Abstract
Removing the spleen prior to ischemic stroke abrogates immunologic response to brain injury and reduces cerebral infarction. However, the effectiveness of splenectomy for neuroprotection after stroke has not been established. Moreover, the risks of the surgical splenectomy in stroke patients create a major obstacle to removing the spleen’s inflammatory response. We hypothesized that acute splenic irradiation will ablate splenic cells and thereby will diminish stroke progression.
Male adult Sprague Dawley rats were subjected to 2-hour middle cerebral artery occlusion (MCAO), then CT scanned for spleen localization and irradiated to the lateral splenic region with 8Gy of Cobalt 60 at 3, 4, 6 or 8 hrs after start of cerebral ischemia. Untreated controls underwent the same procedures except that sham irradiation was applied. At 2 or 7 days after ischemia the rats were euthanized, and brains recovered for the assessment of brain injury and the extent of neuroinflammation.
Irradiation at 3 hrs reduced spleen weight and lymphocyte blood levels after stroke. Splenic irradiation at 3 and 4 hrs after start of ischemia significantly reduced cerebral infarction volumes measured at 48 hrs and 7 days, respectively. The histological analysis on day 7 revealed reduced counts of microglia, infiltrating T cells, and apoptotic neurons in the rats irradiated at 4 hrs.
The noninvasive single-dose procedure of splenic irradiation performed within a time interval of up to 4 hours offers neuroprotection against ischemic stroke possibly by abrogating deployment of splenic cells to the brain.
Keywords: cerebral ischemia, spleen, Cobalt-60, lymphocyte, neuroinflammation, rats
Introduction
Recent studies reported that targeting spleen-derived inflammatory response to ischemic injury limited expansion of brain infarction and increased neuronal survival after stroke [1]. The spleen is a lymphoid organ containing the largest pool of immunological cells in the system [2]. Upon brain ischemia, the spleen is activated via neural sympathetic pathways and deploys immunologic cells to the sites of inflammation in cerebral tissues [1]. It has been demonstrated that removal of the spleen 2 weeks prior to permanent MCAO in the rat significantly reduced neurodegeneration when compared with untreated rats with brain ischemia. Preventive splenectomy also reduced levels of microglia, macrophages, and neutrophils in cerebral tissues [1]. We hypothesized that targeting spleen acutely after stroke will also diminish the progression of brain injury. However, the risks of surgical splenectomy would present a major obstacle to translating this method to clinical management of stroke. Thus, we proposed to explore and develop splenic irradiation in the animal model of ischemic stroke. We hypothesized that splenic irradiation applied at single low dose in the acute ischemic stroke will deplete splenic immunological cells that mediate neuroinflammation and thereby will reduce brain damage.
Methods
Experimental Design
Forty six adult male Sprague Dawley rats weighing 300-350g underwent sham surgery or intraluminal 120-minute MCAO and were allocated (according to the randomization table) to Cobalt-60 irradiation or the sham–irradiation groups. Irradiation was performed at 3 hrs (experiment 1) or 4, 6 and 8 hours (experiment 2) after start of ischemia. The experiments were done in a blinded fashion. All procedures involving animals complied with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Loma Linda University. The rats in the experiment 1 underwent daily neurobehavioral assessment and collection of peripheral blood samples prior to euthanization at 48 hrs after MCAO to determine brain infarction size with 2,3,5-triphenyltetrazolium chloride (TTC) as described previously [3]. In the experiment 2, rats were euthanized on day 7, when brains, lungs, spleens and visceral organs were collected for histology procedures. All euthanization procedures used ketamine/xylazine anesthesia (100/10mg/kg i.p.). Body weight, neurological scores and mortality were recorded daily.
Animal Model of Focal Cerebral Ischemia
Focal cerebral ischemia was done with 4-0 filament occluding MCA for 120 min., as described [3]. During surgery isoflurane anesthesia (4.5% isoflurane for induction and 2% for maintenance in 30% oxygen/medical air) was administered via a nose cone. Every rat was tested for focal neurological deficits at 110 min after the beginning of occlusion. Exclusion criteria comprised lack of neurological deficit, signs of hemorrhage and anesthesia or surgery failure. In total, five rats were excluded from the study based on these criteria.
