Abstract
Studies using the currently available malignant canine mast cell lines and bone marrow-derived cultured mast cells (BMCMCs) have provided an in-depth understanding of normal and neoplastic canine mast cell biology. However, many of the currently available malignant canine mast cell lines possess limitations, including loss of cell surface markers and inability to bind canine IgE. We have recently generated a novel mast cell line, CL1, from an 11-year-old spayed female Labrador retriever diagnosed with systemic mastocytosis and neoplastic effusion. The CL1 cells express KIT, FcεRI, CD44, CD45, CD14, CD11a, CD11b and CD18 as well as chymase. Interestingly, these cells express wild-type KIT, with no evidence of autophosphorylation, but are able to proliferate independently without the addition of exogenous stem cell factor (SCF), KIT ligand. However, stimulation of CL1 cells with SCF induces KIT phosphorylation promoting cell proliferation. The CL1 cells retain functional properties of mast cells, degranulating in a dose-dependent manner in response to both IgE cross-linking and chemical stimulation. Lastly, cytogenetic evaluation revealed several recurrent tumor-associated chromosome copy number imbalances in the CL1 line. In summary, the CL1 cell-line possesses phenotypic and functional properties similar to those found in canine BMCMCs, and will likely be a useful tool to study mast cell biology, factors regulating transformation of mast cells, cytogenetic abnormalities in mast cell tumors, and novel preclinical therapies.
Keywords: mast cell tumor, KIT, cell line
Introduction
Mast cell tumors are the most common skin tumor in canines, and the second most common malignancy found in this species (Brodey, 1970). These tumors range in behavior from benign to extremely malignant resulting in death of affected patients (London and Seguin, 2003). This is in contrast to humans, where the disease often manifests with systemic involvement of various organs including the liver, spleen, and bone marrow (Akin and Metcalfe, 2004; Valent et al., 2005). In canines and humans, malignant mast cell disease is known to be linked to dysregulation of the tyrosine kinase receptor, KIT. Most human patients with systemic mastocytosis carry the D816V mutation in the catalytic domain of KIT, which results in constitutive activation and uncontrolled cell proliferation (Longley et al., 1999; Longley et al., 2000). In contrast, up to 30% of high grade canine mast cell tumors possess an internal tandem duplication (ITD) mutation in the juxtamembrane domain of KIT, which also leads to constitutive activation (Downing et al., 2002; London et al., 1999; Ma et al., 1999; Zemke et al., 2002). More recently, mutations in the exon 8 and exon 9 of cKit have also been reported in a small number of canine mast cell tumors [REF]. These findings have led to the successful application of small molecule KIT inhibitors for the treatment of mast cell disease in both canines and humans (Droogendijk et al., 2006; London et al., 2003).
Studies on the role of KIT dysfunction in mast cell neoplasia have largely relied on a few well-established cell lines including the human HMC1.1 and HMC1.2 mast cell lines and the BR and C2 canine mast cell lines (DeVinney and Gold, 1990; Furitsu et al., 1993; London et al., 1999; Ma et al., 1999). The human lines carry the KIT mutations V560G and V560G/D816V, respectively, and the canine mast cell lines carry mutations in the juxtamembrane domain of KIT (L575P and KIT ITDs, respectively), making them useful tools for studying the biology of KIT dysfunction as well as the application of novel KIT inhibitors. For example, both the human and canine lines have been used to define the biologic activity of drugs such as SU11654, imatinib, sunitinib, 17-AAG, and dasatinib (Fumo et al., 2004; Gleixner et al., 2007; Liao et al., 2002; Ma et al., 2002; Shah et al., 2006).
While the role of KIT mutations in mast cell neoplasia has been well described, little is known regarding the mechanisms responsible for the aggressive biologic behavior of mast cell tumors that do not carry KIT mutations, particularly with respect to canine disease. This is in part due to the lack of readily available canine mast cell lines and reagents to investigate the biology of normal canine mast cell lines. Over the past two years our laboratory has successfully generated canine bone marrow-derived cultured mast cells (BMCMCs) that have been used to investigate the phenotypic and functional properties of normal canine mast cells (Lin and London, 2006; Lin et al., 2006a). These cells have also been used to compare the biologic functions and gene expression patterns of normal and malignant mast cells (unpublished). However, such studies have involved comparisons of BMCMCs with malignant mast cell lines expressing KIT mutations. As 60–70% of malignant canine mast cell tumors do not express activating mutations in KIT, the development of additional mast cell lines not dependent on KIT signaling for survival is important to more thoroughly investigate the biology of malignant mast cell disease. Recently, a novel canine mast cell line, MPT-1 was developed from a canine mast cell tumor for similar purposes (Amagai et al., 2008). The MPT-1 cells express functional high affinity IgE receptors and wild-type Kit. However, the immunophenotype of MPT-1 and associated cytogenetic changes were not reported.
In this study, we describe the generation and characterization of another novel neoplastic canine mast cell line derived from a dog with systemic mastocytosis. This line, CL1, exhibits a similar phenotype and immunophenotype as that found in normal mast cells. Furthermore, the CL1 line also expresses wild-type KIT and retains many functional properties of mast cells, including the ability to degranulate following IgE cross-linking and chemical stimulation. Lastly, cytogenetic evaluation identified several recurrent tumor-associated chromosome copy number imbalances. These data demonstrate that the new CL1 malignant mast cell line will be useful for future studies investigating the biology of canine mast cell disease.
Materials and methods
Reagents
Recombinant canine stem cell factor (rcSCF) was purchased from R&D Systems (Minneapolis, MN). The following reagents and chemicals were obtained from Sigma (St. Louis, MO): calcium ionophore A23187, concanavalin A (ConA), compound 48/80, Tyrode’s basal salt solution, and 4-methylumbelliferyl N-acetyl-β-D-glucosaminide dihydrate. The following antibodies against canine cell surface markers were kindly provided by Dr. Peter Moore (Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, UC Davis): MHC II, CD11a, CD11b, CD11c, CD11d, CD18, and CD45. The antibodies against canine CD14 and CD44 were obtained from AbD Serotec (Raleigh, NC) and rat-anti mouse CD117 (ACK45) conjugated with PE was purchased from BD Biosciences (Franklin Lakes, NJ).
Generation of CL1 line
An 11-year-old spayed female Labrador retriever was presented to the Veterinary Teaching Hospital (VTH) at The Ohio State University (OSU) for evaluation of abdominal distention and labored breathing. The patient was diagnosed as having systemic mastocytosis with neoplastic mast cell effusion, multiple liver masses, and circulating neoplastic mast cells (1,500/μl). The neoplastic effusion was harvested for cell culture. Mononuclear cells were purified from 50 ml abdominal fluid by Ficoll® density gradient purification. Approximately 50% of the cells were cytologically identified as neoplastic mast cells by Wright-Giemsa stain. These mononuclear cells were then cultured at a starting concentration of 107 cells per ml in RPMI medium supplied with 10% FCS, penicillin, streptomycin, and L-glutamine (Invitrogen) for several weeks. Flasks were changed during weekly passages to remove attached cells, including macrophages and fibroblasts.
