Abstract
Aims
We tested the hypothesis that body fat percentage determines cardiac sympathovagal balance in healthy subjects.
Main methods
Heart rate variability (HRV) measurements were made of the standard deviation of the normal– normal RR intervals (SDNN) and the low frequency/high frequency (LF/HF) ratio, from time domain and fast Fourier transform spectral analysis of electrocardiogram RR intervals during trials of uncontrolled and controlled (paced) breathing at 0.2 Hz. Body fat percentage was measured by dual energy x-ray absorptiometric (DEXA) scanning. Significance of differences between uncontrolled and controlled (paced) breathing was determined by analysis of variance and correlations between body fat percentage and HRV measurements by Pearson's coefficient at P<0.05.
Key findings
Percent body fat was negatively correlated with LF/HF during the uncontrolled breathing (r= −0.56, two-tailed P<0.05, one-tailed P<0.01) but not during the paced breathing trial (r=−0.34, (P>0.1).
Significance
We conclude that sympathetic activity produced by paced breathing at 0.2 Hz can obscure the relationship between body fat percentage and sympathovagal balance and that high body fat percentage may be associated with low sympathetic modulation of the heart rate in healthy adolescent/young adult males.
Keywords: Autonomic nervous system, Cardiovascular physiology, Heart rate variability, African-Americans, Males
Introduction
Heart rate variability analyses are a popular means to assess autonomic modulation (Lucini et al. 2002) which make it possible to differentiate a wide variety of conditions with common autonomic etiologies (Vanninen et al. 1996; Narkiewicz et al. 1998; Salo et al. 2000; Gutierrez et al. 2002; Pichon et al. 2004). The use of time domain measurements to delineate autonomic modulation of the heart rate is useful because time domain measures do not require the rigorous acquisition and analysis criteria, i.e. stationarity, when compared to frequency domain analyses. Previous studies have shown correlations between increments in vagal signaling, time domain measurements and high frequency heart rate variability spectral power during paced breathing (De Meersman et al. 1995; Sanderson et al. 1996; Badra et al. 2001). However, the significance of increments in low frequency spectral power during paced breathing remains unclear. We have reported a positive correlation between the respiratory quotient and the low frequency/high frequency ratio of heart rate variability spectral power, measured during paced breathing at 0.2 Hz, in healthy males possessing a wide range of body fat (Millis et al. 2009). Although body fat percentage may be an important determinant of heart rate variability spectral power measured at rest (Nagai et al. 2003; Chen et al. 2008), an earlier study reported no influence of body fat on heart rate variability measurements at rest, only during an autonomic challenge (Matsumoto et al. 1999). Because body fat percentage and paced breathing at 0.2 Hz may affect low frequency and high frequency spectral power independently, we hypothesized that paced breathing at 0.2 Hz could mask the physiological relationship between body fat percentage and heart rate variability measures of autonomic modulation. We, therefore, performed this experiment to determine the effects of paced breathing and body fat percentage on time and frequency domain measures of heart rate variability in healthy adolescent/young adult African-American males.
Materials and methods
Study participants and design
This experimental protocol was approved by the Howard University Human Participants Institutional Review Board, and each subject provided informed consent. A study population of 10 healthy 18–20 year-old African-American male university students was recruited and 8 subjects were included in the experiment. Each subject was studied twice, on separate days. An unsupervised, self reported period of overnight fasting (mean ± SD 12 ± 2 h) was used to limit the potentially confounding effects of diet related differences in autonomic responsiveness that we have described (Millis et al. 2009). Two subjects were excluded because of inadequate fasting as determined by respiratory quotient measurements >0.85. Other criteria for inclusion in the experiment were non-smoking status, absence of alcohol abuse (less than two standard alcohol drinks a day), absence of use of medication that could interfere with autonomic modulation, resting systolic/diastolic blood pressure <140/90 mm Hg. Table 1 summarizes the relevant characteristics of this experimental group. The respiratory quotient indicates utilization of fatty acids as the main energy substrate and the low frequency/high frequency ratio shows a predominance of vagal modulation of the heart rate.
Table 1.
Characteristics of study participants.
| Age (years) | 19 ± 1 |
| Weight (kg) | 82 ±25 |
| Height (cm) | 174 ± 20 |
| Body mass index (kg m–2) | 27±8 |
| Systolic blood pressure (mm Hg) | 130±13 |
| Diastolic blood pressure (mm Hg) | 70±10 |
| Heart rate (beats min–1) | 65±12 |
| Respiratory quotient | 0.75 ±0.05 |
| Energy expenditure (Cal d–1) | 1980 ±369 |
| Body temperature (°F) | 97±1 |
Values in mean ± standard deviation, n =8.
