Abstract
In the United States there are currently two influenza vaccine platforms approved for use in humans – conventional inactivated virus and live-attenuated influenza virus (LAIV). One of the major challenges for influenza A virus (IAV) vaccination is designing a platform that provides protection across strains. Pandemic H1N1 (pH1N1) IAV swept the globe in 2009 and crossed the species barrier, infecting swine in several countries. Pigs are a natural host for IAV and serve as a model for evaluating immune responses following vaccination and challenge. Recently, a temperature-sensitive (ts) LAIV was developed by introducing modifications in the polymerase genes of a swine-like triple reassortant (tr) virus and when paired with pandemic HA and NA, provided sterilizing immunity upon intratracheal challenge with virulent pH1N1 virus. The utility of a ts LAIV is expanded in this report to show vaccination of pigs induced a cell-mediated immune response characterized by an increased number of antigen-specific IFN-γ secreting cells and expanded T cell populations when compared to pigs vaccinated with a whole inactivated virus (WIV) vaccine. Following challenge, there was a significant increase in the percentage of proliferating lymphocytes in the LAIV group compared to the WIV group following restimulation with pH1N1 in vitro. Also, there was an increase in the percentage of CD4/CD8 double-positive memory T cells in LAIV vaccinated pigs compared to WIV vaccinated pigs. Hemagglutination inhibition and serum neutralization titers were significantly higher in the LAIV-vaccinated pigs compared to the WIV vaccinated pigs following the initial dose of vaccine. Taken together, these results indicate the ts LAIV vaccine, generated from a triple reassortant IAV, elicits greater cell-mediated and humoral immune responses in pigs.
Introduction
Contemporary influenza A viruses (IAVs) currently circulating in North American swine are characterized by the triple-reassortant gene (TRIG) cassette, a constellation of genes whose origins trace back to human (PB1), avian (PA and PB2) and swine (NP, M, and NS) lineage IAV [1, 2]. Numerous reassortant events have paired the TRIG with different HA and NA genes, including introductions from circulating human seasonal IAV. Combined with antigenic drift, this has substantially increased the diversity of IAV circulating in pigs, which are represented by numerous H3N2, H1N2 and H1N1 variant viruses [3, 4]. In 2009, a reassortant H1N1 virus emerged in humans that contained the TRIG, though the M and NA gene were characteristic of Eurasian swine lineage IAV [5, 6]. The novel virus spread rapidly through the human population and was declared a pandemic in early June 2009. The virus was classified as a swine-origin influenza virus because six of the RNA gene segments (PB2, PB1, PA, HA, NP and NS) were genetically similar to those in the triple-reassortant viruses circulating in North American swine and the other 2 gene segments (NA and M) were related to those found in Eurasian swine IAV. This genetic grouping for IAV was completely novel and the origin remains unknown [7], though genetic evidence suggests a progenitor virus was not circulating in North American swine prior to the pandemic [3]. Pandemic H1N1 virus was introduced into swine not long after the pandemic emerged in humans, and is now circulating and reassorting with other swine IAV, which highlights the need for prevention and surveillance of IAV in swine [8–10].
Thus, IAV continues to be a problem for swine producers, given the high number of antigenically distinct strains present in pigs and a deficiency in vaccine seed strains matching circulating strains for adequate protection. While these vaccines may reduce disease severity, they do not consistently provide protection from infection or shedding. Many farms have resorted to using autogenous vaccines in an attempt to better protect their herds. IAV vaccines for humans include both inactivated products as well as a temperature-sensitive, live-attenuated influenza virus (LAIV) delivered intranasally; however, only inactivated products are currently licensed for use in pigs.
Humoral and cell-mediated immune responses contribute to immunity to IAV. While antibody plays a major role by protecting against infection, cell-mediated responses are critical for clearing virus-infected cells [11, 12]. Antibody to the surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA) are most associated with protection from infection, though antibody to other viral proteins can be detected following exposure and may provide some protection [11, 13]. In particular, antibodies that block binding of the HA protein to the host cell are the most commonly measured correlate of protection and are typically evaluated in the hemagglutination inhibition (HI) assay. An assay that evaluates virus neutralization, typically utilizing sera or lung lavage fluid, is more sensitive than HI, and evaluates antibody that not only blocks virus entry, but antibody that may neutralize virus at other stages of the replications cycle. IgA provides protection to the mucosal surface, including the upper and lower respiratory tract, sites of IAV infection. IgG, produced systemically, primarily provides protection in the lower respiratory tract but can be detected in nasal secretions, presumably as serum transudate [14, 15]. Antibody mediated protection is effective to homologous IAV strains, but it provides little to no protection against heterologous strains with drifted surface proteins [13]. However, due to their polymeric nature, IgA antibody is believed to be more cross-reactive to drifted IAV when compared to monomeric IgG [16].
Cell-mediated immune responses can be characterized by T helper (Th) lymphocytes (CD4+) and cytotoxic T lymphocytes (CTL, CD8+), each with their respective contributions to host immunity. Th responses are necessary for adequate B cell activation and subsequent antibody production to IAV whereas CTL are important for killing virus-infected cells. A defined direct effector function for CD4 Th cells during influenza virus infection is still lacking [12]. T cell responses to IAV tend to be directed to internal proteins that are more conserved across IAV strains, a property that allows for heterologous cross protection if induced following vaccination (reviewed in [12]). In pigs, a population of memory T cells are characterized by expression of both CD4 and CD8α and are referred to as CD4/CD8 double-positive (DP) T cells, which can expand in response to recall antigen and produce IFN-γ [17]. MHC-I restricted T cells in the pig express the same TCR-αβ as DP cells, but CD8 is a heterodimer of CD8α and CD8β as opposed to the CD8αα homodimer found on DP cells [18].
