Abstract
A diseased heart causes numerous adverse effects on kidney function, and vice versa renal disease can significantly impair cardiac function. Beyond these heart-kidney interrelationships at the clinical level, a reciprocal association has been suggested to exist even in the early stages of those organs' dysfunction. The aim of the present review is to provide evidence of the presence of a preclinical cardiorenal syndrome in the particular setting of essential hypertension, focusing on the subsequent hypertensive sequelae on heart and kidneys. In particular, a plethora of studies have demonstrated not only the predictive role of kidney damage, as expressed by either decreased glomerular filtration or increased urine albumin excretion, for adverse left ventricular functional and structural adaptations but also preclinical heart disease, i.e. left ventricular hypertrophy that is associated with deterioration of renal function. Notably, these reciprocal interactions seem to exist even at the level of microcirculation, since both coronary flow reserve and renal hemodynamics are strongly related with clinical and preclinical renal and cardiac damage, respectively. In this preclinical setting, common pathophysiological denominators, including the increased hemodynamic load, sympathetic and renin-angiotensin system overactivity, increased subclinical inflammatory reaction, and endothelial dysfunction, account not only for the reported associations between overt cardiac and renal damage but also for the parallel changes that occur in coronary and renal microcirculation.
Key Words : Cardiorenal syndrome, Hypertension, Preclinical organ damage
Introduction
Arthur Guyton was the first to extensively describe normal physiological interactions between the control of extracellular fluid volume by the kidney and systemic circulation by the heart. A diseased heart has numerous adverse effects on kidney function, while in parallel, renal disease can significantly impair cardiac function [1].
The so-called ‘cardiorenal syndrome’ is defined as a pathophysiological disorder of the heart and the kidneys in which acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ [2]. Beyond these interrelationships between the heart and the kidneys at the clinical level, a reciprocal association has been suggested to underlie even in the early stages of those organs’ dysfunction. The aim of the present review is to report evidence of the presence of a ‘preclinical cardiorenal syndrome’ in the particular setting of essential hypertension, focusing on the subsequent hypertensive sequelae on heart and kidneys.
Hypertension and Cardiorenal Disease: Epidemiological Data
Hypertension affects nowadays approximately 25% of the global adult population [3] and constitutes a major risk factor that accounts for 54% of the strokes and 47% of events due to ischemic heart disease worldwide [4]. Furthermore, hypertension is both a common cause and complication of chronic kidney disease (CKD). In particular, hypertension is the second leading cause of CKD (second only to diabetes) and is present in up to 80% of individuals with moderate-to-end-stage kidney disease [5].
Beyond end-stage renal disease, CKD, defined by the National Kidney Foundation as the presence of kidney damage or decreased level of kidney function for at least 3 months, is a worldwide public health problem with a rising incidence and prevalence [6]. Currently, over 26 million American adults (approximately 17% of the adult population) have CKD, which is staged according to the glomerular filtration rate (GFR) [7].
Hypertension as a Risk Factor for Subclinical Cardiac Damage
According to the current European Society of Hypertension guidelines, the presence of left ventricular hypertrophy (LVH) constitutes the most established index of hypertensive damage [8,9]. Although the relation between left ventricular mass index and cardiovascular risk is continuous, thresholds of 125 g/m2 for men and 110 g/m2 for women are widely used for conservative estimates of echocardiographic LVH. Concentric hypertrophy (wall-to-radius ratio ≥0.42 in the presence of LVH), eccentric hypertrophy (increased left ventricular mass with a wall-to-radius ratio <0.42), and concentric remodeling (wall-to-radius ratio ≥0.42 with a normal left ventricular mass) all predict an increased incidence of cardiovascular disease, but concentric hypertrophy has consistently been shown to be the condition which most markedly increases the risk [8,9]. Analysis of the data provided by some of the major prospective studies indicates that in hypertensive patients, echocardiographic LVH, particularly of the concentric variety, is associated with an incidence of cardiovascular events ≥20% in 10 years [9,10]. In a prospective study of 1,652 Greek hypertensive patients followed for 6 years, we demonstrated that echocardiographic LVH was significantly associated with either a composite of all-cause mortality and cardiovascular events (hazard ratio 1.53) or with stroke (hazard ratio 2.01), after adjustment for major cardiovascular risk factors [10].