Uncontrolled and paced breathing
The subjects were instrumented and instructed as to the experimental procedures. Subjects were instructed to breathe normally while lying recumbent at 45° in a bed of the General Clinical Research Center (GCRC) at Howard University Hospital. Following the normal uncontrolled breathing protocol, subjects were instructed to perform 5 min of paced breathing by following a visual tracking image on a computer monitor for periodic durations of inspirations and expirations set to 12 breaths min−1 (0.2 Hz). Each subject practiced paced breathing for a period of 1–3 min and was then instructed to perform the paced breathing for the 5 min paced breathing trial during which time the electrocardiogram signal was recorded using a Biopac MP100 data acquisition system (Biopac Systems, Santa Barbara, CA). The electrocardiogram electrodes were placed on the subject's chest in a standard three-lead position with recordings obtained from standard lead II.
Heart rate variability analyses
Heart rate was measured in beats min−1 and vagal modulation of heart rate variability in the time domain was measured as the standard deviation of all normal-to-normal standard electrocardiogram inter-beat intervals (SDNN). Time domain heart rate variability, measured as standard deviation of the RR intervals, was expressed in ms and was computed using data acquisition and analysis software specifically designed to measure heart rate variability in time and frequency domains. Fast Fourier transform analysis of the electrocardiogram RR intervals was used to spectrally decompose heart rate variability in the frequency domain. For the frequency domain analysis, vagal modulation was represented by the area under the high frequency power spectrum (HF: 0.15–0.4 Hz) and low frequency (LF: 0.04–0.14 Hz) expressed as the power in raw ms2 and in normalized units using specialized autonomic neural software (Nevrokard, Version 6.3, Ljubljana, Slovenia).We used the low frequency/high frequency (LF/HF) ratio of heart rate variability power as a measure of cardiac sympathovagal balance. Although this concept has received some criticism (Eckberg 1999; Badra et al. 2001), it is generally accepted that high frequency power measures vagal respiratory components; whereas, low frequency power measures sympathetic and various non-respiratory vagal inputs to heart rate variability (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996). All time and frequency domain analyses were carried out in accordance with the guidelines put forth by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). The very low frequency band (0.001–0.04 Hz) was ignored because, according to the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) guidelines, contribution of this frequency band may be too small to be a reliable indicator during short-term recordings.
Anthropomorphic, cardiovascular and metabolic measurements
Body weight and height were measured (Detecto scale) and these values were used to compute body mass index as the quotient kg body weight/m2 height. Blood pressure was determined using an automated sphygmomanometer (Criticare Systems Model 506DXNT, Waukesha, WI). To validate the effectiveness of overnight fasting, respiratory quotient and resting energy expenditure were measured by indirect calorimetry using an isolated flow-directed breathing chamber (Deltatrac, SensorMedics, Yorba Linda, CA). The participants were taken to the Howard University Exercise Science Laboratory for an assessment of the body fat percentage measured by dual energy x-ray absorptiometric (DEXA) whole body scanning (LUNAR Model DPX-L DEXA, Madison, WI).
Statistical analyses
The study design consisted of a comparison of measurements of resting heart rate variability during trials of uncontrolled versus controlled (paced) breathing at 0.2 Hz in 8 subjects studied twice (n=16). The significance of differences between the uncontrolled and paced breathing trials was evaluated by analysis of variance using a multivariate general linear model with significance set at P<0.05. A correlation analysis between the heart rate variability and body fat percentage measurements after overnight fasting was based on linear regression and Pearson's correlation coefficient during the uncontrolled versus the paced breathing trials with significance at P<0.05. A statistical software package was used for the computations and analyses (SPSS, Chicago, IL).
Results
Table 2 summarizes the heart rate variability data and Table 3 presents the correlations between body fat percentage and heart rate variability for the uncontrolled breathing and for the paced breathing trials. The low frequency power was significantly greater during the paced breathing than during the uncontrolled breathing trial (P<0.05). The differences for heart rate were not significant and for SDNN and total, high frequency and low frequency/high frequency ratio of heart rate variability spectral power measured during the uncontrolled versus the paced breathing trial were marginally significant (P=0.1–0.2), showing a trend toward greater low frequency/high frequency power during the paced breathing trial.
