Abstract
The efficacy of Photodynamic therapy (PDT) for epithelial cancers is increased when PDT is combined with calcitriol (Vit D), a form of Differentiation therapy (DT). Here we describe an underlying mechanism for this effect. Differentiation-promoting agents are known to upregulate CCAAT-enhancer binding proteins (C/EBPs), powerful regulators of cellular differentiation. In subcutaneous A431 tumors in mice, pretreatment with Vit D induced the expression of C/EBPβ isoforms, and of coproporphyrinogen oxidase (CPO), a heme pathway enzyme responsible for the conversion of 5-aminolevulinic acid (ALA) into protoporphyrin IX (PpIX), the principal light-absorbing molecule during PDT. To further investigate this apparent link between C/EBPs and CPO, two cell lines (MEL and LNCaP) were exposed to differentiating agents, and levels of PpIX, C/EBPs, and CPO were measured. Differentiating agents, or transfection of C/EBP expression vectors, increased C/EBP and CPO levels in parallel. Focusing on ~1,300 bp of upstream CPO gene promoter, we tested the ability of recombinant C/EBPα, C/EBPβ, C/EBPδ, and C/EBPζ to bind to CPO gene sequences (EMSA assays) and to affect transcriptional activity (luciferase assays). Multiple C/EBP consensus binding sites were identified (15 for mouse, 18 for human). Individual probes representing each site bound to C/EBPs with characteristic affinities (strong, moderate, or weak), but when sites were inactivated in the context of the native promoter, transcriptional activity was reduced nearly equally for strong or weak sites. Cooperative interactions between regularly spaced C/EBP sites appear critical for CPO transcriptional regulation by DT. These results provide a mechanistic rationale for DT/PDT combination therapy for cancer.
INTRODUCTION
Photodynamic Therapy (PDT) is a relatively new anti-cancer technique in which a photolabile molecule is allowed to selectively accumulate in cancer cells, followed by illumination with high-intensity light to activate the photosensitizer and kill the cells (1, 2). One particularly useful approach is to administer 5-aminolevulinic acid (ALA), a pro-drug selectively taken up by cancer cells and metabolically converted into intracellular porphyrins (3). Porphyrins, mainly protoporphyrin IX (PpIX), are efficient absorbers of visible light. In the presence of oxygen, PpIX is photochemically degraded and forms free radical species that damage membranes and trigger apoptosis (3). PpIX is synthesized from ALA by the sequential action of eight enzymes in the heme-synthetic pathway (4), including coproporphyrinogen oxidase (CPO, sixth enzyme in the cascade). CPO lies upstream of PpIX, and is of particular interest because CPO is rate-limiting for PpIX production under typical PDT conditions (5).
To try to further improve the treatment efficacy of PDT, we have empirically pretreated cells and tumors with various agents prior to ALA-PDT, and found several pretreatments that cause significant elevations of intracellular PpIX levels (6). To date, the following agents have been shown to improve both PpIX accumulation and the therapeutic response: (i) skin or prostate carcinoma cells pretreated with methotrexate (5, 7, 8); (ii) various carcinomas pretreated with vitamin D3 (9, 10); (iii) hormone-responsive prostate cancer cells pretreated with androgens (7); (iv) skin keratinocytes pretreated with calcium (8, 10, 11). In all cases, the pharmacologic pretreatment promoted CPO expression, PpIX levels, and cellular differentiation concurrently (8, 10, 11). We are interested in identifying the mechanisms behind these effects. A positive correlation between agents that induce cellular differentiation and PpIX levels might be only coincidental, unless an underlying mechanism can be proposed and demonstrated.
Consideration of the literature regarding Differentiation Therapy (DT), defined as the use of biological agents to promote cancer cell maturation, offered a clue here. The use of retinoids to drive terminal differentiation of promyelocytic leukemia cells is a familiar example of DT (12, 13); another is the use of IL-15 to eliminate renal cancer stem cells (14). Mechanisms for DT are thought to involve nuclear transcription factors that regulate genes governing proliferation and differentiation. CCAAT-enhancer binding proteins (C/EBPs) are top candidates for this role. C/EBPs were among the first transcription factors identified as powerful regulators of differentiation programs in adipocytes (15, 16), hepatocytes (17, 18), blood cell lineages (19), and keratinocytes (20, 21). More recently, dysregulated expression of C/EBPα and C/EBPβ was shown to be important in the pathogenesis of myeloid, lung, breast, and skin cancers (12, 13, 19, 22–24). Relevant to our work is the fact that many C/EBP family members are implicated in driving cellular differentiation in response to pharmacologic agents such as calcium (25), methotrexate (26), or Vitamin D (27). With this background, we asked whether C/EBP factors might be involved in driving transcription of the CPO gene during combination DT/PDT. Here we describe a molecular investigation in which C/EBPs are shown to regulate CPO gene expression in cancer cells from two species, mouse and human. The evidence strongly suggests that C/EBPs are important regulators of CPO expression and PpIX photosensitizer accumulation, thereby defining an underlying mechanism for DT/PDT combination therapy.
METHODS AND MATERIALS
Subcutaneous A431 tumors in mice, pretreatment with Vitamin D, and measurement of PpIX and various proteins in tissue
Nude mice (female, 6 weeks, Charles River Laboratory) were injected with 2 × 106 A431 cells in the flanks. Mice with visible/palpable tumors (6–10 days post-injection) were preconditioned with systemic vitamin D (5 μg/kg) in PBS or PBS alone (vehicle control) daily for 3 days by an intraperitoneal (i.p.) route. ALA (200 mg/kg) was administered for 4 hours i.p. Tumors were harvested, and PpIX measured in frozen sections by confocal microscopy as described (10). Protein expression was analyzed by immunofluorescent staining or by western blotting as described (10).
Cell culture
MEL erythroleukemia cells, LNCaP prostate carcinoma cells, A431, and COS-7 cells were obtained from American Type Culture Collection (ATCC, Manassas, Virginia), and cultured as recommended by the supplier. Cell lines were tested and authenticated at ATCC by morphological appearance during growth and recovery, cytochrome C oxidase 1 PCR for species specificity, and human origin by short tandem repeat (STR) profiling.
Pretreatment of MEL cells with (DMSO) and measurement of PpIX in cell lysates
MEL cells in 25 cm2 flasks were incubated with or without dimethyl sulfoxide (DMSO, 1% final concentration). After 24 h, medium with 5-aminolevulinic acid (ALA; 1 mM final) was added for 4 hours. Cells were centrifuged and processed for RT-PCR, western blot, or PpIX analysis (spectrofluorimetry). For PpIX measurements, cell pellet were lysed in 1 ml Solvable (Perkin Elmer) and centrifuged at 10,000 x G at 4 °C. PpIX content of 100 μl aliquots was measured in triplicate in 96 well plates (Corning) using a SpectraMAX Gemini-XS spectrofluorimeter (Molecular Devices) at 395 nm excitation and 635 nm emission wavelengths, respectively. PpIX levels were expressed as fluorescence units (FUs) per mg of total protein. Protein concentrations of cell lysates were determined from absorbance at 280 nm rather than with Bradford reagent, which cross-reacts with Solvable.
Pretreatment of LNCaP cells with 1, 25 dihydroxy vitamin D3 (Vit D3) and PpIX analysis by confocal microscopy
LNCaP cells were plated on plastic cover slips in 35 mm dishes (50,000 cells/dish). At 24 h, the medium was changed to fresh medium containing 10−12 –10−6 M Vit D3 or vehicle (ethanol) alone. After 72 h of incubation, medium containing 1 mM ALA was added for 4 h prior to PpIX analysis. PpIX-specific fluorescence in living cells was measured on a confocal laser-scanning microscope and digital images analyzed by image processing as described (8). PpIX levels were expressed as arbitrary fluorescence units per cell.
