Abstract
Exposure to airborne particulate pollutants is intimately linked to vascular oxidative stress and inflammatory responses with clinical relevance to atherosclerosis. Particulate matter (PM) has been reported to induce endothelial dysfunction and atherosclerosis. Here, we tested whether ambient ultrafine particles (UFP, diameter < 200 nm) modulate eNOS activity in terms of nitric oxide (NO) production via protein S-glutathionylation. Treatment of human aortic endothelial cells (HAEC) with UFP significantly reduced NO production. UFP-mediated reduction in NO production was restored in the presence of JNK inhibitor (SP600125), NADPH oxidase inhibitor (Apocynin), anti-oxidant (N-acetyl cysteine), and superoxide dismutase mimetics (Tempol and MnTMPyP). UFP exposure increased the GSSG/GSH ratio and eNOS S-glutathionylation, whereas over-expression of Glutaredoxin-1 (to inhibit S-glutathionylation) restored UFP-mediated reduction in NO production by nearly 80%. Thus, our findings suggest that eNOS S-glutathionylation is a potential mechanism underlying ambient UFP-induced reduction of NO production.
Keywords: Ultrafine Particles/UFP, Oxidative Stress, eNOS, S-Glutathionylation, Endothelial dysfunction, Air Pollution
Introduction
Exposure to ambient particulate matter (PM) is increasingly recognized as a modifiable risk factor to cardiovascular morbidity and mortality [1]. Mice exposed to PM displayed accelerated atherosclerosis and increased lesion size [2]. Both ambient and diesel exhausted particles have been shown to induce endothelial dysfunctions in ApoE-null mice [3,4]. However, the mechanisms underlying UFP-induced endothelial dysfunction remain fairly unexplored.
Endothelial nitric oxide synthase (eNOS) plays a pivotal role in maintaining vascular homeostasis. eNOS is a source of both nitric oxide (NO) and superoxide (O2˙−). In the absence of tetrahydrobiopterin (BH4), eNOS is uncoupled to produce superoxide [5,6,7,8]. In the presence of BH4, NOS activity and NOS-dependent vasodilation were partially restored [5]. These observations led to the investigation of alternative mechanisms underlying the regulation of eNOS activity. Chen et al recently reported that S-glutathionylation uncouples eNOS activity via two mechanisms: 1) exchange of thiol-disulphide with oxidized glutathione, or 2) reaction of oxidant-induced protein thiyl radicals with reduced glutathione [9]. In this context, we sought to assess whether exposure to ambient ultrafine particles (UFPs) modulates eNOS activity.
Ambient ultrafine particles (UFP, diameter < 200 nm), highly enriched in transition metals and redox cycling organic chemicals, harbor potent toxic properties and oxidizing potential [10,11]. UFPs induce vascular oxidative stress via JNK signaling in endothelial cells [12,13], promoting inflammatory responses and reducing anti-oxidant capacity of high-density lipoprotein (HDL) in mouse model of atherosclerosis[2,14]. Oxidative stress modulates cellular protein S-glutathionylation [15,16,17,18], and an increase in reactive oxygen species (ROS) is implicated in a decrease in NO production [19]. However, whether UFP-induced oxidative stress would reduce NO production remain unclear
In this study, we assessed whether exposure to ambient ultrafine particulate pollutants modulated endothelial function via eNOS S-glutathionylation. We demonstrated that UFP significantly increased glutathione oxidation, protein S-glutathionylation, and eNOS S-glutathionylation, leading to a decrease in NO production, whereas over-expression of glutaredoxin-1 (Grx-1) to inhibit protein S-glutathionylation nearly rescued eNOS activity. Thus, S-glutathionylation of eNOS is implied as a potential mechanism underlying UFP-mediated reduction in NO production.
