Abstract
Individuals with cocaine dependence often evidence poor cognitive control. The purpose of this exploratory study was to investigate networks of functional connectivity underlying cognitive control in cocaine dependence and examine the relationship of the networks to the disorder and its treatment. Independent component analysis (ICA) was applied to fMRI data to investigate if regional activations underlying cognitive control processes operate in functional networks, and whether these networks relate to performance and treatment outcome measures in cocaine dependence. Twenty patients completed a Stroop task during fMRI prior to entering outpatient treatment and were compared to 20 control participants. ICA identified five distinct functional networks related to cognitive control interference events. Cocaine-dependent patients displayed differences in performance-related recruitment of three networks. Reduced involvement of a “top-down” fronto-cingular network contributing to conflict monitoring correlated with better treatment retention. Greater engagement of two “bottom-up” subcortical and ventral prefrontal networks related to cue-elicited motivational processing correlated with abstinence during treatment. The identification of subcortical networks linked to cocaine abstinence and cortical networks to treatment retention suggests that specific circuits may represent important, complementary targets in treatment development for cocaine dependence.
Keywords: fMRI, substance use disorders, cocaine dependence, cognitive control, cognitive behavioral therapy
Introduction
Neurocognitive impairments associated with substance use disorders are well-documented (Garavan & Stout, 2005; Lundqvist, 2005; Rogers & Robbins, 2001). Diminished cognitive control may influence the development, perseveration and cessation of addictive behaviors (Buehringer, Goschke, Gottlebe, Kufeld, & Wittchen, 2008). Cocaine dependence (CD) in particular is characterized by deficits in cognitive control at behavioral and neural levels (Barrós-Loscertales, et al., 2011; Beveridge, Gill, Hanlon, & Porrino, 2008; Garavan & Hester, 2007), and these impairments are associated with poor treatment outcome (Aharonovich, Amrhein, Bisaga, Nunes, & Hasin., 2006; Aharonovich et al., 2008; Streeter et al., 2008). Models of addiction suggest that a complimentary process of increasingly impaired “top-down” executive control with a strengthening of “bottom-up” motivational mechanisms may contribute to the development of compulsive addictive behaviors (e.g. Everitt & Robbins, 2005). A better understanding of the distinct neural mechanisms underlying cocaine-related cognitive control impairments and their relationships with outcome measures may aid in understanding the pathophysiology of CD and lead to the development of improved treatment strategies.
Observed across multiple cognitive domains (Goldstein et al., 2007a; Hester & Garavan, 2004; Li et al., 2008; Tucker et al., 2004), abnormal regional brain function, particularly in the prefrontal cortex and mesolimbic structures, may be indicative of deficient executive function and altered motivational and reward processes in cocaine dependence, giving rise to impulsive and compulsive drug-seeking and drug-using behaviors (Everitt et al., 2008; Goldstein & Volkow, 2002; Volkow, Wang, Fowler, Tomasi, & Telang, 2011). Investigations of regional functional connectivity during resting-state in cocaine dependence suggest both “top-down” and “bottom-up” neural mechanisms may contribute to observed cognitive impairments. Deficient top-down prefrontal functional connectivity has been associated with impaired delay discounting and adaptive learning in CD (Camchong et al., 2011). Increased functional connectivity of bottom-up mesocorticolimbic structures with prefrontal regions has been associated with enhanced reactivity to cocaine-related stimuli (Wilcox, Teshiba, Merideth, Ling, & Mayer, 2011). Together, these studies suggest cognitive impairments and addictive behaviors observed in CD may be attributed to differential functioning of brain networks with overlapping regional substrates. Examining how task-related regional brain activity integrates into functional networks may help identify specific neural mechanisms of cognitive control underlying cocaine-related cognitive impairments and allow examination of their relationships to clinically relevant measures like treatment outcome (Potenza, Sofuoglu, Carroll, & Rounsaville, 2011).
The Stroop color-word interference test (Stroop, 1935) is a widely used cognitive-control task (MacLeod, 1991). Using factor analysis, Peterson et al (1999) identified seven brain networks involved in Stroop performance, with each network containing prefrontal regions whose activations related with those in temporal, parietal and striatal structures. Functional relationships between the prefrontal, parietal and temporal cortices have been implicated in modulating conflict adaptation and practice-effects during Stroop performance (Egner & Hirsh, 2005; Harrison et al., 2005). In clinical populations performing the Stroop task, dynamic causal modelling analyses have observed stronger dorsal anterior cingulate effective connectivity within fronto-cingulate attentional networks in depression (Schlosser et al., 2008) and obsessive-compulsive disorder (Schloesser et al., 2010), supporting evidence that cingulate abnormalities observed in both disorders (e.g. Maltby, Tolin, Worhunsky, O'Keefe, & Kiehl, 2005; Wagner et al., 2006) may be attributed to performance monitoring mechanisms rather than affective processing. To date, no study has investigated functional connectivity during Stroop performance in CD.
Few studies have sought to directly determine relationships between brain activity contributing to cocaine-related cognitive impairments and treatment outcome measures. In CD patients performing a working memory task, reduced thalamic activity prior to treatment has been associated with poor treatment outcome (Moeller et al., 2010). Brewer, Worhunsky, Carroll, Rounsaville, and Potenza (2008) found activations in the ventral prefrontal cortex, posterior cingulate cortex and striatum of CD patients during pre-treatment performance of the Stroop task were associated with drug abstinence, and dorsolateral prefrontal cortical activation was associated with treatment retention (Brewer et al., 2008). Although informative, these studies lack examination of how the regional activations integrate into functional networks and how these networks are distinguished in CD and control subjects and relate to treatment outcome measures in CD.
The application of independent component analysis (ICA) to functional magnetic resonance imaging (fMRI) data permits investigation of the global functional organization of regional brain activity (Calhoun et al., 2001a, Calhoun, Liu, & Adali, 2009). ICA is a data-driven technique capable of identifying functionally integrated brain regions through a decomposition of the blood oxygenation level-dependent (BOLD) signal of fMRI into distinct neural systems displaying temporally synchronous activity. In a previous application, this method identified five functional networks associated with correct and erroneous response processing during a Go/No-Go response inhibition task (Stevens, Kiehl, Pearlson, & Calhoun, 2009). Prefrontal regions, particularly the anterior cingulate cortex, that are frequently associated with response inhibition were differentially integrated into all five networks, implicating specific contributions of regional activity in distinct response-processing mechanisms.