LAIV vaccines have been shown to induce immune responses in pigs that provide protection to both homologous and heterologous challenge [19, 20]. However, the attenuation mechanism used in these platforms was not temperature-sensitivity, the attenuation mechanism used in vaccines currently available for humans [21] and horses [22]. Recently, a LAIV using temperature-sensitive (ts) genetic changes for attenuation was developed using a contemporary IAV strain with the triple reassortant backbone and shown to be efficacious in swine [23]. In order to assess the humoral and cellular response associated with this LAIV vaccine in naïve pigs, we evaluated the host response following vaccination, including the local response to the vaccine as well as adaptive response before and after challenge. Results demonstrate LAIV vaccination induces neutralizing antibody and cellular immune responses, evaluated by antigen-specific IFN-γ ELISpot assay, which were significantly increased over pigs vaccinated with a whole-inactivated virus (WIV) preparation. In addition, there was a significant expansion of memory T cells in LAIV vaccinated pigs following challenge which was not detected in pigs vaccinated with WIV. Taken together, these results indicate LAIV vaccination in pigs rapidly elicits both humoral and cellular arms of the adaptive immune response that are significantly increased over responses measured to WIV vaccine.
2. Materials and Methods
2.1 Ethics statement
Animal experiments were approved by the Institutional Animal Care and Use Committee of the National Animal Disease Center in Ames, IA under the approved protocol 3950 (Influenza A virus pathogenesis and host response in swine) and carried out in animal biosafety level 3 (ABSL-3) conditions, the recommended biosafety level for in vivo pH1N1 studies at the time.
2.2 Viruses and vaccines
The IAV vaccines used for this study were previously described in detail. Briefly, reverse-genetics techniques were used to clone all 8 gene segments from A/turkey/Ohio/313053/04 (ty/04, H3N2) and a competent virus was rescued (rg ty/04). The ty/04 strain was isolated from a turkey but is characteristic of a triple reassortant swine IAV [24]. Reverse genetic techniques were used to introduce and rescue a virus with the HA and NA from A/New York/18/09, a pandemic H1N1 isolate. To generate the attenuated ty/04 strain (LAIV), modifications were introduced into the polymerase genes that hindered polymerase activity and rendered the strain temperature-sensitive as described [23]. The rescued strain containing ty/04 backbone with modifications and HA and NA from pH1N1 was in used this study as a vaccine and is the referenced LAIV. A/California/04/09 IAV (Ca/04) was used to generate whole-inactivated virus and was also the strain used in recall assays and for challenge virus.
2.3 Experimental design
To evaluate the protective host immune response to vaccination and the protective efficacy of the LAIV, 40 pigs were randomly distributed into 4 different treatment groups with 10 pigs per group – LAIV, WIV, non-vaccinated/challenge, and non-vaccinated/non-challenged controls. The LAIV group was vaccinated with 105 TCID50/pig by the intranasal route and WT Ca/04 was prepared at 8 HA units per 50 μl, UV-irradiated, and adjuvanted 4:1 (v/v) with Emulsigen-D (MVP Laboratories) and administered as a 2 ml intramuscular dose per pig (WIV). All pigs were vaccinated at approximately 4-weeks of age and boosted 18 days later by the same route with the same respective formulation. Two weeks after the boost, all pigs except the non-vaccinated/non-challenged controls, were challenged by the intratracheal route with 105 TCID50 live Ca/04 virus. Five days after challenge, pigs were humanely euthanized for evaluating macroscopic lung pathology, determining viral titers and cytokine protein levels in the lung, and virus nasal shedding. Blood was collected for serum and or peripheral blood mononuclear cells at times indicated in each figure.
2.4 Sample collection
Blood was collected by venipuncture into BD Vacutainer serum separator tubes (SST) for sera or BD Vacutainer CPT tubes with sodium citrate for peripheral blood mononuclear cell (PBMC) collection according to manufacturer’s recommendations (BD, Franklin Lakes, NJ). Isolated PBMCs were washed once with RPMI-1640 (Invitrogen), passed through a 40 μm screen filter, washed a second time and enumerated for use in the ELISpot and proliferation assay. Nasal swabs were collected as previously described [25] and used to evaluate virus shedding as described below. At necropsy, lungs were removed and an estimate of percent gross lung lesion involvement was determined based on the percentage of each lung lobe affected and the percentage of total lung volume each lobe represented [26, 27]. Lungs were lavaged with 50 ml of minimal essential media (MEM), with recovery of 15–25 ml of fluid. Lavage fluid was used to determine viral load and for cytokine evaluation. For cytokines, 5 ml of lavage fluid was centrifuged at 300 × g for 10 min to pellet cellular debris. The supernatant was stored frozen at −80° C and used to evaluate cytokine levels as described below.
2.5 Virus titration
To determine virus amount in any sample, each sample was titrated on Madin-Darby canine kidney (MDCK) cells to determine TCID50/ml by the method of Reed and Muench [28]. Briefly, ten-fold serial dilutions of each sample were made and added to MDCK cells in triplicate (plated in 96-well plate) in serum-free media containing TPCK-trypsin (Sigma, St. Louis, MO). Samples were incubated with cells for 72 h and supernatant used in an HA assay to determine endpoint viral titer.
2.6 Antibody evaluation
The hemagglutination inhibition (HI) assay was performed as recommended in the WHO animal influenza-training manual using turkey red blood cells with Ca/04 as antigen as previously described [23]. The serum neutralization assay was performed as previously described [29]. The lung lavage neutralization assay was performed the same as the SN assay, with the exception that samples were first treated in a 10 mM dithiothreitol solution for 1 h to break up mucous and two-fold serial dilutions were performed starting at 1:4. Log2 transformations were used for statistical analysis and results reported as the geometric mean titers. Ca/04-specific IgG and IgA titers in the serum and lung lavage were determined by enzyme-linked immunosorbent assays (ELISA) as previously described, with some modifications [25]. Briefly, Ca/04 virus was concentrated and used to coat plates at 100 HAU per well. Non-infected MDCK cell preps were treated the same as CA/04 virus and plates coated the same to evaluate non-specific binding. Plates were blocked with Starting Block Buffer (Thermo Fisher) and subsequently washed. Samples were incubated in PBS/5% bovine serum albumin for 1h at 37d to adsorb non-specific antibody and 50μl added per well in duplicate. After a 1 h incubation with sample, plates were again washed and peroxidase-labeled goat anti-pig IgA (Bethyl Laboratories, Montgomery, TX) or IgG (Kirkgaard and Perry, Gaithersburg, MD) were used as detection antibodies. Sera samples were used at 1:2000 dilution for IgG analysis and 1:4 for IgA. BALF samples were used at 1:4 for both IgG and IgA analysis. Individual antibody levels were determined from average optical density (OD) of duplicate wells for each sample for CA/04 antigen and MDCK antigen and reported as the mean OD for CA/04 minus mean OD for MDCK.
