Abstract
Purpose.
Frequency monitoring of age-related macular degeneration (AMD) and diabetic retinopathy (DR) is crucial for timely intervention. This study evaluated a handheld shape discrimination hyperacuity (hSDH) test iPhone app designed for visual function self-monitoring in patients with AMD and DR.
Methods.
One hundred subjects (27 visually normal, 37 with AMD, and 36 with DR) were included based on clinical documentation and visual acuity of 20/100 or better. The hSDH test was implemented on the iOS platform. A cross-sectional study was conducted to compare the hSDH test with a previously established desktop SDH (dSDH) test and to assess the effect of disease severity on the hSDH test. A user survey was also conducted to assess the usability of the hSDH test on the mobile device.
Results.
The hSDH test and dSDH test were highly correlated (r = 0.88, P < 0.0001). Bland-Altman analysis indicated no significant difference in hSDH and dSDH measurements. One-way ANOVA indicated that the mean hSDH measurement of the eyes with advanced AMD (n = 16) or with severe to very severe nonproliferative DR (NPDR) (n = 12) was significantly worse than that of the eyes with intermediate AMD (n = 11) or with mild to moderate NPDR (n = 11) (P < 0.0001). Ninety-eight percent of 46 patients (10 with AMD and 36 with DR) who completed the usability survey reported that the hSDH test was easy to use.
Conclusions.
This study demonstrated that the hSDH test on a mobile device is comparable to PC-based testing methods. As a mobile app, it is intuitive to use, readily accessible, and sensitive to the severity of maculopathy. It has the potential to provide patients having maculopathy with a new tool to monitor their vision at home.
Keywords: age-related macular degeneration, diabetic retinopathy, shape discrimination, visual acuity, remote vision self-testing
This study demonstrated that a handheld shape discrimination hyperacuity test is intuitive to use, low cost, and sensitive to the severity of maculopathy. This test implemented on a mobile device has the potential to provide patients having maculopathy with a new tool to monitor their vision at home.
Introduction
With effective therapies available for maculopathy such as exudative age-related macular degeneration (AMD)1–3 and diabetic retinopathy (DR),4–7 timely detection of the onset of treatable disease conditions5,8,9 and frequent monitoring of changes in treatable disease conditions2 become crucial for successful, cost-effective intervention.10,11 For instance, it has been shown that many regimens and lifestyle changes could prevent further decline of vision if AMD is treated at an early stage.1,12–14 Landmark DR trials demonstrated that early detection of proliferative changes in the retina would prompt earlier laser treatment and lead to a 90% reduction in severe vision loss from proliferative DR (PDR) and a 50% reduction in diabetic macular edema within 5 years.4,5 A more recent study7 showed that intraocular injections of ranibizumab provided benefit for patients with diabetic macular edema for at least 2 years, and when combined with focal or grid laser treatments, the amount of residual edema was reduced, as well as the frequency of injections needed to control edema.
Unfortunately, even with the current standard of care, many patients with high-risk AMD15 or DR have eye examinations less than once a year on average because of barriers such as cost and time commitment.16,17 The standard of care for monitoring eye disease still depends on the patient visiting the professional caregiver (e.g., an ophthalmologist). However, how often a patient can be seen by a physician is limited because of costs and shortages of eye care professionals.17–19 For instance, according to workforce analyses, each ophthalmologist in the United States would have to work an additional 4 to 6 hours per week to examine every patient with diabetes on an annual basis.17,20 On the other hand, the monthly treatment regimen for patients under active treatment that is suggested by the original ranibizumab clinical trials1,3 is often difficult to be adopted by patients or physicians in practice. Subsequently, a “treat and extend” regimen21 or an as-needed regimen2 was proposed to obtain vision improvement comparable to the original monthly injection protocol. Once the disease condition is stabilized with monthly injections, the interval to the next visit could be extended to 8 weeks or longer. Significant changes in disease conditions that occur between scheduled office visits may not be detected in time, and the patient may lose the optimal window of opportunity for the most effective treatment.