Similarly, we applied ICA to identify how specific brain regions implicated in cognitive control (e.g. the anterior cingulate cortex) function in relation with other brain regions during Stroop performance, and explore how these patterns of integrated activity relate to specific cognitive processes and clinically relevant information. The same sample of CD patients reported in Brewer et al (2008) were studied to investigate the hypotheses that: 1) using ICA, multiple networks related to component processes of cognitive control (e.g. both “top-down” fronto-parietal executive control and “bottom-up” striatal motivational processing) would be identified as underlying Stroop performance; 2) CD participants would display reduced differential engagement in response to incongruent as compared to congruent stimuli than healthy control (HC) participants; 3) Stroop performance measures (e.g., reaction times and error rates) would be associated with motivational networks in CD patients and executive control networks in HC participants; and, 4) greater engagement of executive control networks and reduced engagement of motivation networks would be associated with treatment outcome measures of retention and abstinence in CD patients.
Method
Participants
FMRI data from the 20 treatment-seeking, CD participants previously described (Brewer et al., 2008) and 20 age- and gender-matched HC subjects were studied (Table 1). CD patients met current DSM-IV criteria for CD via structured clinical interviews (First, Spitzer, Gibbon, & Williams, 2002), reported cocaine use in the past 28 days, and could commit to 8 weeks of outpatient treatment. Additional inclusion/exclusion criteria are detailed in the Supplemental Material. CD participants were recruited from two clinical trials, and fMRI procedures were completed prior to treatment. Study 1 investigated a computer-administered cognitive-behavior therapy (CBT) training program in augmenting standard community-based drug program (Carroll et al., 2008). Patients in Study 2 received weekly individual CBT and either disulfiram or placebo, with or without contingency management reinforcing cocaine-free urine screenings. Patients were not significantly different demographically or clinically across the two treatment studies and were combined into a single group for analysis as previously (Brewer et al., 2008). In both studies, scheduled urine screenings were conducted at least twice weekly and self-reported daily substance use calendars were completed. The outcome measures examined during the 8-week treatments included longest duration of self-reported continuous abstinence, percent cocaine-negative urine toxicology screens, and weeks of treatment completed, as previously reported (Brewer et al., 2008). All participants provided written informed consent as approved by the Yale University School of Medicine Human Investigations Committee.
Table 1.
Sample characteristics and Stroop performance for cocaine-dependent and healthy control groups.
| Variable | Cocaine Dependent (N = 20) | Healthy Control (N = 20) | p |
|---|---|---|---|
| Demographics | |||
| Age, years (SD) | 38.6 (9.3) | 36.8 (8.9) | 0.524 |
| Female, % | 40 | 40 | |
| Estimated IQ, mean (SD) | 90.4 (12.8) | 104.4 (11.0) | 0.001 |
| Education, years (SD) | 12.7 (1.2) | 14.8 (2.1) | <0.001 |
| Race: | |||
| White, % | 30 | 70 | 0.017 |
| Black, % | 50 | 30 | |
| Hispanic/Other, % | 20 | 0 | |
| Clinical Characteristics | |||
| Daily tobacco smoker, % | 85 | 20 | <0.001 |
| Cocaine use prior to treatment: mean days out of 28 (SD) | 12.3 (9.5) | - | |
| Last cocaine use prior to scan, mean days (SD) | 5.4 (5.7) | - | |
| Lifetime cocaine use, mean years (SD) | 11.0 (7.9) | - | |
| Treatment Outcome | |||
| Cocaine negative urines, mean % (SD) | 58.4 (46.8) | - | |
| Longest abstinence from cocaine, mean days (SD) | 32.0 (21.4) | - | |
| Weeks in treatment, mean (SD) | 5.5 (3.1) | - | |
| Stroop Performance | |||
| Stroop congruent RT, mean ms (SD) | 579.5 (75.5) | 598 (73.7) | 0.455 |
| Stroop incongruent RT, mean ms (SD) | 792.3 (155.0) | 839.1 (102.3) | 0.288 |
| Stroop Effect, mean ms (SD) | 212.8 (96.5) | 241.1 (85.2) | 0.352 |
| Stroop error rate, mean % (SD) | 27.0 (24.9) | 12.2 (10.4) | 0.014 |
Note: RT=reaction time; ms=milliseconds P-values refer to between-group t-test or Chi square results where appropriate.
Task Design, Imaging Parameters and Processing
The event-related Stroop color-word interference task used has been shown previously to activate brain regions underlying cognitive control (Blumberg et al., 2003; Brewer et al., 2008; Leung, Skudlarski, Gatenby, Peterson, & Gore, 2000; Peterson et al., 2002; Potenza et al., 2003), and is described in Supplemental Materials. Briefly, participants completed six three-minute runs consisting of congruent (e.g., “red” displayed in red font) and incongruent stimuli (e.g., “red” displayed in blue font) during which they were instructed to respond silently, an approach that has been shown to produce activation equivalent to overt response methods (Brown et al., 1999). The incongruent stimuli are characterized as high-conflict events in which individuals must exert cognitive control to inhibit a prepotent response to read the word rather than name the font color as instructed. Prior to scanning, all participants completed two runs of the task aloud with an experimenter present to confirm the task instructions were understood. Immediately following scanning, participants completed a maximum of five additional runs, responding aloud to assess behavioral performance (reaction times, error rates). The Stroop interference effect was calculated as the difference between average incongruent and congruent reaction times.
Images were collected using a Siemens Trio 3T magnetic resonance imaging system (Siemens AG, Erlangen, Germany). Image acquisition parameters, procedures and processing methods have been reported previously (Brewer et al., 2008) and are detailed in Supplemental Materials.
Independent Component Analysis
Group ICA was performed on the fMRI timeseries as described previously (Calhoun et al., 2001a; Erhardt et al., 2011) to examine functional networks activated during performance of the Stroop task. All six functional runs from all 40 participants were included in a single group ICA using the Group ICA of fMRI Toolbox (GIFT v2.0b; http://icatb.sourceforge.net). Data were reduced through principal component analysis (PCA) followed by concatenation (Calhoun, Adali, Pearlson, & Pekar, 2001b; Erhardt et al., 2011). The minimum description length criteria tool available in GIFT estimated a maximum of 24 independent components (Li, Adali, & Calhoun, 2007) which were then extracted from the group aggregate using neural network algorithms designed to maximize the independence of the network outputs (Bell & Sejnowski, 1995). Single-subject component time courses were then reconstructed and the amplitudes were calibrated to reflect percent signal change to facilitate between-subject comparisons.