CT Spleen Localization Procedure and Splenic Irradiation
Thirty minutes prior to irradiation, the animals were anaesthetized with isoflurane (2-3% for induction, 1-1.5% maintenance) and immobilized in a custom-designed holder. Rats underwent a high resolution (512 × 512 image matrix, 0.625 mm slice thickness) CT scan (LightSpeed VCT, GE Healthcare Technologies, Waukesha, WI) and the center of the spleen was localized relative to a longitudinal reference scale (with mm-gradation) and relative to the bottom of the holder in vertical direction. The center of the left ear bar, with a known longitudinal distance to the zero-point of the longitudinal scale, served as visible landmarks in the CT scan. Subsequently, the rats were taken to the Co-60 irradiation unit (Eldorado Cobalt 60 Teletherapy Unit, Theratronics International Ltd., Ottawa, Canada). A 30 mm × 30 mm brass collimator, shielding the nontargeted tissues to better than 3% of the primary dose, was positioned such that the center of the spleen, as determined on CT, coincided with the center of the collimator in longitudinal direction. The vertical height of the collimator was adjusted using a set of 1 mm metal shim plates so that the vertebral column of the rats was shielded, yet more than 80% of the spleen was covered by the primary radiation field. A dose of 8 Gy was delivered to the dose maximum, which was positioned at a depth of 2 mm, coinciding with the depth of the spleen, using an acrylic build-up plate of 3 mm thickness. The dose of 8 Gy was selected based on a series of pilot irradiations, showing that this dose was tolerated well by the rats, yet it provided a significant reduction of spleen size and weight. The length of the cobalt irradiation, performed at a source to axis distance of 800 mm, was adjusted according to the actual source activity, and lasted for about 5 minutes. Untreated controls were exposed to the same CT scan, immobilized for the same length of time, and positioned on the Cobalt irradiator without turning it on (sham irradiation).
Neurological Assessment
Neurobehavioral evaluation utilized a modified Garcia scoring system that tested sensorimotor function and recorded gait abnormalities and beam walking distance [4]. Individual scores could sum up to a maximum 24 points.
Blood Cell Counting
Blood cell counts (leukocytes, lymphocytes, monocytes/macrophages and granulocytes) were done with hematology analyzer using blood from animals’ tail collected for heparin (HESKA Vet ABC-Diff Hematology Analyzer, Heska Corp., Waukesha, WI, USA)[5].
Histological Analysis of Infarction Volume
The animals were euthanized on day 7 after ischemia and subjected to cardiac perfusion with ice cold 0.1 mol/L PBS followed by 10% formalin. The brains were recovered and postfixed in formalin, then cryoprotected, frozen in liquid nitrogen, and cryosectioned into 10 μm slices. 10 samples in total were taken at +2, +1, 0, −1, −2, −3, −4, −5, −6 and −7 millimeter levels with respect to bregma and stained for Nissl substance as described [6], photographed under 1.25x magnification and analyzed using the Adobe Photoshop 6.0 program (Adobe, San Jose, CA). Noninfarcted ipsilateral tissue value was subtracted from the contralateral hemisphere pixel measurement to obtain the infarction size corrected for edema. The infarct volume of all 10 slices was summed up to obtain the infarction volume[3].
Fluorescence Staining and Manual Cell Counting
Fluorescence staining was performed on 4 sections per brain from the central portion of the lesion as previously described [6]. The primary antibodies used were: rabbit anti-Iba1 (Wako), mouse anti-T cell marker (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and mouse anti-NeuN (Millipore, Temecula, CA), each diluted 1:100. The respective secondary antibodies (Jackson Immunoresearch Labs, West Grove, PA) were diluted 1:200. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was done with a kit (Roche, Indianapolis, IN) [6]. Fluorescence photographs were taken under Olympus BX51 microscope digital camera (Olympus America, Center Valley, PA) and merged by means of Magna Fire software (Systat Co., San Jose, CA) [6].