C/EBP expression plasmid constructs, transient transfection of LNCaP cells and PpIX analysis by confocal microscopy
Expression vectors for the C/EBP isoforms were as follows. Rat C/EBPα was cloned in pcDNA3.1, human C/EBPβ1 (LAP*) was cloned in pcDNA3.1/HisA, Rat C/EBPβ2 (LAP) was cloned in pcDNA3.1, rat C/EBPβ3 (LIP) was cloned in pcDNA3.1, mouse C/EBPδ was cloned in pcDNA3.1 and mouse C/EBPζ/CHOP was cloned in pcDNA3.1. Expression vectors for C/EBPδ and C/EBPβ1 (LAP*) were kindly provided by James Dewille (Ohio State University, Columbus, OH) and Linda Sealy (Vanderbilt University, Nashville, TN), respectively.
LNCaP cells were plated on 22 X 22 mm, No. 1 plastic cover slips in a 35 mm dish (50,000 cells/dish). At 24 h cells were transiently transfected with C/EBP expression vectors (10 to 250 ng), or with pcDNA3.1 empty vector, using FuGENE 6 (Roche Diagnostics, Germany) as per the manufacturer’s guidelines. At 24 h post transfection, fresh medium containing 1 mM ALA was applied for 4 hours, then PpIX levels analyzed and quantified as described above.
Preparation of nuclear extracts
Nuclear extracts from COS-7 cells overexpressing individual C/EBP isoforms (α, β, δ and ξ) were prepared as described by (28). Expression of the C/EBP proteins in nuclear extracts was verified by western blotting.
Western blot analysis
Western analyses were performed as described (20, 25). The source and dilution of antisera used were as follows: C/EBPα, C/EBPβ, C/EBPδ, CHOP and GAPDH (Santa Cruz,1:5000); CPO (custom made, 1:5000, ref. (5)); HRP conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, 1:20,000).
Electrophoretic mobility shift assay (EMSA)
Complementary oligonucleotides (~ 43 nt) spanning either one or two C/EBP motifs from the mCPO or hCPO promoters, along with corresponding mutated C/EBP motifs, were synthesized by Integrated DNA Technologies (IDT, San Diego, CA); sequences in Supplementary Table 1, part A. Duplex oligonucleotides were annealed, labeled with [α-32P]dCTP, incubated with nuclear extracts, and the DNA-protein complexes resolved on polyacrylamide gels as described (25). For supershift experiments, 1 μl of antibody was added to the incubation mixture for 15 min prior to addition of labeled oligonucleotides. For competition experiments, a 10 or 100 molar excess of unlabeled oligonucleotides was added to the mixture.
Luciferase reporter plasmid constructs and luciferase assay
Luciferase expression vector pSKLuc (25) containing murine CPO (mCPO) promoter sequences (pSKLuc-946) was constructed by cloning a 946 bp fragment of the promoter (ref. (29); kind gift of Dr. Shigeru Taketani, Kyoto Institute of Technology, Japan) at NotI and XhoI sites. Three of the C/EBP motifs were mutated individually, using the site-specific mutagenesis by overlap extension technique (30). PCR-amplified products were cloned in pCRII-TOPO vector (Invitrogen) and sequenced to verify the mutation. Depending on their orientation in pCRII-TOPO, inserts carrying the C/EBP site mutations were cloned in pSKLuc vector as following; C7m at XhoI and KpnI; C6del, C6m, and C4m at SpeI and XhoI sites. See Supplementary Table 1, part B for details.
LNCaP cells were cultured in 6-well plates (100,000 cells/well) for 24 h, then transfected with 1 μg of luc-expression vectors (pSKLuc or pSKLuc-946) along with 10 to 250 ng of expression plasmid for various C/EBP isoforms (α, β and δ) and 1 μg of pSV- galactosidase expression plasmid (internal control), using FuGENE 6 (Roche Diagnostics, Germany) per manufacturers guidelines. Cells were harvested 24 h post-transfection; light output was measured using a luciferase assay kit (Promega, Madison, WI) and normalized to the activity of beta-galactosidase (β-Gal enzyme assay kit, Promega).
RT-PCR analyses
To detect semiquantitative changes in CPO mRNA levels in MEL cells, the following oligonucleotide (oligos) pairs were used. CPO: sense, 5′-AGGATGCTGTCCATTTCCAC-3′; CPO: antisense, 5′-GGGGAGTCAAGATCGTCAAA-3′; GAPDH: sense, 5′-ACCACAGTCCATGCCATCAC-3′; GAPDH: antisense, 5′-TCCACCACCCTGTTGCTGTA-3′ (IDT DNA technology). RNA (2 μg) extracted from cells using Trizol reagent (Invitrogen) were used for first strand synthesis using gene-specific primers (antisense oligos for the genes mentioned above, 2 pM each). The primers were employed in reactions with SuperScript III H-reverse transcriptase (Invitrogen) as described by the manufacturer. For PCR, amplification, cycles were: 94 °C 2 min, 94 °C 45 s, 60 °C 45 s, 72 C 45 s X 25–28 cycles, and 72 °C for 2 min. Amplification products were analyzed on 1.5% agarose gels along with size markers.
Scoring system for prediction of C/EBP binding at consensus sites in DNA
A comparative sequence approach was taken to identify features of the 17 candidate sites that might correlate with relative binding. In the Results subsection entitled “Nucleotide features of C/EBP sites correlate with observed binding properties,” the sequences were analyzed at the individual nucleotide (NT) level using a scoring system that we devised from the current literature on structural characteristics of optimized C/EBP:DNA complexes. Our scoring system combined available data from random site selection approaches (31, 32) and crystallographic studies (33). C/EBP recognition sites are pseudo-palindromic sequences with a core half-site of GCAAT or very similar; an overall site of 10 bp comprises two abutted dyads, RTTGC•GYAAY, where R= purines A or G, and Y = pyrimidines C or T (32). In double-stranded DNA, at least 12 of the 20 individual nt’s in this 10 bp motif make contact with specific amino acids of the bound C/EBP protein, as shown in studies in which the binding domain of rat C/EBPα was crystallized with a DNA duplex containing the palindrome ATTGC•GCAAT (33). This structural information was used in our combined scoring system to define optimal nucleotides (NT’s) at positions −4 to +4. At positions −5 and +5, for which crystallographic data were unavailable, PCR site-selection data (31, 32) were used to define the optimal nucleotides. In any given half-site, seven key nucleotide locations (at +1, +2, +3, and +5 on the sense strand, and at −2, −3, and −4 on the antisense strand) were evaluated. A candidate C/EBP half-site whose nucleotides match the consensus at all locations would earn a score of 7 out of 7.
RESULTS
Differentiation therapy is associated with upregulation of C/EBPs, CPO gene expression, and PpIX production in tumors in vivo
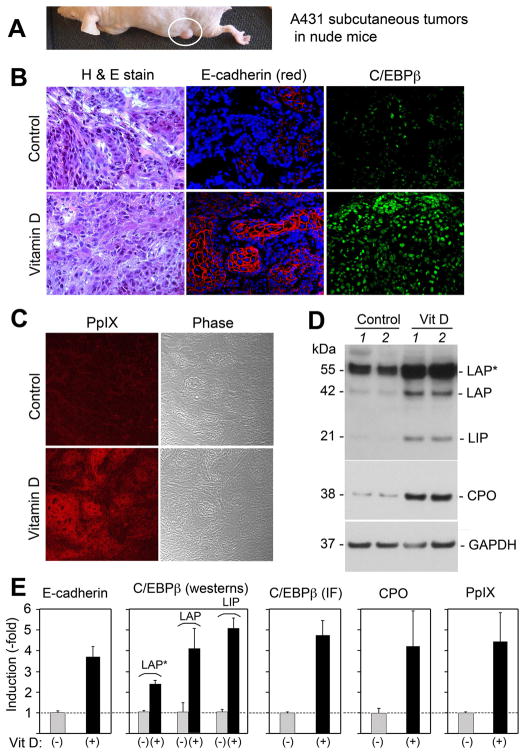
We had previously shown that when subcutaneous A431 squamous tumors in mice (Fig. 1A) are preconditioned for 3 days with calcitriol (Vit D) and then given ALA for 4 hr, the PpIX photosensitizer accumulates to higher levels than in the absence of Vit D, causing more tumor cell death upon exposure to light (10). After Vit D preconditioning and ALA (but before illumination), the histological appearance of tumors appeared unaltered (Fig. 1B, left). By immunostaining, however, levels of E-cadherin (a marker of differentiation, Fig. 1B, middle) and prominent expression of nuclear C/EBPβ (Fig. 1B, right) were observed in the Vit D pretreated tumors. As noted previously, Vit D led to strong accumulation of PpIX (Fig. 1C). Under these same conditions, relative levels of CPO protein were induced 4- to 5 fold (Fig. 1D, E), and C/EBPβ isoforms LIP, LAP, and LAP* were also increased (Fig. 1D, E). Semiquantitative analysis for each of the above molecular targets revealed 3- to 5-fold relative inductions for all (Fig. 1E), supporting the hypothesis that elevated C/EBPβ levels may drive CPO transcription and PpIX synthesis after Vitamin D exposure.