Phenotypic and immunophenotypic analysis
After several weeks of culture, the resultant cell population (termed CL1) was harvested and evaluated by Wright-Giemsa and toluidine blue staining. Chymase activity was detected using a commercially available detection kit (Sigma) using naphthol AS-D chloroacetate as the substrate as previously described (Lin et al., 2006b). Cells were counterstained by hematoxylin. Immunophenotype of the CL1 cells was assessed using flow cytometry. Briefly, cells were washed and incubated with primary antibodies or appropriate isotype control for 1 hour at 4°C. After washing 3 times, cells were incubated with appropriate secondary antibodies for another 30 min at 4°C. Cells were then analyzed by flow cytometry using a FACScalibur with Cell Quest Pro software (BD Biosciences).
Degranulation assay
To analyze response of CL1 cells to chemical stimulation, 1 × 104 cells were collected and suspended in 90 μl of Tyrode’s salt solution and 10 μl of ConA, compound 48/80 or A23187 at various final concentrations as indicated and incubated for 30 min (Lin et al., 2006b). For IgE cross-linking, cells were loaded with 1 μg/ml canine IgE for 2 hours at 37°C. After washing, cells were then incubated with goat anti-dog IgE for 1 hour at 37°C as previously described (Lin et al, 2006a). After incubation with various chemicals and anti-IgE antibodies, 25 μl of cell-free supernatant was collected and the remaining cells were collected and lysed. The concentration of β-hexosminidase was determined using 4-methylumbelliferyl N-acetyl-β-D-glucosaminide as previously described (Lin et al., 2006b). Briefly, 100 μl of 1.2 mM 4-methylumbelliferyl N-acetyl-β-D-glucosaminide in 0.05 M sodium acetate buffer (pH 4.4) was added to 25 μl of supernatant. The reaction was quenched using 175 μl of cold 0.1 M glycine carbonate buffer (pH 10.0) and fluorescence was measured using an ELISA plate reader (Molecular Devices). The β-hexosaminidase released was calculated using the following formula: (fluorescence in cell supernatants − fluorescence in blank)/(fluorescence in cell supernatants − fluorescence in blank + fluorescence in the cell pellet − fluorescence in blank) × 100. All experiments were performed in triplicate.
KIT expression and function
To evaluate the expression and function of KIT on CL1 cells, Western blotting was performed. Briefly, after serum starvation for 2 hours, 1 × 106 CL1 cells were treated with or without 50 ng/ml rcSCF for 15 minutes at room temperature. Cells were then washed and lysed in protein lysis buffer and the protein concentrations were measured. Following SDS-PAGE of 50 μg protein, phosphorylated KIT and total KIT were also evaluated by Western blotting as previously described (Liao et al., 2002); (Lin et al., 2008). To determine the effect of SCF stimulation on CL1 cells, 5 × 104 cells were cultured with or without 50 ng/ml rcSCF for 3 days and cell viability was assessed on day 1, 2 and 3 by the WST-1 assay as previously described (Lin et al., 2006b). Experiments were performed in triplicate.
cKIT sequencing
CL1 cells were evaluated for evidence of cKIT mutation by full length cDNA sequencing as previously described (Lin et al., 2008). The following primer sets were used: P10 (5′-GCA ATT ACA CGT GCA CCA AC-3′); cKIT 1110R (5′-CTG ATA TTA CTT TCA TTG TCA G-3′); cKIT 582F (5′-GCA GGA CGG TGC TGT CCA AG-3′); P5 (5′-CAT GGC CGC ATC CGA CTT AAT CAG-3′); P1 (5′-GAG GAG ATC AAT GGA AAC AAT TAT G-3′); and cDNAR2 (5′-GCT TCA CAC ATC TTC GTG TAC CAG CAG AGG CTG GG-3′). Three large fragments of cKIT cDNA product were amplified, gel purified, then sequenced by the OSU Comprehensive Cancer Center’s Nucleic Acid Shared Resource Center.
Cytogenetic analysis
Microarray-based comparative genomic hybridization analysis (aCGH) was carried out as previously described (Pennington et al., 1992) using a cytogenetically validated array of bacterial artificial chromosome (BAC) clones distributed at one megabase (1Mb) intervals across all dog autosomes and chromosome X (Thomas et al., 2008). Arrayed clones were derived from the CHORI-82 female boxer BAC library from which the dog 7.6x genome sequence assembly (Lindblad-Toh et al., 2005) was constructed (http://bacpac.chori.org, BACPAC Resources, Children’s Hospital Oakland Research Institute, Oakland, CA). DNA was isolated from the CL1 cells and co-hybridized with differentially labeled reference DNA obtained from the peripheral lymphocytes of ten clinically healthy male dogs of mixed breed. A panel of BAC clones represented on the 1Mb array was then selected for direct investigation of their copy number status in the tumor cell population using multicolor fluorescence in situ hybridization analysis. This panel comprised nine clones from seven different chromosomes, representing a range of normal and abnormal genomic copy numbers according to aCGH analysis of CL1. DNA was isolated from each BAC clone and differentially labeled with fluorescent nucleotides as previously described (Breen et al., 2004). The resulting probes were first hybridized onto metaphase chromosome preparations from a clinically normal dog, serving as a control to confirm their normal copy number and genomic specificity. The probes were then applied to CL1 cell preparations and the copy number of each probe was evaluated in no fewer than 30 representative metaphase spreads and interphase nuclei. Two independent investigators assessed the results, and consensus data from the FISH analysis were compared with aCGH data for cross validation.
Statistics
Results were presented as mean ± SD, and a student’s t-test was performed when indicated. A p-value less than 0.05 was considered significant.