T cells were identified as positive for Pan T Cell marker and detected by immunofluorescence with the antibody raised against WAG/Rij spleen cells of rat origin. Absolute numbers were counted in a blinded fashion in the three predetermined peri-infarct regions of the ipsilateral hemisphere at the bregma (0) level then averaged and expressed per square mm, as described [5]. Seven non irradiated and 6 irradiated rats per group were used to arrive at means with standard deviations. The identification of microglial cells was done with antibody against Iba1 and same as above mathematic approach was applied to analyze microglial counts. Spleen cell counts were done in three marginal zone areas in the immediate vicinity of the white pulp of the spleen and expressed in mm−2 units.
Statistical Analysis
Means ± standard deviation were calculated for all relevant parameters. One-way analysis of variance (ANOVA) with Holm-Sidak correction was performed to test for statistical significance of differences between means. When appropriate, a nonparametric Kruskal-Wallis ANOVA with Dunn’s test was employed. Mortality rates were analyzed with a chi square test. Correlation analysis was done with Pearson bivariate correlation test. A type I error at the p<0.05 level was considered statistically significant. The result calculations indicated a sufficient number of animals and/or sample counts were used for all but two variables to achieve power close to 0.80 (0.7 - 0.8). SigmaStat and SigmaPlot 12 software were used for statistical analysis (Systat Software Inc., Chicago, IL).
Results
Quantitative Blood Cell Count
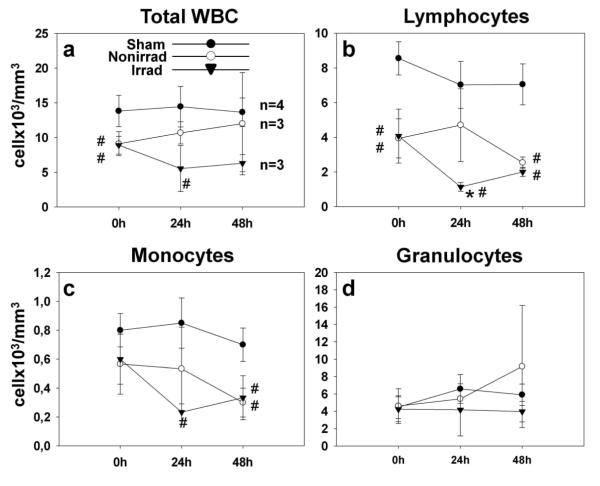
Prior to surgery, white blood cell counts were equivalent in rats assigned to no irradiation ischemic group and irradiation groups; although total WBC and lymphocyte numbers in the ischemic groups at 0hrs were lower than in the sham group (Fig. 1). However at 24 hours, only after splenic irradiation lymphocyte and WBC counts were significantly reduced compared to sham or nonirradiated MCAO (Fig. 1b). Lymphocyte and monocyte counts (Fig. 1c) tended to equalize in nonirradiated and irradiated groups at 48 hours. Granulocyte counts showed an increasing trend in nonirradiated rats, while plateaued in the irradiated group (Fig. 1d). We observed a seemingly apparent correlation between spleen weight and lymphocyte blood cells counts at 48 hrs after surgery, which however did not reach statistical significance (p=0.06; n=3).
Fig. 1.
Peripheral blood leukocyte counts in rats with intact spleens or irradiated 3 hrs after ischemia (a) There was a tendency towards reduced total white blood counts after irradiation as compared to nonirradiated ischemia group. (b) With splenic irradiation lymphocyte levels were significantly reduced at 24 hrs after ischemia. (c) The reduction in the monocyte counts occurred after irradiation with respect to sham however without significant differences between ischemic groups, irradiated or not. (d) Granulocyte levels did not change significantly (*p<0.05 vs. nonirradiated; #p<0.05 vs. sham, ANOVA)
Infarction Volume, Neurological Scores, Body Weight and Mortality
Brain tissues unstained with TTC, consistent with infarction 48 hrs after ischemia, occupy smaller areas in rats with spleen irradiation compared to nonirradiated (Fig. 2ai). The volumetric analysis (Fig. 2aii) demonstrates that splenic irradiation at 3 hrs after MCAO significantly reduced lesion volume measured at 48 hrs after ischemia (p=0.026).
