Abstract
Acute graft-versus-host disease (GVHD) and leukemic relapse are the two major obstacles to successful outcomes after allogeneic bone marrow transplantation (BMT), an effective therapy for hematological malignancies. Several studies have demonstrated that the dysregulation of proinflammatory cytokines and the loss of gastrointestinal tract integrity contribute to GVHD, whereas the donor cytotoxic responses are critical for graft-versus-leukemia (GVL) preservation. Suberoylanilide hydroxamic acid (SAHA) is currently in clinical trials as an antitumor agent; it inhibits the activity of histone deacetylases and at low doses exhibits antiinflammatory effects by reducing the production of proinflammatory cytokines. Using two well characterized mouse models of BMT, we have studied the effects of SAHA on GVHD severity and GVL activity. Administration of SAHA from day +3 to day +7 after BMT reduced serum levels of the proinflammatory cytokines and decreased intestinal histopathology, clinical severity, and mortality from acute GVHD compared with vehicle-treated animals. However, SAHA had no effect on donor T cell proliferative and cytotoxic responses to host antigens in vivo or in vitro. When mice received lethal doses of tumor cells at the time of BMT, administration of SAHA did not impair GVL activity and resulted in significantly improved leukemia-free survival by using two different tumor and donor/recipient combinations. These findings reveal a critical role for histone deacetylase inhibition in the proinflammatory events contributing to GVHD and suggest that this class of pharmacologic agents may provide a strategy to reduce GVHD while preserving cytotoxic T cell responses to host antigens and maintaining beneficial GVL effects.
Allogeneic bone marrow transplantation (BMT) is an effective therapeutic option with a curative potential for many malignant diseases. The therapeutic potential of allogeneic BMT relies on the graft-versus-leukemia (GVL) effect, which eradicates residual malignant cells by immunologic mechanisms (1). Unfortunately, GVL effects are closely associated with graft-versus-host disease (GVHD), the major complication of allogeneic BMT (1). The pathophysiology of GVHD is known to involve donor T cell interactions with host antigen presenting cells and the subsequent induction of proinflammatory cytokines (cytokine storm) and cellular effectors that cause target organ damage (2). Several convergent lines of evidence now implicate a network of proinflammatory cytokines such as IFN-γ, tumor necrosis factor α (TNF-α), and IL-1 as critical mediators of both experimental and clinical GVHD (3, 4). Clinical and experimental observations have demonstrated the importance of donor lymphocytes for a GVL response (1, 5). Attempts to reduce GVHD by T cell depletion have led to significant relapse of malignancy (because of the loss of GVL), failure of engraftment, and a higher rate of infections (1, 6).
Chromatin remodeling by acetylation/deacetylation of histones plays an essential role in the regulation of gene expression (7, 8). Two classes of enzyme affect the acetylation of histones: histone acetyltransferases and histone deacetylases (HDACs) (8). Histone acetyltransferases acetylate histone lysine substrates and open the compacted chromatin, allowing the binding of transcription factors and promoting transcription. By contrast, HDACs decrease the acetylation of histone lysine tails and thereby condense chromatin structure and repress gene transcription (7, 8). HDAC inhibitors result in hyperacetylation and modify gene expression either positively or negatively, depending on the context in a cell-specific manner (8).
Suberoylanilide hydroxamic acid (SAHA) contains the hydroxamic acid moiety that binds to the zinc-containing pocket in the catalytic site of HDAC enzymes and thus causes their reversible inhibition (9). In general, SAHA increases the expression of several genes thought to be constitutively suppressed in tumor cells. Micromolar concentrations of SAHA are required for antitumor effects, whereas at nanomolar concentrations SAHA reduces the secretion of inflammatory cytokines, such as TNF-α, IFN-γ, IL-1β, and IL-12 (10, 11). Because low concentrations of SAHA exhibit significant antiinflammatory properties, and given the central role of proinflammatory cytokines in the pathogenesis of acute GVHD, we investigated the effects of SAHA administration in well characterized murine models of allogeneic BMT. We hypothesized that administration of SAHA during the amplification of the proinflammatory cascade early in the time course of allogeneic BMT would suppress cytokine production and reduce systemic GVHD without eliminating donor T cell responses to host alloantigens that could be important for the beneficial GVL effect.
Materials and Methods
Mice and BMT. Female C57BL/6 (B6Ly5.1, H-2b, CD45.2+), B6D2F1 (H-2bxd, CD45.2+), and BALB/c (H2d) mice were purchased from The Jackson Laboratory, and B6Ly-5.2 (H-2b, CD45.1+) mice were purchased from the National Cancer Institute (Frederick, MD). Mice between the ages of 12 and 20 weeks were used for BMT and in vitro experiments. Mice were given transplants according to a standard protocol as described (12). In brief, cell mixtures of 5 × 106 bone marrow cells supplemented with 2 × 106 splenic T cells from either syngeneic or allogeneic donors were resuspended in Leibovitz's L-15 medium (Life Technologies, Grand Island, NY) and transplanted into recipients by tail-vein infusion (0.25 ml) on day 0. The purity of T cells (CD3+) was consistently >80% after separation with microbeads by Automacs (Miltenyi Biotec, Bergisch Gladbach, Germany). Before transplant, host mice received 13 Gy of total body irradiation (137Cs source) delivered in two fractions or an 11-Gy single dose. Mice were subsequently housed in sterilized microisolator cages and received normal chow and autoclaved hyperchlorinated water for the first 3 weeks after BMT and filtered water thereafter.