Materials and Methods
UFP collection, preparation, and characterization
UFP were collected at the University of Southern California (USC) campus near downtown Los Angeles on 20 × 25 cm Teflon coated filters (PALL Life Sciences, PTFE membrane, 2.0 μm, R2PJO37) using a High-Volume Particle Sampler [20]. PM mass was determined gravimetrically by pre- and post-weighing the high volume sampler filters, as discussed in greater detail in a previous publication [21]. The Teflon-coated glass fiber filters were split into different portions: ¼ was used for chemical analysis, whereas ¾ was used for the preparation of the exposure suspensions, as discussed in subsequent paragraphs. The ¼ of the Teflon filter was split in four equal parts; one part was analyzed by Shimadzu TOC-5000A liquid analyzer [22] for water soluble organic carbon (WSOC) and another one by ion chromatography (IC) technique for their inorganic ion content (i.e. sulfate, nitrate and ammonium). A third portion of the high volume sampler filter was analyzed by gas chromatography-mass spectrometry (GC/MS) for organic compounds [23], whereas the remaining portion was analyzed for UFP bound water-soluble metals and elements by means of Inductively Coupled Plasma – Mass Spectroscopy (ICP-MS) [24]. The chemical composition and size distribution of UFPs are reported in a previous publication by our groups [25](Chemical compositions are provided as a supplemental table). The remaining portion of the high volume samples was used to prepare for the UFP suspension for exposure tests. The filters were first soaked in 10 ml of ultra-pure milli-Q water (USP grade) for 30 minutes in endotoxin-free glass vial, followed by sonication for 30 minutes [26]. After the particle suspension was transferred to endotoxin-free tube, another 10 ml of ultra-pure water was used to repeat the aforementioned process. Our control (i.e. particle – free) suspension was prepared by extracting a blank filter, akin to the ones used for particulate matter (PM) collection in the USP grade water, using the aforementioned procedures. The UFP suspension was aliquoted and stored at −80 °C to maintain chemical stability.
Measurement of NO production
eNOS activity was assessed by measuring NO production using Nitrate/Nitrite Colorimetric Assay Kit from Cayman Chemicals. Due to the presence of nitrate/nitrite in UFP that gave high background, we prepared cell lysate to measure NO production.
Human aortic endothelial cells (HAEC) (Cell Application) were cultured with endothelial cell growth media (Cell Application). The cells were used between passages 5 and 9. For UFP treatment, confluent HAEC were incubated in the presence or absence of UFPs in M199/0.1% FBS (Invitrogen) for 6 hours. After washing with PBS, the cells were lysed with PBS/0.2% Triton X-100 and scraped into 1.5ml tubes. After incubation at 4°C for 30 minutes, the supernatants were collected as lysate for NO production measurement following manufacturer's instruction and for protein assay using BioRad DCP protein assay kit. NO production was normalized to protein concentration relative to control.
To assess NO production in the presence of inhibitors, HAEC were pretreated with JNK inhibitor SP600125 (2μM), NADPH oxidase inhibitor Apocynin (200μM), an anti-oxidant N-acetyl cysteine (NAC, 1mM), or superoxide dismutase mimetics, Tempol (200μM), and MnTMPyP (2μg/mL)) for 30 minutes, followed by treatment with or without 50 μg/mL of UFP in the presence of inhibitors. Cell lysates were then prepared for the measurement of NO production. The inhibitor concentration used in the experiments was lower than that of standard usage and was optimized to minimize the interference with background NO production.
S-glutathionylation of eNOS
Quantification of eNOS S-glutathionylation was performed with a modified protocol from Cayman Chemical. Briefly, HAEC in 100mm dishes were grown to confluent. Cells were treated with or without 50μg/mL of UFP for 6 hours in M199/0.1% FBS. After washing with PBS, cells were collected by trypsinization and re-suspended in 1mL PBS. The cells were then fixed with PBS/3.7% Formaldehyde for 20 min at room temperature, followed by cell lysis. S-glutathionylation proteins were labeled with Biotin following the manufacturer's instructions. The protein lysate was used for ELISA to measure eNOS S-glutathionylation. Briefly, aforementioned protein lysate was added to the 96-well plates coated with NeutrAvidin (Pierce), and incubated for 30 minutes at room temperature. After rinsing with washing buffer (PBS/0.05% Tween-20), anti-eNOS antibody (1:1000, Cell signaling) in Blocking buffer (PBS/1%BSA/0.05% Tween-20) was added. After incubation for 1 hour at room temperature, the wells were rinsed and HRP-anti-Rabbit Ig secondary antibody (1:1000) was added. After 30 minutes, the wells were rinsed and TMB turbo substrate (Pierce) was added. After color development, 2M of sulfuric acid was added to cease the reaction. Optical density at 450nm (OD450) was measured as readout of eNOS S-glutathionylation
Measurement of Glutathione
Levels of glutathione (GSH) were quantified by using Glutathione Assay Kit (Cayman Chemical) according to the manufacturer's instruction. Oxidized glutathione (GSSG) was measured by using GSH derivatizing reagent 2-vinylpyridine using the alternative protocol from the manufacturer. The concentration of GSH and GSSG was normalized to control by protein concentration.