2.7 Cytokine evaluation
Cytokine levels in cell-free lung lavage were determined using a multiplex ELISA assay per manufacturer’s recommendations (Aushon Biosystems). Samples were analyzed in duplicate and results were averaged. Data is reported as the mean ± SEM for pigs in each treatment group.
2.8 IFN-γ ELISpot
ELISpot assay for interferon-gamma secreting cells (IFN-γ SC) was performed as previously described with slight modification [30]. Briefly, 96-well membrane plates (MAIPS4510, Millipore) were prewetted with 35% ethanol, washed, and coated overnight at 4°C with 6μg/ml anti-pIFN-γ (P2G10, BD Biosciences). The next day, the plate was washed and blocked with complete RPMI [RPMI-1640, 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1% antibiotic/antimycotic (Invitrogen), and 50 μg/ml gentamicin] for 2 h at 37° C. The blocking media was removed and 5×105 PBMC were plated per well. Treatments were added to appropriate wells in triplicate in a final volume of 250 μl per well and the plates incubated for 18 h at 37° C 5% CO2. Treatment included UV-inactivated Ca/04 virus at a multiplicity of infection (MOI) = 0.5, control MDCK media, or Concanavalin A at 5μg/ml. After 18 h, plates were washed and incubated with anti-IFN-γ detection antibody (0.5μg/ml, P2C11, BD Biosciences) for 2 h at 37 °C. Plates were washed and developed using ELISpot Blue Color Module (R&D Systems) according to the manufacturer’s recommendations. Plates were scanned and spots enumerated using CTL-ImmunoSpot® S5 UV Analyzer and ImmunoSpot 5 software. The reported values were calculated from the average number of spots counted for wells receiving Ca/04 minus MDCK mock stimulation.
2.9 Proliferation and cell phenotyping
PBMCs were labeled with PKH67 according to manufacturer’s recommendations (Sigma, St. Louis, MO). Labeled cells were cultured at 5×105 cells per well in 96-well round-bottom plates in triplicate for each treatment. Treatment groups included MDCK media-alone or UV-inactivated Ca/04 at a MOI of 0.5 with a final culture volume of 250 μl. Cells were incubated for 5 days at 37° C in 5% CO2. At the end of 5 days, plates were centrifuged at 200 × g for 5 min and supernatant removed. Cells were resuspended with FACS buffer (phosphate-buffered saline with 2% FBS and 0.05% sodium azide) and cells from triplicate wells pooled. Cells were redistributed for phenotypic staining with anti-pig CD4 (74-12-4) and anti-pig CD8α (76-2-11) antibodies (VMRD, Pullman, WA). Secondary antibodies, targeted to murine antibodies, included IgG2b-PE and IgG2a-APC. Data was acquired using CellQuest Pro software (BD Biosciences, San Jose, CA) on an LSRII flow cytometer (Becton Dickinson) and analyzed using FlowJo software (TreeStar, Ashland, OR). Cell proliferation was assessed as the percentage of daughter lymphocytes generated (100% - percentage of parent population remaining) following the incubation period for each treatment group (MDCK media or UV-Ca/04). The percentage of CD4/CD8 double-positive cells and percentages of CD4 single-positive and CD8a single-positive was determined by first gating on the live lymphocyte population and then gating on the population expressing both CD4 and CD8α, CD4-only or CD8α-only, respectively.
2.10 Statistics
All statistical analysis was performed using GraphPad Prism 5 Software (San Diego, CA). A one-way analysis of variance (ANOVA) with a Tukey’s post-test was used for multiple comparisons and a student’s t-test used for comparing two treatment groups. Differences were considered statistically significant at a p-value < 0.05 and each figure legend states which groups were significantly different.
3. Results
3.1 Antibody response following vaccination with LAIV or WIV
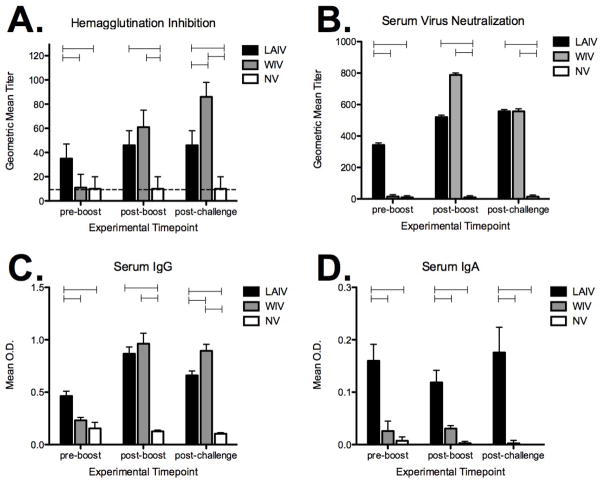
HI titers and SN titers were determined using sera samples collected prior to vaccination, after priming (pre-boost), following boosting (post-boost) and following challenge (Fig. 1A and 1B). Prior to the start of the experiment (vaccination), none of the pigs were positive for influenza virus antibody when evaluated by HI titer using Ca/04 virus as antigen (data not shown). Following priming with a single dose (pre-boost), pigs given the LAIV had significantly higher HI and SN titers when compared to pigs given the WIV vaccine (Fig. 1A and 1B). Although pigs in the LAIV group were boosted, serum HI nor SN titers appeared to increase from titers measured pre-boost. WIV vaccination did not induce significant HI or SN titers following a single dose, but post-boost both HI and SN antibodies were detected. The average SN titer (geometric mean ± SEM) was 15 ± 12 for the WIV group pre-boost, which increased to 788 ± 13 following the boost. Although HI and SN titers did increase post-boost in the WIV group, titers were not significantly different between LAIV and WIV groups at this time point. Pigs in the non-vaccinated group did not seroconvert and were recorded as an HI or SN titer of 10, which represents the lower detection limit of each assay. On day 5 following challenge, there was not a significant increase in HI nor SN titers in either vaccination group; instead, titers were not different than those measured on the day of challenge (post-boost).
Figure 1.