Fig. 2.
Infarct size as determined with TTC and Nissl staining and functional outcomes in the control and experimental groups. (ai) TTC-unstained portion of brain tissue (infarction) is reduced in the animals with spleen irradiated at 3 hrs compared to nonirradiated group. (aii) Significant reduction of infarct size (p<0.05, ANOVA) by 61.46% in rats with spleen irradiated with 8 Gy of Co-60 3 hrs after MCAO compared to nonirradiated rats. (bi) Photographs of Nissl staining reveal reduced cerebral infarction (red arrow) in 4 hrs radiation group 7 days after ischemia (magnification bar=1mm). (bii) The results of volumetric analysis of ischemic lesions determined with Nissl stain confirm infarct volume reduction following spleen irradiation at 4 hrs after MCAO (*p<0.05 vs. nonirradiated, ANOVA). Neurological scores, (c) and body weight loss (d) after splenic irradiation show no significant differences between ischemic groups. Significant deterioration of neuroscores and body weight loss are seen predominantly in 6 and 8 hrs irradiation groups as compared to sham operated rats.†p<0.05 vs. 4hrs irradiation group; ‡ p<0.05 vs. 6hrs irradiation group; §p<0.05 vs. 8hrs irradiation group
Representative photographs of Nissl stained brain sections in nonirradiated controls and in the irradiated group are shown in Fig. 2bi. Reduced extent of infarcted tissue can be seen in rats with spleens irradiated at 4 hrs (right panels). Irradiation at 4 hrs significantly reduced ischemic lesion volume (by 50.83%) (Fig. 2bii). Ischemic volume reduction was insignificant when splenic irradiation was delayed till 6 hrs or 8 hrs (12.07 % and 18.38 %, respectively). Irradiation carried out at 3 hrs after ischemia onset (experiment 1) resulted in a significant amelioration of neuroscores (13.33±1.16 vs.7.67±1.53 p=0.007). However in the experiment 2, splenic irradiation did not improve neurological scores (Fig. 2c) or body weight loss (Fig. 2d) significantly. 7-day mortality in nonirradiated MCAO rats was 12.5%, 14.29% in the 4 hrs irradiation group, and 25 % in 6 hrs and 8 hrs irradiation groups (sham-operated: 0%) without significant intergroup differences.
Reduced Spleen Size and Induction of Apoptosis after Irradiation
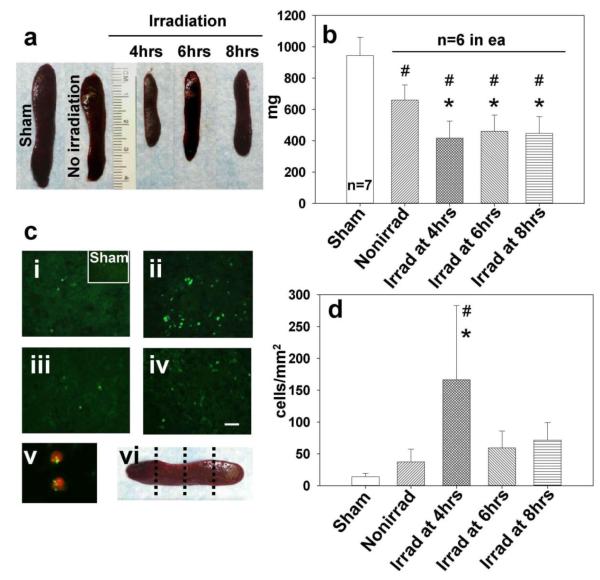
Forty eight hours after MCAO, the mean spleen weight in the nonirradiated MCAO group was 532.33±42.71 mg versus 343.67±27.76 mg in rats irradiated 3 hrs after ischemia (35.44 % reduction, p=0.003). The mean spleen weight was 944.75±116.45 mg in the sham surgery group and 660.0±96.18 mg in the no irradiation MCAO group on day 7 (Fig. 3a). The spleen weight was 416.50±109.99mg (38.24% reduction compared to nonirradiated) when irradiation was done at 4 hrs after ischemia, 460.50±102.92 mg (−30.37%) with 6 hrs delay and 446.83±106.89 mg (−35.16%) after irradiation with 8 hrs delay, as compared to nonirradiated group (p< 0.05 vs. control; Fig. 3b).