Leukemia Induction. In GVL experiments, B6Ly5.2 (CD45.1) mice were used as allogeneic BMT donors and 2,000 P815 tumor cells (H-2d CD45.2, a mastocytoma cell line that is uniformly lethal to syngeneic animals) were added to the bone marrow (BM) inoculum on day 0 (13). Death from P815 tumor was defined by enlargement of the liver and spleen with macroscopic tumor nodules on postmortem examination or hindlimb paralysis. GVHD death was defined as the absence of tumor and the presence of GVHD as determined by the clinical scoring system described below. Minimal residual tumor was determined in surviving animals without gross evidence of tumor by using fluorescence-activated cell sorter analysis of the spleen; sensitivity of tumor detection was 0.2% (13). EL4 leukemia was also used for the GVL experiments as described (14, 15). EL4 is a B6 MHC Class II-/- T cell leukemia/lymphoma EL4 (H2b) and is thus syngeneic (H2b) to B6 hosts and allogeneic to BALB/c (H2d) donors. On day 0, 2,000 EL4 cells were injected into each recipient along with syngeneic (B6) or allogeneic (BALB/c) BM and spleen T cells (14). Death caused by EL4 was defined by enlargement of the liver and spleen, whereas death caused by GVHD was defined as the absence of tumor and the presence of GVHD.
Administration of SAHA. SAHA was obtained from Italfarmaco (Cinisello Balsamo, Italy) as a dry lyophilized powder and was stored at -80°C. Vials of SAHA were first dissolved in 50 μl of DMSO, diluted in sterile H2O, and heated to boiling for complete dissolution before injection. Recipients of allogeneic BMT were given 35 mg/kg SAHA i.p. (0.2 ml) once daily from day +3 to day +7 (five injections), whereas all the controls received sterile H2O.
Systemic and Histopathologic Analysis of GVHD. The degree of systemic GVHD was assessed by a standard scoring system as described (12). A clinical index was generated weekly by summation of five criteria scores: percentage of weight change, posture, activity, fur texture, and skin integrity (12). Acute GVHD was also assessed by histopathologic analysis of the ileum and the ascending colon. Specimens were harvested from animals on day +7, processed, stained with hematoxylin and eosin, and coded for histologic examination. Slides were examined systematically by a single pathologist (C.L.) in a blinded manner by using a semiquantitative scoring system as described (12).
Mixed Lymphocyte Cultures. All culture media reagents were purchased from GIBCO/BRL. For analysis of proliferative response in mixed lymphocyte cultures, splenocytes from individual animals were harvested on day +14 and suspended in 10% FCS/DMEM supplemented with 50 units/ml penicillin, 50 μg/ml streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acid, 0.02 mM β-mercaptoethanol, and 10 mM Hepes (pH 7.75). These cells were normalized for 2 × 105 donor T cells (CD45.1+ CD3+) and cultured in flat-bottomed 96-well Falcon plates (Becton Dickinson) in the presence of irradiated splenocytes obtained from naive B6D2F1 (host) animals. Proliferative response to host antigen was measured by a 1205 Betaplate reader (Wallac Oy, Turku, Finland) after 72 h by incorporation of [3H]thymidine (1 μCi; NEN Life Sciences Products) for the last 24 h of incubation.
51Cr-Release Assays. Tumor targets, 2 × 106 P815 (H-2d) or EL4 (H-2b), were labeled with 100 μCi of 51Cr sodium salt (NEN Life Sciences Products) for 2 h. After washing labeled targets were resuspended and the 51Cr-release assays was performed as described (13). Allogeneic splenocytes were harvested and normalized for donor CD8 (CD45.1+, CD8+) cells on day +14. These preparations were added to quadruplicate wells at varying effector-to-target ratios and incubated for 5 h. Maximal and background release were determined by the addition of Triton-X (Sigma) or media alone to targets, respectively. 51Cr activity in supernatants taken 5 h later were determined in an autogamma counter (Packard) as described (13).
Cell Surface Phenotype Analysis. To analyze cell surface phenotype, splenocytes from naive or transplanted mice were resuspended in PBS and stained with FITC-conjugated mAbs to CD45.1 and phycoerythrin-conjugated CD3+ (Pharmingen) for flow cytometric analysis. In brief, cells (0.5 × 106) were incubated for 20 min at 4°C with mAb 2.4G2 to block nonspecific staining by Fc receptors and then with the appropriate FITC- or phycoerythrin-conjugated mAbs for 30 min at 4°C. They were subsequently washed twice with PBS/0.2% BSA and fixed in 1% paraformaldehyde. Two-color flow cytometric analysis was performed by using a FACScan (Becton Dickinson Immunocytometry Systems; ref. 15).
RNA Isolation and Ribonuclease Protection Assay (RPA). RNA from the splenocytes harvested from the transplanted mice was obtained as described (16). In brief, the harvested cells were homogenized in TRIzol reagent (GIBCO) and incubated at room temperature for 5 min. Cells were then lysed, mixed with 0.2 ml of chloroform, and centrifuged. RNA-containing phase was transferred into a fresh tube, and RNA was precipitated by incubating with isopropyl alcohol. The RNA pellet was washed, air-dried, and dissolved in an appropriate volume of RNase-free water and incubated at 8°C. The RNA quantity was determined from OD260 Spectronic LR45227 (Milton Roy, Ivyland, PA); and the quality by RNA electrophoresis. The RPA was carried out with RiboQuant MultiProbe RPA System per the manufacturer's protocol (Pharmingen). Radiolabeled, single-stranded RNA probes from template mCK-3b were synthesized by using [32P]UTP, 3,000 Ci/mmol, including murine RNA and yeast tRNA (2 μg) as positive and negative controls, respectively. Murine probe sets included templates for TNF-α and IFN-γ. The template sets were used for the T7 RNA polymerase-directed synthesis of a highly specific 32P-labeled antisense riboprobe set. The labeled riboprobe set was hybridized to the total RNA at 56°C for 12-16 h. After hybridization, free riboprobes and single-stranded RNA were digested with an RNase A plus T1 mix at 30°C for 45 min. A proteinase K treatment was used to inactivate the ribonucleases. The remaining protected fragments were purified and separated on denatured gels of 5% acrylamide and 8 M urea. The undigested labeled probe sets were used as markers. The bands were visualized by phosphoimager and the quantity determined by the signal intensity of the protected probe fragment bands by using imagequant software from (Molecular Dynamics). Templates for GAPDH and L32 housekeeping genes allowed the assessment of total RNA levels for normalizing the samples (16).