Ca/04-specific serum antibody responses following vaccination with live-attenuated influenza virus (LAIV) or whole-inactivated influenza virus (WIV). Pigs were bled the day of boosting (pre-boost), prior to challenge (post-boost), and 5 days post-challenge for evaluating Ca/04-specific A) hemagglutination inhibition (HI) titers, B) virus serum neutralization (SN) titers, and C) IgG and D) IgA levels to whole virus. HI and SN titers were log2 converted and reported as the geometric mean ± SEM. Negative HI reactions are at the minimal level of detection (10), denoted by the dotted line. Antibody isotype data is expressed as the mean ± SEM optical density (OD) for 10 pigs per treatment group. A one-way analysis of variance with a Tukey’s post-test was used for statistical analysis and p-values <0.05 are indicated with connecting bars.
Serum levels of IgG and IgA specific for pandemic virus were evaluated following vaccination with LAIV for comparison to levels following vaccination with WIV. IgG and IgA levels to whole-virus (Ca/04) were measured in serum collected after priming (pre-boost) and following boost (post-boost) (Fig. 1C and 1D). Similar to results observed for HI and SN titers, serum Ca/04-specific IgG levels were increased in the LAIV group over the WIV group following priming (Fig. 1C). However, after boosting, IgG levels to virus increased in pigs vaccinated with WIV to levels observed in the LAIV group. Vaccination with the LAIV did induce Ca/04-specific IgA but WIV vaccination did not (Fig. 1D) induce a significant systemic IgA response. Boosting did increase serum levels of Ca/04-specific IgG in the sera, regardless of vaccine platform, but Ca/04-specific serum IgA was only detected in pigs given the LAIV vaccine.
3.2. LAIV vaccination primes a cell-mediated immune response that expands in response to challenge
In order to compare cell-mediated immunity that developed following vaccination with the LAIV versus a WIV, two different assays were performed. An ELISpot assay was used to enumerate IAV-specific interferon-gamma secreting cells (IFN-γ SC) and lymphocyte proliferation was used to evaluate IAV-specific recall responses immediately prior to challenge and 5 days following challenge. Results in Fig. 2A show that pigs given the LAIV vaccine had significantly more IAV-specific cells capable of producing IFN-γ in the periphery when compared to pigs receiving the WIV (118 ± 49 versus 20 ± 26, respectively). Vaccination the WIV failed to increase in the number of IAV-specific IFN-γ SC prior to challenge when compared to non-vaccinated animals (Fig. 2A). Another method used to evaluate antigen-specific recall responses is a proliferation assay, which evaluates the percentage of cells that proliferate following in vitro antigen exposure. After vaccination, but prior to challenge, there was no detectable increase in the percentage of peripheral lymphocytes that proliferated following incubation with UV-Ca/04 virus, regardless of the vaccine administered (data not shown).
Figure 2.
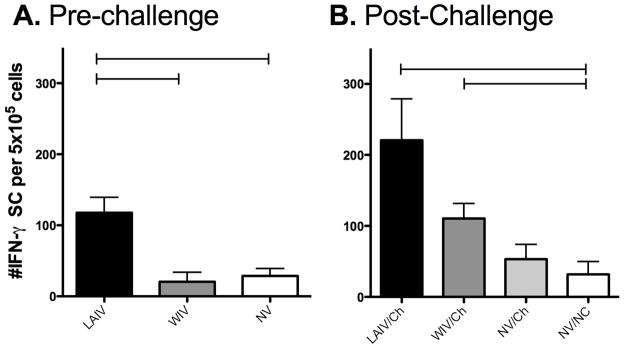
Recall responses to Ca/04 following vaccination. The number of antigen-specific IFN-γ secreting cells (SC) elicited following vaccination with live-attenuated influenza virus (LAIV), whole-inactivated virus (WIV), or cell-culture media (NV). Pigs were given 2 doses of vaccine and peripheral blood mononuclear cells isolated immediately A) prior to challenge or B) five days post-challenge. Cells were restimulated as described in Materials and Methods for each assay. Results are reported as the average number of spots in UV-Ca/04 stimulated wells minus the average number of spots in the media-only wells. The results are the mean ± SEM for each group. A one-way analysis of variance with a Tukey’s post-test was used for statistical analysis and p-values <0.05 are indicated with connecting bars.
In addition to exhibiting IAV-specific IFN-γ responses prior to challenge, pigs in the LAIV group exhibited a stronger cell-mediated immune response following challenge when compared to pigs in the WIV group. The number of IFN-γ SC was significantly increased in the periphery of LAIV vaccinated pigs (Fig. 2B), and there was a significant increase in the percentage of cells that proliferated in response to UV-Ca/04 following challenge (Fig. 3A). In general, the percentage of cells that proliferated following challenge were greater when compared to proliferation prior to challenge even when cells were incubated with MDCK media-alone. This likely indicates activation occurred in vivo from the challenge, and cells from LAIV vaccinated pigs expanded further when incubated with UV-Ca/04 in vitro, though the percentage increase in cells was modest at approximately 6% (%UV-Ca/04 minus media-alone). CD4+/CD8+ double-positive T cells were primed following LAIV vaccination, as there was a significant expansion CD4+/CD8+ double-positive cells following in vitro stimulation with UV-Ca/04 (Fig. 3B). In pigs, CD4+/CD8+ double-positive cells are described as a memory T cell population [17]. No significant changes in single positive CD8 (Fig. 3C) or CD4 (Fig. 3D) populations were detected, regardless of the vaccine platform used. Together, these results indicate the LAIV induced greater cell-mediated immune responses, measureable before and after challenge.
Figure 3.