Hence, there is an increasing need for a new paradigm in patient care that involves remote monitoring of disease conditions to facilitate the management of patients with maculopathy. Recent advances in ophthalmic diagnostics have almost exclusively been focused on digital imaging technologies such as fundus photography, optical coherence tomography (OCT), and scanning laser ophthalmoscopy. However, these technologies are too expensive to purchase and administer for frequent use. The introduction of lower-cost, portable fundus cameras22,23 is important because they promise to lower the cost per test and make testing more available in areas with a shortage of medical professionals,24,25 but they will not significantly improve the frequency of vision screening to more than once every 3 months. It is evident that an effective home visual function monitoring tool is needed for more frequent self-testing by patients with AMD and DR so that clinically significant changes in disease conditions can be detected and treated in time to minimize vision loss in patients with these eye diseases.
Patients with maculopathy often report seeing distortion in visual targets. Given the inhomogeneous nature of abnormal retinal morphology changes in maculopathy,26 it is hypothesized that patients with maculopathy have more difficulty performing visual tasks that require global integration of visual stimuli over a large retinal area than performing a localized task such as visual acuity (VA).27 To test this hypothesis, Wang et al.27 developed a shape discrimination hyperacuity (SDH) test with perfect and distorted circular contours called radial frequency patterns28 as visual stimuli. An important feature of the SDH test is that the optimal performance of this test requires global visual integration.28–32 By measuring SDH, a patient's ability to detect visual distortion and his or her ability to integrate visual information can be quantified.
While SDH is much less affected by normal aging compared with VA and Pelli-Robson letter contrast sensitivity (CS),33 it is significantly reduced in patients with AMD27 and patients with Stargardt disease,34 even though the patients still had normal VA. Recently, a handheld SDH (hSDH) test (myVisionTrack; Vital Art and Science, Inc., Richardson, TX) has been implemented on a mobile platform (iOS; Apple, Inc., Cupertino, CA) (Fig. 1) for use by patients at home for remote monitoring of their visual function.35 The objectives of this study were to compare the hSDH testing protocol with an established desktop SDH (dSDH) testing protocol, to compare hSDH with standard visual function measures such as VA and CS, and to evaluate the effect of the severity of maculopathy on hSDH.
Figure 1. .
Handheld SDH test on an iOS mobile platform.
Methods
Subjects
Thirty-seven patients diagnosed as having AMD (mean ± SD age, 73.9 ± 9.5 years) and 36 patients diagnosed as having DR (mean ± SD age, 60.9 ± 12 years) were recruited for this study. The criteria for patient selection were as follows: (1) AMD or DR with corrected Early Treatment of Diabetic Retinopathy Study (ETDRS) VA of 20/100 or better in at least one eye, (2) ophthalmic evaluation by retina specialists with clinical and spectral-domain (SD)–OCT documentation, (3) no retinal pathology other than AMD or DR, (4) no concurrent systemic illness affecting the retina, and (5) no dementia or other limitation that would prevent the patient from performing a self-test of visual function. Patients with AMD and DR were recruited at various disease stages, including those under active anti–vascular endothelial growth factor treatment. Patients with epiretinal membrane or pigment epithelial detachment were not excluded.
Twenty-seven healthy senior volunteers (mean ± SD age, 68.9 ± 9.4 years) served as a visually normal group. The inclusion criteria for healthy volunteers were (1) corrected VA of 20/32 or better in each eye, (2) normal fundus or OCT images as judged by retina specialists, and (3) no concurrent systemic illness affecting the retina and vision.
Patients with AMD and DR were recruited from the clinic of the Department of Ophthalmology, UT Southwestern Medical Center. Healthy subjects were recruited from the normal subject database of the Retina Foundation of the Southwest. Most subjects (92%) did not own or have prior experience using a handheld touch-screen device. All subjects consented to participate after the study purpose and procedures were explained to them. The study was in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board at UT Southwestern Medical Center.
Table 1 gives the demographic data of the study subjects. Simultaneous comparison among the three groups (visually normal, AMD, and DR) indicated a significant difference in age based on ANOVA (P < 0.0001) and a significant difference in race based on Fisher exact test (P < 0.0001) but no significant difference in sex based on Fisher exact test (P = 0.15).