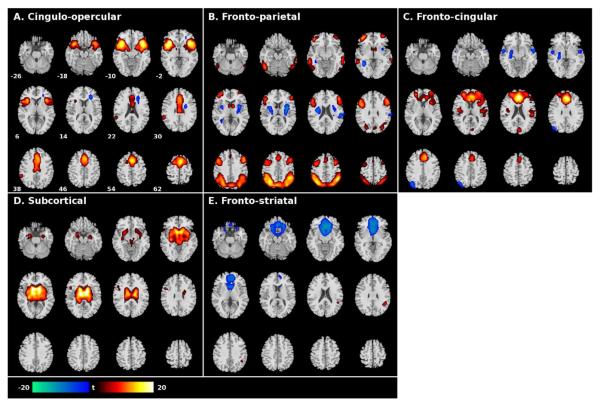
Statistical analyses of the identified components proceeded similar to previous ICA research (Kim et al., 2009a; Kim et al., 2009b). To identify those components most engaged in the cognitive control processes of interest, congruent and incongruent events were modeled using the canonical hemodynamic response function (HRF) in SPM2 (Wellcome Functional Imaging Laboratory, London, UK) to examine the association between component time courses and the two stimulus types. The resulting β-weights, a measure of each component's temporal relation to event-types, were then entered into paired t-tests to identify those components significantly more associated with incongruent as compared to congruent events. Of the 24 extracted components, seven displayed significantly different event-type β-weights (false discovery rate (FDR) corrected p <0.05), five of which displayed greater engagement in response to incongruent as compared to congruent events (Table 2), and were thus included in subsequent analyses as representing interference processing mechanisms. Investigation of the brain regions comprising the five interference components was accomplished using spatial maps of each component. Maps were transformed to z-scores, incorporating a bias term to center each image's distribution of z-scores at zero, and averaged over the six runs to generate one component map per participant. One-sample t-tests were then performed in SPM2 to visualize the brain regions significantly integrated, or displaying patterns of concurrent activity, in each component (and are displayed at a family-wise error corrected p (pfwe) < 0.0001 in Fig 1) across all participants. Due to conventions in the software, the spatial map for component E (Fig 1) was inverted for display purposes to reflect the direction of the hemodynamic response consistent with the other components (i.e. red/yellow indicating increased BOLD signal relative to the task stimulus and blue/green indicating reduced BOLD signal relative to the task stimulus).
Table 2.
Event-relatedness (β) for the five interference components identified by ICA.
| Component event-relatedness (N=40) | CD | HC | |||||
|---|---|---|---|---|---|---|---|
| Component | Incongruent β M (SD) | Congruent β M (SD) | Paired t (p) | β difference M (SD) | Paired t (p) | β difference M (SD) | Paired t (p) |
| A. Cingulo-opercular | 1.25 (0.85) | 0.20 (0.60) | 8.09 (<0.001) | 1.02 (0.97) | 4.69 (<0.001) | 1.16 (0.73) | 7.11 (<0.001) |
| B. Fronto-parietal | 1.42 (1.39) | 0.16 (0.72) | 7.55 (<0.001) | 1.32 (1.16) | 5.08 (<0.001) | 1.69 (1.36) | 5.57 (<0.001) |
| C. Fronto-cingular | 0.76 (1.01) | −0.09 (0.63) | 5.67 (<0.001) | 0.50 (0.64) | 3.52 (0.002) | 0.63 (0.64) | 4.44 (<0.001) |
| D. Subcortical | 0.43 (0.87) | −0.25 (0.65) | 4.41 (<0.001) | 0.33 (0.63) | 2.36 (0.029) | 0.71 (0.82) | 3.86 (0.001) |
| E. Fronto-striatal | −0.51 (1.04) | −0.09 (0.95) | −2.66 (0.011) | −0.23 (0.64) | −1.63 (0.119) | −0.27 (0.56) | −2.12 (0.047) |
Note: CD=cocaine-dependent group, HC=healthy control group.
figure 1.
Spatial maps of the five components associated with cognitive control.
Functionally-integrated brain regions in components associated with incongruent events during a Stroop task. Shown are one-sample t-test (N=40) results of the spatial structure for each component at family-wise corrected (FWE) p < 0.0001. Regions shown with positive t-values (yellow-red) indicate synchronous positive signal change, negative t-values (blue-green) indicate integrated concurrent negative change. Left hemisphere on left. Numbers next to the brain slices in A. indicate the z-coordinate of slices in MNI space used to show all components.
Event-related ß-weight difference values (incongruent ß – congruent ß) were calculated to represent a measure of the interference engagement effect for all subjects. The resulting interference ß-values were entered into univariate analyses to investigate any between-group differences. Correlational analyses were performed using the interference β-values to examine the relationships between network engagement, Stroop performance, clinical characteristics and treatment outcome. Fisher Z transformations were employed to examine between-group differences in component engagement correlations with Stroop performance measures. Inverse β values were used for component E in correlational analyses such that more positive β-values would indicate stronger overall component engagement for consistency in the direction of correlations across all components.
Linear regression and analyses of variance (ANOVA) were performed using SPSS v16.0 (SPSS Inc., Chicago, Illinois, USA) as appropriate to test for main and interaction effects of demographic and clinical characteristics on Stroop performance and ICA measures. Analyses that resulted in p-values greater than 0.05 are reported as non-significant.
Results
Sample Characteristics, Behavioral Performance, and Treatment Outcome
Participant characteristics are summarized in Table 1. The CD and HC groups (Table 1) differed in estimated IQ (p < 0.001), years of education (p<0.001), racial distribution (p=0.02), and smoking status (p < 0.001). Regression analyses examining IQ and years of education did not detect significant influences on Stroop performance or ICA measures across or within groups. There were also no observed within-group main effects of race, or group-by-race interactions on Stroop performance or ICA measures. In HC participants, tobacco smoking was associated with a greater Stroop effect (p=0.05).
All HC and 17 CD participants completed all five post-scan runs. Three CD participants requested to terminate post-scan testing prior to completing all 5 runs, resulting in a CD group average of 4.6 (SD=1.1) runs completed after scanning. Although there were no between-group reaction time differences on the Stroop task, CD patients committed more errors than controls (p=0.01; Table 1). This finding remains significant (p=0.04) in analyses excluding the three patients who did not complete all post-scan runs. Within the HC group and across all participants, age positively correlated with Stroop effect (r=0.47, p=0.05 and r=0.33, p=0.04 respectively); however, all significant results with respect to Stroop effect and ICA measures (Table 3) survive correction for age effects. There were no other significant within- or across-group correlations of behavioral performance measures with demographic characteristics. In CD patients, Stroop effect was inversely correlated with response errors (r=−0.48, p=0.04), a relationship not observed in HC participants.
Table 3.