Table 2.
Heart rate variability measures of sympathovagal balance.
| Heart rate variability measurements | Uncontrolled breathing | Paced breathing at 0.2 Hz |
|---|---|---|
| Total power (nu) | 122±6 | 140 ±19 (N.S.) |
| Low frequency power (nu) | 37±1 | 54 ±5* |
| High frequency power (nu) | 62 ±3 | 67 ±5 (N.S.) |
| Low frequency/high frequency ratio | 0.6 ± 0.09 | 0.8 ±0.3 (N.S.) |
Values in mean±standard error, nu = normalized units, n=16.
N.S. = Differences between uncontrolled and paced breathing not significant (P=0.1– 0.2).
Differences between uncontrolled and paced breathing significant at P<0.05.
Table 3.
Correlations of heart rate variability and body fat percentage.
| Heart rate variability measurements | Uncontrolled breathing | Paced breathing at 0.2 Hz |
|---|---|---|
| Total power (nu) | −0.54* | − 0.54* |
| Low frequency power (nu) | −0.53* | −0.23 |
| High frequency power (nu) | 0.48* | 0.23 |
| Low frequency/high frequency ratio | − 0.56* | −0.34 |
Values in Pearson's correlation coefficient, nu = normalized units, n=16.
Statistically significant at P<0.05.
The correlations between body fat percentage and all the heart rate variability measures of sympathovagal balance were significant for the uncontrolled breathing trial. Only the correlation between body fat percentage and total spectral power was significant for the paced breathing trial. The correlations between body mass index and the heart rate variability measures of sympathovagal balance were not significant. Fig. 1 graphically depicts the results of the linear regression analysis showing the significant negative correlation between the percentage of body fat and the low frequency/high frequency measurements of sympathovagal balance for the uncontrolled, but not for the paced, breathing trials.
Fig. 1.
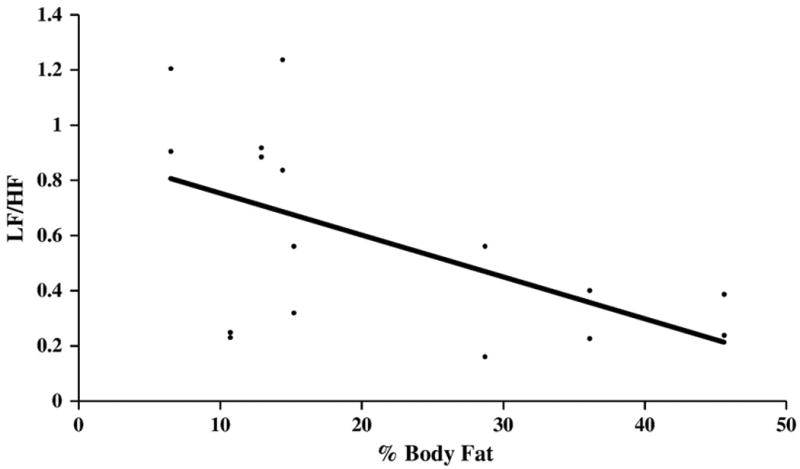
Correlation of body fat percentage and heart rate variability during spontaneous breathing. Linear regression for percent body fat measured by dual x-ray absorptiometric (DEXA) whole body scanning and heart rate variability low frequency/high frequency power ratio (LF/HF) computed from fast Fourier transform analysis of the electrocardiogram RR intervals in normalized units measured during uncontrolled breathing for eight healthy 18–20 year-old African-American male university students studied twice (n=16). Pearson's correlation coefficient, r=−0.56, two-tailed P<0.05, one-tailed P<0.01 for uncontrolled breathing.