Table 1. .
Demographic Data of the Study Subjects
|
Variable |
Visually Normal, n
= 27 |
AMD, n
= 37 |
DR, n
= 36 |
| Age, y | |||
| Mean ± SD | 68.9 ± 9.4 | 73.9 ± 9.5 | 60.9 ± 12.0 |
| Range | 49–84 | 50–93 | 40–83 |
| Sex, n (%) | |||
| Male | 13 (48.1) | 11 (29.7) | 17 (47.2) |
| Female | 14 (51.9) | 26 (70.3) | 19 (52.8) |
| Race, n (%) | |||
| White | 22 (81.5) | 37 (100.0) | 21 (58.3) |
| Black | 1 (3.7) | 0 | 6 (16.7) |
| Hispanic | 2 (7.4) | 0 | 6 (16.7) |
| Asian | 2 (7.4) | 0 | 3 (8.3) |
SDH Test
Stimuli used in dSDH and hSDH testing protocols were distorted and undistorted circular shapes. The amount of distortion from circularity is generated by modulating the radius of a circle sinusoidally. Hence, this type of stimulus is also called a radial frequency pattern.28 Examples of the stimulus patterns are shown in Figure 1. In this shape discrimination test, the threshold to be determined is the minimal radial modulation amplitude that allows a subject to distinguish a distorted circular shape from a perfect one. Because the normal threshold for detecting such radial modulation is typically in the hyperacuity range, this test is called an SDH test. The main parameters describing the stimulus pattern include the following: (1) mean radius (i.e., the radius of undistorted circular contour), (2) radial frequency (the number of modulation cycles per circumference), (3) amplitude of radial modulation (the amount of deformation), (4) peak spatial frequency of radial frequency (RF) patterns (determining the width of the contour), and (5) stimulus contrast.
In the dSDH test, stimuli were generated digitally in MATLAB (The MathWorks, Inc., Natick, MA) and displayed on a gamma-corrected, 8-bit grayscale monitor that was controlled by a PowerMac computer (Apple, Inc.) using the Psychophysics Toolbox,36 which provides high-level access to the C-language VideoToolbox.37 The mean luminance of the monitor was 73 candela (cd)/m2, and the stimulus contrast was 80%. The stimulus screen subtended 18 × 13.5° at the viewing distance of 1.0 m. The peak spatial frequency of the stimuli was 3 cycles per degree (cyc/deg). The radial frequency was 8 cyc/2π, and the mean radius was 1.0°. A temporal two-alternative forced-choice (2AFC) paradigm was used in the dSDH test.27 Subjects were asked to look at a fixation target positioned at the center of the screen, where the stimulus patterns were presented during the experiment. A chin rest was used, and the viewing distance was fixed at 1 m. The instructions for the dSDH test were provided by the tester. In each trial of the temporal 2AFC paradigm, one interval contained a distorted circular shape, and the other interval contained an undistorted one. Subjects were asked to verbally report which interval (one or two) contained the distorted one, and then the tester entered the response by pressing a button on a keyboard. The tester did not have prior knowledge of which interval had the distorted shape. In each stimulus presentation interval, the circular shape was centered at the fixation target. The duration of each stimulus interval was 0.5 seconds. Audio signals were used to prompt the subject before each interval and at the end of each trial, but no feedback about the correctness of responses was provided.
In the hSDH testing protocol, stimuli were generated on an iPod Touch (Apple, Inc.). The instructions for the hSDH test were provided by both the tester and the on-screen prompts. Audio input or guidance was not provided for the hSDH test. The subject was instructed to hold the hSDH device comfortably at a distance of about an arm's length, and the viewing distance was measured by the tester. A spatial 3AFC staircase paradigm was used to control each test run. In each trial, subjects indicated by touch input which of three circular shapes on the iPod Touch (Fig. 1) was distorted. The stimulus patterns stayed on the screen until a touch response was registered. At a viewing distance of 16 in (406 mm), the stimulus parameters were comparable to those of the dSDH protocol.