LVH as a Predictor of Renal Outcome
New data expanded the predictive value of LVH for cardiovascular events to renal end points providing further insights into the pathophysiological connections between the heart and the kidneys [11]. After a follow-up of 14 years in a very large number of patients, a striking elevation in the risk of all the considered renal outcomes (doubling of serum creatinine, an estimated GFR of <30 min/1.73 m2 at follow-up or end-stage renal disease) was observed in patients with LVH. Interestingly, it was shown that concentric LVH, as compared to eccentric LVH, is associated with the worst renal outcomes, in concordance with the unfavorable effect of left ventricular concentric geometry.
In a cohort of 428 untreated essential hypertensives, an increased albumin-to-creatinine ratio (ACR) irrespective of the level of microalbuminuria (MA), in conjunction with pronounced arterial stiffness, is accompanied by augmented left ventricular mass and higher LVH rates [12]. An independent association between GFR estimated either through the MDRD formula or creatinine clearance and left ventricular mass, indexed either for body surface area or for height2.7, was also exhibited in a group of nondiabetic hypertensives free of cardiovascular diseases [13].
In a more recent study by Andrikou et al. [14], the baseline left ventricular mass index was qualified as an accelerator of kidney damage by means of MA in patients with essential hypertension. One of the novel findings of this study is that the baseline left ventricular mass index turned out to be a significant predictor of new-onset MA over a period of >3 years. In addition, the reduction of the left ventricular mass index proved to be a significant negative predictor of MA development, as it reflected an almost halving risk of MA incidence [14]. Therefore, the already established predictive value of left ventricular mass index reduction on cardioprotection is extended to a decreased risk of MA development.
Hypertension as a Risk Factor for Subclinical Renal Damage
According to the current European Society of Hypertension guidelines, diagnosis of hypertension-related renal damage is based on a reduced renal function or an elevated urinary excretion of albumin [8]. Both features of expressing renal disease incur without symptoms and their identification is based solely on the measurement of either serum creatinine or urine albumin excretion [8]. Towards a step further, estimation of the GFR (through the MDRD formula, requiring age, gender, and race) or creatinine clearance (through the Cockroft-Gault formula, requiring also body weight) constitutes a routine procedure. Accordingly, urinary albumin excretion should be measured in all hypertensives in spot urine and related to urinary creatinine excretion [8].
Renal insufficiency is now classified according to the estimated GFR calculated by the abbreviated MDRD formula [15]. Values of estimated GFR <60 ml/min/1.73 m2 indicate CKD stage 3, whilst values <30 and 15 ml/min/1.73 m2 indicate CKD stages 4 and 5, respectively [6]. The Cockcroft-Gault formula is valid in the range >60 ml/min, but it overestimates creatinine clearance in CKD stages 3-5 [6].
Low GFR Predicting Cardiovascular Disease
It is well established that >50% of deaths in end-stage renal disease cohorts are attributed to cardiovascular disease, while even less severe forms of CKD (stage 3) may be associated with significant cardiovascular risk, documenting an adverse relationship between renal function and adverse outcome [16]. In the setting of hypertensive patients, an association between reduced renal function and cardiovascular events based on serum creatinine concentration has been previously demonstrated [17]. ALLHAT participants with a moderate-to-severe reduction in GFR (CKD stages 3-4) exhibited higher 6-year rates for coronary heart disease than for end-stage renal disease (15.4 vs. 6.0%, respectively). In particular, a baseline GFR of <53 ml/min/1.73 m2 was independently associated with a 32% higher risk of coronary heart disease [18]. Furthermore, after an average 8.8-year post-trial follow-up, total mortality was significantly higher in participants with a moderate-to-severe GFR reduction compared to those with a normal and mildly reduced GFR [19]. Based on the VALUE trial, the risk in individuals with CKD stage 3 at baseline was again greater than in those with better renal function; however, GFR calculated with the MDRD formula turned out to be more informative than the estimated creatinine clearance (Cockcroft-Gault) in the prediction of cardiovascular outcomes [20].
In line with these findings, we have verified that asymptomatic renal involvement in a large cohort of hypertensive individuals without overt cardiovascular disease is associated with a very high risk of future cardiovascular events [10]. Interestingly, separate analysis in order to compare the prognostic value of CKD stage 3 for cardiovascular outcomes, based either on estimated GFR (MDRD formula) or estimated creatinine clearance (Cockcroft-Gault), did not exhibit any significant differences in the same study [10].