Figure 1. C/EBP transcription factors, the CPO enzyme, and PpIX are increased in subcutaneous A431 tumors undergoing Vitamin D-induced differentiation in vivo.
Subcutaneous A431 tumors (A) were treated for 3 days with calcitriol (Vitamin D), 5 μg/kg or with saline alone (Control). (B) Tumors were harvested and analyzed as follows: Left panels, Standard histological staining; Middle panels, Immunofluorescence (IF) staining for E-cadherin (red) and nuclear DAPI, counterstain (blue); Right panels, IF for C/EBPβ (green); (C) Confocal imaging for PpIX (red), and the same field shown in phase-contrast; (D) Western analyses for C/EBPβ isoforms, CPO, and GAPDH. Tissues from Vitamin-D treated tumors and control tumors, each from a different mouse, were analyzed. (E) Quantitative analysis of relative expression was analyzed from multiple tumors, in separate graphs as follows (method of analysis in parentheses): E-cadherin (by IF); C/EBPβ, individual isoforms (by westerns); C/EBPβ total levels (by IF); CPO protein (by westerns); PpIX levels (by confocal microscopy). Western analyses were normalized to GAPDH. Bars represent mean ± SD, n = 3 tumors each.
Differentiating agents cause concurrent upregulation of C/EBP transcription factors and CPO gene transcription in vitro
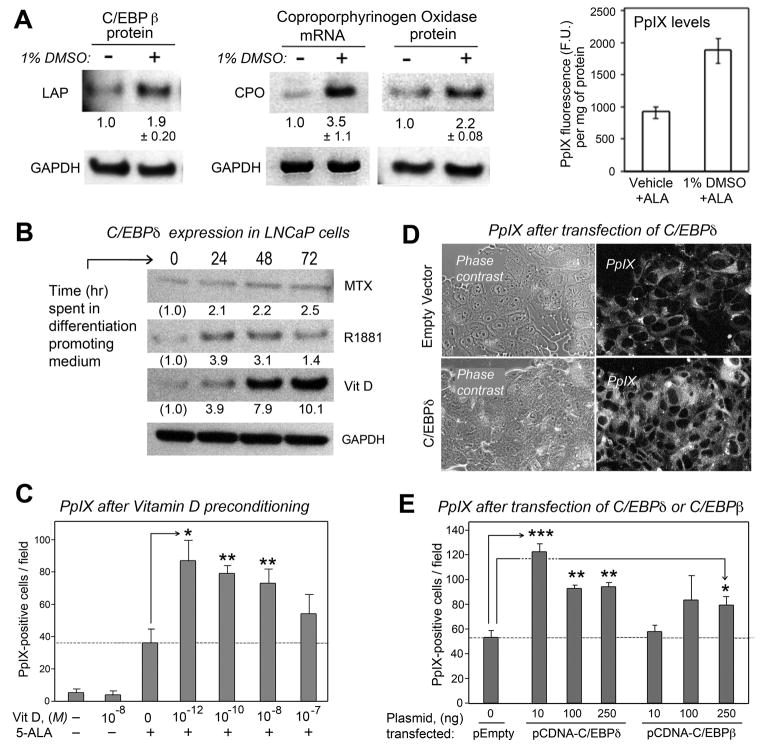
To ask whether upregulation of CPO expression during the differentiation-enhanced PDT regimen might be mediated by C/EBP transcription factors, we turned to cell studies in vitro. Murine erythroleukemia cells (MEL), a well-established line for studying CPO expression, were tried initially. When MEL are incubated in 1% DMSO, large amounts of porphyrins are produced (34) and under these conditions C/EBPβ expression is upregulated (Fig. 2A, left). Concurrently, CPO expression is increased at both mRNA and protein levels (Fig. 2A, middle), and higher levels of PpIX are generated in response to exogenously added ALA (Fig. 2A, right).
Figure 2. C/EBP transcription factors, the CPO enzyme, and PpIX are increased in parallel in cells undergoing differentiation.
(A), Correlation between C/EBPβ expression, CPO expression, and PpIX accumulation in MEL cells after 24 hr in medium with 1% DMSO. (Left panel), C/EBPβ (LAP) is increased in DMSO-treated MEL cells. (Middle panel), CPO mRNA and CPO protein levels are increased in DMSO-treated MEL cells, by semi-quantitative RT-PCR and Western analyses, respectively. Numbers beneath the lanes indicate relative band intensities by densitometry (mean ± half-range of 2 independent experiments). (Right panel), Graph showing relative increase in PpIX levels (arbitrary fluorescence units, F.U.) in MEL cells after a 4 hr incubation in 1 mM ALA; measurement described in Methods. Data are mean ± SD of 3 experiments. (B) Western analyses of LNCaP cells exposed for 72 hr to methotrexate (MTX, 1 μg/ml), the androgen R1881 (10−7 M), or calcitriol (Vit D3, 10−7 M), and probed with antisera to C/EBPδ, or to GAPDH for calcitriol treated cultures. Numbers beneath the lanes indicate fold-induction by densitometry. (C) PpIX expression as measured in LNCaP cultures, following incubation of the cells for 72 hr in calcitriol at the concentrations indicated (M, molar) and then for an additional 4 hr in 1mM ALA. Relative changes in PpIX were measured by analysis of digital confocal images as described in Methods. (D) PpIX levels are increased in cells overexpressing C/EBP transcription factors. Digital confocal images of LNCaP cultures transfected with a C/EBPδ expression vector, or with an empty expression vector, to illustrate increased PpIX expression in C/EBPδ-transfected cultures (lower right panel). (E) Quantitation of relative PpIX expression levels in C/EBPδ- or C/EBPβ-transfected LNCaP cells. Means ± S.D. of 3 dishes per point. Asterisks: Two-sided t-test, (*), p < 0.01; (**), p< 0.005; (***), p < 0.0001.
Because our previous studies on PDT were performed in cells of epithelial origin (6), LNCaP (a human prostate carcinoma cell line) was chosen for further experiments (5, 7). When LNCaP were exposed to MTX, androgen, or calcitriol, C/EBPδ protein levels rose after each of the three differentiation-promoting agents, and most dramatically after calcitriol (Fig. 2B). C/EBPβ levels were not significantly affected (data not shown). Calcitriol-mediated induction of C/EBPδ has been reported before (27), but our goal was to ask whether increased C/EBPδ is functionally associated with CPO induction and PpIX accumulation. Other carcinoma lines respond to calcitriol with increases in CPO and PpIX levels concurrently (9, 10), and LNCaP cells proved to be no exception. PpIX inductions were observed even at very low effective concentrations of Vit D (pM to nM; see Fig. 2C). Forcible overexpression of C/EBPδ (and to lesser extent, C/EBPβ) in LNCaP cells triggered PpIX accumulation, providing further evidence for a link between C/EBPs and the heme pathway (Fig. 2D, E).