Results
Generation and phenotype/immunophenotype of the canine neoplastic mast cell line, CL1
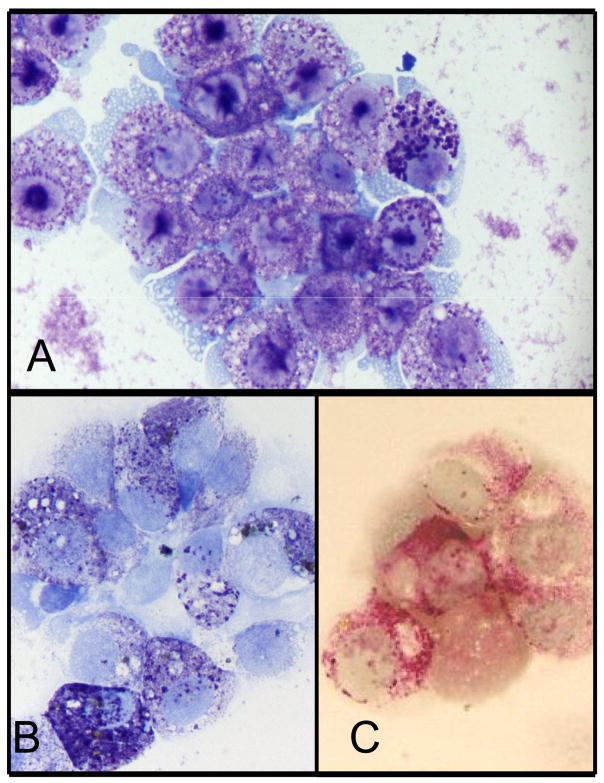
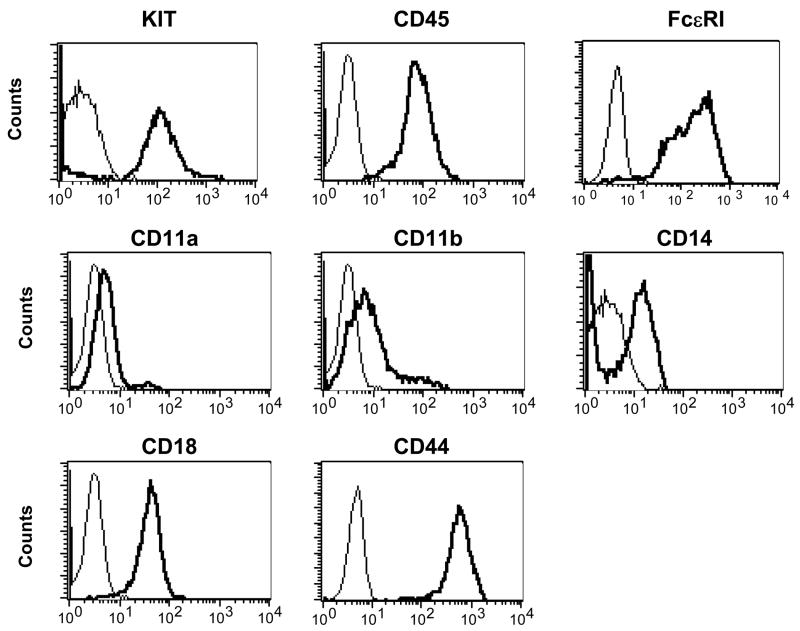
The canine neoplastic mast cell line, CL1, was successfully established from the abdominal effusion of an 11-year-old spayed female Labrador retriever with systemic mastocytosis. The CL1 cells grow as non-adherent cells in standard tissue culture medium containing RPMI and 10% fetal calf serum without needing exogenous SCF for survival. The cells are large round cells which exhibit slight anisocytosis and anisokaryosis, a moderate nuclear to cytoplasm ratio, round nuclei, and variably sized nucleoli (Fig. 1a). They contain variable quantities of small purple cytoplasmic granules which often obscure the nuclear features. As shown in Figs. 1b and 1c, the granules are positive for toluidine blue and chymase activity, respectively. Furthermore, the CL1 cells are positive for CD117 (KIT), CD14, CD44, CD45 and FcεRI, low positive for CD11a and CD11b, but negative for CD11c, CD11d, CD34 and MHC II (Fig. 2 and data not shown). This pattern is compatible with the previously reported phenotype of both human and canine mast cells (Lin et al., 2006a; Welker et al., 2000).
Fig 1. Phenotype of the CL1 mast cell line.
Cell morphology was evaluated by Wright-Giemsa staining (a). These cells are large round cells with a moderate N:C ratio, round nuclei, and variable numbers of purple granules in the cytoplasm. CL1 granules are positive for toluidine blue (b) and exhibit chymase activity (c).
Figure 2. Immunophenotype of the CL1 line.
Immunophenotype of the CL1 cells was evaluated by flow cytometry. The cells express CD117 (KIT), FcεRI, CD44, CD45, CD14, CD11a, CD11b and CD18, but not CD34, CD11c, CD11d and CD1c. Of note, FcεRI was detected indirectly as described previously (Lin et al., 2006a). solid line: isotype control; bold line: specific antibody.
Degranulation of CL1 cells
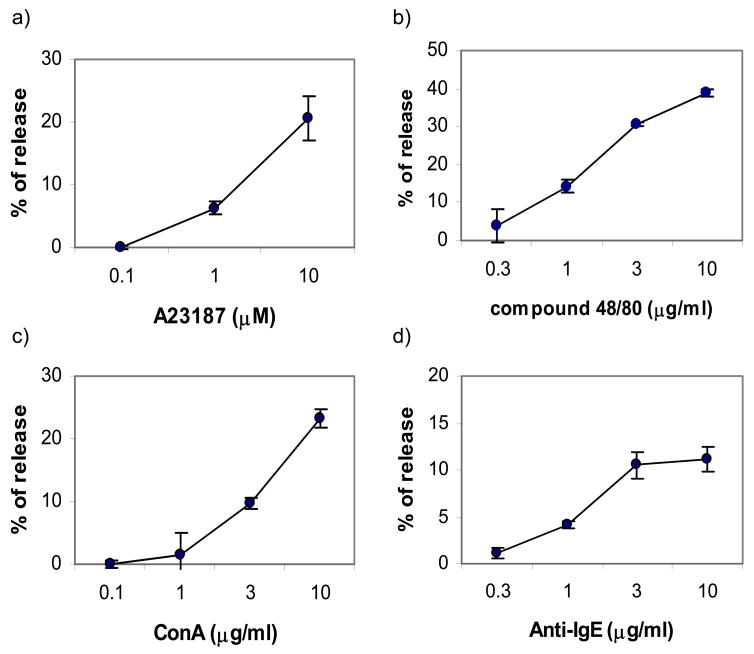
Mast cells contribute to a variety of biological processes in both health and disease through release of several soluble mediators. Despite the fact that neoplastic mast cell lines are often used to study mast cell biology, they frequently lose or do not possess several functional properties. As shown in Fig. 3, the CL1 cells release β-hexosaminidase in response to chemical stimulation with A23187, compound 48/80 and ConA in a dose dependent manner. Additionally, they can be loaded with canine IgE then cross-linked, inducing β-hexosaminidase release. Therefore, the CL1 cells retain their ability to degranulate, a fundamental property of normal mast cells.
Figure 3. CL1 cells degranulate following chemical stimulation or IgE cross-linking.
The CL1 cells were collected and activated by chemical stimulation with ConA, calcium ionophore A23187, or compound 48/80, or activated by IgE crosslinking as previously described (Lin and London, 2006). The magnitude of degranulation was assessed by the percentage of β-hexosaminidase released. The CL1 cells release β-hexosaminidase in a dose dependent manner in response to all forms of activation.