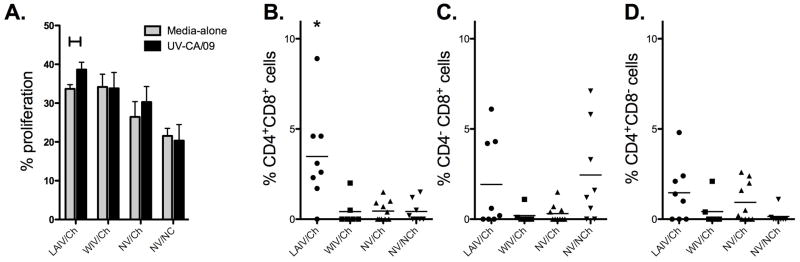
Expansion of CD4+/CD8+ double-positive memory cells following challenge in pigs vaccinated with LAIV. Peripheral blood mononuclear cells were collected from pigs following prime-boost vaccination with live-attenuated influenza virus (LAIV), whole-inactivated virus (WIV), or cell-culture media (NV) 5 days following pH1N1 intratracheal challenge (Ch) or non-challenge controls (NC). PBMCs were labeled with PKH67 and restimulated for five days in vitro with UV-Ca/04 or mock media and the A) percent proliferation and the percent of B) CD4+CD8− C) CD4−CD8+ and D) CD4+/CD8+ double positive cells determined using flow cytometry. Phenotypic data is expressed as the percentage of cells detected in wells following UV-Ca/04 exposure minus wells given media alone. For proliferation, a student’s t-test was used for statistical analysis of UV-Ca/04 stimulated compared to media alone and for phenotypic data a one-way analysis of variance with a Tukey’s post-test was used and p-values <0.05 are indicated with a connecting bar or asterisk.
3.3. Increased levels of T cell associated cytokines in the lungs of vaccinated pigs following challenge
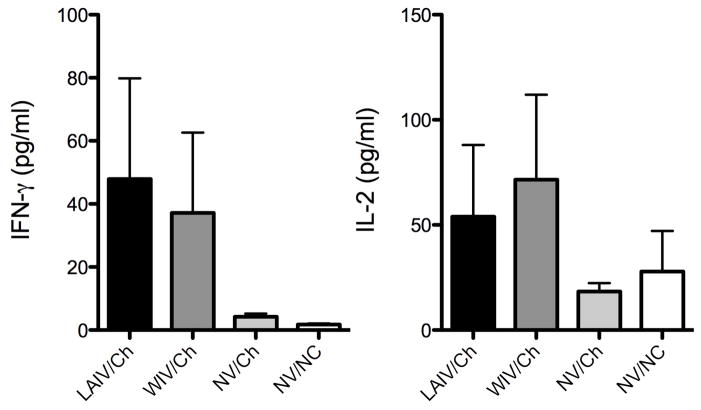
Memory T cells, elicited by vaccination and expanded upon challenge, would encounter virus-infected cells primarily in the lungs. IL-2 and IFN-γ are associated with T cell responses and are often measured to evaluate T cell activation. The levels of IL-2 and IFN-γ in lung lavage 5 days following pH1N1 challenge were evaluated to gauge T cell activity in the lungs. Levels of IFN-γ and IL-2 were increased in the lungs of vaccinated pigs, but not non-vaccinated pigs, 5 days following challenge (Fig. 4). There was no significant difference between IL-2 or IFN-γ levels in the lung lavage of pigs vaccinated with the LAIV compared to the WIV.
Figure 4.
Levels of IL-2 and IFN-γ in the lung lavage of pigs previously vaccinated with live-attenuated influenza virus (LAIV), whole-inactivated virus (WIV), or mock-vaccinated (NV) collected 5 days after pH1N1 challenge (Ca/04) or mock challenge (NC). The results are expressed as the mean ± SEM for each group.
3.4 Increased antibody levels in the lungs of LAIV vaccinates compared to WIV vaccinates following homologous challenge
Lung lavage was performed on day 5 following homologous challenge and assayed for levels of neutralizing antibody and total Ca/04-specific IgG and IgA. Fig. 5 indicates that IgG and IgA antibody levels in the lung lavage of LAIV vaccinated pigs was significantly increased over levels in WIV vaccinated pigs following challenge. In addition, the antibody detected in the lung lavage of the LAIV/Ch group was able to neutralize Ca/04 virus in a neutralization assay.
Figure 5.
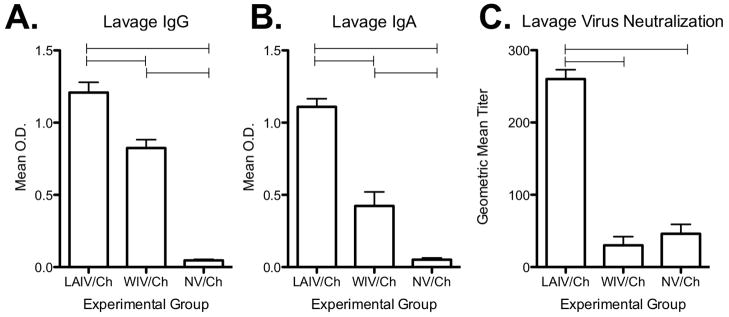
Levels of Ca/04-specific antibody in the lung lavage of pigs previously vaccinated with live-attenuated influenza virus (LAIV), whole-inactivated virus (WIV), or mock-vaccinated (NV) 5 days after pH1N1 challenge (Ca/04). Lung lavage was collected on day 5 following challenge and assayed for A) IgG and B) IgA levels specific to Ca/04 virus and C) Ca/04 neutralization titer. Neutralization titers were log2 converted and reported as the geometric mean ± SEM. Antibody isotype data is expressed as the mean ± SEM optical density (OD) for 10 pigs per treatment group. A one-way analysis of variance with a Tukey’s post-test was used for statistical analysis and p-values <0.05 are indicated with connecting bars.
Discussion
There are several factors to consider when evaluating and comparing host immune responses to different IAV vaccines. These include, but are not limited to, the immune status of the individual at the time of vaccination, the vaccine platform (inactivated, attenuated, etc), adjuvant, the route of administration, and the relatedness of the IAV in the vaccine versus IAV used as antigen or challenge virus. The current study was aimed at assessing the immune response of IAV-naïve pigs following intramuscular vaccination with a monovalent, adjuvanted WIV or intranasal vaccination with a temperature-sensitive LAIV. Humoral and cell-mediated immune responses to homologous virus (pH1N1) were evaluated at various times post vaccination or 5 days following challenge. While both vaccine platforms elicited responses to vaccine virus, the characteristics and magnitude of the responses were unique between the two vaccine types.