Stroop performance correlation (Pearson r) with component event β-weight difference (incongruent β – congruent β) and between-group correlation Z-score
| Component | Congruent RT | Incongruent RT | Stroop Effect | Percent errors | |
|---|---|---|---|---|---|
| A | CD | 0.34 | 0.28 | 0.18 | −0.28 |
| HC | −0.17 | −0.09 | 0.03 | −0.02 | |
| ZDiff | 1.48 | 1.07 | 0.42 | −0.77 | |
|
| |||||
| B | CD | 0.24 | 0.21 | 0.15 | −0.17 |
| HC | −0.05 | −0.61** | −0.69** | −0.18 | |
| ZDiff | 0.84 | 2.59** | 2.79** | 0.01 | |
|
| |||||
| C | CD | 0.50* | 0.47* | 0.36 | −0.24 |
| HC | −0.02 | −0.49* | −0.58** | −0.15 | |
| ZDiff | 1.60 | 2.97** | 2.94** | −0.27 | |
|
| |||||
| D | CD | 0.41 | 0.46* | 0.42 | −0.10 |
| HC | 0.42 | −0.10 | −0.49* | −0.13 | |
| ZDiff | −0.05 | 1.70 | 2.78** | 0.11 | |
|
| |||||
| E | CD | −0.04 | −0.12 | −0.17 | 0.23 |
| HC | −0.04 | −0.20 | −0.21 | −0.29 | |
| ZDiff | 0.01 | 0.23 | 0.12 | 1.52 | |
Note: CD=cocaine-dependent group, HC=healthy control group, RT=reaction time, A=cingulo-opercular network, B=fronto-parietal network, C=fronto-cingular network, D=subcortical network; E=fronto-striatal network,.
p<0.05,
p<0.01.
Co-occurring disorders and previous treatment histories in the patient group are listed in Supplemental Table S1. Briefly, half of the CD sample met criteria for a lifetime depressive disorder, eleven reported a lifetime alcohol use disorder, and four had not previously received treatment for a substance use disorder. There were no main effects of co-occurring disorders or previous treatment history on Stroop performance or treatment outcomes. On average, patients reported last using cocaine 5.4 (SD=5.7) days prior to scanning with a range of 1–20 days, remained in treatment 5.5 (SD=3.1) weeks, reported 32.0 (SD=21.4) consecutive days of abstinence, and provided 58.4% (SD=46.8) cocaine-free urine samples during treatment. Across treatment studies, pre-treatment cocaine use (days of the 28 prior to treatment) inversely correlated with cocaine-negative urine toxicology (r = −0.65, p < 0.01). Correcting for pre-treatment use utilizing analyses of covariance that included pre-treatment use as a covariate, there were no main effects of study or treatment condition on any of the outcome measures. There were no observed correlations of lifetime or pre-treatment cocaine use with Stroop performance or ICA measures, and no observed demographic correlates of treatment outcome.
Functionally Integrated Brain Activity
The mean β-weights resulting from the temporal regression of component time courses with the SPM model of expected event-related HRFs are summarized for the five components of interest in Table 2. These β-weights represent how well a functional network is “engaged” or “recruited” in response to an event type, with higher absolute values indicating a stronger association between the component and stimulus type. The average group interference β-values are shown in Table 2, with greater absolute values indicating a greater difference in network engagement in response to incongruent as compared to congruent stimuli. The spatial structures of the five interference-related components identified by ICA are shown in Fig 1A–E, with respective brain regions, peak-voxel coordinates and t-scores listed in Supplemental Table S2. The spatial maps represent the extent of brain regions integrated within the functional network. No significant between-group differences in interference β-values were observed. Results of the interference β-value correlations with Stroop performance and between-groups tests of differences are shown in Table 3, and results of correlations with treatment outcome are displayed in Table 4. The ICA results are described in more detail for each component below.
Table 4.
Relationship (Pearson r) between component event β-weight differences (incongruent β – congruent β), clinical characteristics and outcomes for cocaine dependent patients (N=20).
| Outcome Variable | A | B | C | D | E |
|---|---|---|---|---|---|
| Lifetime cocaine use | −0.08 | 0.18 | −0.21 | 0.00 | −0.18 |
| Pre-treatment cocaine use | 0.31 | 0.25 | 0.23 | −0.14 | −0.35 |
| Longest contiguous abstinence | −0.18 | −0.23 | −0.16 | 0.09 | 0.30 |
| Weeks in treatment | −0.05 | −0.01 | −0.48* | −0.28 | 0.01 |
| Negative urine toxicology | −0.24 | −0.06 | 0.05 | 0.46* | 0.51* |
Note: A=cingulo-opercular network, B=fronto-parietal network, C=fronto-cingular network, D=subcortical network; E=fronto-striatal network,.
p<0.05.
Component A: Cingulo-opercular network
Functionally-integrated activation was observed in the dorsal anterior cingulate extending to the medial superior frontal cortex and bilateral frontal operculum extending into the insula and superior temporal cortex. No significant relationship with engagement of component A was observed with Stroop performance or treatment outcome; however, there was a significant reduction in interference β-values in CD participants reporting at least one previous treatment episode (p=0.001).
Component B: Fronto-parietal network
Bilateral activity in the ventrolateral and dorsolateral prefrontal cortex, inferior temporal cortex, and parietal cortex were integrated with activity in the dorsomedial frontal cortex in this component. Concurrent negative signal in the right postcentral gyrus and bilaterally in the putamen and extending into the insula was observed. Greater incongruent as compared to congruent engagement of this component was inversely associated with incongruent reaction times (r=−0.61, p=0.006) and Stroop effect (r=−0.69, p=0.001) in HC participants, which were significantly different than patients (Z=2.59, p=0.009 and Z=2.79, p=0.005 respectively; Table 3), and survive controlling for smoking status. No associations with treatment outcome were observed for component B.
Component C: Fronto-cingular network
Activity centered on the anterior cingulate and extending across medial regions of the middle and superior frontal cortex was integrated with activity in bilateral dorsal regions of the insula in component C. Negative signal in the left putamen and left precuneus along with bilateral regions of the middle and superior temporal cortex were also integrated into this component. There was a significant group-by-smoking status interaction effect observed in the engagement of component C (p=0.01), with tobacco smoking being associated with decreased component engagement (p=0.01) in HC participants. In the CD group, there was a main effect of a lifetime marijuana use disorder on increasing differences in engagement of component C (p=0.03). Greater interference β-values were positively correlated with congruent and incongruent reaction times in CD patients (r=0.50, p=0.03 and r=0.47, p=0.04 respectively; Table 3). In HC participants, greater interference β-values were inversely correlated with incongruent reaction times (r=−0.49, p=0.03) and Stroop effect (r=−0.58, p=0.01), which was significantly different than patients (Z=2.97, p=0.003 and Z=2.94, p=0.003 respectively; Table 3) and survive controlling for smoking status. In CD patients, engagement of component C was inversely associated with the number of weeks of treatment completed (r=−0.48, p=0.03; Table 4).