Discussion
One of the aims of this experiment was to show the effects of paced breathing at 0.2 Hz, compared to uncontrolled breathing, on time and frequency domain measures of heart rate variability (Figs. 1 and 2). The results showed a marginally significant trend for paced breathing at 0.2 Hz to increase heart rate variability in the time domain, measured by SDNN, and to shift sympathovagal balance, measured by lowfrequency/high frequency spectral power, toward greater sympathetic activity. Because of the respiration-related variability (respiratory sinus arrhythmia) of electrocardiogram inter-beat (RR) intervals, the necessity of controlling respiratory frequency during measurements of heart rate variability has been demonstrated (De Meersman et al. 1995; Sanderson et al. 1996; Badra et al. 2001). Several mechanisms have been attributed to this observation, e.g., the respiratory sinus arrhythmia might be amplified due to increased tidal volume (De Meersman et al. 1995). A higher low frequency/high frequency ratio of spectral power was also found during paced breathing. Detailed studies performed in a group of nine, predominantly male, healthy supine adults have shown that changes in low frequency power resulted from intrinsic sympathetic oscillations and that controlling respiratory frequency at 0.25 Hz, near to that of our subjects, had no significant effect on low frequency power (Badra et al. 2001). In a group of ten normal supine and upright adults, respiratory frequencies controlled at 0.17 Hz, 0.25 Hz and 0.33 Hz modulated high frequency power but had no effect on low frequency power; however, the postural change supine to upright increased low frequency power (Sanderson et al. 1996). Tidal volume is also reported to be a modulator of the heart rate variability spectrum by increasing high frequency power (Grossman et al. 2004; Pöyhönen et al. 2004) and 0.2 Hz paced breathing usually increases tidal volume (Pinna et al. 2006) which is what we observed visually in the actions of the respiratory muscles. Although our subjects were lying recumbent during both the paced and uncontrolled breathing conditions studied, it is plausible that sympathetic oscillations, akin to those associated with changing posture, may have systematically increased low frequency power and brought about a higher low frequency/high frequency ratio of heart rate variability spectral power during paced breathing. Such modulation of low frequency power has been shown to occur in association with an increased respiratory rate during conditions of mental stress (Bernardi et al. 2000) and could have occurred in the present experiment because of differences in tidal volumes during the paced breathing and/or because of differences in respiratory frequency during the uncontrolled breathing trials. One of the limitations of this study is the absence of tidal volume and respiratory frequency data for the paced and uncontrolled breathing trials. In the absence of such data, we cannot know whether the negative correlation between the percentages of body fat and the heart rate variability is because an obese person has faster respiratory frequency and lower tidal volume during the uncontrolled breathing. At the 0.2 Hz (12/min) paced breathing, tidal volume may have been, systematically, higher than during the uncontrolled breathing. The paced breathing may just have corrected the tidal volume variation between the high body fat and low body fat subjects. A future study correlating the body fat percentage with respiratory frequency and tidal volume will help to address this issue. In the absence of tidal volume data, we used paced breathing at 0.2 Hz to control for changes in respiratory frequency, the main modulator of high frequency spectral power. We found that the difference in high frequency power between the paced and spontaneous breathing trials was not significant. This finding suggests that the increase in low frequency power associated with paced breathing at 0.2 Hz is, likely, indicative of an increase in sympathetic, rather than a decrease in vagal modulation of the heart rate. We also found that the negative correlation between body fat percentage and low frequency/high frequency spectral power was significant only for the spontaneous breathing trials. These findings suggest that the effects of a greater low frequency power during paced breathing may have counteracted those of a lesser low frequency power associated with a greater percentage of body fat on the low frequency/high frequency measure of sympathovagal balance.
Fig. 2.
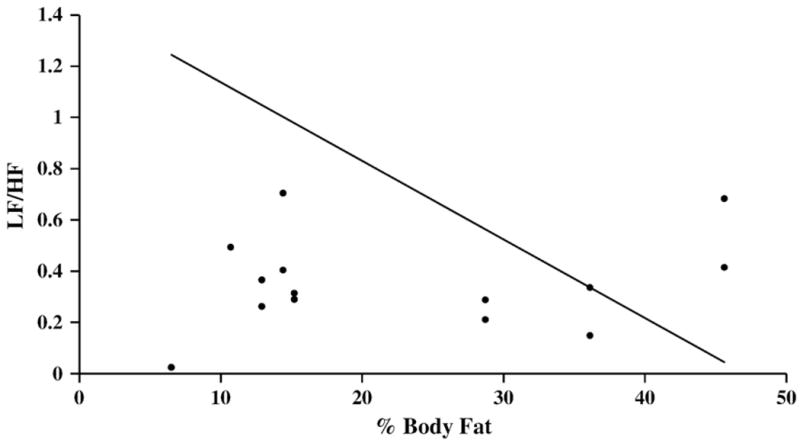
Correlation of body fat percentage and heart rate variability during paced breathing. Linear regression for percent body fat measured by dual x-ray absorptiometric (DEXA) whole body scanning and heart rate variability low frequency/high frequency power ratio (LF/HF) computed from fast Fourier transform analysis of the electrocardiogram RR intervals in normalized units measured during paced breathing at 0.2 Hz for eight healthy 18–20 year-old African-American male university students studied twice (n=16). Pearson's correlation coefficient for controlled paced breathing at 0.2 Hz was not significant (r=−0.34).