HI and SN titers measured in this experiment were greatest in the pigs that received the WIV; however, a single dose of LAIV was sufficient to induce production of neutralizing antibody whereas a single dose of WIV vaccine was not (Fig. 1A). Accordingly, Ca/04-specific IgG and IgA were measurable in the sera of LAIV vaccinates following a single administration of vaccine as well (Fig. 1C & 1D). HI titers were not detectable following priming (pre-boost) for the majority of the pigs in the WIV group and Ca/04-specific IgG was not detected in the sera of WIV vaccinates at this same time point (Fig. 1C). Serum IgG to whole-virus increased following administration of the WIV boost (Fig. 1C), as did neutralization titers (Fig. 1A & 1B), whereas boosting with the LAIV did not seem to significantly increase serum antibody levels. Taken together, a single dose of LAIV induced production of neutralizing antibodies, but boosting did not significantly increase circulating antibody levels. On the contrary, boosting was required to induce the production of neutralizing antibody in pigs receiving WIV vaccine. Our results are different than those described for humans, as LAIV vaccination in humans has been shown to induce lower HI titers when compared to HI titers following WIV vaccination [31]. However, those studies primarily analyzed responses to vaccine in individuals previously exposed to IAV antigen, either by infection or vaccination. Instead, research has shown that children more frequently exhibit antibody responses following LAIV vaccination when compared to adults, suggesting prior immune status plays an important role in serologic response to vaccine. Also, LAIV vaccines are more efficacious than inactivated products in children [32, 33]. Lastly, there are reports in children in which shedding is appreciated following the first dose of LAIV, but not the second dose, suggesting immunity from the first dose significantly decreases the amount of antigen encountered upon boosting [34]. This may be one explanation for the lack of an anamnestic response following boosting in the LAIV vaccinated group. The pigs used in this study are more apt to model children given both their age and naïve status to IAV antigen prior to vaccination.
Also in agreement with our results is previous work that showed inoculation of naïve pigs with wild-type IAV induced HI antibody, and anti-influenza IgG and IgA antibody that, after peaking on day 14 post-inoculation, decreased overtime. In addition, reinoculation with the same virus did not induce an anamnestic increase in antibody titers when evaluated 14 days after secondary exposure [35]. This result is similar to results described here, in that HI antibody titers were significantly increased following priming with the LAIV vaccine, but an anamnestic response was not appreciated when HI titers were evaluated 2 weeks post-boost or 5 days following homologous challenge. Thus, in evaluating the efficacy of a specific vaccine, the time following vaccination in which the response is evaluated may need to be adjusted based on the target population.
In addition to HI antibody, vaccination with the LAIV induced an antigen-specific IFN-γ response that was measurable in the periphery prior to challenge. LAIV vaccination has been shown to induce a robust cell-mediated immune response, particularly in children [34, 36]. In the current study, there was a significant number of circulating peripheral blood mononuclear cells primed to produce IFN-γ in response to IAV (Fig. 2A) following LAIV vaccination. The day of challenge was the only time point before challenge in which IAV-specific IFN-γ SCs were enumerated in the periphery; thus, it is difficult to determine how quickly following LAIV vaccination cell-mediated immunity is detectable.
Although IFN-γ SCs were measurable in the periphery prior to challenge, there was not a significant increase in the percentage of peripheral blood mononuclear cells that proliferated following incubation with UV-Ca/04 prior to challenge (data not shown). It’s difficult to discern why responses were measurable in the IFN-γ SC ELISpot but not proliferation assay, but could be due to several factors, not excluding assay sensitivity. First, expression of viral proteins that would be recognized by memory T cells for proliferative responses were not presented because inactivated virus was used as antigen. It’s also possible that secondary signals required for adequate expansion of antigen-specific lymphocytes, such as IL-2 or co-stimulation between an antigen presenting cell and T cell, were not supplied. Or, too few progenitor memory cells were present in the peripheral blood. Nonetheless, following challenge of LAIV vaccinated pigs, there was measurable expansion of peripheral blood cells following in vitro stimulation with UV-Ca/04 (Fig. 3A). All pigs challenged with pH1N1, regardless of vaccination, had an expansion of peripheral blood cells in vitro (Fig. 3A). This was likely due to activation in vivo associated with the active infection, as proliferative responses in non-vaccinated/non-challenged pigs were similar to responses observed for all pigs, regardless of vaccine group, when evaluated the day of challenge, in which about 20% of cells proliferated (Fig. 3A and data not shown). However, in vitro exposure of peripheral cells from LAIV vaccinated pigs to UV-Ca/04 induced a significant increase in the percentage of cells that proliferated over media-alone stimulation, though this level may be considered modest with only about a 6% increase in antigen-specific responses.
Immunization with LAIV primed for expansion of CD4/CD8α double-positive (DP) memory T cells upon homologous challenge (Fig. 3B). Though DP memory T cells in pigs are MHC II restricted, they can express perforin and be cytolytic against virus-infected target cells [37, 38]. CD4−CD8α+ cells did not display a significant expansion upon in vitro stimulation with UV-Ca/04 regardless of vaccination, though expansion was detected for a few of the animals (Fig. 3C). Because inactivated virus was used as antigen, it’s possible that antigen was primarily presented via MHC-II and not MHC-I, limiting recall responses in the CD4-CD8α+ population. However, recent work from our group indicates that there is not a significant difference in responses using live versus inactivated virus in effector recall assays (Kappes et al, 2011). CD4−CD8α+ cells from several of the pigs in the NV/NCh group did expand upon in vitroUV -Ca/04 exposure (Fig. 3C). In addition to being expressed on classical CTL, in pigs, CD8α is also expressed on natural killers cells as well as γδ T cells [18, 39]. Flow cytometry staining used for this experiment did not discern between these populations; thus, it’s possible that NK or γδ T cells were responding to virus in vitro. This trend for the NV/NCh group was not observed in either of the other populations examined (CD4+CD8α − or CD4+CD8α+). It was surprising that LAIV vaccination did not prime for expansion of CD4−CD8α+ cells, which we expected to detect after challenge. It’s possible that this population of cells was not present in the periphery, but instead had homed to the respiratory tract. Additional research is warranted to further characterize the populations of lymphocytes responding to each vaccine platform and where these cells migrate to following vaccination and challenge. Our data underscore that the type of assay used to evaluate CMI is important and several measures may need to be used to adequately evaluate vaccine immunogenicity and/or efficacy.