Cytokine ELISAs. Concentrations of TNF-α, IL-1β, and IFN-γ were measured in serum and culture supernatants by ELISA with specific anti-mouse mAbs for capture and detection, and the appropriate standards were purchased from Pharmingen (IFN-γ, IL-1β, and TNF-α). Assays were performed according to the manufacturer's protocol. Samples were diluted 1:4, and samples and standards were run in duplicate. ELISA plates were read at 450 nm by using a microplate reader (Bio-Rad).
Histone Isolation and Immunoblotting. Histones were isolated as described (11). In brief, splenocytes were harvested from the recipient mice 7 days after BMT and lysed in 1 ml of ice-cold buffer (10 mM Tris·HCl, pH 6.5/50 mM sodium bisulfite/1% Triton X-100/10 mM MgCl2/8.6% sucrose) followed by centrifugation. Nuclei were washed, pelleted, and resuspended in ice-cold water. After acidification with H2SO4, samples were incubated on ice for 1 h. Proteins were precipitated by overnight incubation (20°C) with 1 ml of acetone and resuspended in distilled water. Protein concentrations were determined and normalized with a Bio-Rad protein assay kit, and equivalent amounts from each group were loaded. Samples in loading buffer were boiled and electrophoresed in 15% polyacrylamide gels and transferred to nitrocellulose membranes and blocked in 5% milk/Tris-buffered saline with Tween (TBST) followed by overnight incubation in polyclonal rabbit antiacetylated histone H3 (1:1,000). The blots were incubated for 1 h in goat anti-rabbit horseradish peroxidase (1:25,000 in block) and washed extensively in Tris-buffered saline/TBST; bands were visualized by using the enhanced chemiluminescence system according to the manufacturer's directions (Pierce) (11).
Statistical Considerations. All values are expressed as the mean ± SEM. Statistical comparisons between groups were completed by using the nonparametric, unpaired, Mann-Whitney test, except for analyzing survival data, when the Wilcoxon rank test was used.
Results
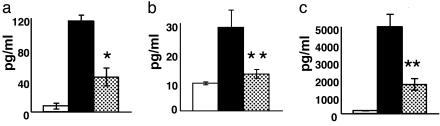
SAHA Inhibits Proiflammatory Cytokines After Allogeneic BMT. We first tested the HDAC inhibitor, SAHA, in a dose-response experiment for its effects on the secretion of proinflammatory cytokines in a B6 (H-2b) → B6D2F1 (H-2b/d) mouse model of acute GVHD. Lethally irradiated B6D2F1 mice received BM and splenic T cells from allogeneic (B6) or syngeneic (B6D2F1) donors, as described in Materials and Methods. BMT recipients were injected i.p. with different doses (10, 20, or 35 mg·kg-1·day-1) of either SAHA or vehicle from day +3 to day +7. This schedule was chosen to modulate the inflammatory cascade of acute GVHD that reaches peak severity by day +7 after BMT in this model but not interrupting the initial donor T cell interaction with host antigen presenting cells in the first 72 h after BMT (12, 17, 18). Administration of 10 or 20 mg/kg had no significant effects (data not shown), whereas 35 mg/kg SAHA significantly reduced serum levels of TNF-α, IL-1, and IFN-γ (Fig. 1, P < 0.03). We also analyzed the effect of SAHA administration on the cellular production of cytokines by phenotyping splenocytes on day +7 after BMT for intracytoplasmic IFN-γ by fluorescence-activated cell sorter analysis. SAHA reduced the percent of donor cells secreting IFN-γ by >50% (18 ± 5.6 vs. 39 ± 4.2, P < 0.03).
Fig. 1.
Injection of SAHA inhibits in vivo proinflammatory cytokine production after BMT. B6D2F1 mice were given 1,300 cGy of total body irradiation and received transplants of 5 × 106 T cell-depleted BM cells and 2 × 106 T cells from either allogeneic (B6) or syngeneic (B6D2F1) donors as in Materials and Methods. Each F1 recipient of the allogeneic cells were injected i.p. with 35 mg·kg-1·day-1 SAHA or the diluent control from day +3 to day +7 after transplantation. Sera from the recipient animals (n = 3 per group) were obtained by retroorbital venous puncture on day 7 after BMT and analyzed, as described in Materials and Methods. (a) Serum TNF-α levels are reduced in the recipients treated with SAHA. Shown are syngeneic (□) and allogeneic controls (▪) vs. SAHA allogeneic (allo) recipients ( ); *, P < 0.05. Results from one of two similar experiments are shown. (b) Serum IL1-β levels are reduced. Shown are syngeneic (□), allo controls (▪) vs. SAHA-treated allo recipients (
); *, P < 0.05. Results from one of two similar experiments are shown. (b) Serum IL1-β levels are reduced. Shown are syngeneic (□), allo controls (▪) vs. SAHA-treated allo recipients ( ); **, P < 0.02. Data are from one of two similar experiments. (c) Serum IFN-γ levels are reduced. Shown are syngeneic (□), allo controls (▪) vs. SAHA-treated allo recipients (
); **, P < 0.02. Data are from one of two similar experiments. (c) Serum IFN-γ levels are reduced. Shown are syngeneic (□), allo controls (▪) vs. SAHA-treated allo recipients ( ); **, P < 0.02. Data from one of three similar experiments are shown.
); **, P < 0.02. Data from one of three similar experiments are shown.