Both dSDH and hSDH tests were controlled by a two-down, one-up staircase procedure38 and ended after eight and six reversals for the dSDH and hSDH testing protocols, respectively. Because the chance level of the 2AFC dSDH test was 50%, while that of the 3AFC hSDH test was 33% in each trial, the 3AFC hSDH test could reach threshold level sooner than the 2AFC dSDH test for a given test variability, and fewer reversals could be used in the 3AFC hSDH test. A maximum-likelihood fitting procedure was used to fit a Weibull function39,40 to data obtained from each test run. The estimated modulation threshold was defined as the stimulus level at the inflection point of the Weibull psychometric function, which corresponded to 82% and 75% correct responses for the dSDH (2AFC) and hSDH (3AFC) testing protocols, respectively.
Study Design
A cross-sectional study was designed and conducted. In addition to dSDH and hSDH tests, the subject performed the ETDRS VA test (E-ETDRS41) and the Pelli-Robson letter CS test. The order of visual function tests was VA, CS, dSDH test, training for the hSDH test, and performing the hSDH test. Subjects used current spectacle or contact lens corrections to perform the psychophysical tests. All tests were performed monocularly in a visual function testing laboratory, and a complete test session took approximately 1½ hours. Each time the subject took a test, the right eye was always tested first with the left eye covered by an eye patch, followed by testing of the left eye with the right eye covered by an eye patch. For the dSDH and hSDH tests, each eye was tested twice (test–retest). A third test was required for an eye if the scores of the first and second tests differed by 0.30 logarithm of the minimum angle of resolution (logMAR) or more. The average of the two tests (or three if there was a third one) was used as a mean SDH estimate for the test eye. In addition, SD-OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany) 31-line macular volume scans were obtained from the participants.
Usability Survey
A usability survey was conducted among 46 patients with AMD (n = 10) or DR (n = 36) after they finished all testing. The survey included the following rating scale questions: (1) Do you understand how to use the hSDH device to test your vision? (2) Is the hSDH test easy to use? (3) Do you feel confident that you can test your own vision with the hSDH test device? For each question, the following five ratings were available to choose from: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, or (5) strongly agree.
Data Analysis
The results obtained from one eye of each subject were used for data analyses as described below. Because one of the main objectives of the study was to compare the hSDH testing protocol with the dSDH testing protocol, diseased eyes with VA of 20/100 or better were selected because these eyes were still able to perform temporal forced-choice paradigms used by the dSDH protocol at the viewing distance of 1.0 m. In addition, the hSDH test used in this study had a peak spatial frequency of 3 cyc/deg at a designed test distance of 16 in (406 mm). Eyes with VA of 20/100 had a cutoff spatial frequency (resolution limit) of 6 cyc/deg, twice the peak spatial frequency of the circular shapes used in the SDH test. Given the one-octave spatial frequency bandwidth of the circular shape,28 all spatial frequency components of the stimulus pattern were within the resolution limit and were visible to the eyes with VA of 20/100 or better. When performing the hSDH test on the iPod Touch (Apple, Inc.), the measured average viewing distance of the maculopathy eyes with VA of 20/100 or better was a mean ± SD of 15.7 ± 2.5 in (398.8 ± 63.5 mm), resulting in comparable stimulus parameters in both testing protocols.
Another rationale for selecting which eye from each patient to use for data analyses was to cover a larger range of VA. For the visually normal subjects, the eye with better VA, or the left eye if both eyes had the same VA, was chosen. For the patients, the criteria for study eye selection were as follows: (1) the eye with worse VA was selected if both eyes were 20/100 or better and (2) the left eye was selected if both eyes had the same VA of 20/100 or better. Table 2 gives the selected eyes and their clinical evaluation (grading). The classification of AMD in Table 2 was according to the Age-Related Eye Disease Study grading scale.
Table 2. .