Component D: Subcortical network
Limbic structures including the thalamus, striatum, amygdala, and hippocampus displayed integrated activity bilaterally in this component with concurrent activity in left inferior frontal cortex. Interference β-values for component D were positively correlated with incongruent reaction times in CD patients (r=0.46, p=0.05), and inversely correlated with Stroop effect in HC participants (r=−0.49, p=0.03) which differed significantly than CD patients (Z=2.78, p=0.005; Table 3). Greater incongruent as compared to congruent engagement of component D was positively correlated with greater cocaine-free urine toxicology during treatment in CD patients (r=0.46, p=0.04; Table 4).
Component E: Ventral fronto-striatal network
Component E was comprised of negative signal in the ventral medial prefrontal cortex extending into ventral striatal structures and subgenual and rostral anterior cingulate. Concurrent positive signal in the right supramarginal gyrus was also integrated into this component. Though significant between-event-type engagement was observed across all subjects (t=−2.66, p=0.011), by-group analysis revealed component E was more strongly associated with incongruent than congruent events in the HC group only (t=−2.12, p=0.05; Table 2). There was a significant group-by-smoking status interaction effect observed in the engagement of component E (p=0.04), with a trend toward smoking decreasing engagement in HC participants (p=0.06). In CD patients, depression and alcohol use history were associated with increased interference β-values (p=0.005 and p=0.05, respectively). No relationships with Stroop performance were observed for the interference β-values of component E (Table 3); however, greater incongruent as compared to congruent engagement of this component was positively correlated with greater cocaine-free urine toxicology during treatment (r=0.51, p=0.02; Table 4). This relationship between engagement and abstinence survives controlling for co-occurring depression and alcohol use disorders.
Discussion
The current exploratory study used ICA to examine functional brain networks in CD patients and HC participants during performance of a Stroop task. Consistent with the first hypothesis, ICA identified five interference-related functional networks associated with top-down and bottom-up mechanisms of cognitive control. These networks were largely consistent with previous research indicating that multiple parallel and distinct mechanisms of integrated cingulate, frontal and subcortical regions are involved in Stroop performance (Peterson, et al. 1999). No between-group differences in overall component engagement were observed to support the second hypothesis. With respect to the third hypothesis of between-group differences in component associations with Stroop performance measures, greater engagement of top-down and bottom-up networks was associated with impaired performance in CD patients and improved performance in HC participants. Contrary to the final hypothesis, reduced engagement of a functional network related to top-down conflict monitoring mechanisms of cognitive control was associated with treatment retention, while greater engagement of two bottom-up networks related to motivational and incentive processing were associated with abstinence during treatment. Biological and clinical implications are discussed below.
Functionally-integrated Cognitive Control Networks
Consistent with hypotheses, ICA identified multiple functional networks contributing to cognitive control during Stroop performance, including circuitry previously implicated in executive control, conflict monitoring, and motivational and incentive processing. Although no between-group differences were observed in overall component engagements, differential contributions to improved Stroop performance were detected. While attributing specific functional roles to each network is challenging, observed relationships with performance and previous research allow the interpretation of potential contributions of each component in cognitive control mechanisms. For example, the anterior cingulate cortex, frequently implicated in cognitive control and cocaine dependence, was differentially integrated into three of the five distinct networks identified by ICA, a finding consistent with prior Stroop research identifying functional subdivisions of the cingulate contributing to different aspects of cognitive control including conflict monitoring, stimulus saliency, and emotional regulation (Milham & Banich, 2005; Mohanty et al., 2007; van Veen & Carter, 2005).
The current findings are consistent with intrinsic connectivity models of executive control systems. The fronto-parietal and cingulo-opercular functional networks of components A and B fit well with models of complementary, top-down executive control mechanisms. Dosenbach, Fair, Cohen, Schlaggar, and Petersen (2008) proposed these two networks work in parallel, with the fronto-parietal circuit responsible for rapid, cue-elicited initiation of control mechanisms, while the cingulo-opercular circuit is associated with adaptive control over repetitions. Seeley et al (2007) identified similar brain networks, again attributing initiation of executive control to the fronto-parietal network, while characterizing the cingulo-opercular network as a saliency network sensitive to stimulus-relevance. In Stroop research, regions in both networks have been associated with performance monitoring, response inhibition, and error processing (Blasi et al., 2006; Ullsperger & von Cramon, 2001), further supporting interpretations that these networks are integral to executive cognitive control mechanisms.
Component C is consistent with a medial fronto-cingular system associated with evaluative functions of the rostral anterior cingulate that include conflict monitoring and error-processing (Kelly et al., 2009). In the current study, engagement of this network was not associated with errors in either group or across all subjects; however, this observation is limited by the collection of performance measures after scanning procedures, and the contribution of this network to error-processing during Stroop performance requires further investigation. Previous research into oddball effects in Stroop paradigms indicates the infrequency of incongruent stimuli may increase BOLD response in regions associated with cognitive conflict (Leung et al., 2000; Melcher & Gruber, 2006; Milham, Banich, & Barad, 2003). However, as this network is not significantly engaged by oddball stimuli (Calhoun, Kiehl, & Pearlson., 2008), activity observed in the current study may be contributing specifically to the conflict inherent to incongruent stimuli rather than their infrequency, though this requires further study particularly as individuals with CD display reduced attentional response to oddball stimuli (Gooding, Burroughs, & Boutros, 2008; Moeller et al., 2004). Engagement of component C was sensitive to co-occurring substance use in both groups (i.e. marijuana use history in CD patients and tobacco use in HC participants), and may represent a target for future research into addictive behaviors.
Component D displayed integrated activity in bilateral subcortical regions including the thalamus, hippocampus, amygdala and striatum. These regions are widely implicated in various stimulus- and valence-based processes (Morgane, Galler, & Mokler, 2005). In the domain of cognitive control, a network comprised of these regions may be involved in the bottom-up motivational reactivity to unexpected or infrequent and salient stimuli (Redgrave, Prescott, & Gurney, 1999; Wittmann, Daw, Seymour, & Dolan, 2008; Yamagata, Yamaguchi, & Kobayashi, 2004). In CD, thalamic hypo-activity has been associated with cognitive impairment (Tomasi et al., 2007) and poor treatment outcomes (Moeller et al., 2010). An overall reduction in interference engagement of component D in CD as compared to HC participants was not significant but approached trend level (p=0.11). As such, while the current preliminary study does not provide support for substantial between-group differences related to thalamic circuitry during Stroop interference responses, it is possible that future studies employing larger samples may identify such contributions.