Urine Albumin Excretion Predicting Cardiovascular Disease
While an elevated serum creatinine concentration points to a reduced rate of glomerular filtration, an increased rate of urinary albumin or protein excretion points to a derangement in the glomerular filtration barrier. The presence of overt proteinuria generally indicates the existence of an established renal parenchymatous damage [21]. Focusing on an asymptomatic preclinical level, MA even below the threshold values currently considered [8], has been shown to predict cardiovascular events in both diabetic and nondiabetic hypertensive patients [21,22]. Furthermore, a continuous relationship between cardiovascular, as well as noncardiovascular, mortality and urinary ACR ≥3.9 mg/g in men and 7.5 mg/g in women has been reported in several studies [23,24]. Additionally, in high-risk individuals participating in the HOPE study, it was indicated that any degree of albuminuria is a risk factor for cardiovascular events and that the risk increases with the ACR, starting well below the MA cutoff [25].
In the I-SEARCH study, a recent large cross-sectional study, the relationship between the number of cardiovascular comorbidities and the prevalence of MA was investigated in 21,867 high-risk hypertensives [26]. One third of those patients (32%) suffered from at least one comorbidity, and the prevalence of MA increased from 54% in patients without cardiovascular comorbidity to 74% in patients with ≥3 comorbidities. Particularly, the presence of ≥3 cardiovascular comorbidities nearly doubled the risk of MA, while patients with LVH had the highest prevalence of MA (68%). Additionally, Tsioufis et al. [27] have demonstrated an association between MA and unfavorable cardiac geometric adaptations in essential hypertensive subjects. Furthermore, MA has been shown to be a marker of preclinical diastolic dysfunction in never-treated essential hypertensives [28]. Likewise, it has also been shown than LVH is highly prevalent in hypertensive patients with CKD stages 2-5. In this population, LVH is often inappropriate and characterized by the simultaneous increase of wall thicknesses and diameters [29].
In general, the finding of impaired renal function in a hypertensive patient, expressed as any of the abnormalities mentioned above, is frequent and constitutes a very potent predictor of future cardiovascular events and death even in treated patients [8]. There are also some data showing that normalization of MA might reflect a trend for reduction in cardiovascular events and that treatment with albuminuria-lowering properties is associated with beneficial clinical outcome [30,31]. Specifically, in a LIFE sub-study it was shown that changes in ACR during antihypertensive treatment over time translated to changes in risk of cardiovascular morbidity and mortality irrespective of the in-treatment level of blood pressure [32]. However, ONTARGET showed that in patients with increased cardiovascular risk, the reduction of MA was not accompanied by nephroprotection [33]. On the contrary, no studies have specifically assessed the value of any kind of normalization of mild asymptomatic renal impairment (stage 3 to stage 2 or 1), while in a recent study persistence/development of CKD stage 3 from baseline to follow-up was identified as an independent predictor of cardiovascular events (adjusted hazard ratio 1.94) in hypertensive patients free of cardiovascular disease [34].
Parallel Changes in Cardiac and Renal Microcirculation
Predictive Role of Coronary Circulation in Renal Damage
Research on coronary flow reserve (CFR), an index of coronary microvascular dysfunction, has shown that it is usually impaired in various clinical settings, including hypertension, despite the presence of angiographically normal coronary arteries [35]. Reduced CFR is associated with an increased left ventricular mass, abnormal left ventricular geometry and diastolic function in essential hypertension [36,37].
Beyond these direct associations at the level of the heart, impaired CFR, through invasive assessment, was shown in patients with reduced renal function and nonobstructive coronary artery disease [38]. Specifically, it was Ragosta [38] who first used a FloWire for direct measurement of CFR, demonstrating significantly decreased CFR values in patients with diabetic nephropathy compared to diabetics and those with normal renal function (1.6 vs. 2.7 vs. 2.8, respectively). Additionally, the results of the so far largest study using invasive measurement of CFR demonstrated significantly increased CFR values in patients with normal renal function compared to those with CKD stage 3 (3.0 ± 0.8 vs. 2.6 ± 0.6, p < 0.001, respectively) [39]. Accordingly, when CFR was estimated by transthoracic echocardiography, lower values were exhibited in patients with end-stage renal disease as compared to kidney transplant patients and controls [40]. Towards the same direction, lower values of transthoracically measured CFR were identified in patients with nephrotic syndrome [41].