KIT expression and function on CL1 cells
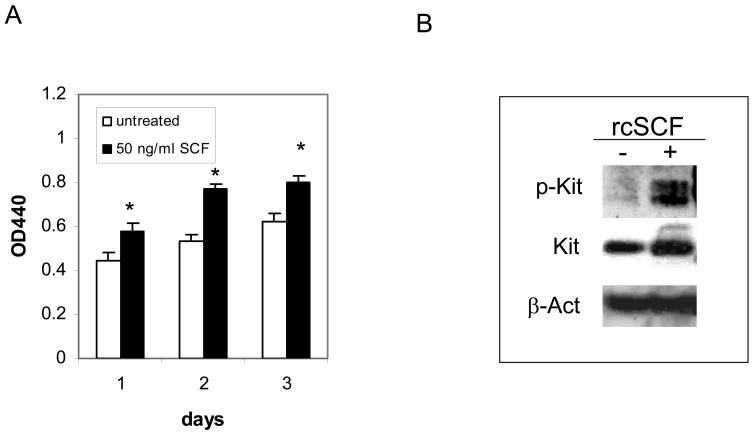
To evaluate the role of KIT on the CL1 cells, cells were cultured in the WST-1 assay on days 1, 2 and 3 of culture. As shown in Fig. 4a, the CL1 cells proliferate without the need for rcSCF, although exogenous rcSCF significantly enhances this proliferation by day 3 of culture. KIT is not constitutively phosphorylated in the absence of rcSCF stimulation, but can be activated following a short incubation with this cytokine (Fig. 4b). The lack of constitutive KIT phosphorylation is supported by the fact that no mutations were identified in the cKIT sequence derived from CL1 cells. In summary, the CL1 cells are independent of SCF for their survival and proliferation, although SCF stimulation does enhance their proliferation.
Figure 4. CL1 cells respond to rcSCF stimulation.
(a) CL1 cells were cultured with or without 50 ng/ml rcSCF for 3 days and cell viability was assessed on days 1, 2 and 3 of culture. The CL1 cells are viable in the absence of SCF stimulation, but the addition of SCF significantly enhances their viability (* P<0.05, t-test). (b) CL1 cells were serum-starved for 2 hours then left untreated or incubated with SCF for 15 minutes. Cells were harvested and phosphorylated KIT and total KIT were evaluated by Western blotting. CL1 cells express KIT without evidence of constitutive phosphorylation, although KIT phosphorylation can be induced by rcSCF stimulation.
Cytogenetic analysis
Genome-wide aCGH analysis of the CL1 cell line revealed several recurrent chromosome copy number changes in the tumor genome, including gain of dog chromosome (CFA) 13, 21, 35 and 37, and partial loss of CFA 34 (Fig. 5). The inclusion on the aCGH array of BAC clones representing 53 key cancer-associated genes (Thomas et al., submitted) revealed that these chromosome aberrations included copy number gains corresponding to the MYC and KIT oncogenes, located on CFA 13, as well as gain of the PAX3 gene (CFA 37), and loss of the tumor suppressor genes CDKN2A (CFA 11) and BCR (CFA 26).
Figure 5. Whole genome aCGH profile of CL1 cells.
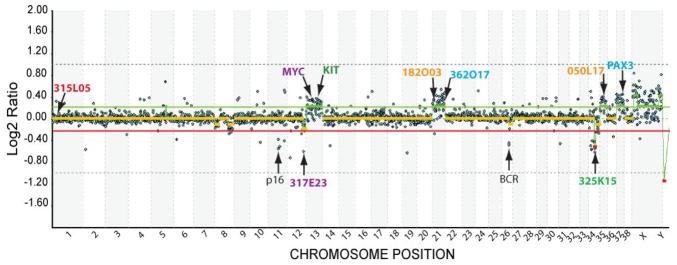
A whole genome aCGH profile of CL1 was generated using a 1Mb assembly-integrated, cytogenetically validated dog BAC array. Data are plotted as the median, block-normalized and background-subtracted log2 ratio of the replicate spots for each BAC clone on the array. Clones representing key genes of interest are arrowed. Clones identified by colored text (either BAC address or gene name) represent the nine clones that were used for multicolor single locus probe (SLP) analysis of cells from the CL1 line to verify results of the aCGH data (see Fig. 6). Log2 ratios representing genomic gain and loss are indicated by horizontal bars above (green line) and below (red line) the midline (orange line) representing normal copy number. Regions corresponding to chromosome gain or loss are indicated by thick green and red lines, respectively, as determined by the aCGH Smooth algorithm (Jong et al., 2004).
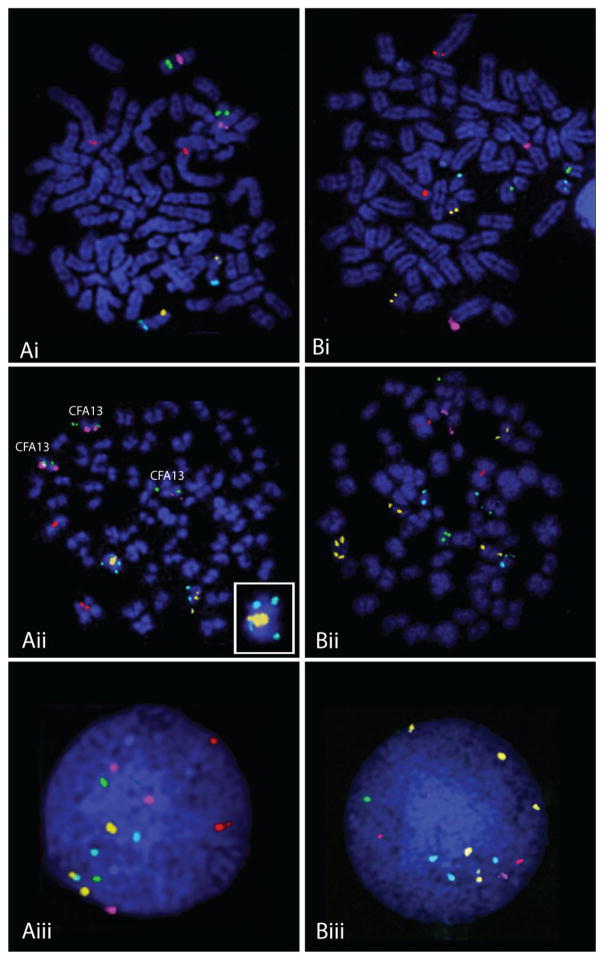
FISH analysis of CL1 tumor chromosomes using clones selected from the array showed excellent correlation with aCGH data (Fig 6). Over 90% of cells analyzed showed the expected normal copy number (n=2) for a clone located on CFA 1. This clone was selected as an internal control for genomic balance. FISH data indicated increased copy number for clones representing CFA 13 (MYC and KIT), CFA 21, CFA 35 and CFA 37 (PAX3), in concordance with aCGH data. FISH analysis also revealed structural rearrangements involving CFA 21 and CFA 35 (Fig. 6). The additional copies of CFA 21 and CFA 35 independently exhibited translocations onto different chromosomes, resulting in the generation of derivative chromosomes significantly longer than normal. This appears to be the result of centric fusion combined with chromosomal amplification. In accordance with aCGH data, FISH analysis detected copy number decreases of loci on the distal end of CFA 12 and the proximal half of CFA 34 in 44% and 78% of CL1 cells, respectively. A quantitative summary of the FISH data for each of the nine loci is shown in Fig. 7.