Grading of the Study Eyes in the Visually Normal, AMD, and DR Groups
|
Grade |
n |
VA, Mean ± SD, logMAR |
Clinical Findings |
| Visually normal | |||
| 1 | 27 | 0.02 ± 0.07 | No signs of retinal disease |
| AMD | |||
| 2 | 10 | 0.11 ± 0.20 | Early AMD (medium-size drusen) |
| 3 | 11 | 0.23 ± 0.20 | Intermediate AMD (large-size drusen or pigment change) |
| 4 | 16 | 0.41 ± 0.18 | Advanced AMD (geographic atrophy or exudation) |
| DR | |||
| 2 | 11 | 0.19 ± 0.20 | Mild to moderate NPDR |
| 3 | 12 | 0.26 ± 0.13 | Severe to very severe NPDR or pre-PDR |
| 4 | 13 | 0.55 ± 0.14 | PDR or NPDR affecting the fovea |
Comparing dSDH and hSDH Testing Protocols.
Linear regression analysis was conducted to evaluate the level of agreement between dSDH and hSDH testing protocols. Bland-Altman analysis was similarly performed.
Comparing hSDH With VA or CS.
Linear regression analysis was conducted to evaluate the level of agreement between hSDH and VA or CS. Correlation coefficients between SDH and VA or letter CS threshold were calculated, and the significance of correlation coefficients was assessed.
Evaluating the Effect of Disease Severity on SDH.
The eyes with AMD or DR were classified into three groups based on their ophthalmic evaluation and the grading of the disease severity (Table 2). Visual function test results were compared with the grading of AMD or DR. One-way ANOVA was carried out to determine if there was any difference between the mean thresholds of the eyes in different groups.
Usability Survey.
A usability survey was administered. Percentage responses in all scales of each question were calculated.
Results
Comparison of the hSDH Test With dSDH Testing Protocols
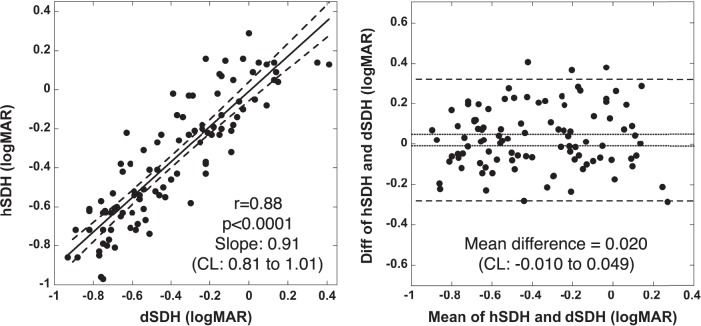
Figure 2 (left) shows the SDH plots in logMAR obtained with the hSDH testing protocol compared with those obtained with a dSDH testing protocol. It is evident that the results obtained with these two protocols are highly correlated (r = 0.88, P < 0.0001). The slope of linear regression is 0.91 (95% confidence interval [CI], 0.81–1.01), including slope one, suggesting no significant difference in SDH measurements by these two testing protocols.
Figure 2. .
Left: Correlation of hSDH and dSDH testing protocols. The solid line is the linear regression. The dashed lines represent the band of 95% CI of the linear regression. Right: Bland-Altman analysis of hSDH and dSDH testing protocols. The dotted horizontal lines represent the 95% CI of the mean difference. The dashed horizontal lines represent the mean difference of ±1.96 SD.
Figure 2 (right) shows the results of Bland-Altman analysis, which plots the difference in hSDH and dSDH measurements versus their mean. The mean difference is 0.020 logMAR. This bias is not significantly different from zero because the 95% CI (−0.010 to 0.049 logMAR) of the mean difference includes zero.
The average time to obtain a self-measurement of hSDH was a mean ± SD of 92 ± 43 seconds. By comparison, the average time to obtain a dSDH measurement was a mean ± SD of 130 ± 34 seconds. A third test was required 9.3% of the time; this happened when the within-session results of the hSDH test differed by 0.30 logMAR or more.
hSDH Versus VA or Letter CS
Figure 3 (left) shows the hSDH plots versus VA obtained from 100 study eyes of 100 participants. The solid line is the linear fit for all data points. The correlation coefficient is 0.78 (P < 0.0001), indicating that hSDH is significantly correlated with VA. The slope of linear fit is 1.05 (95% CI, 0.88–1.22), close to unity and suggesting that logMAR changes in hSDH are comparable to those in VA. However, this fitted line does not go through the points of the normal mean VA (0.02 logMAR) and the normal mean hSDH (−0.69 logMAR) (indicated by the cross in Fig. 3). Hence, when referenced to the normal means, the change in hSDH may be different from that of VA. The finding that the fitted line shifts upward relative to a unity line that goes through the point of the normal means suggests that hSDH may reveal additional functional deficit that VA could not.