The murine CPO gene upstream region contains many competent C/EBP binding sites
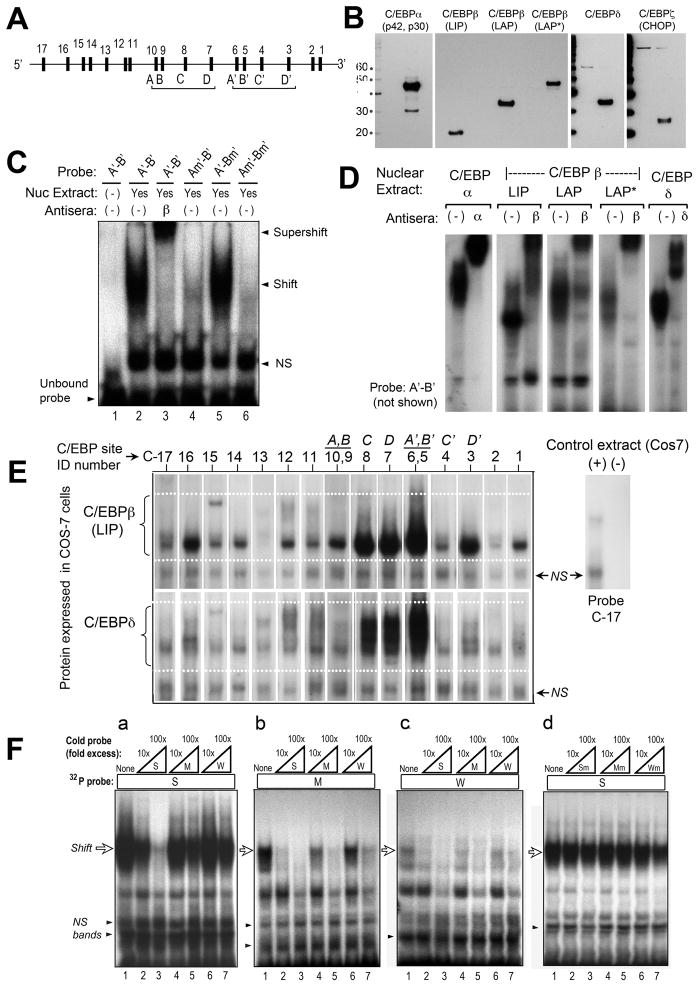
We now asked whether C/EBP factors can regulate CPO transcription by binding to DNA elements in the mCPO promoter/enhancer region. When our studies began, the mouse CPO gene had been cloned but little was known about its regulation (29, 35). Using pattern-recognition software (36), 946 bp of mCPO upstream sequence were analyzed; two repeated clusters of sites were identified containing NT motifs highly homologous to the presumed C/EBP binding consensus (31, 37). These candidate sites were named (ABCD) and (A′B′C′D′); see Fig. 3A. To test C/EBP binding, EMSA was performed using nuclear extracts from COS-7 cells overexpressing each of the major C/EBP isoforms, confirmed by western analyses (Fig. 3B). The relative ability of each site to bind C/EBPs was determined, as a function of DNA sequence and as a function of C/EBP isoform. First, the ability of one C/EBP isoform to bind each DNA motif was tested. Nuclear extracts containing C/EBPβ(LAP) were incubated with a 32P-labeled dsDNA probe harboring one candidate motif, site A′ (later renamed C6) (Fig. 3C). C/EBPβ(LAP) bound to A′, forming a specific protein-DNA complex (lane 2, shift) that was recognized by an anti-C/EBPβ antibody (lane 3, supershift). Mutation of site A′ abolished C/EBPβ binding (lane 4). In this instance, another potential site (B′) was located only 16 nucleotides away from A′ in the mCPO promoter, so the dsDNA probe used in Fig. 3C contained both sites (Probe A′-B′). To ask whether site A′ or site B′ contributed more to the observed binding, probes were created in which A′ or B′ was disrupted by mutation. C/EBPβ(LAP) bound preferentially to site A′ and not site B′ (Fig. 3C, lane 5 vs. 4).
Figure 3. Characterization of putative C/EBP binding sites in the murine CPO promoter, by electrophoretic mobility shift analysis (EMSA).
(A) Schematic locations of the 17 candidate sites. (See Fig. 5a for exact locations.) (B) Western blots to verify proper expression of C/EBP isoforms. COS-7 cells were transfected with expression vectors encoding each of the C/EBP isoforms indicated, then analyzed on western blots using specific antisera. A 10 kDa molecular weight ladder was run in parallel, to establish the relative sizes of the visualized bands (kDa, on left). For the three C/EBPβ isoforms (LIP, LAP, and LAP*) which are translated from alternative translation start sites, a single antibody to the C-terminal end of C/EBPβ was used. (C) Demonstration of specific binding at site A’. Nuclear extracts containing C/EBPβ were incubated with the 32P-labeled double-stranded DNA probe indicated above each lane. Each probe contained two candidate sites (A’ and B’), located 16 bp apart. For probe Am’-B’ or A’-Bm’, the sites A’ or B’ (respectively) were disrupted by mutation. NS, nonspecific bands. (D) Binding of different C/EBP isoforms to site A’. The test probe, A’-B’, was incubated with nuclear extracts overexpressing different C/EBP isoforms. Antibodies to C/EBPα, C/EBPβ, or C/EBPδ were added in some of the binding reactions to demonstrate specificity of the DNA-protein complexes (supershifts). (E) EMSA to test the relative binding of C/EBP isoforms to candidate sites in the mCPO gene. EMSA was performed with double-stranded DNA probes representing each of the 17 candidate sites. In the figure, specific DNA-protein complexes formed with C/EBPβ-LIP (upper panel), or with C/EBPδ (lower panel), are illustrated. The results of experiments with all of the C/EBP isoforms are summarized in Table 1. (F) Cross-competition analysis to test a predicted hierarchy of binding affinities. Three binding probes with relative affinities of 40% (site C6), 6% (site C3), and 3% (site C10) were chosen and for simplicity renamed strong (S), medium (M), and weak (W), respectively. Binding of C/EBPβ-LAP to these 32P-labeled probes was tested by EMSA in the absence or presence of excess amounts (10-fold or 100-fold) of unlabeled probe, in order to assess the ability of unlabeled competitors to diminish 32P-probe binding. Subpanels a, b, and c: Unlabeled competitors were able to displace 32P-probe binding as predicted by the relative order of binding affinities predicted from Table 1. Subpanel d: mutated sites were unable to compete (negative controls).
We next asked whether site A′ can bind other C/EBP isoforms. C/EBPα, C/EBPβ(LIP), C/EBPβ(LAP), C/EBPβ(LAP*), and C/EBPδ were shown to bind specifically to site A′, as demonstrated by supershift experiments (Fig. 3D). C/EBPζ(CHOP) failed to bind (data not shown), an expected result since CHOP has an atypical bZIP region and does not bind conventional C/EBP consensus sites (38). CHOP was not examined further here.
C/EBP consensus sites have been historically difficult to recognize and define, due to sequence degeneracy and overlap with other transcription factor recognition sites (31, 32, 39). Computer models could miss unconventional sites; a safer approach was to use a looser definition of nucleotides for consensus binding, and test a broad array of candidate sites experimentally. All potential motifs containing “GCAA”, “CCAA”, “ACAA”, or “GTAA” were visually identified in a survey of 1300 bp of mCPO promoter now available on http://www.ncbi.nlm.nih.gov; seventeen candidate sites were identified (Fig. 3A). To test the binding ability of each site, dsDNA probes (~40 bp, containing different consensus sites but identical flanking regions) were allowed to bind recombinant C/EBP proteins under identical reaction conditions. For example, Fig. 3E shows EMSA analyses in which C/EBPβ(LIP), or C/EBPδ, were tested for binding at each of 17 sites. Each transcription factor bound very strongly to three sites (C6, C7, and C8), but bound differentially elsewhere; C/EBPβ(LIP) formed prominent complexes at most sites whereas C/EBPδ bound more weakly (Fig. 3E). Similar experiments for all C/EBP isoforms are summarized in Table 1. Candidate consensus sites in Table 1 were classifiable into four groups: strong (3 sites), moderate (6 sites), weak (6 sites), and nonbinding (2 sites). Three strong sites (C6, C7, and C8) accounted for ~75% of relative binding overall. However, the 25% contribution from moderate and weak sites might also be important. To be sure that the relative affinity data in Table 1 are functionally relevant, we asked whether they can successfully predict a rank order of binding when sites are tested in competitive binding assays (Fig. 3F). Three binding probes (sites C6, C3, and C10) with decreasing affinity (S, M, and W, respectively) were chosen for competition analyses (Fig. 3F). For example, cross-competition experiments in panel B showed that C/EBPβ binding to a moderate-affinity 32P-labeleled probe (M) is reduced by coincubation with a 10-fold excess of unlabeled S or M competitor probe, but not with the weakest (W) probe. In other panels of Fig. 3F, displacement of binding occurred as expected, relative to the predicted affinity.