Fig. 3.
The effect of irradiation on spleen 7 days after stroke. (a) Representative photographs of spleens from sham surgery group and nonirradiated MCAO (right panel) and irradiated spleens. (b) Graph bars indicate reduced weight of irradiated spleens after ischemia, most profoundly at 4 hrs time of delay (*p<0.05 vs. nonirradiated, #p<0.05 vs. sham, ANOVA). (ci) In the sham operated and nonirradiated ischemic group only a limited number of TUNEL-positive cells was found (magnification bar: 30μm). (cii) Strong TUNEL positivity of splenic cells was associated with radiation at 4 hrs post-ischemia. Splenic irradiation performed at 6 hrs (ciii) and 8 hrs (civ) post ischemia resulted in fewer TUNEL positive cells than seen in the 4 hrs irradiation group. (cv) The photograph (blown up X4) of double stain involving TUNEL (fluorescein) and T cell marker (Texas Red) indicates T cells as apoptotic spleen cells following irradiation at 4 hrs. (cvi) Levels of spleen sectioning for immunofluorescence and cell count. (d) Bar graphs showing significant increase in the number of splenic apoptotic cells in the 4 hrs group vs. nonirradiated (*p<0.05) and vs. sham-operated rats (#p<0.05)
Only scarcely distributed apoptotic cells were present in spleen at 7 days after sham surgery (Fig. 3ci inset) or MCAO alone (Fig. 3ci). 7 days after ischemia with irradiation at 4 hrs, many apoptotic cells were seen (Fig. 3cii) and the double staining revealed TUNEL positive T cells (Fig. 3cv). Few apoptotic cells were present in spleens of animals irradiated at 6 and 8 hrs after ischemia (Fig. 3ciii and 3civ), which may suggest earlier deployment of spleen cells. In spleen areas adjacent to white pulp the number of apoptotic cells increased significantly only in 4 hrs group, by 445.86 %, as compared to nonirradiated group (p<0.05; Fig. 3d). There was also a significant inverse correlation between spleen weight and number of apoptotic cells within spleens on day 7 (r=−0.491; p<0.01).
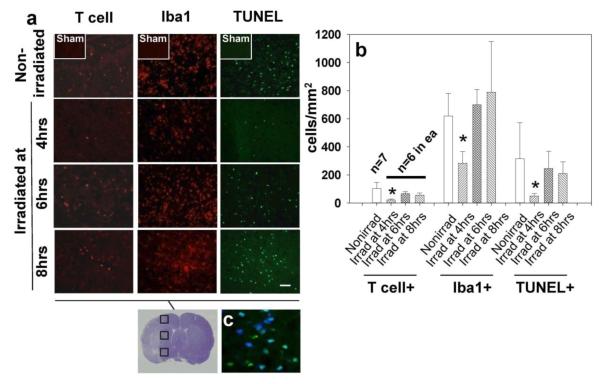
Suppression of Brain Inflammation in Rats Irradiated at 4 hrs
The first column of histological panels in Fig. 4a shows a reduction in brain-invading T cells in the 4 hrs irradiation group compared to the nonirradiated group and groups with irradiation at 6 and 8 hrs. Reductions in Iba1 and TUNEL-positive cells (columns 2 and 3, respectively) were also seen in the 4 hrs irradiation group. The majority of apoptotic cells displayed neuronal marker NeuN (Fig. 4b). In the 4 hrs irradiation group, the reduction in T cell numbers was by 78.23 % compared to the control group, while it was reduced by 35.07 % and 46.48 % in the 6 hrs and 8 hrs irradiation groups, respectively (Fig. 4c). Notably, in all ischemic group there was a significant positive correlativity between spleen weight and invading lymphocyte numbers in the brain (correlation coefficient r =.618; p<0.01). The Iba1+ cells count was significantly reduced (by 54.73%) in the 4-hours group but not in other irradiation groups. The number of TUNEL+ cells in the 4 hrs irradiation group was reduced to 15.73 % of that in the nonirradiated group (p<0.05 ANOVA), while insignificant reductions, to 77.85% and 66.55 %, were also detected in the 6 hrs and 8 hrs irradiation groups, respectively.