SAHA Increases Histone Acetylation After Allogeneic BMT. The hydroxamic acid moiety of SAHA binds to the zinc-containing pocket of HDAC enzymes, preventing the deacetylation of histones and resulting in hyperacetylation of histones (8). Analysis of histone H3 acetylation in splenocytes harvested from the recipient mice 7 days after BMT showed increased acetylation in mice treated with SAHA, confirming the inhibition of HDAC enzymes (Fig. 2a). To determine whether SAHA regulated the transcription of inflammatory cytokine genes, we next determined the mRNA expression of TNF-α and IFN-γ after BMT by RPA on day +7 after BMT, as described in Materials and Methods. Consistent with the increase in histone H3 acetylation, mice treated with SAHA showed a significant reduction in the transcription of TNF-α and IFN-γ by ≈50%. Thus, brief administration of SAHA after allogeneic BMT increased histone acetylation and reduced the production of proinflammatory cytokines at systemic, cellular protein, and transcription levels.
Fig. 2.
SAHA increases the acetylation of histone H3 and down-regulates the transcription of TNF-α and IFN-γ after allogeneic (allo) BMT. B6D2F1 animals were irradiated and transplanted with cells from either syngeneic (syn) or allo donors as in Fig. 1. Allo recipients were injected with either SAHA or the control diluent for 5 days, from day +3 to day +7 after BMT. (a) On day +7 after BMT the recipient splenocytes were harvested (n = 3 per group), and histones were isolated by acid extraction, as described in Materials and Methods. Immunoblot analysis was performed to detect acetylation of histones by using antiacetylated H3 Ab. (b) Total RNA was extracted from the splenocytes harvested and pooled together on day +7 (n = 3 per group; □, syngeneic; ▪, allo controls , SAHA allo recipients) after BMT as above. The expression of TNF-α and IFN-γ mRNA levels were analyzed by RPA, as described in Materials and Methods. The TNF-α and the IFN-γ mRNA transcript levels were reduced in the recipients treated with SAHA. Shown are controls (▪) vs. SAHA allo recipients (
, SAHA allo recipients) after BMT as above. The expression of TNF-α and IFN-γ mRNA levels were analyzed by RPA, as described in Materials and Methods. The TNF-α and the IFN-γ mRNA transcript levels were reduced in the recipients treated with SAHA. Shown are controls (▪) vs. SAHA allo recipients ( ); *, P < 0.05. Results are from one of two similar experiments.
); *, P < 0.05. Results are from one of two similar experiments.
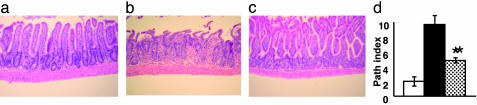
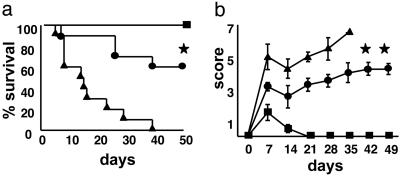
Inhibition of Proinflammatory Cytokine Secretion Decreases the Severity of Intestinal and Systemic GVHD After Allogeneic BMT. TNF-α is a critical inflammatory mediator of gastrointestinal toxicity after allogeneic BMT (19), therefore we analyzed whether the reduction in TNF-α levels seen after SAHA administration affected the pathology of gastrointestinal tract, a major target organ of acute GVHD. Samples of small and large bowel were harvested on day +7 after BMT and evaluated for histopathologic changes, as described in Materials and Methods. Examination of small intestine from syngeneic BMT recipients showed almost complete restoration of normal villous architecture (Fig. 3a). By contrast, recipients of allogeneic BMT and the vehicle exhibited severe villous blunting, crypt destruction, and epithelial attenuation with intense inflammation of the lamina propria, all characteristics of acute GVHD (Fig. 3b). SAHA administration significantly reduced the gastrointestinal tract damage when compared with allogeneic controls (Fig. 3c), although scores remained higher than the syngeneic recipients (Fig. 3d; P = 0.02). We next evaluated the effects of SAHA administration on the clinical severity and mortality of acute GVHD. SAHA treatment produced a highly significant survival advantage (Fig. 4a; P < 0.01) and reduced the severity of clinical GVHD scores (Fig. 4b; P < 0.05). All surviving mice showed greater complete donor chimerism by fluorescence-activated cell sorter analysis (data not shown), ruling out graft failure or mixed chimerism as a cause for the GVHD protection (20, 21).
Fig. 3.
Treatment with SAHA results in decreased intestinal histopathology after allogeneic (allo) BMT. B6D2F1 animals received syngeneic BMT or allo BMT from C57BL/6 donors as in Fig. 1. Allo recipients were injected with either SAHA or the control diluent for 5 days, from day +3 to day +7 after BMT. On days +7 to +8, small-bowel samples from BMT recipient mice (n = 4 per group) were obtained and analyzed microscopically, as described in Materials and Methods. (a) Syngeneic BMT recipients demonstrate reestablishment of intestinal architecture with villi of near-normal length and without significant cellular infiltration into the lamina propria. Regenerative change is evident by focally increased nuclear staining and increased nuclear/cytoplasmic ratios. (b) Recipients of allo BMT show severe intestinal toxicity, including surface erosion, villous blunting, epithelial attenuation, and an intense cellular infiltration in the lamina propria. (c) Animals treated with SAHA exhibit partial restoration of small intestinal villous architecture, regenerative change, and little inflammatory infiltration. (Original magnification, ×200.) (d) Coded slides were scored semiquantitatively for pathological damage, as described in Materials and Methods. BMT recipients of diluent (▪, n = 4) or SAHA ( , n = 4) from allo donors and of syngeneic donors (□, n = 4) are shown. Total GVHD score: mean ± SE of the sum of scores for small bowel and colon from individual animals in each group. *, P < 0.05, control (▪) vs. the SAHA (
, n = 4) from allo donors and of syngeneic donors (□, n = 4) are shown. Total GVHD score: mean ± SE of the sum of scores for small bowel and colon from individual animals in each group. *, P < 0.05, control (▪) vs. the SAHA ( ) allo group.