Another aim of this experiment was to demonstrate the relationship between percentage of body fat and time and frequency domain measures of heart rate variability. The results showed significant, negative correlations between the total and regional percentages of body fat (but not the body mass index) and the heart rate variability spectral measures of sympathovagal balance during the condition of uncontrolled, but not during that of 0.2 Hz paced, breathing. These findings suggest that, in healthy young adult males, the sympathetic activation produced by paced breathing at 0.2 Hz may mask the association of low sympathetic modulation of the heart rate with high percentage of body fat. In a previous study, we used paced breathing at 0.2 Hz to limit the variations in respiratory frequency which could contribute to differences in low frequency/high frequency heart rate variability spectral power and sympathovagal balance for differentiating the effects of metabolizing fat versus those of metabolizing carbohydrate (Millis et al. 2009). We designed this experiment to determine the correlation between percentage of body fat and sympathovagal balance. Interestingly, we found that body fat may have influenced the measures of sympathovagal balance oppositely to that of paced breathing. Whereas paced breathing at 0.2 Hz produced a marginally significant increase in low frequency/high frequency heart rate variability spectral power, an increased percentage of body fat was associated with a decreased low frequency/high frequency ratio. Hence, paced breathing at 0.2 Hz could have masked or otherwise obscured the relationship between percentage of body fat and the heart rate variability measures of sympathovagal balance. Consequently, the physiological relationship between adiposity and sympathovagal balance was found to be significant during uncontrolled breathing but not during paced breathing at 0.2 Hz.
The association of a low percentage of body fat and a shift in heart rate variability spectral power toward greater vagal modulation reported in this study may be similar to the association of shifts in sympathovagal balance toward greater vagal modulation during the ingestion of water in healthy young subjects (Routledge et al. 2002). Our finding is in contrast to the association of a high percentage of body fat and a shift in heart rate variability spectral power toward greater sympathetic modulation observed in this study and shifts in sympathovagal balance toward greater sympathetic modulation reported in children, adolescents and adults after ingesting food and during orthostatic tilting (Piccirillo et al. 1998; Paolisso et al. 2000; Martini et al. 2001; Rabbia et al. 2003; Guízar et al. 2005; Kaufman et al. 2007a,b; Nagai and Moritani 2004) and in adult males studied after myocardial infarction (Piestrzeniewicz et al. 2008). Low frequency/high frequency heart rate variability spectral power is an accepted measure of cardiac sympathovagal balance and it may also reflect the autonomic sympathetic–parasympathetic balance at skeletal muscle and adipose tissues. High body mass index appears to be associated with a shift in cardiac sympathovagal balance toward greater sympathetic activity with higher plasma leptin values, greater orthostatic responsiveness (Paolisso et al. 2000) and greater lipolytic activity (Berlan et al. 2002; Tentolouris et al. 2008). In light of these associations, our present findings of a negative correlation between percentage of body fat, but not of body mass index, and sympathetic modulation suggest physiological adaptations favoring greater adiposity and a limitation to lipolysis and/or adipokinesis in apparently healthy young adult African-American males.
Conclusion
In summary, frequency domain measures of heart rate variability suggested a trend toward greater sympathetic activity during paced breathing at 0.2 Hz compared to uncontrolled spontaneous breathing in healthy adolescent/young adult males. These measures of heart rate variability, indicative of a shift in cardiac sympathovagal balance, toward greater vagal activity, were associated with a high percentage of body fat.