Figure 6. aCGH targeted single locus probe (SLP) analysis of CL1.
Panels A and B each shows the co-hybridization of five differentially labeled BAC clones. Panel A shows signal resulting from clones specific for CFA1 at 12Mb (315L05, red signal), CFA13 at 28Mb (396J05[MYC], purple signal) and 50Mb (265L22[KIT], green signal) and CFA21 at 4Mb (182O03, orange signal) and 54Mb (362O17, aqua signal), while panel B shows signal for CFA1 at 12Mb (315L05, red signal) and also for CFA 12 at 75Mb (317E23, purple signal), CFA34 at 13Mb (325K15, green signal), CFA 35 at 29Mb (050L17, orange signal) and CFA37 at 31Mb (57H23[PAX3], aqua signal). Panels Ai and Bi show hybridization of the BAC clones to metaphase preparations from a clinically healthy dog, revealing the expected copy number (n=2) of each locus per cell. Panels Aii and Bii show the same clones hybridized to metaphase preparations generated from the line CL1. Panels Aiii and Biii show the probes hybridized to interphase nuclei derived from CL1. Analysis of the copy number status of the five probes in Aii reveals a copy number of n=3 for both MYC and KIT (CFA13) and also an apparent n=3 362017 (aqua signal) (CFA21;54 Mb). A closer look at one of the homologues of CFA21 indicates it is bi-armed due to the presence of a centric fusion of CFA21 (see inset) with an oversized signal for the centromeric clone 182O03 (orange signal). In the CL1 interphase nucleus shown in Aiii, while three copies of MYC are evident (purple signal), there are two copies of KIT (green signal) in this particular cell. There are three copies each of both cones from CFA21. In panels Bii and Biii, it is evident that there are copy number losses for the clones representing CFA12 and CFA34 (both present at n=1) and copy number gains for the clones representing CFA 35 (n=5) and CFA37 (n=3). A summary of the frequency of copy number for each clone is shown in Fig 7.
Figure 7. Frequency of copy number variation for the nine BAC clones selected to verify the aCGH data for CL1.
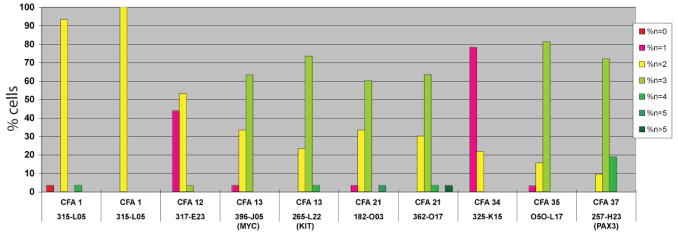
Clone 315L05 was a common BAC to both groups of five clones and data are thus presented twice. Overall the copy number changes for each clone was in complete concordance with that indicated by aCGH analysis.
Discussion
Neoplastic mast cell diseases are rare in people and often present as systemic mastocytosis, which usually occurs without cutaneous lesions and frequently involves diffuse infiltration of the bone marrow, liver, spleen, and abdominal lymph nodes with neoplastic mast cells (Akin and Metcalfe, 2004; Butterfield, 2006). In contrast, canine mast cell tumors are the second most common tumor in canines and usually present as cutaneous tumors that may spread to local lymph nodes, liver and spleen (London and Seguin, 2003). Most cases of human mastocytosis are known to be linked to dysregulation of the receptor tyrosine kinase KIT. This receptor acts primarily through mutation in the catalytic domain that promotes uncontrolled growth (Longley et al., 1999; Longley et al., 2001; Roskoski, 2005). In contrast, only a portion of canine mast cell tumors are known to possess activating KIT mutations, most of which occur in the juxtamembrane domain of the receptor (Downing et al., 2002; London et al., 1999; Ma et al., 1999; Zemke et al., 2002). More recently, point mutations in exons 8 and 9, encoding part of the extracellular domain, have been identified in a small number of tumors (Letard et al., 2008). These mutations also appear to be activating in nature, although to a far lesser degree than the juxtamembrane domain mutations. Little is known about the mechanisms that contribute to malignant transformation of canine mast cell tumors that do not possess KIT mutations.
The currently available canine mast cell lines include C2, BR, CM-MC, and VI-MC, and more recently MPT-1 (Liao et al., 2002; Takahashi et al., 2001); (Amagai et al., 2008). The BR cells have been used to examine the ability of malignant mast cells to respond to various chemicals (Garcia et al., 1998), while the CM-MC cells have been used to demonstrate that mast cells can degranulate upon cross-linking of human IgG1 or IgG4 bound to the low-affinity IgG receptor (Sato et al., 2004). Both the C2 and BR cells possess KIT mutations in the juxtamembrane domain that cause constitutive activation, and as such these lines have been used to explore the use of targeted therapeutics directed at KIT inhibition (Liao et al., 2002; London et al., 1999; Ma et al., 1999). However, several of these lines have been cultured for years and have undergone multiple passages, and seemingly may not be representative of the original tumors from which they were derived. For example, the BR cells have expression of FcεRI but fail to respond to IgE cross-linking (Garcia et al., 1998). Also, several of the mast cell lines do not have distinct mast cell granules and contain low levels of histamine (DeVinney and Gold, 1990; Garcia et al., 1998; Takahashi et al., 2001). The MPT-1 canine mast cell line expresses IgE receptors and wild-type Kit and is dependent on the PI3K pathway-dependent for cell proliferation (Amagai et al., 2008). As there are only a limited number of malignant canine mast cell lines, it would be useful to have additional lines, especially without KIT mutation, that could be used to evaluate the biology of mast cell disease.
In this study, we developed a new mast cell line, CL1, from a dog with systemic mastocytosis. The CL1 cells exhibit an immunophenotype similar to that found in both normal human and canine mast cells (Lin et al., 2006a; Welker et al., 2000). Interestingly, the CL1 cells express CD14, the receptor for lipopolysaccharide (LPS) which combined with TLR4, contributes to recognition of bacterial pathogens. Bone marrow derived cultured canine mast cells and cord blood derived human mast cells do not express CD14, while human bone marrow derived mast cells do (Escribano et al., 1998; Inomata et al., 2005; Lin et al., 2006a). This inconsistent expression is most likely due to differences in culture conditions used to generate mast cells in vitro. Indeed, evidence suggests that expression of CD14 by mast cells in vivo is linked to their local tissue microenvironment (Escribano et al., 1998). Importantly, CL1 is the first canine mast cell line with confirmed expression of CD14 and may therefore be used to investigate the role of canine mast cell responses to bacterial challenge.