In the setting of essential hypertension, Bezante et al. [42] studied 76 newly diagnosed hypertensive patients by transthoracic echocardiography for CFR estimation. Impaired CFR, based on a cutoff value of 2, was present in 10% of the study population. These patients demonstrated also a higher prevalence of CKD stage 3 as well as of MA. Notably, a low GFR turned out to be an independent predictor of the presence of a low CFR [42]. Recently, we have provided evidence of a significant and independent association between ACR levels and CFR values in a cohort of asymptomatic untreated essential hypertensives [43]. In our study, CFR was measured invasively through FloWire and using a cutoff value of 2.5. It was demonstrated that patients with impaired CFR had also greater ACR levels and a higher prevalence of MA. Moreover, according to a ROC analysis, a value of ACR >13 mg/g (at levels lower than the MA definition) was accompanied by impaired CFR (<2.5), providing high sensitivity (77.3%) and specificity (86.7%) [43].
Focusing on the relationship between structure of the coronary epicardial vessels and renal damage, it has been suggested that increased urine albumin excretion is associated not only with coronary artery calcification but also with impaired endothelium-dependent dilatation of the coronary arteries in patients with type 2 diabetes mellitus [44,45]. In particular, Yamagami et al. [44] in 2005 studied calcification of coronary arteries and aorta in 177 diabetics (106 with normoalbuminuria and 71 with MA) and in 79 nondiabetic patients. Coronary calcium score was significantly increased in diabetics as compared to those without diabetes, while calcification was even more pronounced in diabetics with MA. An independent association was observed between MA and coronary calcification in this study, while no difference occurred between the study groups as regards calcification of the aorta. Of great importance is also the study conducted in 2006 by Cosson et al. [45], aiming to investigate the relationship of MA with endothelium-dependent (by cold pressor testing) and endothelium-independent (through intracoronary administration of nitrates) vasodilatation of the coronary arteries in 84 patients with type 2 diabetes mellitus. While there was no difference between diabetics with and those without MA regarding endothelium-independent vasodilatation of the coronary arteries, endothelium-dependent vasodilatation was significantly impaired in diabetic patients with MA.
Predictive Role of Renal Circulation in Cardiac Damage
Renal Doppler sonography permits noninvasive assessment of intrarenal hemodynamics in addition to evaluation of anatomic information. Intrarenal arterial waveforms recorded by Doppler sonography have been widely used to evaluate renal function. Previous studies have explored the capacity of the resistive index calculated from blood flow velocity in renal vessels to predict the progression of renal function in patients with hypertension, diabetes mellitus, or chronic nephropathy [46]. In addition, histological studies demonstrated that the renal resistive index not only reflects changes in intrarenal perfusion and renovascular resistance but also was increased in several pathological conditions, such as renal atherosclerosis and tubulointerstitial damage [47].
In studies conducted in patients with essential hypertension, a higher renal resistive index is associated with carotid wall thickening, LVH, and albuminuria, regardless of other known cardiovascular risk factors [48]. Raff et al. [49] also reported the clinical utility of the measurement of the renal resistive index in addition to albuminuria to evaluate target organ damage in therapy-resistant hypertension.
As regards the prognostic value of the renal resistive index, initially this has been examined in chronic nephropathy, elderly, or heart failure patients. Recently, in a cohort of 426 essential hypertensives without previous cardiovascular disease, it was demonstrated that there is a significant relationship between a high renal resistive index and cardiovascular and renal outcomes (including all-cause death, myocardial infarction, stroke, congestive heart failure requiring hospitalization, aortic dissection, and end-stage renal failure requiring regular hemodialysis) which persists after multivariate Cox regression analysis, including traditional risk factors [50]. Moreover, even in the subgroup with a GFR <60 ml/min per 1.73 m2, a high renal resistive index was a significant predictor of the primary composite end points. The combination of high renal resistive index and low GFR constitutes a powerful independent predictor of worse outcome [50].
Pathophysiologic Mechanisms Underlying Preclinical Cardiorenal Damage
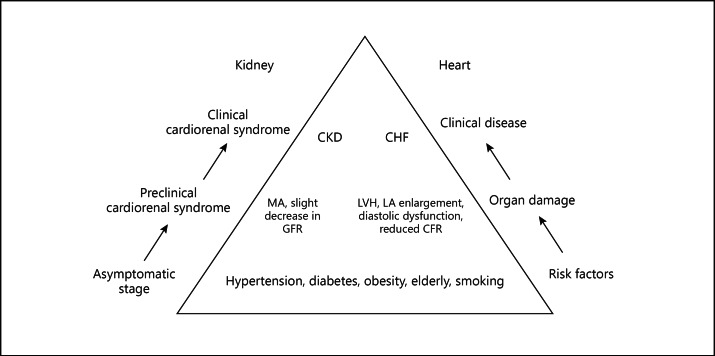
The most obvious underlying mechanism that may explain either the parallel progression of cardiac and renal damage or the unfavorable interaction between these two vital organs is the fact that traditional cardiovascular risk factors act adversely towards the heart and the kidneys. Prolonged exposure to these risk factors, namely hypertension and diabetes, affect both organs and induce development of LVH and renal disease, i.e. MA and/or CKD (fig. 1).