TABLE 1.
Relative Binding Affinity of C/EBP Isoforms at Candidate Consensus Sites in the Murine CPO Upstream Gene
| Site Name | Location of Site (nt) (a) | Relative Affinity of Binding to Individual Isoforms (%)(b) | Characteristics of the Binding Site | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C/EBPα | C/EBPβ (LAP) | C/EBPβ (LIP) | C/EBPβ (LAP*) | C/EBPδ | Mean Relative Affinity (%) (c) | Isoform Score (d) | Strength Category (e) | ||
| C1 | −339 | 1.1 | 3.8 | 3.4 | 0.0 | 1.9 | 2.0% | 4 | Moderate |
| C2 | −362 | 0.0 | 0.3 | 0.3 | 0.0 | 0.0 | 0.1% | 2 | Weak |
| C3 | −483 | 9.5 | 7.7 | 10.4 | 0.0 | 4.0 | 6.3% | 4 | Moderate |
| C4 | −570 | 0.5 | 0.8 | 2.6 | 0.0 | 0.0 | 0.8% | 3 | Weak |
| C5 | −631 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0% | 0 | No binding |
| C6 | −647 | 34.4 | 32.2 | 26.6 | 70.8 | 35.5 | 39.9% | 5 | Strong |
| C7 | −754 | 25.4 | 18.4 | 15.0 | 11.4 | 18.9 | 17.8% | 5 | Strong |
| C8 | −836 | 23.4 | 15.4 | 14.8 | 10.4 | 18.2 | 16.5% | 5 | Strong |
| C9 | −910 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0% | 0 | No binding |
| C10 | −930 | 0.9 | 3.6 | 5.7 | 3.1 | 3.8 | 3.4% | 5 | Moderate |
| C11 | −1018 | 0.0 | 3.2 | 2.2 | 3.3 | 5.8 | 2.9% | 4 | Moderate |
| C12 | −1027 | 1.1 | 3.9 | 3.0 | 0.9 | 5.9 | 3.0% | 5 | Moderate |
| C13 | −1096 | 0.0 | 0.4 | 0.0 | 0.0 | 1.0 | 0.3% | 2 | Weak |
| C14 | −1152 | 0.0 | 1.1 | 2.2 | 0.0 | 0.0 | 0.7% | 2 | Weak |
| C15 | −1177 | 0.0 | 1.3 | 2.2 | 0.0 | 0.0 | 0.7% | 2 | Weak |
| C16 | −1220 | 3.5 | 6.7 | 8.0 | 0.0 | 3.8 | 4.4% | 4 | Moderate |
| C17 | −1297 | 0.2 | 1.1 | 3.7 | 0.0 | 1.2 | 1.2% | 4 | Weak |
| Total signal in binding assay: | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | ||||
Location upstream of ATG in the mCPO promoter. The nucleotide shown is +1 in each 10 bp C/EBP-binding motif as defined in Figure 4.
For each C/EBP protein isoform, binding affinities were estimated from the signal intensity of specific bands on EMSA autoradiograms. (Nuclear proteins from C/EBP-transfected COS-7 cells were bound to individual oligonucleotide probes encompassing each CPO promoter site). For each band, relative affinity was expressed as a percentage of the total signal intensity for that isoform, summed from all 17 promoter sites.
Mean binding affinity, averaged across all five C/EBP isoforms.
Isoform binding score: Number of C/EBP isoforms that showed detectable binding to that particular DNA sequence.
Binding Strength Category of the nucleotide sequence, defined as follows:
| Strong: | Mean binding affinity is > 10%; the site can bind to all five C/EBP isoforms. |
| Moderate: | Mean binding affinity is at least 2%; the site can bind to 4 or more isoforms. |
| Weak: | Mean binding affinity is less than 2%; the site can bind to at least 2 isoforms. |
| Non-binding | The site fails to bind to any of the C/EBP isoforms. |
Nucleotide features of the C/EBP sites that are predictive of observed binding properties
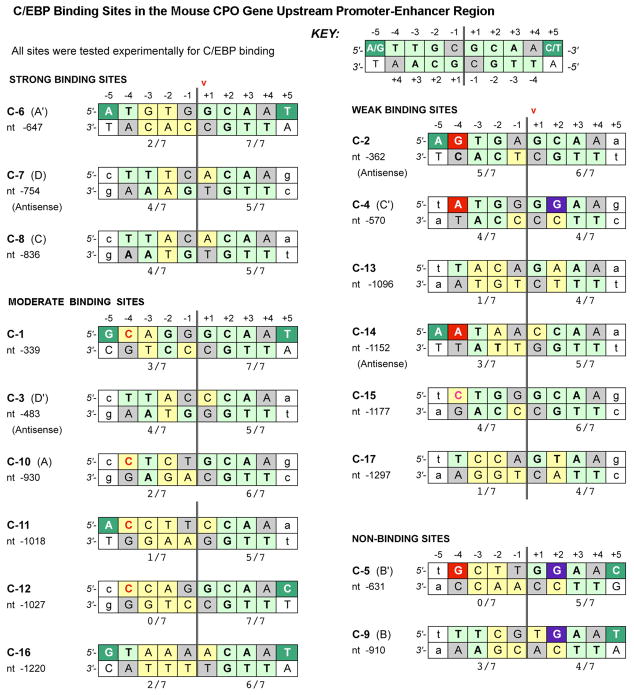
A comparative sequence analysis of the 17 candidate sites was undertaken to identify NT features that might correlate with relative binding. Details of the scoring system used in Fig. 4 are described in Methods and in the figure legend itself. Briefly, the scoring scheme generates a consensus score, based upon published sequence-selection data (31, 32) and crystallographic studies (33) that indicate the optimal NT expected at any given location within each half-site of the C/EBP binding motif. This consensus score (the numerical value shown beneath each half-site in Fig. 4) indicates the “goodness of fit” to the data of Johnson et al. (33); note that 7 out of 7 indicates a perfect match. Examination of Fig. 4 suggests that certain features of the consensus sequence may correlate with the hierarchy of relative C/EBP binding strength that was determined in Table 1, but the relationships are not simple. Broadly speaking, each half-site to the right of the dyad axis of symmetry (as displayed in Fig. 4) determines the overall eligibility of the site to bind C/EBPs, whereas the left half-site determines the ultimate strength of binding. In support of this statement, one can see that all bona fide C/EBP-binding sites have similar consensus scores for the right half-site. a value of at least 5/7 for strong or moderate sites, and 4/7 for weak sites. Thus, a right-hand consensus score of 5/7 or higher is necessary but not sufficient for moderate-to-strong binding. To test this notion, we applied our scoring system to 25 additional gene promoters taken from the literature, each of which contained a proven C/EBP binding site, and in all but two cases the best half-site score was 5/7 or greater (Supplementary Fig. 1).
Figure 4. Comparative sequence analysis of C/EBP sites in the murine CPO upstream region.