Western Blots
HAEC were grown to confluence, and treated with or without UFP. Cell lysate preparation and western blots were performed as previously described [13]. To assess the role of oxidative stress on eNOS S-glutathionylation, we infected HAEC with glutaredoxin-1 (Grx-1) recombinant adenovirus (kindly provided by Dr. Mieyal at Case Western Reserve University) or with the control LacZ recombinant adenovirus overnight. The levels of Grx-1 expression were assessed by western blot with anti Grx-1 antibody (Abcam).
Immunohistochemistry
LDLR-null mice were exposed to filtered air (FA) or UFP as described[14]. After exposure for ten weeks, the heart tissues of mice were dissected and paraffin blocks were made. The heart sections were stained with anti-GSH antibody (1:100) (Virogen) for visualizing protein S-glutathionylation with standard immunohistochemistry procedure as described previously[27].
Statistical Analysis
All of the experiments were performed for three or more trials. Data were expressed as mean ± standard deviation (SD). Student t-test was used for significance analysis. A P value of < 0.05 was considered statistically significant.
Results
UFP-induced oxidative stress reduced endothelial NO production
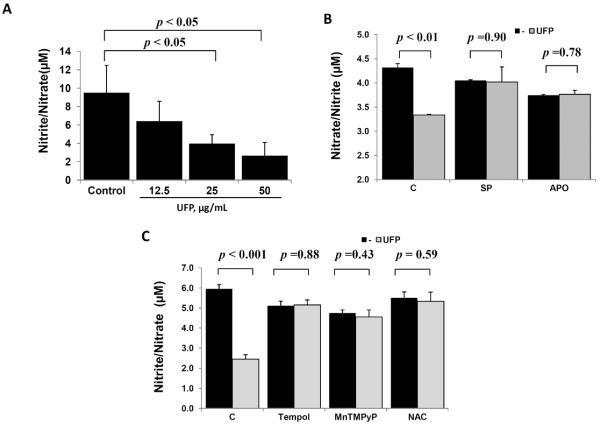
UFP induced JNK-dependent oxidative stress in vascular cells [13]. Here, we demonstrate that significantly reduced NO production in terms of nitrite/nitrate concentration in a dose dependent manner (control=9.5±3.0 μM; UFP at 12.5μg/mL=6.4 ±2.2 μM; UFP at 25μg/mL 4.0±1.0, p < 0.05 vs. control; UFP at 50μg/mL 2.7±1.4, p < 0.05 vs. Control; n = 3) (Fig. 1A). For this reason, UFP concentration at 50 μg/mL was arbitrarily used for the subsequent studies.
Fig. 1. UFP-induced oxidative stress reduced NO production.
(A) HAEC were treated with an incremental concentration of UFP for 6 hours. NO production was assessed in terms of Nitrite /Nitrate concentration in cell lysate. (B) and (C) HAEC were pre-treated with 2μM of SP600125 (SP) or 200 μM of Apocynin (APO), and with SOD1 mimetics, Tempol, at 200μM, SOD2 mimetic, MnTMPyP, at 2μg/mL, or an antioxidant N-acetyl Cysteine (NAC, 1mM) for 30 minutes, followed by co-treatment with or without 50μg/mL of UFP for 6 hours. Inhibition of JNK or NADPH oxidase in (B) as well as mitigation of ROS by antioxidants in (C) restored UFP-induced inhibition on NO production (n=3).