) allo group.
Fig. 4.
Treatment of allogeneic (allo) recipients with SAHA attenuates acute GVHD mortality and morbidity. B6D2F1 mice were given 1,300 cGy of total body irradiation and transplants of 5 × 106 T cell-depleted BM cells and 2 × 106 T cells from allo B6 donors as in Materials and Methods. Syngeneic recipients (▪, n = 5) received transplants similarly with cells from F1 donors. Each allo recipient was injected i.p. with either 35 mg·kg-1·day-1 SAHA (•, n = 10) or the control vehicle (▴, n = 10) for 5 days from day +3 to day +7. Transplanted animals were monitored daily for survival and assessed weekly for clinical severity of acute GVHD, as described in Materials and Methods. Data from one of two similar experiments are shown. (a) Percent survival after BMT. • vs. ▴, *, P < 0.002 by Wilcoxon rank test. (b) Animals scored for clinical GVHD, as described in Materials and Methods. Data are expressed as mean ± SEM. • vs. ▴, **, P < 0.05 by Mann-Whitney U test from day 7 to day 35.
To test the concept that HDAC inhibition by SAHA is a generalizable strategy for GVHD prophylaxis, we administered SAHA in a second well characterized mouse model of acute GVHD directed against both MHC and minor histocompatibility antigens, BALB/c (H-2d) → B6 (H-2b) (15). Recipient B6 mice were conditioned and received transplants of cells from either syngeneic (B6) or allogeneic (BALB/c) donors, as described in Materials and Methods. SAHA administration at a dose of 35 mg/kg from day +3 to day +7 significantly improved survival (60% vs. 0%, P < 0.02) and reduced its clinical severity compared with controls, although some GVHD was still observed in SAHA-treated animals (see Fig. 7, which is published as supporting information on the PNAS web site). Together these data demonstrate that SAHA reduced but did not completely eliminate acute GVHD in two different allogeneic BMT models.
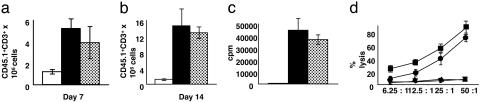
SAHA Administration Does Not Impair T Cell Responses to Host Antigens After BMT. During GVHD, donor T cells respond to host histocompatibility antigens within 72 h of injection after BMT (3, 17, 18). Our rationale to treat BMT recipients with SAHA from day +3 to day +7 was to permit initial donor T cell activation while preventing the amplification of the systemic inflammation of acute GVHD. Because inflammatory cytokines, such as TNF-α, IL-1, and IFN-γ, can enhance the activation and expansion of T cells (4, 19), it was possible that inhibition of these cytokines by SAHA administration could reduce donor T cell responses to host antigens. We therefore measured multiple parameters of donor T cell function in the spleens of BMT recipients. In these experiments we used B6 Ly5.2 (CD45.1) mice as donors. The overall splenic cell counts in the SAHA- and control-treated allogeneic mice were equivalent (data not shown). Furthermore, as shown in Fig. 5 a and b, SAHA did not significantly alter the expansion of donor CD45.1+CD3+ lymphocytes either at day +7 or +14, the period of maximal donor T cell expansion in this model. Proliferation of donor T cells to irradiated host F1 stimulators in vitro was also equivalent between animals treated with SAHA and control on day +14 (Fig. 5c). Furthermore, T cells demonstrated vigorous lysis of host-type P815 (H-2d) tumor targets with only a small reduction in killing at lower effector/target ratios on day +14 (Fig. 5d). However, when the splenocytes harvested after BMT were stimulated in vitro with lipopolysaccharide, members of the SAHA-treated group secreted significantly less TNF-α and IFN-γ (data not shown). Furthermore, the proliferative responses of donor T cells to F1 stimulators taken 7 days after BMT from SAHA- and control-treated animals were also equivalent (27,899 ± 5,113 vs. 32,549 ± 3,677 cpm, P = NS). These data thus demonstrate that SAHA administration at this dose and schedule after BMT did not affect donor T cell responses to host antigens, and its ability to reduce GVHD was primarily due to its inhibition of inflammatory cytokines.
Fig. 5.
Effect of SAHA administration on donor T cell functions after BMT. B6 Ly5.2 (CD45.1+) donor cells were transplanted into irradiated syngeneic (B6 Ly 5.1, CD 45.2) or allogeneic (allo) (B6D2F1) recipients injected with SAHA or the vehicle as in Materials and Methods. Splenocytes were harvested from the recipients on days +7 (a) and +14 (b) after BMT (n = 4 per group) and labeled with anti-CD3 phycoerythrin and anti-CD45.1 FITC. The number of donor T cells (CD45.1+ CD3+) were determined [allo plus control ( ) vs. allo plus SAHA (▪), P = NS]. Recipients of syngeneic BMT (□) demonstrated lower numbers of donor T cells at both time points. Data from one of three similar experiments are shown. (c) Harvested splenocytes normalized for donor T cells (CD45.1+ and CD3+) were restimulated in quadruplicate with irradiated naive host (syngeneic B6 Ly5.2 and allo F1) splenocytes in mixed lymphocyte reaction cultures for 48 h. Proliferation was determined by incubation of cells with [3H]thymidine (1 μCi per well) for an additional 24 h. T cells from SAHA-treated animals showed equivalent proliferation (
) vs. allo plus SAHA (▪), P = NS]. Recipients of syngeneic BMT (□) demonstrated lower numbers of donor T cells at both time points. Data from one of three similar experiments are shown. (c) Harvested splenocytes normalized for donor T cells (CD45.1+ and CD3+) were restimulated in quadruplicate with irradiated naive host (syngeneic B6 Ly5.2 and allo F1) splenocytes in mixed lymphocyte reaction cultures for 48 h. Proliferation was determined by incubation of cells with [3H]thymidine (1 μCi per well) for an additional 24 h. T cells from SAHA-treated animals showed equivalent proliferation ( vs.▪, P = NS). Results from one of three similar experiments are shown. (d) SAHA treatment preserves cytotoxic T lymphocyte function after BMT. Splenocytes harvested from allo animals on day +14 after BMT were pooled (n = 3 per group), normalized for donor CD8+ cells, and used in a 51Cr-release assay. Cytotoxic T lymphocyte activity against allo targets (P815) in control (
vs.▪, P = NS). Results from one of three similar experiments are shown. (d) SAHA treatment preserves cytotoxic T lymphocyte function after BMT. Splenocytes harvested from allo animals on day +14 after BMT were pooled (n = 3 per group), normalized for donor CD8+ cells, and used in a 51Cr-release assay. Cytotoxic T lymphocyte activity against allo targets (P815) in control ( ) and SAHA (▪) groups was similar, whereas no significant lysis of syngeneic targets (EL-4) by both groups (▴ and ▾) occurred. Data from one of two similar experiments are shown.