Figure 3. .
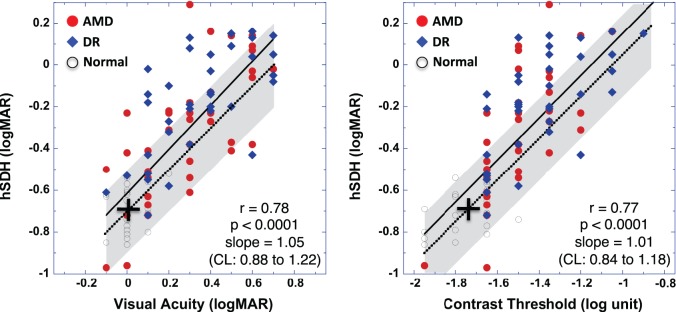
Correlation of hSDH with VA (left) or with CS (right) for data obtained from the visually normal subjects (open circles), AMD (closed red circles), and DR (closed blue diamonds) groups. The solid lines represent the linear fit for all data points. The cross indicates a point at the normal mean hSDH (−0.69 logMAR) and the normal mean VA (0.02 logMAR) (left) or the normal mean CS (−1.72 log unit) (right). The dotted lines go through the normal mean points (crosses) and have a slope of 1. The shaded area indicates a range of ±0.20 logMAR (estimated 95% CI) from the dotted line along both the vertical and horizontal axes.
To further illustrate that hSDH may reveal more deficits than VA, a dotted line with the slope equal to unity through the point of the normal means is also shown in Figure 3. Data points falling on this dotted line would represent equal amounts of deficits in hSDH and VA relative to the normal means, data points above this dotted line would indicate more deficit in hSDH than in VA, and data points below this line would indicate less deficit in hSDH than in VA. To consider the fact that test variability can lead to the varied test results, a shaded area is shown in Figure 3 to indicate an estimated ±95% range of test variability (CI) centered at the dotted line. For the visually normal subjects, the SD of the mean hSDH was 0.10 logMAR as found in this study. Preliminary analysis of test variability showed that for patients with VA of 20/100 or better the average SD of the mean hSDH was also around 0.10 logMAR (Wang Y-Z, et al. IOVS 2012;53:ARVO E-Abstract 2914). Hence, the ±95% range of the shaded area corresponds to ±0.20 logMAR for hSDH. This ±95% range is also comparable to the finding of VA test-retest variability.41 Therefore, while the data points in this shaded area can be accounted for by test variability only (i.e., comparable loss in VA and hSDH), the data points outside this shaded area would indicate additional disease-caused changes revealed by one test or the other. It is evident that most data points outside the test variability area are above, supporting the argument that hSDH can reveal additional visual function deficits to which VA may not be sensitive.
The correlation between hSDH and VA is also examined for the three individual groups of visually normal, AMD, and DR. Handheld SDH is significantly correlated with VA for AMD (r = 0.69, P < 0.0001, slope of 0.95) and DR (r = 0.66, P < 0.0001, slope of 0.75) but was not significantly correlated with VA for the visually normal subjects (r = 0.29, P > 0.138, slope of 0.41).
Similar results are obtained when comparing hSDH with letter CS as shown in Figure 3 (right), where hSDH is plotted as a function of letter contrast threshold, with a linear regression slope of 1.01 (95% CI, 0.84–1.18; r = 0.77, P < 0.0001) for all data points (solid line). Further analysis shows that hSDH can reveal additional visual function deficits that CS cannot.