In the presence of JNK inhibitor (SP600125) and NADPH oxidase inhibitor (Apocynin), UFP-mediated reduction in NO production was restored (Fig. 1B). Similarly, superoxide dismutase mimetics, both Tempol (SOD1) and MnTMPyP (SOD2), as well as anti-oxidant, N-acetyl cysteine (NAC), rescued UFP-mediated reduction in NO production (Fig. 1C). Thus, UFP-induced oxidative stress diminished endothelial NO production.
UFP exposure increased eNOS S-glutathionylation
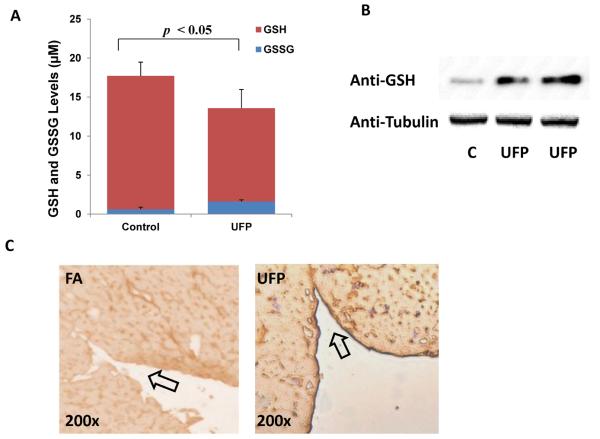
Protein thiols can be glutathionylated by oxidized glutathione (GSSG) through disulfide exchange that modulates protein function [9]. To further elucidate the mechanism underlying UFP-induced reduction in NO production, we assessed the relative levels of oxidized (GSSG) and reduced glutathione (GSH) in response to UFP exposure. In HAEC exposed to UFP, GSH levels were decreased (control=17.1±1.8 μM, UFP=12.0 ±2.4 μM, n=4, p < 0.05), whereas GSSG levels were increased (control=0.62±0.26 μM, UFP=1.60 ±0.20 μM, n=4, p < 0.05) (Fig. 2A). The GSSG/GSH ratio was 0.134 in UFP-exposed cells versus 0.036 in control cells. In corollary, UFP treatment increased protein S-glutathionylation as evidenced by the bands to anti-GSH antibody (Fig. 2B) in consistent with S-glutathionylated Actin in response to oxidative stress [18]. Increased protein S-glutathionylation was further recapitulated in mice exposed to UFP. The endothelium of endocardium of LDLR-null mice exposed to UFP revealed prominent staining to anti-GSH antibody (Fig. 2C). Thus, increased GSSG/GSH ratio was in parallel with the elevated protein S-glutathionylation in response to UFP exposure.
Fig. 2. UFP increased protein S-glutathionylation in HAEC.
(A) HAEC were treated with or without 50μg/mL of UFP for 6 hours, cellular levels of GSH and GSSG were measured as described in Methods. UFP decreased GSH level, but increased GSSG level. (n=4) (B) HAEC were treated with or without 50μg/mL of UFP for 6 hours and protein lysates were collected. Western blots with antibody against GSH revealed an increase in a dominant band to S-Glutathionylated Actin. The western blot with anti-Tubulin was performed as loading reference. (C) Sections of endocardium from LDLR-null mice exposed to filtered air (FA) or UFP for 10 weeks were stained with anti-GSH antibody for visualization of protein S-glutathionylation. UFP exposure led to a prominent staining in the endothelium of endocardium (Arrow).
To examine if the reduction in NO production is associated with protein S-glutathionylation, we assessed eNOS S-glutathionylation in response to UFP exposure. By performing biotin-labeling of S-glutathionylation proteins and ELISA for eNOS, we demonstrated that UFP significantly increased eNOS S-glutathionylation in HAEC (control=0.008±0.001, UFP=0.042±0.006, BCNU (1,3-cis(2-chloroethyl)-1-nitrosourea, a positive control) = 0.032 ±0.004, n=3, p < 0.01) (Fig. 3).
Fig. 3. UFP stimulated eNOS S-glutathionylation.