Both human and canine mast cell lines have been extremely useful for studying normal mast cell biology, as it is difficult to obtain large numbers of normal mast cells directly from tissues (Brazis et al., 2002; Brazis et al., 2000). However, as previously discussed, many mast cell lines lose their original phenotype after long-term culture, resulting in an absence of observable cytoplasmic granules or loss of FcεRI function or expression. Like the MPT-1 canine mastocytoma cell line, the CL1 cells possess large numbers of cytoplasmic granules and degranulate upon chemical stimulation and IgE cross-linking, retaining a critical functional property of normal mast cells. As such, the CL1 cells may be useful for evaluating biologic properties of canine mast cells and for testing therapeutics targeting mast cell mediators.
The CL1 cell line was generated from a dog exhibiting a clinical presentation consistent with systemic mastocytosis. We found that CL1 cells are independent of SCF stimulation for their survival and proliferation, do not express mutations in the coding region of cKIT, do not exhibit KIT auto-phosphorylation in the absence of SCF stimulation, and yet retain responsiveness to SCF stimulation. Therefore, this growth factor independent malignant canine mast cell line represents a novel resource for evaluating the molecular biology of malignant mast cell disease lacking Kit mutation in the dog. Studies are currently underway to determine the mechanism of growth factor independence in the CL1 line.
Cytogenetic analysis of the CL1 cell line revealed several recurrent genomic imbalances, including both whole and partial chromosome gains and losses. Targeted FISH analysis of CL1 tumor chromosomes demonstrated a strong correlation with the copy number indicated by aCGH analysis. FISH data confirmed copy number gains of MYC and KIT oncogenes on CFA 13 and also PAX3 on CFA 37. Gain of CFA 13 is a highly recurrent event in a wide range of dog primary tumors and cell lines (Kisseberth et al., 2007; Thomas et al., 2007; Thomas et al., 2003; Winkler et al., 2006). Over-expression of KIT may result in increased binding SCF thereby enhancing mast cell proliferation and survival, potentially contributing to malignant transformation or progression. While there was no evidence of KIT autophosphorylation in the CL1 line, it is possible that these cells are hypersensitive to SCF in vivo and that this contributes to an aggressive biologic behavior through enhanced proliferative capacity. Indeed, the addition of SCF to CL1 cultures did augment their in vivo proliferation.
MYC is believed to regulate the expression of approximately 15% of all genes (Knoepfler, 2007; Patel et al., 2004) and dysregulation of MYC occurs in over 80% of human cancers (Popescu and Zimonjic, 2002). In general, this dysregulation occurs through gene amplification, resulting in the inappropriate expression of several genes involved in cell proliferation and development (Popescu and Zimonjic, 2002). Gain in copy number of the KIT and MYC oncogenes on CFA 13, and deletion of the regions of CFA 11 and CFA 26 harboring the tumor suppressor genes CDKN2A and BCR, suggest that these events may contribute to the genetic instability of the CL1 cell line, although further expression level analysis will be required to determine the exact influence of these copy number alterations.
In summary, we have established and characterized a novel canine neoplastic mast cell line, CL1, from a dog with systemic mastocytosis. CL1 has a phenotype similar to that of normal human and canine mast cells and retains functional FcεR1 expression. Moreover, the CL1 line is not dependent on rcSCF for survival and proliferation, yet does not express activating KIT mutations, suggesting that molecular abnormalities other than those associated with KIT contributed to its growth factor independence. Studies evaluating these potential molecular abnormalities are ongoing.
Acknowledgments
This work was supported in part by a grant from the AKC Canine Health Foundation. Canine molecular cytogenetics at NCSU is generously supported by grants awarded to MB from the NIH, AKC Canine Health Foundation and the Morris Animal Foundation. We thank Cordelia Langford, Peter Ellis and Anne Evans for help with generating the 1Mb array used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–432. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- Amagai Y, Tanaka A, Ohmori K, Matsuda H. Establishment of a novel high-affinity IgE receptor-positive canine mast cell line with wild-type c-kit receptors. Biochem Biophys Res Commun. 2008;366:857–861. doi: 10.1016/j.bbrc.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Brazis P, De Mora F, Ferrer L, Puigdemont A. IgE enhances Fc epsilon RI expression and IgE-dependent TNF-alpha release from canine skin mast cells. Vet Immunol Immunopathol. 2002;85:205–212. doi: 10.1016/s0165-2427(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Brazis P, Queralt M, de Mora F, Ferrer LI, Puigdemont A. Stem cell factor enhances IgE-mediated histamine and TNF-alpha release from dispersed canine cutaneous mast cells. Vet Immunol Immunopathol. 2000;75:97–108. doi: 10.1016/s0165-2427(00)00188-4. [DOI] [PubMed] [Google Scholar]
- Breen M, Hitte C, Lorentzen TD, Thomas R, Cadieu E, Sabacan L, Scott A, Evanno G, Parker HG, Kirkness EF, Hudson R, Guyon R, Mahairas GG, Gelfenbeyn B, Fraser CM, Andre C, Galibert F, Ostrander EA. An integrated 4249 marker FISH/RH map of the canine genome. BMC Genomics. 2004;5:65. doi: 10.1186/1471-2164-5-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodey RS. Canine and feline neoplasia. Adv Vet Sci Comp Med. 1970;14:309–354. [PubMed] [Google Scholar]
- Butterfield JH. Systemic mastocytosis: clinical manifestations and differential diagnosis. Immunol Allergy Clin North Am. 2006;26:487–513. doi: 10.1016/j.iac.2006.05.006. [DOI] [PubMed] [Google Scholar]
- DeVinney R, Gold WM. Establishment of two dog mastocytoma cell lines in continuous culture. Am J Respir Cell Mol Biol. 1990;3:413–420. doi: 10.1165/ajrcmb/3.5.413. [DOI] [PubMed] [Google Scholar]