Fig. 4.
The effect of splenic irradiation on the immune invasion and inflammatory response in the post-ichemic brain. (a) Reduced number of invading T-cells, ameboid microglia, and apoptotic cells in the postischemic brain after splenic irradiation (magnification bar: 30μm). (b) Graph showing significantly reduced cell counts associated with irradiation at 4 hrs but not at 6 and 8 hrs after ischemia; *p<0.05 vs. nonirradiated, ANOVA. (c) The photograph (blown up X3) showing co-localization of TUNEL and neuronal marker, NeuN (blue color: NeuN, AMCA; green: TUNEL, fluorescein. Turquoise color results from merging AMCA and fluorescein component images)
Microstructure of Visceral Organs
Lung tissue samples were collected 7 days after ischemia and stained with hematoxylin and eosin (H&E). Microscopic observations revealed no detectable thickening of alveolar walls and granulocyte infiltrates that would be consistent with increased microbial load as a consequence of splenic irradiation [7]. No structural differences were seen between lung samples obtained from the nonirradiated ischemia group (Fig. 5ai) and from 4 hrs radiation group (Fig. 5aii).
Fig. 5.

Photomicrographs of the (a) lung, (b) kidney (magnification bar: 200 μm), (c) liver (magnification bar: 100 μm), and (d) small intestine (magnification bar=300 μm) from nonirradiated rats with MCAO (ai-di) and irradiated on the spleen at 4 hrs after ischemia (aii-dii). All organs were harvested at the time of euthanization 7 days post ischemia. (aii) The histological analysis showed no signs of increased microbial load in the lungs after splenic irradiation. (bii-dii) Absence of tissue damage in the abdominal organs after splenic irradiation
Examination of H&E stained histological preparations revealed no signs of radiation injury to the abdominal organs at 7 days after splenic irradiation. The kidneys showed well preserved glomeruli and tubules (Fig. 5bi and Fig. 5bii). Similarly, the lobuli and central vein of the liver showed normal morphology regardless irradiated or not (Fig. 5ci and 5cii). The histological preparations of the small intestine (known to be sensitive to acute radiation effects) were negative for the signs of radiation injury (e.g. flattening of the intestinal villi) (Fig. 5dii).
Similarly, well preserved morphology of visceral organs was found in the groups of rats irradiated at 6 and 8 hrs after ischemia (data not shown).
Discussion
Irradiation of the spleen with 8 Gy of Cobalt-60 gamma rays resulted in spleen size reduction and death of spleen cells via apoptosis. To our knowledge, this is the first demonstration that post–ischemic ablation of the spleen by gamma irradiation reduced the volume of cerebral infarction. Moreover, statistical analysis confirmed that spleen weight and number of lymphocytes invading the ischemic brain were correlated with each other. This suggests that smaller spleens go along with fewer infiltrating cells and further corroborates the benefit of splenic irradiation for brain protection against neuroinflammation. Negative correlation between spleen weights and number of apoptotic spleen cells may then indicate that splenic irradiation eliminates spleen immunocytes through apoptosis. Finally, our observation that lowered lymphocyte blood levels tended to accompany reduction in irradiated spleen weight, might suggest that targeting spleen with radiation compromises its ability of releasing immunocytes in response to ischemic brain insult. Concordantly, at the ischemic border numbers of infiltrating lymphocytes, microglia and apoptotic cells were reduced by splenic irradiation done 4 hrs after the onset of ischemia. The therapeutic window for splenic irradiation seems to end somewhere between 3 and 4 hours after ischemic onset, considered there was no functional improvement for irradiation at 4h, 6h or 8h after ischemia. The relatively short therapeutic window for splenic irradiation may appear suprising given that spleen immunocytes can enter the brain within hours and days after injury. Such result however may only indicate that splenic irradiation need to target early deployment of these cells to provide neuroprotective effect.