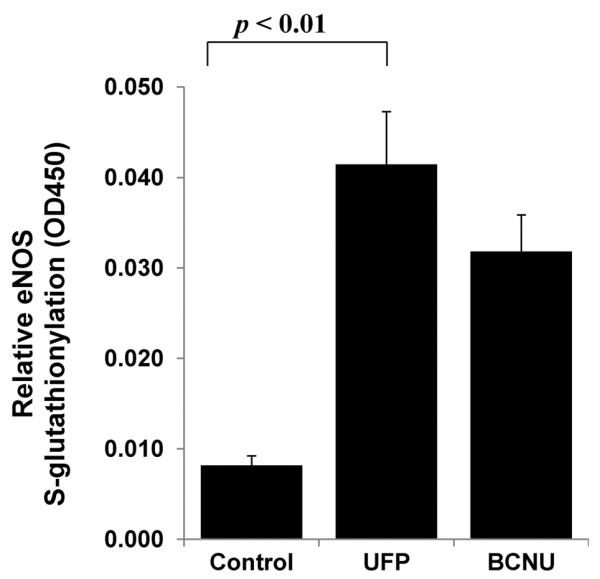
HAEC were treated with 50μg/mL of UFP or 25μM BCNU (a positive control) for 6 hours. eNOS S-glutathionylation was measured as described in Methods. UFP significantly increased eNOS S-glutathionylation (n=3, p < 0.01).
S-glutathionylation mediated UFP-reduced NO Production
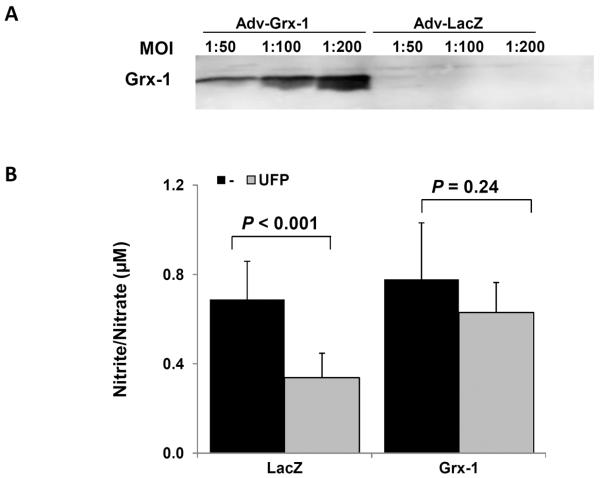
To assess whether eNOS S-glutathionylation mediated a reduction in NO production in response to UFP exposure, we over-expressed Glutaredoxin-1(Grx-1), an inhibitor of protein S-glutathionylation. While NO production was lower in general in adenovirus-infected endothelial cells, over-expression of Grx-1 with recombinant adenoviruses (Fig. 4A) significantly attenuated UFP-mediated reduction in NO production by nearly 80% as compared to over-expression of control gene LacZ (control/LacZ=0.69±0.17; LacZ/UFP=0.34±0.11; Grx=0.78±0.25, Grx/UFP=0.63±13; LacZ/UFP vs. LacZ p < 0.001; Grx/UFP vs. Grx, p = 0.24; n=6) (Fig. 4B). Thus our findings corroborated UFP-mediated protein S-glutathionylation in modulating NO production.
Fig. 4. S-glutathionylation mediated UFP-reduced NO Production.
(A) HAEC were infected with control (Adv-LacZ) or Glutaredoxin-1(Adv-Grx-1) adenoviruses at different multiple of infection (MOI) overnight. Grx-1 expression was assessed by western blot. (B) HAEC were infected with Adv-LacZ or Adv-Grx-1 at MOI of 1:100 overnight. The cells were then treated with or without 50μg/mL of UFP for 6 hours. NO production was measured. Over-expression of Grx-1 attenuated UFP-mediated inhibition in NO production (n=6).
Discussion
In this study, we elucidated a novel mechanism by which ambient ultrafine particulate pollutants reduced vascular endothelial NO production via eNOS S-glutathionylation, whereas over-expression of glutaredoxin-1 nearly rescued UFP-mediated reduction in NO production. Exposure to particulate matter (PM) has been shown to promote endothelial dysfunctions in ApoE−/− mice [3,4]. Here, we demonstrate that eNOS S-glutathionylation is a novel mechanism underlying UFP-mediated reduction in NO production.