The correlation between hSDH and CS is also examined for the three individual groups of visually normal, AMD, and DR. Handheld SDH is significantly correlated with CS for AMD (r = 0.72, P < 0.0001, slope of 1.24), DR (r = 0.62, P < 0.0001, slope of 0.62), and visually normal subjects (r = 0.51, P < 0.007, slope of 0.42).
Effect of Macular Edema on hSDH and VA
Figure 4 shows the hSDH as well as VA obtained from the visually normal, AMD, and DR groups as a function of the average thickness of the central subfield of the ETDRS macular grid measured by SD-OCT. The vertical dotted lines represent the normal range of the central subfield thickness from the visually normal group in this study. Excluded in this figure are eyes with atrophy as defined by the central subfield being thinner than the normal lower 95% CI. Similar to VA (r = 0.56, P < 0.0001), it is evident that the loss of SDH is significantly correlated with the central subfield thickness (r = 0.58, P < 0.0001). However, these data also suggest that visual function can change significantly, even though the central subfield thickness is within the normal range.
Figure 4. .
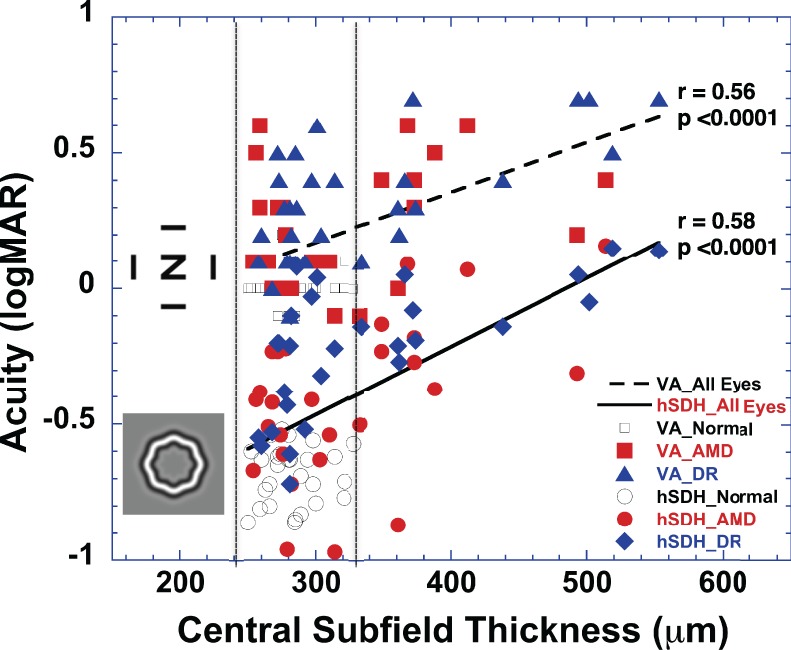
Handheld SDH and VA obtained from the visually normal subjects (open circles and squares), AMD (red closed circles and squares), and DR (blue closed diamonds and triangles) groups as a function of the average thickness of the central subfield of the ETDRS macular grid. The two vertical dotted lines represent the ±95% range (mean ± SD, 284 ± 23 mm) of the central subfield thickness obtained from the visually normal group in this study. In this plot, the eyes with a central subfield thinner than the normal ±95% range (<240 mm) were excluded. The dashed line is the linear fit for VA, while the solid line is the linear fit for hSDH.
Effect of Disease Severity on hSDH and VA
Figure 5 shows one-way ANOVA results of comparing the mean hSDH (filled red circles) and the mean VA (filled red squares) for each of the four groups of visually normal senior (grade 1), early AMD (grade 2), high-risk intermediate AMD (grade 3), and advanced AMD (grade 4). It is evident that the loss of SDH increased with increased severity of AMD. One-way ANOVA indicated that the mean SDHs of four groups were significantly different (P < 0.0001). Pairwise t-test revealed that any two of three AMD groups showed a significant difference in SDH (P < 0.013). Compared with VA, hSDH also showed a larger dynamic range for scoring from early AMD to advanced AMD (0.6 logMAR for SDH vs. 0.3 logMAR for VA) with a comparable 95% CI for the group mean.
Figure 5. .