) and SAHA (▪) groups was similar, whereas no significant lysis of syngeneic targets (EL-4) by both groups (▴ and ▾) occurred. Data from one of two similar experiments are shown.
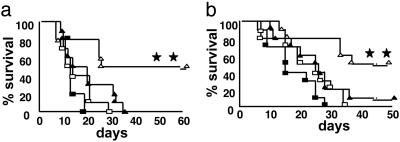
Inhibition of HDACs by SAHA Preserves GVL Activity After Allogeneic BMT. The maintenance of donor T cell responses to host antigens after SAHA treatment suggested that this approach might also preserve the beneficial GVL activity. To test this hypothesis directly, we added 2,000 P815 (H-2d, CD45.2+) mastocytoma cells to the BM inoculum to model residual disease at the time of BMT. As shown previously, when injected intravenously into B6D2F1 (H-2bxd) hosts, this dose of P815 is uniformly lethal and behaves like a leukemia, causing massive infiltration and enlargement of the liver and spleen and hindlimb paralysis from infiltration of the spinal cord (13). All syngeneic BMT recipients treated with SAHA or the control showed evidence of tumor infiltration at necropsy and did not exhibit prolonged survival, ruling out a significant direct antitumor effect from this dose and schedule of SAHA (Fig. 6a). In recipients of allogeneic BMT and 2,000 P815 cells, SAHA administration resulted in 50% survival, a significant survival advantage compared with controls (Fig. 6a; P < 0.03). Necropsy performed either on the day of death or at the end of the observation period showed no evidence of tumor in the recipients of allogeneic BMT regardless of SAHA treatment, confirming the graft-versus-tumor (GVL) effect. We confirmed these observations by using a BALB/c → B6 allogeneic BMT model of GVL. We added 2,000 EL-4 (T cell leukemia/lymphoma) cells (H-2b, CD45.2+) to the BM inoculum on the day of transplant. EL-4 cells also cause massive infiltration and enlargement of liver and spleen, and an injection of as few as 500 cells is uniformly fatal (14). Syngeneic BMT recipients again showed 100% mortality with evidence of tumor infiltrating into liver and spleen regardless of SAHA treatment (Fig. 6b). By contrast, allogeneic recipients injected with SAHA showed 50% survival on day +50 that was significantly greater than controls (Fig. 6b; P < 0.01) with no evidence of tumor in the liver and spleen on necropsy. Thus, the maintenance of T cell function by HDAC inhibition with SAHA preserved GVL effects and improved leukemia-free survival in two separate BMT tumor models.
Fig. 6.
Treatment with SAHA preserves GVL activity and improves leukemia-free survival. (a) B6D2F1 mice receiving transplants of T cells from syngeneic donors and injected with either SAHA (□, n = 10) or control vehicle (▪, n = 10) or from allogeneic (allo) B6Ly5.2 donors and injected with SAHA (▵, n = 10) or vehicle (▴, n = 10) as described in Fig. 1. P815 cells (2,000) were added to the BM inoculum at day 0, as described in Materials and Methods. Animals were monitored daily for survival, and necropsy was performed on all dying animals to determine whether death was caused by leukemia or GVHD. **, P < 0.05 for allo plus SAHA (▵) vs. allo plus vehicle (▴) and P < 0.02 for allo plus SAHA (▵) vs. syngeneic plus SAHA (□). Data shown are from one of two similar experiments. (b) Total-body-irradiated B6 Ly5.2 mice received transplants of 5 × 106 TCD BM and 2 × 106 splenic T cells from allo BALB/c donors and were treated with SAHA (▵, n = 11) or control (▴, n = 10) or from syngeneic B6 donors and were injected with control (▪, n = 10) or SAHA (□, n = 11) as in Materials and Methods. All the recipient mice were injected i.v. with 2,000 EL4 tumor cells on day 0. **, P < 0.05 for ▵ vs. ▴ and P < 0.01 for ▵ vs. □.
Discussion
HDAC inhibitors such as SAHA and trichostatin possess antitumor properties at higher concentrations and have recently been shown to suppress proinflammatory cytokine secretion at much lower concentrations (10, 11). Our data show that brief inhibition of HDAC enzymes with SAHA from day +3 to day +7 after allogeneic BMT significantly suppressed serum levels of TNF-α, IL-1, and IFN-γ and thus modulated the cytokine storm and reduced acute GVHD by clinical and histologic parameters. However, SAHA treatment did not alter T cell activity to host antigens either in vivo or in vitro and preserved GVL effects, resulting in significantly higher leukemia-free survival in two separate models.