Increased activity in the ventral frontal regions integrated in component E is associated with the default network (Buckner, Andrews-Hanna, & Schacter, 2008). The decreased activity in this ventral fronto-striatal network observed in the current study has been associated with recruitment of global cognitive demands, being particularly sensitive to quickly presented stimuli and stimulus complexity (McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003). Decreased activity in a comparable network was associated with correct and erroneous responses during performance of a response inhibition task (Stevens et al., 2009). Together, these data suggest that a ventral fronto-striatal network may contribute to bottom-up cue-elicited attentional processes involved in cognitive control.
Cognitive Control Networks and Stroop Performance in Cocaine Dependence
Stroop performance measures were differentially associated with network engagement between CD and HC participants, suggesting a dysregulation of cognitive control processes in CD. Among HC participants, greater engagement in response to incongruent as compared to congruent events of top-down executive control and conflict processing networks (components B and C) and a bottom-up motivational network (component D) was associated with reduced Stroop-interference reaction-time effects. However, in CD patients, there was no observed relationship between the engagement of any component and Stroop effect, though conflict processing and motivational networks (components C and D) were associated with slower reaction times in response to incongruent events. It is hypothesized that in healthy interference processing, integration of executive control and conflict monitoring processes with motivational mechanisms contribute to efficiently resolving conflict prior to engaging downstream response processes (Egnar, 2008; Schulz, Bedard, Czarnecki, & Fan, 2011; Nee, Wager, & Jonides, 2007). In CD patients, dysregulation of this system may produce conflicted response processing, resulting in increased errors. Future research acquiring performance measures during fMRI could help identify the potential influence of conflict on response mechanisms in CD.
Cognitive Control Networks and Treatment Outcome in Cocaine Dependence
Three of the cognitive control components identified by ICA were associated with treatment outcome in CD patients. There was an inverse relationship between treatment retention and engagement of the medial fronto-cingular network (component C) in response to incongruent as compared to congruent stimuli. More robust engagement of this component, proposed to contribute to top-down conflict monitoring, was associated with slower overall performance in CD patients, suggesting that hyperactivity in this component may be associated with increased experiences of conflict and indecision in CD patients. This finding in conjunction with the observed relationship between greater incongruent as compared to congruent engagement of this component and poorer retention supports conflict-relapse models of cocaine-seeking (Cooper, Barnea-Ygael, Levy, Shaham, & Zangen,2007). It could be postulated that CD patients with more robust engagement of this network may find specific conflict-related demands of behavioral therapy (e.g., learning to modify responses to drug cues) particularly difficult, leading to disengagement from treatment and attrition. Alternatively, hyperactivity of this network may reflect inefficient processing during conflict-related situations, and for CD patients who are ambivalent about treatment, engagement might lead to treatment drop-out. Research into retention in substance use programs has shown mixed and complex social and personal factors are associated with treatment drop-out (Ball, Carroll, Canning-Ball, & Rounsaville, 2006), and future studies assessing reasons for discontinuing treatment in relation to cognitive impairments and their neural correlates are needed.
Greater incongruent as compared to congruent engagement of bottom-up subcortical and ventral fronto-striatal networks (components D and E) was associated with increased abstinence, measured by negative urine toxicology screens during treatment. As discussed, these components may represent mechanisms of stimulus-driven motivational processes and cue reactivity. The positive association between component D and abstinence is consistent with previous findings that CD patients displaying greater thalamic activity are more abstinent during treatment (Moeller et al., 2010). Individuals with CD display increased functional connectivity between ventral striatal and orbital frontal regions integrated into component E, which may contribute to the increased responsiveness to drug cues observed in CD (Wilcox et al., 2011). Their relationship with abstinence suggests CD patients with more robust (or healthier) bottom-up integration of sensory information with motivational and incentive control processes may be more responsive to behavioral therapies. The extent to which specific components of therapy (e.g., those aimed at recognizing, adapting and controlling appetitive reactivity to drug-related cues and cravings) might mediate the relationship between these components and drug abstinence requires additional investigation.
Extension of Previous Research
The current study extends previous research utilizing standard fMRI analyses to investigate cognitive control in cocaine dependence by identifying distinct functional networks that are differentially engaged in CD participants. Findings suggest a network-based functional restructuring of neural mechanisms involved in cognitive control processes may underlie cognitive impairments observed in CD. Whether this functional reorganization pre-exists cocaine dependence, or is perhaps a consequence of chronic drug use, requires further research, particularly as structural brain abnormalities related to response inhibition have been observed in unaffected relatives of individuals with stimulant dependence (Ersche et al, 2012). The correlation of components involving the striatum with abstinence is consistent with previous findings in this sample using conventional fMRI analysis (Brewer et al., 2008). The previous report found interference-related activity in the right striatum was associated with both negative urine toxicology and longest self-reported contiguous abstinence measures. Employing ICA, this study extends the previous findings with the observation that striatal activity as integrated into networks involved in bottom-up motivational mechanisms, rather than top-down executive control processes, is important in maintaining abstinence in cocaine dependence. This observation is restricted to urine toxicology in the current study as there were no significant relationships between component engagement and longest contiguous abstinence. Representing control over urges and use behaviors over time, maintaining extended periods of abstinence may involve a complex set of cognitive, emotional and motivation processes not captured by any individual network identified in the current study. Also as in the prior report, an association between dorsal prefrontal activity and treatment retention was observed; furthermore, the current study implicated a medial fronto-cingular network, suggesting functional connectivity between lateral, medial and anterior cingulate cortical regions may more aptly be linked to retention.
In contrast to the prior study, the current study included a HC comparison group to identify between-group differences related to both task performance and its neural correlates. Importantly, the decomposition of BOLD signal into functionally integrated activations by ICA allows for the identification of neural networks underlying Stroop performance, how these networks are differentially recruited between CD and HC groups, and the relationship between the networks and treatment outcome.
In comparison to previous connectivity research in CD, the current study identifies brain networks associated with cognitive impairments and related to clinical outcome. Examining resting-state mesocorticolimbic connectivity, Gu et al (2010) found decreased regional connectivity between midbrain and subcortical structures was associated with increased years of cocaine use. A similar finding of decreased midbrain, thalamic, and cortical functional connectivity has been reported in cocaine abusers during a sustained attention task, and while also correlated with years of use, connectivity differences were not associated with performance impairments (Tomasi et al., 2010). Although none of the functional networks in the current study were associated with use history, greater engagement of these mesolimbic structures as integrated within a subcortical network (component D) was associated with improved treatment outcome. Future research could explore the regional connectivity of these structures with respect to observed engagement of this functional network, and the association with clinical outcomes.