Oxidative stress regulates enzymatic activity of eNOS, and is intimately linked with production of reactive oxygen species (ROS) via eNOS uncoupling [28]. In this study, UFP-mediated reduction in NO production was restored in the presence of antioxidants; namely, N-acetyl cysteine (NAC), Tempol, and MnTMPyP, as well as NADPH oxidase inhibitor, Apocynin, and JNK inhibitor, SP600125. These findings support the previously published reports that UFP induced vascular oxidative stress via NADPH oxidase and JNK activation [13,29].
Oxidative stress was reported to increase protein S-glutathionylation [18], which has recently been reported to uncouple eNOS [9]. UFP increased the intensity of a dominant band by Western analysis, consistent with S-glutathionylated Actin [18]. We employed a modified S-glutathionylated protein Biotin labeling/ELISA methodology to establish the role of UFP in eNOS S-glutathionylation. Over-expression of glutaredoxin-1 (Grx-1) to inhibit protein S-glutathionylation corroborated the role of S-glutathionylation in the regulation of eNOS activity [30]. Oxidative stress was also implicated in the reduced bioavailability of BH4, a cofactor of eNOS, resulting in eNOS uncoupling [31]. Thus, UFP-mediated reduction in NO production may be influenced by both S-glutathionylation and BH4 reduction.
Various mechanisms are involved in protein S-glutathionylation. In the presence of ROS, cysteines form thiyl radicals which, in turn, react with glutathione (GSH). Alternatively, protein thiols can be glutathionylated by oxidized glutathione (GSSG) through disulfide exchange [9]. In this study, we revealed that UFPs increased intracellular GSSG levels as the potential underlying mechanism in the increase in eNOS S-glutathionylation. However, the precise mechanisms whereby UFP mediate eNOS S-glutathionylation and reduction in eNOS activity are in need of further investigation.
In summary, we demonstrate that ambient UFP collected in the urban environment of Los Angeles reduced vascular endothelial NO production via S-glutathionylation. In light of NO's role in vascular homeostasis, S-glutathionylation in eNOS may further influence UFP-mediated reduction in HDL anti-oxidant capacity [14] with clinical relevance to accelerated atherosclerosis [2].
Supplementary Material
Highlights
Ultrafine particles (UFP) reduced nitric oxide (NO) production
UFP-induced oxidative stress mediated the reduction in NO production
UFP induced eNOS S-glutathionylation
Inhibition of S-glutathionylation attenuated UFP-induced reduction in NO production
Acknowledgements
The authors would like to express gratitude to Dr. Mieyal at Case Western Reserve University for providing the Gluaredoxin-1 adenovirus. This study was supported by the National Heart Lung and Blood Institute, R01HL083015 (TKH) and R01HL111437 (TKH), and by the Southern California Particle Center, funded by EPA under STAR program through award number-2145 G GB139 (CS) and South Coast Air Quality Management District Award # 11527 (CS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflict of interest.
References
- [1].Brooks MM, Chung SC, Helmy T, Hillegass WB, Escobedo J, Melsop KA, Massaro EM, McBane RD, Hyde P, Hlatky MA. Health Status After Treatment for Coronary Artery Disease and Type 2 Diabetes Mellitus in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial. Circulation. 2010;122:1690–1699. doi: 10.1161/CIRCULATIONAHA.109.912642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hansen CS, Sheykhzade M, Moller P, Folkmann JK, Amtorp O, Jonassen T, Loft S. Diesel exhaust particles induce endothelial dysfunction in apoE−/− mice. Toxicol Appl Pharmacol. 2007;219:24–32. doi: 10.1016/j.taap.2006.10.032. [DOI] [PubMed] [Google Scholar]
- [4].Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- [5].Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci U S A. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- [7].Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- [9].Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- [11].Zhang Y, Schauer JJ, Shafer MM, Hannigan MP, Dutton SJ. Source apportionment of in vitro reactive oxygen species bioassay activity from atmospheric particulate matter. Environmental Science & Technology. 2008;42:7502–7509. doi: 10.1021/es800126y. [DOI] [PubMed] [Google Scholar]