Each double-stranded DNA site for C/EBP binding consists of two 5 bp half-sites centered on a dyad axis of symmetry (vertical double-lines). Boxes are shaded as follows: Light green: this nucleotide (NT) is identical to the NT shown to contact specific amino acids of C/EBP in the crystallographic studies of Miller et al (33). Yellow: Deviations from the NT shown in Miller et al, 2003. Dark green: A pyrimidine is the NT that occurs most commonly at position (+5) in natural promoters (31, 32). Red: purine at position −4 is sterically unfavorabe. Purple: a guanine is normally never observed at position +2, except in weak or nonbinding sites. Gray, the NT at this location is not critical for binding (33), and therefore not considered in the scoring system. Arrow, this nucleotide (+1) defines the location of the site in the mCPO promoter. Numerals beneath each 5 bp half-site: This is the consensus score, i.e., the number of NTs that match the theoretically perfect consensus sequence; 7 out of a possible 7 is perfect.
If the right half-site determines binding eligibility, then the role of the left half-site may be to determine strong vs. moderate vs. weak affinity through the presence (or absence) of certain nucleotides that are unfavorable or inhibitory for binding. In moderate binding sites, a pyrimidine-to-pyrimidine substitution (C instead of T) is tolerated at position −4 (Fig. 4, red font), although C is clearly less effective than the optimal T. For weak binding sequences, substitution of a relatively bulky purine (A or G) at position −4 for the optimal pyrimidine (T) is commonly observed (Fig. 4, red boxes) and appears to be inhibitory. Such pyrimidine-to-purine substitutions are highly unfavorable not only at position −4, but also at position +2 (Fig. 4, purple boxes). These substitutions are never observed in any of the C/EBP sites from the literature which we assume to be strong or moderate-affinity sites (Supplementary Fig. 1). Interestingly, all C/EBP sites in murine CPO are asymmetric, with wide divergence between the two half-sites (a substantial departure from a perfect palindrome; for example, site C-6). Asymmetry is also true for C/EBP sites in our literature survey, with rare exceptions; perfect symmetry was found only in the murine leptin and Ob promoters (Supplementary Fig. 1).
The arrangement of C/EBP sites in the upstream regions of mouse and human CPO favor cooperative transcriptional regulation
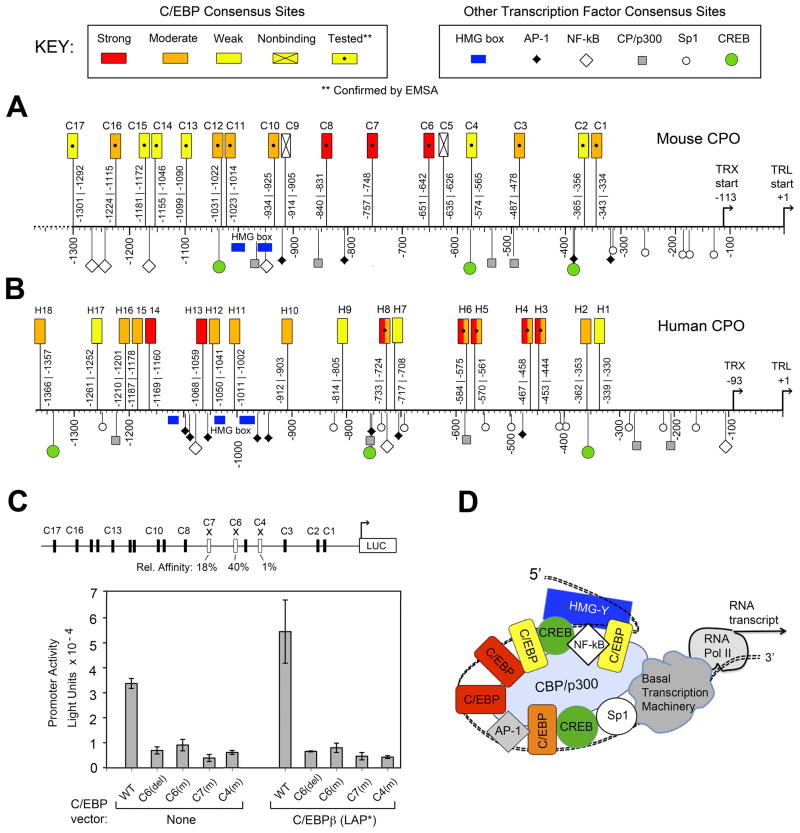
Fifteen functional C/EBP binding motifs in the mCPO promoter (Fig. 5A, and Table 1) is a substantial number, among the largest reported for any gene. To assess whether abundant C/EBP sites are unique to murine CPO, we analyzed a similar length of the human CPO upstream region (~1.3 kb). C/EBP sites were identified by inspection, and binding was tested for a subset of these (Supplementary Fig. 2 and Fig. 5B). Eighteen sites were identified in hCPO; all were predicted to bind C/EBPs with moderate or higher affinity based upon the criteria in Fig. 4. Five sites were randomly selected for EMSA, and shown to bind with strong-to-moderate affinity (Supplementary Fig. 2). Overall, the number and arrangement of C/EBP sites in CPO genes from both species appears to be remarkably similar (Fig. 5A, B).
Figure 5. Locations of C/EBP binding sites, and evidence for cooperative interactions, in the upstream regulatory region of mouse and human CPO genes.
Alignments of the (A) mouse CPO, and (B) human CPO regulatory upstream gene regions. Each promoter sequence is aligned relative to the translation (TRL) and transcription (TRX) start sites. Locations of the C/EBP sites are indicated by rectangles. Locations of some other transcription factor binding motifs (identified by Transfac search algorithm) are shown by other symbols. See Key for explanation. (C) Mutation of individual C/EBP sites within an intact 946 bp mCPO promoter abrogates transcriptional activity. When a wildtype reporter-luciferase reporter construct (pSK-946Luc) is transfected into LNCaP cells, cotransfection with C/EBPβ further induces transcriptional activity (compare bars 1 and 6). When C/EBP sites are deleted (del) or disrupted by single-site mutations (m), overall activity is dramatically reduced. Error bars, range of duplicates. Schematic (top) shows the mCPO promoter sites chosen for mutation (open rectangles), with their relative binding affinities prior to mutation (from Table 1) shown beneath. Mutated sequences are found in Supplementary Table 1. (D) Hypothetical model of promoter-enhancer system (enhanceosome) regulating CPO transcription; see text for details.
One might predict that strong-affinity sites are functionally more important than weaker sites for regulating transcriptional activation of the full-length mCPO promoter. Alternatively, moderate and weak sites might play a larger than anticipated role because they occur at regular spatial intervals along the mCPO sequence. To distinguish between these possibilities, three C/EBP sites with different binding strengths (strong, moderate, or weak) were randomly chosen for mutagenesis experiments. A full length promoter-reporter construct, comprising 946 bp of intact, wildtype mCPO upstream sequence linked to a luciferase reporter, showed robust transcriptional activity and was induced by C/EBPβ cotransfection as expected (Fig. 5C). However, when C/EBP site-specific mutations were introduced into promoter-reporter constructs, all constructs showed similarly reduced expression, independent of the inherent binding affinities of individual sites (Fig. 5C). These results were not due to technical issues; all constructs were verified by DNA sequencing. Rather, loss of inducibility due to the promoter mutations occurred reproducibly with all C/EBP isoforms (Supplementary Table 2), arguing that CPO promoter activity depends upon C/EBP site location, and not simply upon inherent binding affinity. A hypothetical model (Fig. 5D) to support and interpret these findings is presented in the Discussion.