- Downing S, Chien MB, Kass PH, Moore PE, London CA. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c-kit in mast cell tumors of dogs. Am J Vet Res. 2002;63:1718–1723. doi: 10.2460/ajvr.2002.63.1718. [DOI] [PubMed] [Google Scholar]
- Droogendijk HJ, Kluin-Nelemans HJ, van Doormaal JJ, Oranje AP, van de Loosdrecht AA, van Daele PL. Imatinib mesylate in the treatment of systemic mastocytosis: a phase II trial. Cancer. 2006;107:345–351. doi: 10.1002/cncr.21996. [DOI] [PubMed] [Google Scholar]
- Escribano L, Orfao A, Villarrubia J, Diaz-Agustin B, Cervero C, Rios A, Velasco JL, Ciudad J, Navarro JL, San Miguel JF. Immunophenotypic characterization of human bone marrow mast cells. A flow cytometric study of normal and pathological bone marrow samples. Anal Cell Pathol. 1998;16:151–159. doi: 10.1155/1998/341340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumo G, Akin C, Metcalfe DD, Neckers L. 17-Allylamino-17-demethoxygeldanamycin (17-AAG) is effective in down-regulating mutated, constitutively activated KIT protein in human mast cells. Blood. 2004;103:1078–1084. doi: 10.1182/blood-2003-07-2477. [DOI] [PubMed] [Google Scholar]
- Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayamo H, Koshimizu U, Sugahara H, Butterfield JH, Ashman LK, Kanayama Y, Matsuzawa Y, Kitamura Y, Kanakura Y. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand independent activation of c-kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G, Brazis P, Majo N, Ferrer L, de Mora F, Puigdemont A. Comparative morphofunctional study of dispersed mature canine cutaneous mast cells and BR cells, a poorly differentiated mast cell line from a dog subcutaneous mastocytoma. Vet Immunol Immunopathol. 1998;62:323–337. doi: 10.1016/s0165-2427(97)00166-9. [DOI] [PubMed] [Google Scholar]
- Gleixner KV, Rebuzzi L, Mayerhofer M, Gruze A, Hadzijusufovic E, Sonneck K, Vales A, Kneidinger M, Samorapoompichit P, Thaiwong T, Pickl WF, Yuzbasiyan-Gurkan V, Sillaber C, Willmann M, Valent P. Synergistic antiproliferative effects of KIT tyrosine kinase inhibitors on neoplastic canine mast cells. Exp Hematol. 2007;35:1510–1521. doi: 10.1016/j.exphem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Inomata N, Tomita H, Ikezawa Z, Saito H. Differential gene expression profile between cord blood progenitor-derived and adult progenitor-derived human mast cells. Immunol Lett. 2005;98:265–271. doi: 10.1016/j.imlet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Jong K, Marchiori E, Meijer G, Vaart AV, Ylstra B. Breakpoint identification and smoothing of array comparative genomic hybridization data. Bioinformatics. 2004;20:3636–3637. doi: 10.1093/bioinformatics/bth355. [DOI] [PubMed] [Google Scholar]
- Kisseberth WC, Nadella MV, Breen M, Thomas R, Duke SE, Murahari S, Kosarek CE, Vernau W, Avery AC, Burkhard MJ, Rosol TJ. A novel canine lymphoma cell line: a translational and comparative model for lymphoma research. Leuk Res. 2007;31:1709–1720. doi: 10.1016/j.leukres.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS. Myc goes global: new tricks for an old oncogene. Cancer Res. 2007;67:5061–5063. doi: 10.1158/0008-5472.CAN-07-0426. [DOI] [PubMed] [Google Scholar]
- Letard S, Yang Y, Hanssens K, Palmerini F, Leventhal PS, Guery S, Moussy A, Kinet JP, Hermine O, Dubreuil P. Gain-of-function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Mol Cancer Res. 2008;6:1137–1145. doi: 10.1158/1541-7786.MCR-08-0067. [DOI] [PubMed] [Google Scholar]
- Liao AT, Chien MB, Shenoy N, Mendel DB, McMahon G, Cherrington JM, London CA. Inhibition of constitutively active forms of mutant kit by multitargeted indolinone tyrosine kinase inhibitors. Blood. 2002;100:585–593. doi: 10.1182/blood-2001-12-0350. [DOI] [PubMed] [Google Scholar]
- Lin TY, Bear M, Du Z, Foley KP, Ying W, Barsoum J, London C. The novel HSP90 inhibitor STA-9090 exhibits activity against Kit-dependent and -independent malignant mast cell tumors. Exp Hematol. 2008 doi: 10.1016/j.exphem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TY, London CA. A functional comparison of canine and murine bone marrow derived cultured mast cells. Vet Immunol Immunopathol. 2006;114:320–334. doi: 10.1016/j.vetimm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Lin TY, Rush LJ, London CA. Generation and characterization of bone marrow-derived cultured canine mast cells. Vet Immunol Immunopathol. 2006a;113:37–52. doi: 10.1016/j.vetimm.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Lin TY, Rush LJ, London CA. Generation and characterization of bone marrow-derived cultured canine mast cells. Vet Immunol Immunopathol. 2006b doi: 10.1016/j.vetimm.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ, 3rd, Zody MC, Mauceli E, Xie X, Breen M, Wayne RK, Ostrander EA, Ponting CP, Galibert F, Smith DR, DeJong PJ, Kirkness E, Alvarez P, Biagi T, Brockman W, Butler J, Chin CW, Cook A, Cuff J, Daly MJ, DeCaprio D, Gnerre S, Grabherr M, Kellis M, Kleber M, Bardeleben C, Goodstadt L, Heger A, Hitte C, Kim L, Koepfli KP, Parker HG, Pollinger JP, Searle SM, Sutter NB, Thomas R, Webber C, Baldwin J, Abebe A, Abouelleil A, Aftuck L, Ait-Zahra M, Aldredge T, Allen N, An P, Anderson S, Antoine C, Arachchi H, Aslam A, Ayotte L, Bachantsang P, Barry A, Bayul T, Benamara M, Berlin A, Bessette D, Blitshteyn B, Bloom T, Blye J, Boguslavskiy L, Bonnet C, Boukhgalter B, Brown A, Cahill P, Calixte N, Camarata J, Cheshatsang Y, Chu J, Citroen M, Collymore A, Cooke P, Dawoe T, Daza R, Decktor K, DeGray S, Dhargay N, Dooley K, Dooley K, Dorje P, Dorjee K, Dorris L, Duffey N, Dupes A, Egbiremolen O, Elong R, Falk J, Farina A, Faro S, Ferguson D, Ferreira P, Fisher S, FitzGerald M, Foley K, Foley C, Franke A, Friedrich D, Gage D, Garber M, Gearin G, Giannoukos G, Goode T, Goyette A, Graham J, Grandbois E, Gyaltsen K, Hafez N, Hagopian D, Hagos B, Hall J, Healy C, Hegarty R, Honan T, Horn A, Houde N, Hughes L, Hunnicutt L, Husby M, Jester B, Jones C, Kamat A, Kanga B, Kells C, Khazanovich D, Kieu AC, Kisner P, Kumar M, Lance K, Landers T, Lara M, Lee W, Leger JP, Lennon N, Leuper