Fig. 1.
Common pathophysiological denominators affect in parallel cardiac and renal function and structure both at the preclinical setting and at expression of overt disease. LA = Left atrial.
The diagnosis of hypertension does not occur in isolation for most patients with a high blood pressure. Indeed, hypertension is typically found alongside other well-known cardiac risk factors, including glucose intolerance, obesity and dyslipidemia. Furthermore, <15% of coronary events in hypertensive patients occur in the absence of additional risk factors [51]. As noted earlier, patients with CKD are far more likely to die of cardiovascular disease than to progress to end-stage renal disease [6,16]. This increased risk is often attributed to a litany of traditional cardiovascular risk factors – hypertension, diabetes, tobacco abuse, and ad-vanced age – that frequently accompany reduced renal function. Among patients with CKD, the presence of hypertension increases the risk of new or recurrent cardiovascular events by about twofold. Of the traditional cardiac risk factors, only diabetes appears to confer more of an increased risk (about threefold) [52].
Common pathophysiological denominators that characterize classical risk factors and modulate the latter relationship include the increased hemodynamic load, sympathetic and renin-angiotensin system overactivity, increased subclinical inflammatory reaction, endothelial dysfunction and production of reactive oxygen species, that in combination or separately induce fibrotic phenomena to the heart and the kidneys [18,53,54]. These mechanisms seem to account not only for the reported associations between ACR, GFR, and cardiovascular adaptations (i.e. LVH, left ventricular geometry and aortic stiffness) but also between ACR and abnormal exercise parameters in untreated hypertensives, such as peak exercise systolic blood pressure and exercise capacity [55], or for the parallel changes that occur in coronary and renal microcirculation as reflected by the association between ACR and CFR [43].
Besides these common underlying mechanisms that affect in parallel cardiac and renal function and structure, in recent years, evidence has accumulated supporting a role for elevated renal venous pressure and intra-abdominal pressure in the development of renal dysfunction in patients with heart failure [56]. Accordingly, the ESCAPE trial found a significant relation between right atrial pressure measured during pulmonary artery catheterization and serum creatinine, indicating the important role of renal congestion [57]. In patients with LVH, and in particular in the presence of concentric geometry, a more severe impairment of the diastolic function and an increase in the left ventricular filling pressures may contribute to a more rapid decline of the renal function. Another proposed mechanism is the progressive reduction in renal blood flow in patients with LVH, particularly in those with concentric geometry, accompanying a hemodynamic pattern with reduced stroke volume and increased peripheral resistance. Recent data indicate that the media-to-lumen ratio of small resistance arteries in hypertensive patients is not only related with indices of renal function, but also predicts changes in renal function over time [58].
Conclusion
The definition and the classification of the cardiorenal syndrome in 2008 by Ronco et al. [2] represent a great achievement, despite the existing debate on how precise and realistic this terminology is. This complex reciprocal association between the heart and the kidneys starts from the very early stages contributing to the appearance of what we named ‘preclinical cardiorenal syndrome’ at least, but not limited, in hypertensive patients. A large number of direct and indirect effects of each organ's subclinical dysfunction can initiate and perpetuate the combined disorder of both organs through a complex interplay of neurohumoral feedback mechanisms. A multidisciplinary approach towards new diagnostic, preventive and therapeutic strategies for hypertensive patients suffering from combined disorders of the heart and the kidneys even at the subclinical level should be initiated.
