in vascular smooth muscle cells. J. Biol. Chem. 2011;286:28312–28321. doi: 10.1074/jbc.M111.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng Y, Liu X, Yang J, Lin Y, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y-S, Wang H-YJ, Liao Y-C, Tsai P-C, et al. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc. Res. 2012;95:517–526. doi: 10.1093/cvr/cvs223. [DOI] [PubMed] [Google Scholar]
- 100.Torella D, Iaconetti C, Catalucci D, Ellison GM, et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo/novelty and significance. Circ. Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 101.Kang H, Davis-Dusenbery BN, Nguyen PH, Lal A, et al. Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating MicroRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J. Biol. Chem. 2012;287:3976–3986. doi: 10.1074/jbc.M111.303156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang M, Li W, Chang G-Q, Ye C-S, et al. MicroRNA-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arteriosclerosis Thrombosis, Vasc. Biol. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 104.Sarkar J, Gou D, Turaka P, Viktorova E, et al. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am. J. Physiol. – Lung Cell. Mol. Physiol. 2010;299:L861–L871. doi: 10.1152/ajplung.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu X, Cheng Y, Zhang S, Lin Y, et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larsson E, Fuchs PF, Heldin J, Barkefors I, et al. Discovery of microvascular miRNAs using public gene expression data: MiR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009:1. doi: 10.1186/gm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi Z-D, Tarbell J. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann. Biomed. Eng. 2011;39:1608–1619. doi: 10.1007/s10439-011-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang DM, Tarbell JM. Modeling interstitial flow in an artery wall allows estimation of wall shear stress on smooth muscle cells. J. Biomech. Eng. 1995;117:358–363. doi: 10.1115/1.2794192. [DOI] [PubMed] [Google Scholar]
- 109.Shi Z-D, Ji X-Y, Qazi H, Tarbell JM. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. Am. J. Physiol. – Heart Circ. Physiol. 2009;297:H1225–H1234. doi: 10.1152/ajpheart.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tada S, Tarbell JM. Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am. J. Physiol. – Heart Circ. Physiol. 2000;278:H1589–H1597. doi: 10.1152/ajpheart.2000.278.5.H1589. [DOI] [PubMed] [Google Scholar]
- 111.Lee AA, Graham DA, Dela Cruz S, Ratcliffe A, Karlon WJ. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J. Biomech. Eng. 2002;124:37–43. doi: 10.1115/1.1427697. [DOI] [PubMed] [Google Scholar]
- 112.Opitz F, Schenke-Layland K, Cohnert TU, Stock UA. Phenotypical plasticity of vascular smooth muscle cells—effect of in vitro and in vivo shear stress for tissue engineering of blood vessels. Tissue Eng. 2007;13:2505–2514. doi: 10.1089/ten.2006.0424. [DOI] [PubMed] [Google Scholar]
- 113.Papadaki M, McIntire LV, Eskin SG. Effects of shear stress on the growth kinetics of human aortic smooth muscle cells in vitro. Biotechnol. Bioeng. 1996;50:555–561. doi: 10.1002/(SICI)1097-0290(19960605)50:5<555::AID-BIT10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 114.Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J. Vasc. Res. 2012;49:463–478. doi: 10.1159/000339151. [DOI] [PubMed] [Google Scholar]
- 115.Park JS, Chu JSF, Cheng C, Chen F, et al. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol. Bioeng. 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 116.Tock J, Van Putten V, Stenmark KR, Nemenoff RA. Induction of SM-α-actin expression by mechanical strain in adult vascular smooth muscle cells is mediated through activation of JNK and p38 MAP kinase. Biochem. Biophys. Res. Commun. 2003;301:1116–1121. doi: 10.1016/s0006-291x(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 117.Qu MJ, Liu B, Wang HQ, Yan ZQ, et al. Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J. Vasc. Res. 2007;44:345–353. doi: 10.1159/000102278. [DOI] [PubMed] [Google Scholar]
- 118.Liu B, Qu M-J, Qin K-R, Li H, et al. Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophys. J. 2008;94:1497–1507. doi: 10.1529/biophysj.106.098574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Joki N, Kaname S, Hirakata M, Hori Y, et al. Tyrosine-kinase dependent TGF-β and extracellular matrix expression by mechanical stretch in vascular smooth muscle cells. Hypertens. Res. 2000;23:91–99. doi: 10.1291/hypres.23.91. [DOI] [PubMed] [Google Scholar]
- 120.O’Callaghan CJ, Williams B. Mechanical strain–induced extracellular matrix production by human vascular smooth muscle cells. Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 121.Isenberg BC, Tranquillo RT. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann. Biomed. Eng. 2003;31:937–949. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 122.Jeong SI, Kwon JH, Lim JI, Cho S-W, et al. Mechano-active tissue engineering of vascular smooth muscle using pulsatile perfusion bioreactors and elastic PLCL scaffolds. Biomaterials. 2005;26:1405–1411. doi: 10.1016/j.biomaterials.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 123.Wang C, Cen L, Yin S, Liu Q, et al. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials. 2010;31:621–630. doi: 10.1016/j.biomaterials.2009.09.086. [DOI] [PubMed] [Google Scholar]
- 124.Xu ZC, Zhang WJ, Li H, Cui L, et al. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials. 2008;29:1464–1472. doi: 10.1016/j.biomaterials.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 125.Thakar RG, Cheng Q, Patel S, Chu J, et al. Cell-shape regulation of smooth muscle cell proliferation. Biophys. J. 2009;96:3423–3432. doi: 10.1016/j.bpj.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thakar RG, Ho F, Huang NF, Liepmann D, Li S. Regulation of vascular smooth muscle cells by micropatterning. Biochem. Biophys. Res. Commun. 2003;307:883–890. doi: 10.1016/s0006-291x(03)01285-3. [DOI] [PubMed] [Google Scholar]
- 127.Williams C, Brown XQ, Bartolak-Suki E, Ma H, et al. The use of micropatterning to control smooth muscle myosin heavy chain expression and limit the response to transforming growth factor β1 in vascular smooth muscle cells. Biomaterials. 2011;32:410–418. doi: 10.1016/j.biomaterials.2010.08.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29:2597–2607. doi: 10.1016/j.biomaterials.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moussallem MD, Olenych SG, Scott SL, Keller TCS, Schlenoff JB. Smooth muscle cell phenotype modulation and contraction on native and cross-linked polyelectrolyte multilayers. Biomacromolecules. 2009;10:3062–3068. doi: 10.1021/bm9007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y, Chan-Park MB. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials. 2010;31:1158–1170. doi: 10.1016/j.biomaterials.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 131.Robinson HC, Baker AH. How do microRNAs affect vascular smooth muscle cell biology? Curr. Opin. Lipidol. 2012;23:405–411. doi: 10.1097/MOL.0b013e32835719a1. [DOI] [PubMed] [Google Scholar]
- 132.Švorčík V, Kolářová K, Slepička P, Macková A, et al. Modification of surface properties of high and low density polyethylene by Ar plasma discharge. Polym. Degrad. Stab. 2006;91:1219–1225. [Google Scholar]
- 133.Bailey SRM, Flavahan S, Bergdall VK, Flavahan NA. In vivo endothelial denudation disrupts smooth muscle caveolae and differentially impairs agonist-induced constriction in small arteries. J. Cardiovasc. Pharm. 2007;49:183–190. doi: 10.1097/FJC.0b013e318031d5dd. [DOI] [PubMed] [Google Scholar]