L, LeVine S, Liu J, Liu X, Lokyitsang Y, Lokyitsang T, Lui A, Macdonald J, Major J, Marabella R, Maru K, Matthews C, McDonough S, Mehta T, Meldrim J, Melnikov A, Meneus L, Mihalev A, Mihova T, Miller K, Mittelman R, Mlenga V, Mulrain L, Munson G, Navidi A, Naylor J, Nguyen T, Nguyen N, Nguyen C, Nguyen T, Nicol R, Norbu N, Norbu C, Novod N, Nyima T, Olandt P, O’Neill B, O’Neill K, Osman S, Oyono L, Patti C, Perrin D, Phunkhang P, Pierre F, Priest M, Rachupka A, Raghuraman S, Rameau R, Ray V, Raymond C, Rege F, Rise C, Rogers J, Rogov P, Sahalie J, Settipalli S, Sharpe T, Shea T, Sheehan M, Sherpa N, Shi J, Shih D, Sloan J, Smith C, Sparrow T, Stalker J, Stange-Thomann N, Stavropoulos S, Stone C, Stone S, Sykes S, Tchuinga P, Tenzing P, Tesfaye S, Thoulutsang D, Thoulutsang Y, Topham K, Topping I, Tsamla T, Vassiliev H, Venkataraman V, Vo A, Wangchuk T, Wangdi T, Weiand M, Wilkinson J, Wilson A, Yadav S, Yang S, Yang X, Young G, Yu Q, Zainoun J, Zembek L, Zimmer A, Lander ES. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, Geissler EN. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp Hematol. 1999;27:689–697. doi: 10.1016/s0301-472x(98)00075-7. [DOI] [PubMed] [Google Scholar]
- London CA, Hannah AL, Zadovoskaya R, Chien MB, Kollias-Baker C, Rosenberg M, Downing S, Post G, Boucher J, Shenoy N, Mendel DB, Cherrington JM. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs wtih spontaneous malignancies. Clin Cancer Res. 2003;9:2755–2768. [PubMed] [Google Scholar]
- London CA, Seguin B. Mast cell tumors in the dog. Vet Clin North Am Small Anim Pract. 2003;33:473–489. v. doi: 10.1016/s0195-5616(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Longley BJ, Jr, Metcalfe DD, Tharp M, Wang X, Tyrrell L, Lu SZ, Heitjan D, Ma Y. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci U S A. 1999;96:1609–1614. doi: 10.1073/pnas.96.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley BJ, Ma Y, Carter E, McMahon G. New approaches to therapy for mastocytosis. A case for treatment with kit kinase inhibitors. Hematol Oncol Clin North Am. 2000;14:689–695. doi: 10.1016/s0889-8588(05)70302-6. [DOI] [PubMed] [Google Scholar]
- Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001;25:571–576. doi: 10.1016/s0145-2126(01)00028-5. [DOI] [PubMed] [Google Scholar]
- Ma Y, Longley BJ, Wang X, Blount JL, Langley K, Caughey GH. Clustering of activating mutations in c-KIT’s juxtamembrane coding region of canine mast cell neoplasms. J Invest Dermatol. 1999;112:165–170. doi: 10.1046/j.1523-1747.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zeng S, Metcalfe DD, Akin C, Dimitrijevic S, Butterfield JH, McMahon G, Longley BJ. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–1744. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- Patel JH, Loboda AP, Showe MK, Showe LC, McMahon SB. Analysis of genomic targets reveals complex functions of MYC. Nat Rev Cancer. 2004;4:562–568. doi: 10.1038/nrc1393. [DOI] [PubMed] [Google Scholar]
- Pennington DW, Lopez AR, Thomas PS, Peck C, Gold WM. Dog mastocytoma cells produce transforming growth factor beta 1. J Clin Invest. 1992;90:35–41. doi: 10.1172/JCI115853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu NC, Zimonjic DB. Chromosome-mediated alterations of the MYC gene in human cancer. J Cell Mol Med. 2002;6:151–159. doi: 10.1111/j.1582-4934.2002.tb00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr Structure and regulation of Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- Sato Y, Teshima R, Nakamura R, Takagi K, Sasaki N, Sawada J, Kitani S. Canine mast cell activation via human IgG1 and IgG4. Int Arch Allergy Immunol. 2004;135:154–160. doi: 10.1159/000080659. [DOI] [PubMed] [Google Scholar]
- Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kitani S, Nagase M, Mochizuki M, Nishimura R, Morita Y, Sasaki N. IgG-mediated histamine release from canine mastocytoma-derived cells. Int Arch Allergy Immunol. 2001;125:228–235. doi: 10.1159/000053820. [DOI] [PubMed] [Google Scholar]
- Thomas R, Duke SE, Bloom SK, Breen TE, Young AC, Feiste E, Seiser EL, Tsai PC, Langford CF, Ellis P, Karlsson EK, Lindblad-Toh K, Breen M. A cytogenetically characterized, genome-anchored 10-Mb BAC set and CGH array for the domestic dog. J Hered. 2007;98:474–484. doi: 10.1093/jhered/esm053. [DOI] [PubMed] [Google Scholar]
- Thomas R, Duke SE, Karlsson EK, Evans A, Ellis P, Lindblad-Toh K, Langford CF, Breen M. A genome assembly-integrated dog 1Mb BAC microarray: a cytogenetic resource for canine cancer studies and comparative genomic analysis. Cytogenet Genome Res. 2008 doi: 10.1159/000163088. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Smith KC, Ostrander EA, Galibert F, Breen M. Chromosome aberrations in canine multicentric lymphomas detected with comparative genomic hybridisation and a panel of single locus probes. Br J Cancer. 2003;89:1530–1537. doi: 10.1038/sj.bjc.6601275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent P, Akin C, Sperr WR, Mayerhofer M, Fodinger M, Fritsche-Polanz R, Sotlar K, Escribano L, Arock M, Horny HP, Metcalfe DD. Mastocytosis: pathology, genetics, and current options for therapy. Leuk Lymphoma. 2005;46:35–48. doi: 10.1080/10428190400010775. [DOI] [PubMed] [Google Scholar]
- Welker P, Grabbe J, Zuberbier T, Guhl S, Henz BM. Mast cell and myeloid marker expression during early in vitro mast cell differentiation from human peripheral blood mononuclear cells. J Invest Dermatol. 2000;114:44–50. doi: 10.1046/j.1523-1747.2000.00827.x. [DOI] [PubMed] [Google Scholar]
- Winkler S, Reimann-Berg N, Murua Escobar H, Loeschke S, Eberle N, Hoinghaus R, Nolte I, Bullerdiek J. Polysomy 13 in a canine prostate carcinoma underlining its significance in the development of prostate cancer. Cancer Genet Cytogenet. 2006;169:154–158. doi: 10.1016/j.cancergencyto.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Zemke D, Yamini B, Yuzbasiyan-Gurkan V. Mutations in the juxtamembrane domain of c-KIT are associated with higher grade mast cell tumors in dogs. Vet Pathol. 2002;39:529–535. doi: 10.1354/vp.39-5-529. [DOI] [PubMed] [Google Scholar]