References
- 1.Berl T, Henrich W. Kidney-heart interactions: epidemiology, pathogenesis, and treatment. Clin J Am Soc Nephrol. 2006;1:8–18. doi: 10.2215/CJN.00730805. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. http://www.ncbi.nlm.nih.gov/pubmed/17563527. [DOI] [PubMed] [Google Scholar]
- 9.Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 10.Tsioufis C, Vezali E, Tsiachris T, Dimitriadis K, Taxiarchou E, Chatzis D, Thomopoulos C, Syrseloudis D, Stefanadi E, Mihas C, Katsi V, Papademetriou V, Stefanadis C. Left ventricular hypertrophy versus kidney disease as predictors of cardiovascular events in hypertension: a Greek 6 years follow-up study. J Hypertens. 2009;27:744–752. doi: 10.1097/HJH.0b013e32832401ff. [DOI] [PubMed] [Google Scholar]
- 11.Tsioufis C, Kokkinos P, MacManus C, Thomopoulos C, Faselis C, Doumas M, Stefanadis C, Papademetriou V. Left ventricular hypertrophy as a determinant of renal outcome in patients with high cardiovascular risk. J Hypertens. 2010;28:2299–2308. doi: 10.1097/HJH.0b013e32833d95fe. [DOI] [PubMed] [Google Scholar]
- 12.Andrikou E, Tsioufis C, Dimitriadis K, Flessas D, Chatzistamatiou V, Grassos C, Papavasiliou M, Papadopoulos D, Stefanadis C. Parallel deterioration of albuminuria, arterial stiffness and left ventricular mass in essential hypertension: integrating target organ damage. Nephron Clin Pract. 2011;119:c27–c34. doi: 10.1159/000324215. [DOI] [PubMed] [Google Scholar]
- 13.Cerasola G, Nardi E, Mulè G, Palermo A, Cusimano P, Guarneri M, Arsena R, Giammarresi G, Carola Foraci A, Cottone S. Left ventricular mass in hypertensive patients with mild-to-moderate reduction of renal function. Nephrology (Carlton) 2010;15:203–210. doi: 10.1111/j.1440-1797.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- 14.Andrikou E, Tsioufis C, Thomopoulos C, Andrikou I, Kasiakogias A, Leontsinis I, Kordalis A, Katsimichas T, Tousoulis D, Stefanadis C. Left ventricular mass index as a predictor of new-onset microalbuminuria in hypertensive dubjects: a prospective study. Am J Hypertens. 2012;25:1195–1201. doi: 10.1038/ajh.2012.109. [DOI] [PubMed] [Google Scholar]
- 15.Hallan S, Asberg A, Lindberg M, Johnsen H. Validation of the modification of diet in renal disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am J Kidney Dis. 2004;44:84–93. doi: 10.1053/j.ajkd.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 17.Schillaci G, Reboldi G, Verdecchia P. High-normal serum creatinine concentration is a predictor of cardiovascular risk in essential hypertension. Arch Intern Med. 2001;161:886–891. doi: 10.1001/archinte.161.6.886. [DOI] [PubMed] [Google Scholar]
- 18.Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT, Jr, Whelton PK, Barzilay J, Batuman V, Eckfeldt JH, Farber MA, Franklin S, Henriquez M, Kopyt N, Louis GT, Saklayen M, Stanford C, Walworth C, Ward H, Wiegmann T. ALLHAT Collaborative Research Group: Cardiovascular outcomes in high-risk hypertensive patients stratified by baseline glomerular filtration rate. Ann Intern Med. 2006;144:172–180. doi: 10.7326/0003-4819-144-3-200602070-00005. [DOI] [PubMed] [Google Scholar]
- 19.Rahman M, Ford CE, Cutler JA, Davis BR, Piller LB, Whelton PK, Wright JT, Jr, Barzilay JI, Brown CD, Colon PJ Sr, Fine LJ, Grimm RH, Jr, Gupta AK, Baimbridge C, Haywood LJ, Henriquez MA, Ilamaythi E, Oparil S, Preston R. ALLHAT Collaborative Research Group: Long-term renal and cardiovascular outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants by baseline estimated GFR. Clin J Am Soc Nephrol. 2012;7:989–1002. doi: 10.2215/CJN.07800811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruilope LM, Zanchetti A, Julius S, McInnes GT, Segura J, Stolt P, Hua TA, Weber MA, Jamerson K. VALUE Investigators: Prediction of cardiovascular outcome by estimated glomerular filtration rate and estimated creatinine clearance in the high-risk hypertension population of the VALUE trial. J Hypertens. 2007;25:1473–1479. doi: 10.1097/HJH.0b013e328133246c. [DOI] [PubMed] [Google Scholar]
- 21.Tsioufis C, Dimitriadis K, Antoniadis D, Stefanadis C, Kallikazaros I. Inter-relationships of microalbuminuria with the other surrogates of the atherosclerotic cardiovascular disease in hypertensive subjects. Am J Hypertens. 2004;17:470–476. doi: 10.1016/j.amjhyper.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Jensen JS, Feldt-Rasmussen B, Strandgaard S, Schroll M, Borch-Johnsen K. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35:898–903. doi: 10.1161/01.hyp.35.4.898. [DOI] [PubMed] [Google Scholar]