Limitations, Strengths and Future Directions
The current study is limited by a small number of subjects in each group and a diverse treatment regimen in the patient group. Behavioral performance was not assessed during scanning, and any differences in reaction times or error rates that may have contributed to or influenced the observed component engagements could not be directly investigated. Potential between-group differences of practice and repetition effects may have influenced fMRI and behavioral measures (Goldstein et al., 2007b) and require further investigation. Also, while self-reported last use prior to scanning did not significantly correlate with ICA measures, these dates could not be objectively verified and the possible influences of residual cocaine and acute or chronic withdrawal states including cravings could not be accounted for at the time of scan. Most CD patients and very few HC participants were daily tobacco smokers, had a history of depressive disorders, and reported lifetime alcohol and marijuana use disorders. Although all primary findings reported survive corrections for these conditions, the possible influences of past depressive episodes, tobacco and other substance use including alcohol require further investigation. The current study did not directly examine potential socioeconomic factors or personality traits (e.g. impulsivity) or disorders (e.g., antisocial personality disorder) that may have influenced treatment performance and imaging results. The methodology used represents one application of ICA for fMRI data, and performance of ICA on the groups independently may yield slightly different results. The selection of a single ICA across groups allowed comparison of all subjects projected into the same space, and back-reconstruction maps and timecourses using this methodology have been shown to be very accurate in preserving and representing individual subject variations (Erhardt et al., 2011). Due to these limitations and the exploratory nature of these investigations, corrections for multiple comparisons were not performed post-hoc on the correlational analyses.
Strengths of the study include the use of a data-driven approach (ICA) to identify functionally integrated brain activations underlying a widely used cognitive task (Stroop), the performance of which has been linked to clinically relevant measures like treatment outcome in CD (Streeter et al., 2008). Furthermore, the identification of specific networks demonstrating between-diagnostic-group differences and their associations with performance and treatment outcome measures provide not only a richer understanding of the pathophysiology of CD, but also a stronger foundation for investigating the mechanisms underlying effective treatment (see Potenza et al., 2011). As regional changes in Stroop-related brain activity have been observed following treatment of substance use disorders (Devito, et al., in press), future studies may also investigate changes in functional brain networks following treatment for cocaine dependence. Future investigations addressing the temporal interaction and effectiveness of these functional networks could help in identifying the specific contributions of each mechanism and the consequences of their dysregulation on cognitive control. Also, examination of the components in relation to clinically relevant intermediary pheonotypes (e.g., facets of impulsivity) and in other substance and behavioral addictions may aid in developing specialized target processes for cognitive and pharmacological interventions. Arguably most importantly, applying this approach to larger samples in randomized clinical trials might help to understand better how specific treatments work, for whom they work best, and in what ways treatments may be improved for specific groups.
Extending on previous research of cognitive control in cocaine dependence, the current study suggests a network-based functional restructuring may underlie cognitive impairments in CD. Utilizing ICA, regional activation patterns during Stroop performance were associated with five interference-related functional mechanisms proposed to represent distinct component processes of cognitive control. Three of these functional networks were correlated with treatment outcomes in CD patients, with findings suggesting that recruitment of `top-down' control processes are associated with retention, while `bottom-up' motivational mechanisms are associated with achieving and maintaining abstinence during treatment.
Supplementary Material
Acknowledgments
This study was funded by the following Grants: National Institute on Drug Abuse (NIDA) P50-DA09241 (BJR/KMC), R01-DA020908 (MNP), R37 DA15969 (KMC), K05-DA00457 (KMC), P20-DA027844 (MNP), R01-DA020709 (GP), R01-EB000840 (VDC), K05-DA00089 (BJR), and the Veterans Integrated Service Network 1 Mental Illness Research, Education, and Clinical Center (MIRECC). Data for this report were drawn from clinical trials registered with ClinicalTrials.gov (www.clinicaltrials.gov). Registry Identifiers: NCT00350870 and NCT00350610.
References
- Abbott C, Kim D, Sponheim SR, Bustillo J, Calhoun VD. Decreased default mode neural modulation with age in schizophrenia. American Journal of Geriatric Psychiatry. 2010;18:897–907. doi: 10.1097/JGP.0b013e3181e9b9d9. doi:10.1097/JGP.0b013e3181e9b9d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Amrhein PC, Bisaga A, Nunes EV, Hasin DS. Cognition, commitment language, and behavioral change among cocaine-dependent patients. Psychology of Addictive Behaviors. 2008;22:557–562. doi: 10.1037/a0012971. doi:10.1037/a0012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and Alcohol Dependence. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. doi:10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Ball SA, Carroll KM, Canning-Ball M, Rounsaville BJ. Reasons for dropout from drug abuse treatment: symptoms, personality, and motivation. Addictive Behaviors. 2006;31:320–330. doi: 10.1016/j.addbeh.2005.05.013. doi:10.1016/j.addbeh.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Bustamante J, Ventura-Campos N, Llopis J, Parcet M, Ávila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Research: Neuroimaging. 2011;194:111–118. doi: 10.1016/j.pscychresns.2011.05.001. doi:10.1016/j.pscychresns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Computation. 1995;7:1129–59. doi: 10.1162/neco.1995.7.6.1129. doi:10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Beveridge TJR, Gill KE, Hanlon CA, Porrino LJ. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philosophical Transactions of the Royal Society B. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. doi:10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, Bertolino A, Callicott JH, Weinberger DR, Mattay VS. Brain regions underlying response inhibition and interference monitoring and suppression. European Journal of Neuroscience. 2006;23:1658–64. doi: 10.1111/j.1460-9568.2006.04680.x. doi:10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung H-C, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Archives of General Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. doi:10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Brewer J, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. doi:10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Kinderman SS, Siegle GJ, Granholm E, Wong EC, Buxton RB. Brain activation and pupil response during covert performance of the Stroop Color Word task. Journal of the International Neuropsychological Society. 1999;5:308–319. doi: 10.1017/s1355617799544020. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. doi:10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buehringer G, Goschke T, Gottlebe K, Kufeld C, Wittchen HU. Why people change? The role of cognitive-control processes in the onset and cessation of substance-abuse disorders. International Journal of Methods in Psychiatric Research. 2008;17(S1):S4–S15. doi: 10.1002/mpr.246. doi:10.1002/mpr.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, McGinty VB, Pekar JJ, Watson TD, Pearlson GD. fMRI activation in a visual-perception task: Network of areas detected using the general linear model and independent components analysis. Neuroimage. 2001a;14:1080–1088. doi: 10.1006/nimg.2001.0921. doi:10.1006/nimg.2001.0921. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001b;14:140–51. doi: 10.1002/hbm.1048. doi:10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping. 2008;29:828–838. doi: 10.1002/hbm.20581. doi:10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:163–172. doi: 10.1016/j.neuroimage.2008.10.057. doi:10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, Nelson B, Bell C, Mueller BA, Specker S, Lim KO. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biological Psychiatry. 2011;69:1117–23. doi: 10.1016/j.biopsych.2011.01.008. doi:10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted delivery of cognitive-behaviorial therapy for addiction: a randomized trial of CBT4CBT. American Journal of Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. doi:10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. 