Our irradiation-at-4 hrs results may suggest that when protective treatment does not reduce brain lesion below certain critical volume, the functional improvement does not occur. Earlier authors reported that favorable neurological outcomes cannot be anticipated if more than 80 cc of the human brain is infracted [8].
The neuroprotective effect of splenic irradiation on day 7 was associated with decreased apoptotic cell counts in the brain. Significant portion of TUNEL positive cells correlated with neuronal marker NeuN. However, there were quite numerous non neuronal TUNEL positive cells as well. We want to point out that astrocytes might account for nonneuronal cells exhibiting TUNEL. The astrocytes have all components needed to execute intrinsic and extrinsic pathways of apoptotic cell death [9]. Studies have shown marked increases in the number of astrocytes with DNA damage 7 days after focal cerebral ischemia [10]. Although further studies are warranted it cannot be ruled out at this point that astrocytic apoptosis was present on day 7 after fulminate ischemic brain insult while splenic irradiation worked to the rescue of astrocytes in this setting.
The results of experiment 1 indicate that the acute brain protection, demonstrated by a reduced infarction size and improved neuroscores at 48 hrs, may also involve reduced blood lymphocyte numbers after irradiation. The initially observed difference in WBC and lymphocyte numbers between ischemic groups at 0hrs and the sham group appears accidental and stems from physiological variations. The reported levels of WBC in normal male SD rats were 12.97 +/− 2.4 ×109/L and the range was rather broad, as it spanned from 8.1 to 21.5 ×109/L, while lymphocyte percentage ranged from 37.4 to 91.9% [11]. Later time point measurements confirmed no effect of sham surgery on the level of WBC and lymphocytes, while there was a marked decrease in those levels in the irradiated group; especially lymphocyte numbers (1.33+/− 0.25 ×103 cells/mm3 at 24hrs after irradiation) dropped below the normal range.
Lymphocytes, accounting for the majority of cells released by the spleen, have been implicated in the development of neuroinflammation, thrombosis and neurological impairment in ischemic stroke [12]. Studies have shown that activated lymphocytes, which excessively produce inflammatory cytokines, can enter the brain, and adhere to and kill neurons [13, 14]. The role of cytokines in apoptosis have been well established in the literatures [15]. Cytokines released by lymphoid cells can also cause neurotoxicity indirectly, via reactive oxygen species and excess of nitric oxide released from cytokine-activated glial cells [16]. Consequently, in T and B cell deficient mice, displaying reduced cytokine levels, total infarct volumes were significantly reduced at 22 hrs after 90 min MCAO [17].
Splenic irradiation is highly effective in reducing spleen size in patients with lymphoepithelial and hemopoietic diseases associated with splenomegaly [18]. Spleen-derived immunologic cells are known to be very sensitive to irradiation and undergo apoptosis after relatively low doses of a few Gy [19]. Megavoltage radiation can easily penetrate lymphoid organs and provide almost immediate destruction of targeted cells. Thus, splenic irradiation is a simple and well tolerated procedure and could be performed on stroke patients.
In contrast, surgical splenectomy is an invasive procedure carrying the risk of hemorrhage and splenosis [20]. Especially in the elderly, in whom the surgery itself can precipitate stroke-like complications, surgical splenectomy does not seem to make a good treatment option for stroke [21].
It has been reported that after irradiation with doses below 10 Gy, splenic function can be regained by repopulation with cells from other lymphoid tissues within a matter of weeks. Conversely, doses larger than 10 Gy may induce stromal changes such as fibrosis, which may prevent complete restoration of splenic function [22]. The relatively short time line of our study did not allow for the evaluation of long-term radiation effects in the spleen after a single dose of 8 Gy. To this end, further study is needed in order to examine the spleen recovery and long-term neurological outcomes.
It has been postulated that immunodepression occurring after stroke can compromise patients’ outcomes [7]. In the present study, we did not find any histological signs of lung injury that could point towards increased microbial load following splenic irradiation. The plausible explanation is that the other lymphoid organs could compensate for immune functions of the spleen.