The induction of acute GVHD is a direct consequence of donor T cell responses to host alloantigens and the dysregulation of inflammatory cytokine cascades (4). Inflammatory cytokines, such as IL-1 and TNF-α, are now known to play a central role in the pathogenesis of many transplant-related complications in clinical and experimental GVHD (3, 22). Injury to the gastrointestinal tract initiated by BMT-conditioning regimens allows passage of lipopolysaccharide into the circulation where it triggers cytokine release by mononuclear cells that have been primed by IFN-γ. These proinflammatory cytokines further damage gut mucosa and perpetuate the translocation of additional lipopolysaccharide into the systemic circulation, thus establishing a positive feedback loop for progressive gastrointestinal tract injury and systemic inflammation (4). Although clinical gene polymorphism studies of several inflammatory cytokines, including TNF-α and IFN-γ, have been associated with increased GVHD severity after allogeneic BMT (22), strategies aimed at inhibiting these cytokines individually have not yielded encouraging clinical results (22, 23). In the current study, inhibition of HDAC enzymes with SAHA reduced GVHD, suggesting that perhaps inhibiting several proinflammatory cytokines simultaneously might be a possible strategy for this intractable complication of BMT.
The antiinflammatory effects of HDAC inhibition observed in our study are consistent with several recent reports of this activity in other disease models. For example, the combination of HDAC inhibitors trichostatin and butyrate have been shown to reduce IFN-γ production by T cells (24). Leoni et al. (10) have demonstrated significant reductions of TNF-α, IL-1β, and IFN-γ in experimental endotoxemia and ConA-induced hepatic injury models with lower doses of SAHA than usually used as an antitumor agent. Trichostatin has also been recently shown to reduce the inflammation and clinical manifestations of systemic lupus erythematosus in a murine model (11). Another recent study demonstrated that inhibition of HDAC enzymes reduced the activation of both CD4 and CD8 T cells and lessened the severity of autoimmune diabetes (25). However the lack of significant effect on donor T cell responses to host antigens after BMT at the dose and schedule used in our experiments shows that this level of HDAC inhibition was not immunosuppressive despite its clear antiinflammatory effects. In support of this differential mechanism of action, consistent low concentrations of SAHA (100 nM) did not significantly alter T cell proliferation in a mixed lymphocyte reaction (25,911 ± 2,835 vs. 29,357 ± 3,843 cpm, P = NS) but reduced TNF-α secretion by naïve splenocytes after lipopolysaccharide stimulation. Furthermore, SAHA has been shown to have no effect on anti-CD3+-induced T cell activation at nanomolar concentrations in vivo (10). Taken together with our study, these data would suggest that the extent of HDAC inhibition may produce qualitatively different effects in terms of tumor cell proliferation/apoptosis, T cell activation/proliferation, and production of inflammatory cytokines. Studies are needed to further delineate the potential differences.
Histone acetylation is critical in regulating gene expression for many immune processes (8). Although the exact mechanisms of the suppression of proinflammatory cytokines remain to be determined, the inhibition of cytokines by SAHA temporally correlated with enhanced acetylation of H3 histones. These parallel findings suggest that chromatin remodeling may play a role in decreased cytokine production perhaps by increasing the expression of genes that down-regulate cytokine production, such as the CIS/SOCS/SSI family proteins (26). It is also possible that HDAC inhibitors could increase the production of antiinflammatory cytokines such as TGF-β (27) or regulate nitric oxide (NO) secretion that could dampen cytokine secretion (10). Alteration of the acetylation status of other nonhistone proteins might also indirectly contribute to the effects of SAHA on cytokine secretion. Further studies will need to determine whether these multiple potential mechanisms of action contribute to the differential effects observed in cell death, proliferation, and cytokine secretion.
The toxicity of GVHD is difficult to separate from GVL effects, a well recognized benefit of immunotherapy for malignancies (1). Inflammatory cytokines significantly contribute to the toxicity of GVHD but have a more limited role in the eradication of residual leukemia, which is primarily mediated by donor cytotoxic T lymphocytes and NK cells in experimental models by using established tumor cell lines (5). The maintenance of such lines for extended periods in vitro may cause significant shifts in tumor cell biology, and, thus, they may not accurately reflect all aspects of spontaneously occurring malignancies, but such tumor systems have been useful in elucidating effector mechanisms of immunotherapy and GVL activity (13, 14, 28). Thus, SAHA administration in two different tumor models leads to disruption of inflammatory cytokine cascades while maintaining cytotoxic T lymphocytes and thereby successfully separates GVHD and GVL. HDAC inhibitors can reduce the proliferation of transformed cells in vitro and in vivo (9, 29), but at the lower doses used in our study, SAHA did not independently reduce tumor-related mortality in the syngeneic controls even though it increased H3 acetylation (data not shown). Experiments are needed to determine whether the concentrations of SAHA sufficient for antitumor activity will also suppress immunologically mediated GVL effects.
In summary, our findings demonstrate a role for the HDAC inhibitor SAHA in reducing the early inflammatory responses contributing to GVHD while preserving donor T cell responses and GVL effects. Because SAHA is relatively nontoxic and is currently being tested in phase I/II clinical trials (30), it may soon be testable in allogeneic BMT recipients as an antiinflammatory adjunct to standard GVHD prophylaxis and treatment.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants K08 AI052863-01 (to P.R.) and CA 49542 (to J.L.M.F.).
Abbreviations: BM, bone marrow; BMT, bone marrow transplantation; GVHD, graft-versus-host disease; GVL, graft-versus-leukemia; SAHA, suberoylanilide hydroxamic acid; TNF-α, tumor necrosis factor α; HDAC, histone deacetylase; RPA, ribonuclease protection assay.