- 23.Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D'Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 24.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE. Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group: Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 25.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. HOPE Study Investigators: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 26.Mahfoud F, Ukena C, Pöss J, Bramlage P, Volpe M, Thoenes M, Schmieder R, Böhm M. Microalbuminuria independently correlates to cardiovascular comorbidity burden in patients with hypertension. Clin Res Cardiol. 2012;101:761–766. doi: 10.1007/s00392-012-0459-8. [DOI] [PubMed] [Google Scholar]
- 27.Tsioufis C, Stefanadis C, Toutouza M, Kallikazaros I, Toutouzas K, Tousoulis D, Pitsavos C, Papademetriou V, Toutouzas P. Microalbuminuria is associated with unfavourable cardiac geometric adaptations in essential hypertensive subjects. J Hum Hypertens. 2002;16:249–254. doi: 10.1038/sj.jhh.1001379. [DOI] [PubMed] [Google Scholar]
- 28.Grandi AM, Santillo R, Bertolini A, Imperiale D, Broggi R, Colombo S, Selva E, Jessula A, Guasti L, Venco A. Microalbuminuria as a marker of preclinical diastolic dysfunction in never-treated essential hypertensives. Am J Hypertens. 2001;14:644–648. doi: 10.1016/s0895-7061(01)01305-x. [DOI] [PubMed] [Google Scholar]
- 29.Nardi E, Palermo A, Mule G, Cusimano P, Cottone S, Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertension. 2009;27:633–641. doi: 10.1097/HJH.0b013e3283220ecd. [DOI] [PubMed] [Google Scholar]
- 30.Schrader J, Luders S, Kulschewski A, Hammersen F, Zuchner C, Venneklaas U, Schrandt G, Schnieders M, Rangoonwala B, Berger J, Dominiak P, Zidek W. MARPLE Study Group: Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: final results of a prospective long-term study (MARPLE Study) J Hypertens. 2006;24:541–548. doi: 10.1097/01.hjh.0000209991.48928.c4. [DOI] [PubMed] [Google Scholar]
- 31.Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, de Zeeuw D, de Jong PE, van Veldhuisen DJ, van Gilst WH. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 32.Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlöf B, Devereux RB, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wan Y. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 33.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 34.Salvetti M, Muiesan ML, Paini A, Monteduro C, Agabiti-Rosei C, Aggiusti C, Bertacchini F, Stassaldi D, Castellano M, Agabiti-Rosei E. Left ventricular hypertrophy and renal dysfunction during antihypertensive treatment adversely affect cardiovascular prognosis in hypertensive patients. J Hypertens. 2012;30:411–420. doi: 10.1097/HJH.0b013e32834e90d8. [DOI] [PubMed] [Google Scholar]
- 35.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 36.Galderisi M, Cicala S, Caso P, De Simone L, D'Errico A, Petrocelli A, de Divitiis O. Coronary flow reserve and myocardial diastolic dysfunction in arterial hypertension. Am J Cardiol. 2002;90:860–864. doi: 10.1016/s0002-9149(02)02708-x. [DOI] [PubMed] [Google Scholar]
- 37.Galderisi M, de Simone G, Cicala S, De Simone L, D'Errico A, Caso P, de Divitiis O. Coronary flow reserve in hypertensive patients with appropriate or inappropriate left ventricular mass. J Hypertens. 2003;21:2183–2188. doi: 10.1097/00004872-200311000-00029. [DOI] [PubMed] [Google Scholar]
- 38.Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ, Powers ER. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J. 2004;147:1017–1023. doi: 10.1016/j.ahj.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 39.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int. 2006;69:266–271. doi: 10.1038/sj.ki.5000031. [DOI] [PubMed] [Google Scholar]
- 40.Caliskan Y, Oflaz H, Demirturk M, Yazici H, Turkmen A, Cimen A, Elitok A, Yildiz A. Coronary flow reserve dysfunction in hemodialysis and kidney transplant patients. Clin Transplant. 2008;22:785–793. doi: 10.1111/j.1399-0012.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 41.Oflaz H, Sen F. Reduced Coronary Flow Reserve and Early Diastolic Filling Abnormalities in Patients with Nephrotic Syndrome. Renal Failure. 2008;30:914–920. doi: 10.1080/08860220802353819. [DOI] [PubMed] [Google Scholar]