In addition, we did not observe any signs of radiation injury in the abdominal organs. This finding is consistent with the notion that a dose of 8 Gy in the spleen, with lower doses in downstream organs, was below the threshold for an acute radiation syndrome.
Conclusions
Splenic irradiation done up to 4 hours after focal brain ischemia provides histological protection associated with widespread apoptotic cell death in the spleen and reduced brain infiltration by immunologic cells. This sizable neuroprotection was not accompanied by side effects from immune system or acute radiation syndrome. Splenic irradiation for cerebral ischemia, if further substantiated in the long term outcome studies, may carry a promise for the clinical management of ischemic stroke.
Acknowledgements
This work was supported by grants NS43338 and HD43120 from the National Institutes of Health to JH Zhang.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Ajmo CT, Jr., Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86(10):2227–34. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraal G. Cells in the marginal zone of the spleen. International review of cytology. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41(2):368–74. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–35. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 5.Titova E, Ostrowski RP, Adami A, Badaut J, Lalas S, Ghosh N, et al. Brain irradiation improves focal cerebral ischemia recovery in aged rats. J Neurol Sci. 2011;306(1-2):143–53. doi: 10.1016/j.jns.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42(2):484–90. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198(5):725–36. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogatsky GG, Shifrin EG, Mayevsky A. Physiologic and biochemical monitoring during hyperbaric oxygenation: a review. Undersea Hyperb Med. 1999;26(2):111–22. [PubMed] [Google Scholar]
- 9.Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol. 2004;72(2):111–27. doi: 10.1016/j.pneurobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda S, Umeda M, Kato H, Araki T. Glial damage after transient focal cerebral ischemia in rats. J Mol Neurosci. 2009;38(2):220–6. doi: 10.1007/s12031-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 11.Petterino C, Argentino-Storino A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol. 2006;57(3):213–9. doi: 10.1016/j.etp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Leonardo CCP, K.R. The Splenic Response to Ischemic Stroke: What Have We Learned from Rodent Models? Transl Stroke Res. 2011;2:328–38. doi: 10.1007/s12975-011-0075-3. [DOI] [PubMed] [Google Scholar]
- 13.Giuliani F, Goodyer CG, Antel JP, Yong VW. Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol. 2003;171(1):368–79. doi: 10.4049/jimmunol.171.1.368. [DOI] [PubMed] [Google Scholar]
- 14.Lee EUJ, L.-J., Lee GK. Entry of Lymphocytes into the Brain and Expression of ICAM-1 on the Brain Endothelium. Korean J Anat. 2004;37:431–9. [Google Scholar]
- 15.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–9. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Peterson PK, Chao CC. Cytokine-mediated neuronal apoptosis. Neurochem Int. 1997;30(4-5):427–31. doi: 10.1016/s0197-0186(96)00078-2. [DOI] [PubMed] [Google Scholar]
- 17.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27(11):1798–805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schratter-Sehn AU, Cerveny M, Simmel H, Schlogl E, Schratter A. Short-time splenic irradiation for splenomegaly. Onkologie. 2003;26(1):21–4. doi: 10.1159/000069859. [DOI] [PubMed] [Google Scholar]
- 19.Filippi AR, Franco P, Galliano M, Ricardi U. Peripheral blood complete remission after splenic irradiation in mantle-cell lymphoma with 11q22-23 deletion and ATM inactivation. Radiat Oncol. 2006;1:35. doi: 10.1186/1748-717X-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro RAK, J.E., Philips EH. Complications of laparoscopic splenectomy. Semin Laparosc Surg. 1997;4:182–9. [Google Scholar]
- 21.Lu YH, Liaw WJ, Chang WT, Wong CS, Ho ST. Stroke-like complication after abdominal surgery: a case report of chronic subdural hematoma. Chin Med J. 1994;54(2):141–4. [PubMed] [Google Scholar]
- 22.Weinmann M, Becker G, Einsele H, Bamberg M. Clinical indications and biological mechanisms of splenic irradiation in autoimmune diseases. Strahlenther Onkol. 2001;177(2):105–11. doi: 10.1007/pl00002384. [DOI] [PubMed] [Google Scholar]