References
- 1.Appelbaum, F. R. (2001) Nature 411, 385-389. [DOI] [PubMed] [Google Scholar]
- 2.Antin, J. H. & Ferrara, J. L. M. (1992) Blood 80, 2964-2968. [PubMed] [Google Scholar]
- 3.Teshima, T., Ordemann, R., Reddy, P., Gagin, S., Liu, C., Cooke, K. R. & Ferrara, J. L. (2002) Nat. Med. 8, 575-581. [DOI] [PubMed] [Google Scholar]
- 4.Reddy, P. & Ferrara, J. L. (2003) Blood Rev. 17, 187-194. [DOI] [PubMed] [Google Scholar]
- 5.Riddell, S. R., Murata, M., Bryant, S. & Warren, E. H. (2002) Int. J. Hematol. 76, Suppl. 2, 155-161. [DOI] [PubMed] [Google Scholar]
- 6.Thomas, E., Blume, K. & Forman, S. (1999) Hematopoietic Cell Transplantation (Blackwell Science, Malden, MA).
- 7.Roth, S. Y., Denu, J. M. & Allis, C. D. (2001) Annu. Rev. Biochem. 70, 81-120. [DOI] [PubMed] [Google Scholar]
- 8.Marks, P., Rifkind, R. A., Richon, V. M., Breslow, R., Miller, T. & Kelly, W. K. (2001) Nat. Rev. Cancer 1, 194-202. [DOI] [PubMed] [Google Scholar]
- 9.Marks, P. A., Miller, T. & Richon, V. M. (2003) Curr. Opin. Pharmacol. 3, 344-351. [DOI] [PubMed] [Google Scholar]
- 10.Leoni, F., Zaliani, A., Bertolini, G., Porro, G., Pagani, P., Pozzi, P., Dona, G., Fossati, G., Sozzani, S., Azam, T., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 2995-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra, N., Reilly, C. M., Brown, D. R., Ruiz, P. & Gilkeson, G. S. (2003) J. Clin. Invest. 111, 539-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill, G. R., Crawford, J. M., Cooke, K. J., Brinson, Y. S., Pan, L. & Ferrara, J. L. M. (1997) Blood 90, 3204-3213. [PubMed] [Google Scholar]
- 13.Teshima, T., Hill, G., Pan, L., Brinson, Y., van den Brink, M. R., Cooke, K. & Ferrara, J. (1999) J. Clin. Invest. 104, 317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang, Y. G., Sergio, J. J., Pearson, D. A., Szot, G. L., Shimizu, A. & Sykes, M. (1997) Blood 90, 4651-4660. [PubMed] [Google Scholar]
- 15.Reddy, P., Teshima, T., Hildebrandt, G., Williams, D. L., Liu, C., Cooke, K. R. & Ferrara, J. L. (2003) Blood 101, 2877-2885. [DOI] [PubMed] [Google Scholar]
- 16.Ichiba, T., Teshima, T., Kuick, R., Misek, D. E., Liu, C., Takada, Y., Maeda, Y., Reddy, P., Williams, D. L., Hanash, S. M. & Ferrara, J. L. (2003) Blood 102, 763-771. [DOI] [PubMed] [Google Scholar]
- 17.Reddy, P., Teshima, T., Kukuruga, M., Ordemann, R., Liu, C., Lowler, K. & Ferrara, J. L. (2001) J. Exp. Med. 194, 1433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, Y., Louboutin, J. P., Zhu, J., Rivera, A. J. & Emerson, S. G. (2002) J. Clin. Invest. 109, 1335-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, G. R. & Ferrara, J. L. (2000) Blood 95, 2754-2759. [PubMed] [Google Scholar]
- 20.Murphy, W. J., Kumar, V. & Bennett, M. (1987) J. Exp. Med. 166, 1499-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sykes, M. (2001) Immunity 14, 417-424. [DOI] [PubMed] [Google Scholar]
- 22.Holler, E. (2002) Curr. Opin. Hematol. 9, 479-484. [DOI] [PubMed] [Google Scholar]
- 23.Antin, J. H., Weisdorf, D., Neuberg, D., Nicklow, R., Clouthier, S., Lee, S. J., Alyea, E., McGarigle, C., Blazar, B. R., Sonis, S., et al. (2002) Blood 100, 3479-3482. [DOI] [PubMed] [Google Scholar]
- 24.Saemann, M. D., Bohmig, G. A., Osterreicher, C. H., Burtscher, H., Parolini, O., Diakos, C., Stockl, J., Horl, W. H. & Zlabinger, G. J. (2000) FASEB J. 14, 2380-2382. [DOI] [PubMed] [Google Scholar]
- 25.Skov, S., Rieneck, K., Bovin, L. F., Skak, K., Tomra, S., Michelsen, B. K. & Odum, N. (2003) Blood 101, 1430-1438. [DOI] [PubMed] [Google Scholar]
- 26.Alexander, W. S. (2002) Nat. Rev. Immunol. 2, 410-416. [DOI] [PubMed] [Google Scholar]
- 27.Letterio, J. J. & Roberts, A. B. (1998) Annu. Rev. Immunol. 16, 137-161. [DOI] [PubMed] [Google Scholar]
- 28.Edinger, M., Hoffmann, P., Ermann, J., Drago, K., Fathman, C. G., Strober, S. & Negrin, R. S. (2003) Nat. Med. 9, 1144-1150. [DOI] [PubMed] [Google Scholar]
- 29.Marks, P. A., Rifkind, R. A., Richon, V. M. & Breslow, R. (2001) Clin. Cancer Res. 7, 759-760. [PubMed] [Google Scholar]
- 30.Kelly, W. K., Richon, V. M., O'Connor, O., Curley, T., MacGregor-Curtelli, B., Tong, W., Klang, M., Schwartz, L., Richardson, S., Rosa, E., et al. (2003) Clin. Cancer Res. 9, 3578-3588. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.