- 42.Bezante GP, Viazzi F, Leoncini G, Ratto E, Conti N, Balbi M, Agosti S, Deferrari L, Deferrari G, Pontremoli R. Coronary flow reserve is impaired in hypertensive patients with subclinical renal damage. Am J Hypertens. 2009;22:191–196. doi: 10.1038/ajh.2008.351. [DOI] [PubMed] [Google Scholar]
- 43.Tsiachris D, Tsioufis C, Dimitriadis K, Syrseloudis D, Rousos D, Kasiakogias A, Papademetriou V, Tousoulis D, Stefanadis C. Relation of impaired coronary microcirculation to increased urine albumin excretion in patients with systemic hypertension and no epicardial coronary arterial narrowing. Am J Cardiol. 2012;109:1026–1030. doi: 10.1016/j.amjcard.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 44.Yamagami K, Hosoi M, Yamamoto T, Fukumoto M, Yamakita T, Miyamoto M, Yoshioka K, Ishii T, Sato T, Tanaka S, Fujii S. Coronary arterial calcification is associated with albuminuria in type 2 diabetic patient. Diabetes Obes Metab. 2005;7:390–396. doi: 10.1111/j.1463-1326.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 45.Cosson M, Pham I, Valenci P, Paries J, Attali JR, Nitenberg A. Impaired coronary endothelium-dependent vasodilation is associated with microalbuminuria in patients with type 2 diabetes and angiographically normal coronary arteries. Diabetes Care. 2006;29:107–112. doi: 10.2337/diacare.29.1.107. [DOI] [PubMed] [Google Scholar]
- 46.Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699–703. doi: 10.1161/hy0202.103782. [DOI] [PubMed] [Google Scholar]
- 47.Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H, Suzuki S, Miura S. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis. 2005;46:603–609. doi: 10.1053/j.ajkd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Tedesco MA, Natale F, Mocerino R, Tassinario G, Calabrò R. Renal resistive index and cardiovascular organ damage in a large population of hypertensive patients. J Hum Hypertens. 2007;21:291–296. doi: 10.1038/sj.jhh.1002145. [DOI] [PubMed] [Google Scholar]
- 49.Raff U, Schmidt BM, Schwab J, Schwarz TK, Achenbach S, Bär I, Schmieder RE. Renal resistive index in addition to low-grade albuminuria complements screening for target organ damage in therapy-resistant hypertension. J Hypertens. 2010;28:608–614. doi: 10.1097/HJH.0b013e32833487b8. [DOI] [PubMed] [Google Scholar]
- 50.Doi Y, Iwashima Y, Yoshihara F, Kamide K, Hayashi S, Kubota Y, Nakamura S, Horio T, Kawano Y. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension. 2012;60:770–777. doi: 10.1161/HYPERTENSIONAHA.112.196717. [DOI] [PubMed] [Google Scholar]
- 51.Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13:3S–10S. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 52.Rucker D, Tonelli M. Cardiovascular risk and management in chronic kidney disease. Nat Rev Nephrol. 2009;5:287–296. doi: 10.1038/nrneph.2009.42. [DOI] [PubMed] [Google Scholar]
- 53.Tsioufis C, Dimitriadis K, Andrikou E, Thomopoulos C, Tsiachris D, Stefanadi E, Mihas C, Miliou A, Papademetriou V, Stefanadis C. ADMA, C-reactive protein, and albuminuria in untreated essential hypertension: a cross-sectional study. Am J Kidney Dis. 2010;55:1050–1059. doi: 10.1053/j.ajkd.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Dimitriadis K, Tsioufis C, Selima M, Tsiachris D, Miliou A, Kasiakogias A, Andrikou E, Tousoulis D, Stefanadis C. Independent association of circulating resistin with glomerular filtration rate in the early stages of essential hypertension. J Hum Hypertens. 2009;23:668–673. doi: 10.1038/jhh.2009.12. [DOI] [PubMed] [Google Scholar]
- 55.Tsiachris D, Tsioufis C, Syrseloudis D, Thomopoulos C, Mpafakis I, Michaelides A, Redon J, Stefanadis C. Impaired exercise tolerance is associated with increased urine albumin excretion in the early stages of essential hypertension. Eur J Cardiovasc Prev Rehabil. 2012;19:452–459. doi: 10.1177/1741826711402739. [DOI] [PubMed] [Google Scholar]
- 56.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 57.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions-insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 58.Boari GE, Rizzoni D, De Ciuceis C, Porteri E, Avanzi D, Platto C, Mazza M, Brignani A, Rosei CA, Ricotta D, Caimi L, Rosei EA. Structural alterations in subcutaneous small resistance arteries predict changes in the renal function of hypertensive patients. J Hypertens. 2010;28:1951–1958. doi: 10.1097/HJH.0b013e32833c2177. [DOI] [PubMed] [Google Scholar]