DISCUSSION
Differentiation-enhanced photodynamic therapy (DT/PDT) is a new cancer treatment concept (6) in which cellular differentiation accompanies an increased in expression of CPO, a major enzyme for protoporphyrin accumulation. This report establishes C/EBP transcription factors as a mechanistic link between enhanced differentiation and increased CPO expression in cancer cells. In several models, exposure to differentiation-promoting agents stimulates expression of C/EBPs, CPO mRNA, CPO protein, and PpIX. Direct, vector-based overexpression of C/EBPs also stimulates this pathway. A comprehensive examination of mouse and human CPO upstream gene regions revealed an astonishing number of C/EBP binding motifs (15 sites for mCPO, 18 for hCPO), each capable of binding major C/EBP isoforms with high specificity. Tested in isolation, each DNA site showed a characteristic binding affinity (strong, moderate, or weak), but in the context of a fully intact promoter, mutational inactivation of individual sites (regardless of affinity) completely abolished C/EBP-mediated transactivation. These findings, together with the remarkable regularity of C/EBP site spacing (suggesting the importance of site location), suggests that these sites regulate transcription in a cooperative fashion.
Cooperative interactions between multiple C/EBP recognition sites has been described before. For example, the keratin K10 and elafin gene promoters each contain three C/EBP sites that are all required for optimal promoter activity (25, 40). Interestingly, 2 to 3 C/EBP sites are typically reported for many target genes (see Supplementary Figure 1). In this regard the three strongest C/EBP sites identified in the mCPO gene (C-6, C-7, and C-8) seem consistent with previous reports. What is novel here is the discovery of many additional medium- and low-affinity C/EBP sites in CPO genes that play a role in transcriptional regulation.
Evidence for a high degree of cooperativity, together with a similar distribution of C/EBP binding sites and related transcription factor sites in mouse and human CPO genes (Fig. 5A, B), leads us to interpret our data within the framework of a transcriptional “enhanceosome”. Enhancers are cis-acting DNA regulatory elements that control transcription, whereas enhanceosomes are higher-order complexes of enhancers and transcription factors assembled together on DNA, functioning together to modify local chromatin architecture and recruit the RNA polymerase machinery to the promoter (41). CPO upstream gene sequences display several key features required for an enhanceosome (42). First, precise spacing between binding sites enables all protein components in the enhanceosome to contact each other and/or to contact the DNA, conferring great stability to the final complex. Consequently, deletion or mutation of a single binding site is often sufficient to inactivate the entire complex, as demonstrated here. A second key feature is a dependence upon high mobility group proteins, i.e. “architectural” factors such as HMG I(Y) that bind to AT-rich sequences and facilitate DNA bending (43). Interestingly, AT-rich sequences are present at approximately −1000 bp in both the mouse and human CPO genes. Third, enhanceosomes involve the participation of many classes of transcription factors simultaneously. All the factors highlighted in Fig. 5 (not only C/EBPs but also NF-κB, Sp1, AP-1, and CREB) are known to be expressed in LNCaP cells (44, 45), and all of them can undergo protein-protein bridging interactions with co-activators (CBP/p300) and HMG proteins, as determined in studies with IL-6 (46) or the insulin receptor gene (47). In our study of the CPO gene, only the C/EBP consensus sites were actually tested by EMSA (as opposed to identification by DNA sequence homology). However, this still allows us to make the point that in many genes, C/EBP sites have now been reported to lie in close proximity to AP-1, NF-κB, and the other binding motifs of Fig. 5 (40, 48). C/EBPs can heterodimerize not only with themselves, but also with other basic leucine-zipper transcription factors, including C/ATF and NF-κB, when binding at composite recognition elements (49, 50). This raises the possibility that some of the moderate or weak sites identified here may in fact be composite elements. Growing evidence indicates that “every piece of real estate” on mammalian gene promoters is occupied by binding proteins, as shown for the IFN-β enhanceosome (42). Such apparent complexity of molecular interactions offers opportunities for finely-tuned combinations of transcription factors (differentially activated via receptor signaling pathways) to coordinately regulate CPO gene transcription.
In conclusion, these results support a new therapeutic platform that involves C/EBP-mediated cellular differentiation for a combination treatment of cancer. Further work is justified to fully explore the breadth and variety of cancers that may benefit from this approach.
Supplementary Material
Acknowledgments
Financial support: NIH grant P01-CA84203
We thank Dr. Shigeru Taketani (Kansai Medical University, Osaka, Japan) for the gift of the murine 5′ promoter (946 bp-mCPO luciferase constructs), Dr. James Dewille (Ohio State University, Columbus, OH) for the C/EBPδ construct, and Dr. Linda Sealy (Vanderbilt University, Nashville TN) for the C/EBPβ-LAP* construct. We thank Dr. Bernhard Ortel (Univeristy of Chicago) for help with preparation of constructs, Dr. Judy Drazba (Cleveland Clinic) for help with digital imaging, and Mr. Kishore Rollakanti (Cleveland State University) for experimental assistance.
GRANT SUPPORT
This work was supported by the National Cancer Institute at the National Institutes of Health, P01 CA84203 to T. Hasan and E. Maytin, and a grant from the Dermatology Foundation to S. Anand.
Abbreviations used
- ALA
5-aminolevulinic acid
- C/EBPs
CCAAT/enhancer binding proteins
- CPO
coproporphyrinogen oxidase
- DT
differentiation therapy
- EMSA
electrophoretic mobility shift assay
- H&E
hematoxylin and eosin
- LNCaP
a prostate cancer cell line
- MEL
a murine erythroleukemia line
- PDT
photodynamic therapy
- PpIX
protoporphyrin IX
Footnotes
Conflict of interest statement: The authors have no actual, potential, or perceived conflict of interests with regard to this manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors have no competing financial interests to declare.
References
- 1.Hasan T, Ortel B, Solban N, Pogue BW. Photodynamic therapy of cancer. In: Kufe D, Bast R, Hait W, et al., editors. Cancer Medicine. 7. Hamilton, Ontario: BC Decker, Inc; 2006. pp. 537–48. [Google Scholar]
- 2.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110:2795–838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990;6:143–8. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275–92. doi: 10.1016/1011-1344(92)85108-7. [DOI] [PubMed] [Google Scholar]
- 5.Sinha AK, Anand S, Ortel BJ, Chang Y, Mai Z, Hasan T, et al. Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells. Br J Cancer. 2006;95:485–95. doi: 10.1038/sj.bjc.6603273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Letters. 2012;326:8–16. doi: 10.1016/j.canlet.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortel B, Sharlin D, O’Donnell D, Sinha AK, Maytin EV, Hasan T. Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br J Cancer. 2002;87:1321–7. doi: 10.1038/sj.bjc.6600575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand S, Honari G, Hasan T, Elson P, Maytin EV. Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin Cancer Res. 2009;15:3333–43. doi: 10.1158/1078-0432.CCR-08-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato N, Moore BW, Keevey S, Drazba JA, Hasan T, Maytin EV. Vitamin D enhances ALA-induced protoporphyrin IX production and photodynamic cell death in 3-D organotypic cultures of keratinocytes. J Invest Dermatol. 2007;127:925–34. doi: 10.1038/sj.jid.5700595. [DOI] [PubMed] [Google Scholar]