While IL-2 is not an effector cytokine, it is necessary for expansion of CD4 and CD8 T cells. IFN-γ can be produced by a variety of cell types, including NK cells, γδ T cells, as well as αβ T cells. In order to indirectly gauge T cell activity in the lungs following homologous challenge, we evaluated cytokine levels in the lung lavage 5 days following pH1N1 challenge. While not significantly different, there was a trend for increased levels of IL-2 and IFN-γ in the lungs of vaccinated pigs when compared to non-vaccinated pigs (Fig. 4). While an indirect measure, this does suggest T cell activity in the lungs of these pigs. Wide variations in the cytokine levels were detected, which is often true when evaluating immune responses in pigs. Like humans, pigs are an outbred population of animals; thus, variation in the immune response is typically higher as compared to inbred lines of laboratory animals.
In this particular study, LAIV vaccination elicited sterilizing immunity following intratracheal pH1N1 challenge. However, in the WIV vaccine group, a few pigs did not demonstrate full immunity and virus was isolated from nasal swabs and lung lavage following challenge [23]. HI and SN antibodies were detected in the WIV group on the day of challenge, although they were not significantly elevated over the LAIV group. The gold standard for protection to IAV is a HI titer of 40 and titers in both groups were above this on the day of challenge. Peripheral antibody, particularly IgG from circulation, can play a significant role in protection to IAV-infection by neutralizing virus in the lower respiratory tract [40]. However, one advantage of intranasal LAIV vaccination is thought to be the induction of local responses, including induction of IgA production in the upper and lower respiratory tract. In the current study, Ca/04-specific IgG and IgA was detected in the lungs of both LAIV and WIV vaccinated pigs 5 days following challenge; however, antibody levels were significantly greater in the LAIV vaccinated group (Fig. 5A & 5B). In addition, neutralizing antibody was detectable in the lung lavage of LAIV/Ch pigs on day 5 following challenge (Fig. 5C). Thus, local antibody induced following LAIV vaccination may have been involved in protection following challenge, which would not have been present in WIV vaccinated pigs. Additional work by our research group is aimed at evaluating antibody in the respiratory tract prior to challenge to determine its role in protection.
Results from this work show that LAIV vaccination primes T cells; however, LAIV vaccination likely induced a local antibody response that contributed significantly to protection as well. However, samples were not collected in this study to evaluate IgA levels in the respiratory tract prior to challenge. On day 5 post-challenge, Ca/04-specific IgA and neutralizing antibody in the lungs were significantly higher in pigs that received the LAIV compared to the WIV. As T cells primed by LAIV vaccination contribute primarily to clearance of virus-infected cells, local IgA may have prevented infection of cells in the respiratory tract of LAIV vaccinated, challenged pigs. Thus, the contribution of primed T cells to protection following homologous challenge is unclear, though challenge with homologous virus likely served as another boost. IL-2 and IFN-γ levels in the lung lavage suggest T cell activity, though further work is warranted to clarify the role and activation of local T cell responses. Lastly, the superiority of LAIV vaccination is believed to be not only the induction of local immunity, but cross-protection to heterologous virus [41]. The internal genes of the temperature-sensitive LAIV are more conserved across contemporary swine IAV; therefore, we anticipate cross-reactive T cell responses between viruses even with antigenic drift in surface proteins. Research in our group is ongoing to evaluate the cross-reactive responses (IgA and T cell) elicited following LAIV vaccination in pigs and how these responses contribute to protection.
Highlights.
Vaccine comparison in pigs.
Live-attenuated influenza virus vaccination elicits greater antibody response.
Live-attenuated vaccination elicits peripheral antigen-specific IFN-γ responses.
Influenza virus immunogenicity in pigs.
Acknowledgments
We thank Gwen Nordholm, Michelle Harland, Zahra Olson, Theresa Olter-Marth, and Andrea Ferrero for excellent technical assistance and Brian Pottebaum and Jason Huegel for assistance with animal work. Thanks to Dr. Ray Waters for critical review and insight on manuscript preparation.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendations or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity employer. DRP and LP were funded by CSREES/NIFA-USDA 2006-01587, 2007-04981, and 2008-00909 and NIAID-NIH NNSN266200700010C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses a North American perspective. Adv Virus Res. 2008;72:127–54. doi: 10.1016/S0065-3527(08)00403-X. [DOI] [PubMed] [Google Scholar]
- 2.Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, et al. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. Journal of virology. 1999 Oct;73(10):8851–6. doi: 10.1128/jvi.73.10.8851-8856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorusso A, Vincent AL, Harland ML, Alt D, Bayles DO, Swenson SL, et al. Genetic and antigenic characterization of H1 influenza viruses from United States swine from 2008. The Journal of general virology. 2011 Apr;92(Pt 4):919–30. doi: 10.1099/vir.0.027557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent AL, Ma W, Lager KM, Gramer MR, Richt JA, Janke BH. Characterization of a newly emerged genetic cluster of H1N1 and H1N2 swine influenza virus in the United States. Virus genes. 2009 Jul 14; doi: 10.1007/s11262-009-0386-6. [DOI] [PubMed] [Google Scholar]
- 5.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. The New England journal of medicine. 2009 Jun 18;360(25):2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 6.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009 Jul 10;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009 Jun 25;459(7250):1122–5. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 8.Lam TT, Zhu H, Wang J, Smith DK, Holmes EC, Webster RG, et al. Reassortment Events Among Swine Influenza A Viruses in China: Implications for the Origin of the 2009 Influenza Pandemic. Journal of virology. 2011 Jul 27; doi: 10.1128/JVI.05262-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nfon CK, Berhane Y, Hisanaga T, Zhang S, Handel K, Kehler H, et al. Characterization of H1N1 Swine Influenza Viruses Circulating in Canadian Pigs in 2009. Journal of virology. 2011 Sep;85(17):8667–79. doi: 10.1128/JVI.00801-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starick E, Lange E, Fereidouni S, Bunzenthal C, Hoveler R, Kuczka A, et al. Reassorted pandemic (H1N1) 2009 influenza A virus discovered from pigs in Germany. The Journal of general virology. 2011 May;92(Pt 5):1184–8. doi: 10.1099/vir.0.028662-0. [DOI] [PubMed] [Google Scholar]