Acknowledgments
Supported in part by grants from the Cooperative Extension Service of the University of the District of Columbia & United States Department of Agriculture, Center for Diet, Nutrition & Health, from the Mordecai Wyatt Johnson Fund and by the Howard University General Clinical Research Center grant [M01 RR10284]), funded through the National Institutes of Health National Center for Research Resources, and the VIDDA foundation.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Respiratory modulation of human autonomic rhythms. American Journal of Physiology. Heart and Circulatory Physiology. 2001;280(6):H2674–H2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- Berlan M, Verhaeghe S, Pavy-Le Traon A, Thalamas C, Lafontan M, Marques MA, Sennard JM, Parent M, Galitzky J. Yohimbine administration prevents over-responsiveness to epinephrine induced by simulated microgravity. Aviation Space and Environmental Medicine. 2002;73(8):735–742. [PubMed] [Google Scholar]
- Bernardi L, Wdowczyk-Szulc J, Valoenti C, Castoldi S, Passino C, Spadacini G, Sleight P. Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. Journal of the American College of Cardiology. 2000;35(6):1462–1469. doi: 10.1016/s0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]
- Chen GY, Hsiao TJ, Lo HM, Kuo CD. Abdominal obesity is associated with autonomic nervous derangement in healthy Asian obese subjects. Clinical Nutrition. 2008;27(2):212–217. doi: 10.1016/j.clnu.2007.11.004. [DOI] [PubMed] [Google Scholar]
- De Meersman RE, Reisman SS, Daum M, Zorowitz R, Leifer M, Findley T. Influence of respiration on metabolic, hemodynamic, psychometric, and R–R interval power spectral parameters. American Journal of Physiology. 1995;269(4Pt2):H1437–H1440. doi: 10.1152/ajpheart.1995.269.4.H1437. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Mathematical treatment of autonomic oscillations. Circulation. 1999;100(15):63–64. doi: 10.1161/01.cir.100.15.e63. [DOI] [PubMed] [Google Scholar]
- Grossman P, Wilhelm FH, Spoerle M. Respiratory sinus arrhythmia, cardiac vagal control, and daily activity. American Journal of Physiology. Heart and Circulatory Physiology. 2004;287(2):H728–H734. doi: 10.1152/ajpheart.00825.2003. [DOI] [PubMed] [Google Scholar]
- Guízar JM, Ahuatzin R, Amador N, Sánchez G, Romer G. Heart autonomic function in overweight adolescents. Indian Pediatrics. 2005;42(5):464–469. [PubMed] [Google Scholar]
- Gutierrez J, Santiesteban R, Garcia H, Voustianiouk A, Freeman R, Kaufmann H. High blood pressure and decreased heart rate variability in the Cuban epidemic neuropathy. Journal of Neurology, Neurosurgery and Psychiatry. 2002;73(1):71–72. doi: 10.1136/jnnp.73.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CL, Kaiser DR, Steinberger J, Dengel DR. Relationships between heart rate variability, vascular function, and adiposity in children. Clinical Autonomic Research. 2007b;17(3):165–171. doi: 10.1007/s10286-007-0411-6. [DOI] [PubMed] [Google Scholar]
- Kaufman CL, Kaiser DR, Steinberger J, Kelly AS, Dengel DR. Relationships of cardiac autonomic function with metabolic abnormalities in childhood obesity. Obesity (Silver Spring) 2007a;15(5):1164–1171. doi: 10.1038/oby.2007.619. [DOI] [PubMed] [Google Scholar]
- Lucini D, Guzzetti S, Casaraghi S, Pagani M. Correlation between baroreflex gain and 24-h indices of heart rate variability. Journal of Hypertension. 2002;20(8):1625–1631. doi: 10.1097/00004872-200208000-00026. [DOI] [PubMed] [Google Scholar]
- Martini G, Riva P, Rabbia F, Molini V, Ferrero GB, Cerutti F, Carra R, Veglio F. Heart rate variability in childhood obesity. Clinical Autonomic Research. 2001;11(2):87–91. doi: 10.1007/BF02322051. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Miyawaki T, Ue H, Kanda T, Zenji C, Moritani T. Autonomic responsiveness to acute cold exposure in obese and non-obese young women. International Journal of Obesity and Related Metabolic Disorders. 1999;23(8):793–800. doi: 10.1038/sj.ijo.0800928. [DOI] [PubMed] [Google Scholar]
- Millis RM, Austin RE, Bond V, Faruque M, Goring KL, Hickey BM, Blakely R, DeMeersman RE. Effects of high-carbohydrate and high-fat dietary treatments on measures of heart rate variability and sympathovagal balance. Life Sciences. 2009;85(3–4):141–145. doi: 10.1016/j.lfs.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Nagai N, Matsumoto T, Kita H, Moritani T. Autonomic nervous system activity and the state and development of obesity in Japanese school children. Obesity Research. 2003;11(1):25–32. doi: 10.1038/oby.2003.6. [DOI] [PubMed] [Google Scholar]