2007;194:117–125. doi: 10.1007/s00213-007-0827-7. doi:10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and Alcohol Dependence. doi: 10.1016/j.drugalcdep.2011.10.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends in Cognitive Sciences. 2008;12:374–380. doi: 10.1016/j.tics.2008.07.001. doi:10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsh J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;23:539–547. doi: 10.1016/j.neuroimage.2004.09.007. doi:10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping. 2011 doi: 10.1002/hbm.21170. [Epub ahead of print]. doi:10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PJ, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. doi:10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. doi:10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-patient Edition. SCID-I/NP. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychology Review. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. doi:10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends in Cognitive Sciences. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. doi:10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. Neuroimage. 2007b;35:194–206. doi: 10.1016/j.neuroimage.2006.12.004. doi:10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007a;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. doi:10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Burroughs S, Boutros NN. Attentional deficits in cocaine-dependent patients: converging behavioral and electrophysiological evidence. Psychiatry Research. 2008;160:145–154. doi: 10.1016/j.psychres.2007.11.019. doi:10.1016/j.psychres.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. doi:10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Shaw M, Yucel M, Purcell R, Brewer WJ, Strother SC, Egan GF, Olverh JS, Nathana PJ, Pantelis C. Functional connectivity during Stroop task performance. Neuroimage. 2005;24:181–191. doi: 10.1016/j.neuroimage.2004.08.033. doi:10.1016/j.neuroimage.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. Journal of Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. doi:10.1523/jneurosci.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. doi:10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Ford JM, Gollub RL, White T, Wible C, Belger A, Bockholt HJ, Clark VP, Lauriello J, O'Leary D, Mueller BA, Lim KO, Andreasen N, Potkin SG, Calhoun VD. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Human Brain Mapping. 2009a;30:3795–3811. doi: 10.1002/hbm.20807. doi:10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Mathalon D, Ford JM, Mannell M, Turner J, Brown G, Belger A, Gollub RL, Lauriello J, Wible CG, O'Leary D, Lim K, Potkin S, Calhoun VD. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophrenia Bulletin. 2009b;35:67–81. doi: 10.1093/schbul/sbn133. doi:10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H-C, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cerebral Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. doi:10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Yan P, Bhagawar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine dependent men. Neuropsychopharmacology. 2008;33:1798–1807. doi: 10.1038/sj.npp.1301568. doi:10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Adali T, Calhoun VD. Estimating the number of independent components for fMRI data. Human Brain Mapping. 2007;28:1251–1266. doi: 10.1002/hbm.20359. doi:10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacology Biochemistry and Behavior. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. doi:10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. doi:10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. doi:10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. doi:10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Melcher T, Gruber O. Oddball and incongruity effects during Stroop task performance: A comparative fMRI study on selective attention. Brain Research. 2006;1121:136–149. doi: 10.1016/j.brainres.2006.08.120. doi:10.1016/j.brainres.2006.08.120. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. doi:10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex's involvement in top-down control: an event-related fMRI study of the stroop task. Cognitive Brain Research. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. doi:10.1016/S0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Fischer CJ, Dougherty DM, Reilly EL, Mathias CW, Swann AC. P300 event-related potential amplitude an impulsivity in cocaine-dependent subjects. Neuropsychobiology. 2004;50:167–173. doi: 10.1159/000079110. doi:10.1159/000079110. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, ramer LA, Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Research. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. doi:10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ringo Ho MH, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. doi:10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Progress in Neurobiology. 2005;75:143–60. doi: 10.1016/j.pneurobio.2005.01.001. doi:10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience. 2008;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung H-C, May J, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cognitive Brain Research. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. doi:10.1016/S0926-6410(02)00054-X. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biological Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. doi:10.1016/S0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung H-C, Blumberg HP, Fulbright RK, Skudlarski P, Lacadie CM. An FMRI Stroop study of pathological gamblers. American Journal of Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. doi:10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends in Neuroscience. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. doi:10.1016/S0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. doi:10.1016/S0959-4388(00)00204-X. [DOI] [PubMed] [Google Scholar]
- Schlosser RGM, Wagner G, Koch K, Dahnke R, Reichenbach JR, Sauer H. Fronto-cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage. 2008;43:645–655. doi: 10.1016/j.neuroimage.2008.08.002. doi:10.1016/j.neuroimage.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Schlosser RGM, Wagner G, Schachtzabel C, Peikert G, Koch K, Reichenbach JR, Sauer H. Fronto-cingulate effective connectivity in obsessive compulsive disorder: A study with fMRI and dynamic causal modeling. Human Brain Mapping. 2010;31:1834–1850. doi: 10.1002/hbm.20980. doi:10.1002/hbm.20980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Bédard AC, Czarnecki R, Fan J. Preparatory activity and connectivity in dorsal anterior cingulate cortex for cognitive control. Neuroimage. 2011;57(1):242–250. doi: 10.1016/j.neuroimage.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. doi:10.1523/JNEUROSCI.5587-06.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behavioural Brain Research. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. doi:10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Brain network dynamics during error commission. Human Brain Mapping. 2009;30:24–37. doi: 10.1002/hbm.20478. doi:10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. doi:10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–661. reprint doi:10.1037/0096-3445.121.1.15. [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Research. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrilo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS ONE. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. doi:10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KA, Potenza MN, Beauvais JE, Browndyke JN, Gottschalk PC, Kosten TR. Perfusion abnormalities and decision making in cocaine dependence. Biological Psychiatry. 2004;56:527–530. doi: 10.1016/j.biopsych.2004.06.031. doi:10.1016/j.biopsych.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. doi:10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Separating semantic conflict and response conflict in the Stroop task: A functional MRI study. Neuroimage. 2005;27:497–504. doi: 10.1016/j.neuroimage.2005.04.042. doi:10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG. Cortical inefficiency in patients with unipolar depression: an event-related fMRI study with the Stroop task. Biological Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. doi:10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug and Alcohol Dependence. 2011;115:137–44. doi: 10.1016/j.drugalcdep.2011.01.009. doi:10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Daw ND, Seymour B, Dolan RJ. Striatal activity underlies novelty-based choice in humans. Neuron. 2008;58:967–973. doi: 10.1016/j.neuron.2008.04.027. doi:10.1016/j.neuron.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata S, Yamaguchi S, Kobayashi S. Impaired novelty processing in apathy after subcortical stroke. Stroke. 2004;35:1935–1940. doi: 10.1161/01.STR.0000135017.51144.c9. doi:10.1161/01.STR.0000135017.51144.c9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.