- 11.Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. Journal of virology. 1997 Jun;71(6):4347–55. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerging infectious diseases. 2006 Jan;12(1):48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozdzanowska K, Maiese K, Furchner M, Gerhard W. Treatment of influenza virus-infected SCID mice with nonneutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology. 1999 Feb 1;254(1):138–46. doi: 10.1006/viro.1998.9534. [DOI] [PubMed] [Google Scholar]
- 14.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. Journal of clinical microbiology. 1986 Jul;24(1):157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. Journal of clinical microbiology. 1986 Jan;23(1):66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asahi Y, Yoshikawa T, Watanabe I, Iwasaki T, Hasegawa H, Sato Y, et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. Journal of immunology. 2002 Mar 15;168(6):2930–8. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 17.Zuckermann FA. Extrathymic CD4/CD8 double positive T cells. Veterinary immunology and immunopathology. 1999 Dec 15;72(1–2):55–66. doi: 10.1016/s0165-2427(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 18.Gerner W, Kaser T, Saalmuller A. Porcine T lymphocytes and NK cells--an update. Developmental and comparative immunology. 2009 Mar;33(3):310–20. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Masic A, Booth JS, Mutwiri GK, Babiuk LA, Zhou Y. Elastase-dependent live attenuated swine influenza A viruses are immunogenic and confer protection against swine influenza A virus infection in pigs. Journal of virology. 2009 Oct;83(19):10198–210. doi: 10.1128/JVI.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent AL, Ma W, Lager KM, Janke BH, Webby RJ, Garcia-Sastre A, et al. Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine. Vaccine. 2007 Nov 19;25(47):7999–8009. doi: 10.1016/j.vaccine.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus research. 2004 Jul;103(1–2):177–85. doi: 10.1016/j.virusres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Townsend HG, Penner SJ, Watts TC, Cook A, Bogdan J, Haines DM, et al. Efficacy of a cold-adapted, intranasal, equine influenza vaccine: challenge trials. Equine veterinary journal. 2001 Nov;33(7):637–43. doi: 10.2746/042516401776249354. [DOI] [PubMed] [Google Scholar]
- 23.Pena L, Vincent AL, Ye J, Ciacci-Zanella JR, Angel M, Lorusso A, et al. Modifications in the polymerase genes of a swine-like triple-reassortant influenza virus to generate live attenuated vaccines against 2009 pandemic H1N1 viruses. Journal of virology. 2011 Jan;85(1):456–69. doi: 10.1128/JVI.01503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Lee CW, Zhang Y, Senne DA, Dearth R, Byrum B, et al. Isolation and characterization of H3N2 influenza A virus from turkeys. Avian diseases. 2005 Jun;49(2):207–13. doi: 10.1637/7288-101304R. [DOI] [PubMed] [Google Scholar]
- 25.Vincent AL, Ciacci-Zanella JR, Lorusso A, Gauger PC, Zanella EL, Kehrli ME, Jr, et al. Efficacy of inactivated swine influenza virus vaccines against the 2009 A/H1N1 influenza virus in pigs. Vaccine. 2010 Mar 24;28(15):2782–7. doi: 10.1016/j.vaccine.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 26.Loving CL, Brockmeier SL, Vincent AL, Palmer MV, Sacco RE, Nicholson TL. Influenza virus coinfection with Bordetella bronchiseptica enhances bacterial colonization and host responses exacerbating pulmonary lesions. Microb Pathog. 2010 Nov;49(5):237–45. doi: 10.1016/j.micpath.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995 Nov;32(6):648–60. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 28.Reed LJaHM. A simple method for estimating fifty percent endpoints. American Journal of Hygeine. 1938;27:493–7. [Google Scholar]
- 29.Vincent AL, Lager KM, Ma W, Lekcharoensuk P, Gramer MR, Loiacono C, et al. Evaluation of hemagglutinin subtype 1 swine influenza viruses from the United States. Vet Microbiol. 2006 Dec 20;118(3–4):212–22. doi: 10.1016/j.vetmic.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Zuckermann FA, Husmann RJ, Schwartz R, Brandt J, Mateu de Antonio E, Martin S. Interleukin-12 enhances the virus-specific interferon gamma response of pigs to an inactivated pseudorabies virus vaccine. Veterinary immunology and immunopathology. 1998 May 15;63(1–2):57–67. doi: 10.1016/s0165-2427(98)00082-8. [DOI] [PubMed] [Google Scholar]
- 31.Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002 Jan 31;20(9–10):1340–53. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- 32.Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. The New England journal of medicine. 1998 May 14;338(20):1405–12. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 33.Johnson PR, Jr, Feldman S, Thompson JM, Mahoney JD, Wright PF. Comparison of long-term systemic and secretory antibody responses in children given live, attenuated, or inactivated influenza A vaccine. Journal of medical virology. 1985 Dec;17(4):325–35. doi: 10.1002/jmv.1890170405. [DOI] [PubMed] [Google Scholar]
- 34.Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. The Journal of infectious diseases. 2011 Sep;204(6):845–53. doi: 10.1093/infdis/jir436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen DL, Karasin A, Zuckermann F, Olsen CW. Systemic and mucosal immune responses to H1N1 influenza virus infection in pigs. Veterinary Microbiology. 2000 May 22;74(1–2):117–31. doi: 10.1016/s0378-1135(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 36.He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. Journal of virology. 2006 Dec;80(23):11756–66. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Bruin TG, Van Rooij EM, De Visser YE, Bianchi AT. Cytolytic function for pseudorabies virus-stimulated porcine CD4+ CD8dull+ lymphocytes. Viral immunology. 2000;13(4):511–20. doi: 10.1089/vim.2000.13.511. [DOI] [PubMed] [Google Scholar]
- 38.Denyer MS, Wileman TE, Stirling CM, Zuber B, Takamatsu HH. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Veterinary immunology and immunopathology. 2006 Apr 15;110(3–4):279–92. doi: 10.1016/j.vetimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Charerntantanakul W, Roth JA. Biology of porcine T lymphocytes. Animal Health Research Review. 2006;7:81–96. doi: 10.1017/S1466252307001235. [DOI] [PubMed] [Google Scholar]
- 40.Ramphal R, Cogliano RC, Shands JW, Jr, Small PA., Jr Serum antibody prevents lethal murine influenza pneumonitis but not tracheitis. Infection and immunity. 1979 Sep;25(3):992–7. doi: 10.1128/iai.25.3.992-997.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. Journal of immunology. 2007 Jan 15;178(2):1030–8. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]