- Nagai N, Moritani T. Effect of physical activity on autonomic nervous system function in lean and obese children. International Journal of Obesity and Related Metabolic Disorders. 2004;28(1):27–33. doi: 10.1038/sj.ijo.0802470. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98(20):1071–1077. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- Piestrzeniewicz K, Łuczak K, Lelonek M, Wranicz JK, Goch JH. Obesity and heart rate variability in men with myocardial infarction. Cardiology Journal. 2008;15(1):43–49. [PubMed] [Google Scholar]
- Paolisso G, Manzella D, Montano N, Gambardella A, Varricchio M. Plasma leptin concentrations and cardiac autonomic nervous system in healthy subjects with different body weights. Journal of Clinical Endocrinology and Metabolism. 2000;85(5):1810–1814. doi: 10.1210/jcem.85.5.6511. [DOI] [PubMed] [Google Scholar]
- Piccirillo G, Vetta F, Viola E, Santagada E, Ronzoni S, Cacciafesta M, Marigliano V. Heart rate and blood pressure variability in obese normotensive subjects. International Journal of Obesity and Related Metabolic Disorders. 1998;22(8):741–750. doi: 10.1038/sj.ijo.0800650. [DOI] [PubMed] [Google Scholar]
- Pichon AP, De Bisschop C, Roulaud M, Pichon AP, De Bisschop C, Roulaud M. Spectral analysis of heart rate variability during exercise in trained subjects. Medicine and Science in Sports and Exercise. 2004;36(10):1702–1708. doi: 10.1249/01.mss.0000142403.93205.35. [DOI] [PubMed] [Google Scholar]
- Pinna GD, Maestri R, La Rovere MT, Gobbi E, Fanfulla F. Effect of paced breathing on ventilatory and cardiovascular variability parameters during short-term investigations of autonomic function. American Journal of Physiology. Heart and Circulatory Physiology. 2006;290(1):H424–H433. doi: 10.1152/ajpheart.00438.2005. [DOI] [PubMed] [Google Scholar]
- Pöyhönen M, Syväoja S, Hartikainen J, Ruokonen E, Takala J. The effect of carbon dioxide, respiratory rate and tidal volume on human heart rate variability. Acta Anaesthesiologica Scandinavica. 2004;48(1):93–101. doi: 10.1111/j.1399-6576.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- Rabbia F, Silke B, Conterno A, Grosso T, De Vito B, Rabbone I, Chiandussi L, Veglio F. Assessment of cardiac autonomic modulation during adolescent obesity. Obesity Research. 2003;11(4):541–548. doi: 10.1038/oby.2003.76. 2004. [DOI] [PubMed] [Google Scholar]
- Routledge HC, Chowdhary S, Coote JH, Townend JN. Cardiac vagal response to water ingestion in normal human subjects. Clinical Science (London) 2002;103(2):157–162. doi: 10.1042/cs1030157. [DOI] [PubMed] [Google Scholar]
- Salo TM, Jula AM, Piha JS, Kantola IM, Pelttari L, Rauhala E, Metsala TH, Jalonen JO, Voipio-Pulkki LM, Viikari JS. Comparison of autonomic withdrawal in men with obstructive sleep apnea syndrome, systemic hypertension, and neither condition. American Journal of Cardiology. 2000;85(2):232–238. doi: 10.1016/s0002-9149(99)00638-4. [DOI] [PubMed] [Google Scholar]
- Sanderson JE, Yeung LY, Yeung DT, Kay RL, Tomlinson B, Critchley JA, Woo KS, Bernardi L. Impact of changes in respiratory frequency and posture on power spectral analysis of heart rate and systolic blood pressure variability in normal subjects and patients with heart failure. Clinical Science (London, England) 1996;91(1):35–43. doi: 10.1042/cs0910035. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology the North American Society. Heart rate variability, standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Tentolouris N, Pavlatos S, Kokkinos A, Perrea D, Pagoni S, Katsilambros N. Diet-induced thermogenesis and substrate oxidation are not different between lean and obese women after two different isocaloric meals, one rich in protein and one rich in fat. Metabolism. 2008;57(3):313–320. doi: 10.1016/j.metabol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Vanninen E, Tuunainen A, Kansanen M, Uusitupa M, Lansimies E. Cardiac sympathovagal balance during sleep apnea episodes. Clinical Physiology (Oxford, England) 1996;16(3):209–216. doi: 10.1111/j.1475-097x.1996.tb00569.x. [DOI] [PubMed] [Google Scholar]