- [12].Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radical Biology and Medicine. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li R, Ning Z, Cui J, Khalsa B, Ai L, Takabe W, Beebe T, Majumdar R, Sioutas C, Hsiai T. Ultrafine particles from diesel engines induce vascular oxidative stress via JNK activation. Free Radic Biol Med. 2009;46:775–782. doi: 10.1016/j.freeradbiomed.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li R, Navab M, Pakbin P, Ning Z, Navab K, Hough G, Morgan TE, Finch CE, Araujo JA, Fogelman AM, Sioutas C, Hsiai T. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res. 2013;54:1608–1615. doi: 10.1194/jlr.M035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jin X, Yu L, Wu Y, Zhang S, Shi Z, Chen X, Yang Y, Zhang X, Jiang C. S-Glutathionylation underscores the modulation of the heteromeric Kir4.1-Kir5.1 channel in oxidative stress. J Physiol. 2012;590:5335–5348. doi: 10.1113/jphysiol.2012.236885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mieyal JJ, Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation. Antioxid Redox Signal. 2012;16:471–475. doi: 10.1089/ars.2011.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun R, Eriksson S, Wang L. Oxidative stress induced S-glutathionylation and proteolytic degradation of mitochondrial thymidine kinase 2. J Biol Chem. 2012;287:24304–24312. doi: 10.1074/jbc.M112.381996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- [19].Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23:222–231. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Misra C, Kim S, Shen S, Sioutas C. A high flow rate, very low pressure drop impactor for inertial separation of ultrafine from accumulation mode particles. Journal of Aerosol Science. 2002;33:735–752. [Google Scholar]
- [21].Li R, Mittelstein D, Kam W, Pakbin P, Du Y, Tintut Y, Navab M, Sioutas C, Hsiai T. Atmospheric Ultrafine Particles Promote Vascular Calcification via the NF-kappaB Signaling Pathway. Am J Physiol Cell Physiol. 2012 doi: 10.1152/ajpcell.00322.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Decesari S, Facchini MC, Matta E, Lettini F, Mircea M, Fuzzi S, Tagliavini E, Putaud JP. Chemical features and seasonal variation of fine aerosol water-soluble organic compounds in the Po Valley, Italy. Atmospheric Environment. 2001;35:3691–3699. [Google Scholar]
- [23].Schauer JJ, Fraser MP, Cass GR, Simoneit BR. Source reconciliation of atmospheric gas-phase and particle-phase pollutants during a severe photochemical smog episode. Environ Sci Technol. 2002;36:3806–3814. doi: 10.1021/es011458j. [DOI] [PubMed] [Google Scholar]
- [24].Lough GC, Schauer JJ, Park JS, Shafer MM, Deminter JT, Weinstein JP. Emissions of metals associated with motor vehicle roadways. Environmental Science & Technology. 2005;39:826–836. doi: 10.1021/es048715f. [DOI] [PubMed] [Google Scholar]
- [25].Li R, Mittelstein D, Kam W, Pakbin P, Du Y, Tintut Y, Navab M, Sioutas C, Hsiai T. Atmospheric ultrafine particles promote vascular calcification via the NF-kappaB signaling pathway. Am J Physiol Cell Physiol. 2013;304:C362–369. doi: 10.1152/ajpcell.00322.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morgan TE, Davis DA, Iwata N, Tanner JA, Snyder D, Ning Z, Kam W, Hsu YT, Winkler JW, Chen JC, Petasis NA, Baudry M, Sioutas C, Finch CE. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Perspect. 2011;119:1003–1009. doi: 10.1289/ehp.1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ai L, Rouhanizadeh M, Wu JC, Takabe W, Yu H, Alavi M, Li R, Chu Y, Miller J, Heistad DD, Hsiai TK. Shear stress influences spatial variations in vascular Mn-SOD expression: implication for LDL nitration. Am J Physiol Cell Physiol. 2008;294:C1576–1585. doi: 10.1152/ajpcell.00518.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal. 2011;14:1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mo Y, Wan R, Chien S, Tollerud DJ, Zhang Q. Activation of endothelial cells after exposure to ambient ultrafine particles: the role of NADPH oxidase. Toxicol Appl Pharmacol. 2009;236:183–193. doi: 10.1016/j.taap.2009.01.017. [DOI] [PubMed] [Google Scholar]
- [30].Chrestensen CA, Starke DW, Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- [31].Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.