- 10.Anand S, Wilson C, Hasan T, Maytin EV. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011;71:6040–50. doi: 10.1158/0008-5472.CAN-11-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortel B, Chen N, Brissette J, Dotto GP, Maytin E, Hasan T. Differentiation-specific increase in ALA-induced protoporphyrin IX accumulation in primary mouse keratinocytes. Br J Cancer. 1998;77:1744–51. doi: 10.1038/bjc.1998.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duprez E, Wagner K, Koch H, Tenen DG. C/EBPbeta: a major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells. Embo J. 2003;22:5806–16. doi: 10.1093/emboj/cdg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YJ, Jones LC, Timchenko NA, Perrotti D, Tenen DG, Kogan SC. CCAAT/enhancer binding proteins alpha and epsilon cooperate with all-trans retinoic acid in therapy but differ in their antileukemic activities. Blood. 2006;108:2416–9. doi: 10.1182/blood-2006-02-003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzi S, Bruno S, Giron-Michel J, Clay D, Devocelle A, Croce M, et al. Differentiation therapy: targeting human renal cancer stem cells with interleukin 15. J Natl Cancer Inst. 2011;103:1884–98. doi: 10.1093/jnci/djr451. [DOI] [PubMed] [Google Scholar]
- 15.Umek RM, Friedman AD, McKnight SL. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–92. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 16.Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. 1999;266:677–83. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- 17.Diehl AM, Michaelson P, Yang SQ. Selective induction of CCAAT/enhancer binding protein isoforms occurs during rat liver development. Gastroenterology. 1994;106:1625–37. doi: 10.1016/0016-5085(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 18.Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part II: the C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol Rev. 2004;56:291–330. doi: 10.1124/pr.56.2.5. [DOI] [PubMed] [Google Scholar]
- 19.Koschmieder S, Halmos B, Levantini E, Tenen DG. Dysregulation of the C/EBPalpha differentiation pathway in human cancer. J Clin Oncol. 2009;27:619–28. doi: 10.1200/JCO.2008.17.9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maytin EV, Habener JF. Transcription factors C/EBPalpha, C/EBPbeta, and CHOP (Gadd153) expressed during the differentiation program of keratinocytes in vitro and in vivo. J Invest Dermatol. 1998;110:238–46. doi: 10.1046/j.1523-1747.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 21.Oh HS, Smart RC. Expression of CCAAT/enhancer binding proteins (C/EBP) is associated with squamous differentiation in epidermis and isolated primary keratinocytes and is altered in skin neoplasms. J Invest Dermatol. 1998;110:939–45. doi: 10.1046/j.1523-1747.1998.00199.x. [DOI] [PubMed] [Google Scholar]
- 22.Bundy LM, Sealy L. CCAAT/enhancer binding protein beta (C/EBPbeta)-2 transforms normal mammary epithelial cells and induces epithelial to mesenchymal transition in culture. Oncogene. 2003;22:869–83. doi: 10.1038/sj.onc.1206216. [DOI] [PubMed] [Google Scholar]
- 23.Sterneck E, Zhu S, Ramirez A, Jorcano JL, Smart RC. Conditional ablation of C/EBP beta demonstrates its keratinocyte-specific requirement for cell survival and mouse skin tumorigenesis. Oncogene. 2006;25:1272–6. doi: 10.1038/sj.onc.1209144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh WJ, Rishi V, Orosz A, Gerdes MJ, Vinson C. Inhibition of CCAAT/enhancer binding protein family DNA binding in mouse epidermis prevents and regresses papillomas. Cancer Res. 2007;67:1867–76. doi: 10.1158/0008-5472.CAN-06-2746. [DOI] [PubMed] [Google Scholar]
- 25.Maytin EV, Lin JC, Krishnamurthy R, Batchvarova N, Ron D, Mitchell PJ, et al. Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev Biol. 1999;216:164–81. doi: 10.1006/dbio.1999.9460. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PM, Barnett SK, Atillasoy ES, Milstone LM. Methotrexate induces differentiation of human keratinocytes. Proc Natl Acad Sci U S A. 1992;89:594–8. doi: 10.1073/pnas.89.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikezoe T, Gery S, Yin D, O’Kelly J, Binderup L, Lemp N, et al. CCAAT/enhancer-binding protein delta: a molecular target of 1,25-dihydroxyvitamin D3 in androgen-responsive prostate cancer LNCaP cells. Cancer Res. 2005;65:4762–8. doi: 10.1158/0008-5472.CAN-03-3619. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi S, Taketani S, Akasaka JE, Kobayashi A, Hayashi N, Yamamoto M, et al. Differential regulation of coproporphyrinogen oxidase gene between erythroid and nonerythroid cells. Blood. 1998;92:3436–44. [PubMed] [Google Scholar]
- 30.Pogulis RJ, Vallejo AN, Pease LR. In vitro recombination and mutagenesis by overlap extension PCR. Methods Mol Biol. 1996;57:167–76. doi: 10.1385/0-89603-332-5:167. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PF. Identification of C/EBP basic region residues involved in DNA sequence recognition and half-site spacing preference. Mol Cell Biol. 1993;13:6919–30. doi: 10.1128/mcb.13.11.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osada S, Yamamoto H, Nishihara T, Imagawa M. DNA binding specificity of the CCAAT/enhancer-binding protein transcription factor family. J Biol Chem. 1996;271:3891–6. doi: 10.1074/jbc.271.7.3891. [DOI] [PubMed] [Google Scholar]
- 33.Miller M, Shuman JD, Sebastian T, Dauter Z, Johnson PF. Structural basis for DNA recognition by the basic region leucine zipper transcription factor CCAAT/enhancer-binding protein alpha. J Biol Chem. 2003;278:15178–84. doi: 10.1074/jbc.M300417200. [DOI] [PubMed] [Google Scholar]
- 34.Orkin SH. Differentiation of murine erythroleukemic (Friend) cells: an in vitro model of erythropoiesis. In Vitro. 1978;14:146–54. doi: 10.1007/BF02618181. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe A, Furukawa T, Ogawa Y, Yamamoto M, Hayashi N, Tokunaga R, et al. Involvement of the transcriptional factor GATA-1 in regulation of expression of coproporphyrinogen oxidase in mouse erythroleukemia cells. Biochem Biophys Res Commun. 1997;233:729–36. doi: 10.1006/bbrc.1997.6532. [DOI] [PubMed] [Google Scholar]
- 36.Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26:362–7. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faisst S, Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992;20:3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–53. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 39.McKnight SL. CCAAT/Enhancer inding Protein. In: McKnight SL, Yamamoto KR, editors. Transcriptional Regulation. Cold Spring Harbor Laboratory Press; 1992. pp. 771–95. [Google Scholar]
- 40.Yokota T, Bui T, Liu Y, Yi M, Hunt KK, Keyomarsi K. Differential regulation of elafin in normal and tumor-derived mammary epithelial cells is mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2007;67:11272–83. doi: 10.1158/0008-5472.CAN-07-2322. [DOI] [PubMed] [Google Scholar]
- 41.Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 42.Panne D. The enhanceosome. Curr Opin Struct Biol. 2008;18:236–42. doi: 10.1016/j.sbi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- 44.Ripple MO, Henry WF, Schwarze SR, Wilding G, Weindruch R. Effect of antioxidants on androgen-induced AP-1 and NF-kappaB DNA-binding activity in prostate carcinoma cells. J Natl Cancer Inst. 1999;91:1227–32. doi: 10.1093/jnci/91.14.1227. [DOI] [PubMed] [Google Scholar]
- 45.Huang PH, Wang D, Chuang HC, Wei S, Kulp SK, Chen CS. alpha-Tocopheryl succinate and derivatives mediate the transcriptional repression of androgen receptor in prostate cancer cells by targeting the PP2A-JNK-Sp1-signaling axis. Carcinogenesis. 2009;30:1125–31. doi: 10.1093/carcin/bgp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baccam M, Woo SY, Vinson C, Bishop GA. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: involvement of NF-kappa B, AP-1, and C/EBP. J Immunol. 2003;170:3099–108. doi: 10.4049/jimmunol.170.6.3099. [DOI] [PubMed] [Google Scholar]
- 47.Foti D, Iuliano R, Chiefari E, Brunetti A. A nucleoprotein complex containing Sp1, C/EBP beta, and HMGI-Y controls human insulin receptor gene transcription. Mol Cell Biol. 2003;23:2720–32. doi: 10.1128/MCB.23.8.2720-2732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kagan BL, Henke RT, Cabal-Manzano R, Stoica GE, Nguyen Q, Wellstein A, et al. Complex regulation of the fibroblast growth factor-binding protein in MDA- MB-468 breast cancer cells by CCAAT/enhancer-binding protein beta. Cancer Res. 2003;63:1696–705. [PubMed] [Google Scholar]
- 49.Vallejo M, Ron D, Miller CP, Habener JF. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci U S A. 1993;90:4679–83. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diehl JA, Hannink M. Identification of a C/EBP-Rel complex in avian lymphoid cells. Mol Cell Biol. 1994;14:6635–46. doi: 10.1128/mcb.14.10.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.