One-way ANOVA of hSDH (closed circles and diamonds) and VA (closed squares and triangles) versus the grading of AMD (red closed circles and squares) or DR (blue closed diamonds and triangles). Error bars denote 95% CI.
Similar results were obtained from patients with DR as shown in Figure 5, where hSDH (closed blue diamonds) and VA (closed blue triangles) were obtained from the four groups of visually normal senior (grade 1), mild to moderate nonproliferative DR (NPDR) (grade 2), severe to very severe NPDR or pre-PDR (grade 3), and PDR or NPDR affecting the fovea (grade 4). It is evident that the loss of SDH increased with increased severity of DR. One-way ANOVA indicated that the mean SDHs of four groups were different. Pairwise t-test revealed that grade 3 and grade 4 DR groups showed significantly higher hSDH than DR group 2 (P < 0.0002).
After controlling for disease category (visually normal, AMD, and DR) and disease grading, multiple linear regression analyses indicated that age, sex, and race were not significantly associated with VA, hSDH, or OCT central subfield thickness. Sex and race were also not significantly associated with CS, but age showed a significant association with CS (P = 0.002; slope of 0.005 [95% CI, 0.002–0.008] log unit contrast threshold increase per year).
Usability Survey Findings
Among 46 patients with AMD or DR who answered the usability rating scale questions, 37% agreed and 63% strongly agreed that they understood how to use the hSDH device to test their visual function. Furthermore, 24% of patients agreed and 74% strongly agreed that the hSDH test device was easy to use, while 2% (one patient) answered neutral. In addition, 26% agreed and 72% strongly agreed that they felt confident that they could test their own vision with the hSDH test device, while 2% (one patient) answered neutral.
Discussion
The findings in this study demonstrated that the hSDH test implemented on a touch-screen mobile device for visual function self-testing is intuitive, easy to use, and comparable to the previously established dSDH test for a wide range of VA (20/100 or better). Furthermore, the cross-sectional results showed that the hSDH of the patients with advanced AMD or with very severe NPDR or PDR was significantly reduced compared with that of patients having high-risk early AMD or having mild to moderate NPDR. The SDH difference between more advanced and earlier stages of disease was 0.25 to 0.30 logMAR for AMD or DR (Fig. 5). While this difference was from a cross-sectional study of patients with various degrees of AMD or DR severity, it provides evidence to suggest that the hSDH test could potentially detect a change from earlier to more advanced stages of disease during longitudinal follow-up of patients with maculopathy and can potentially document change in visual function to help assess treatment effects. Future longitudinal studies are needed to establish the thresholds for detecting clinically significant SDH changes in individual patients and to evaluate the sensitivity and specificity of the test to detect such changes.
The results of this study also showed that for VA of 20/100 or better hSDH is significantly correlated with ETDRS VA or Pelli-Robson letter CS (Fig. 3). From the ANOVA results in Figure 5, one may conclude that both hSDH and VA are comparable in their capability to differentiate more advanced from earlier stages of disease conditions. However, as a visual function self-monitoring tool for home use, the SDH test has many advantages over VA or CS. While VA is the standard visual function test for clinical use, it is impractical to use distance VA for self-testing at home, and near VA measurement requires accurate determination of the viewing distance during self-testing, which is difficult to achieve. On the other hand, it is well known that aging and change in luminance significantly affect CS in laboratory testing conditions.42 In addition, CS is sensitive to nonretinal factors such as the deterioration of the eye's optical system because the optics have a major contribution to CS.43 While at-home PC-based CS self-testing may be reliable in terms of test-retest variability at a viewing distance of 1500 mm as demonstrated in a study44 of retinitis pigmentosa, it remains to be seen if a handheld version of the CS test at a much closer viewing distance would work reliably for self-testing at home by patients with AMD or DR.
By comparison, previous studies using various dSDH testing protocols have shown the following characteristics of the SDH test: (1) it is a hyperacuity test and is spatial frequency band limited28 around the peak CS (3 cyc/deg in the hSDH test) so that it is much less affected by the deterioration of the eye's optical system than VA,33 (2) it is much less affected by normal aging than VA or CS,33 and (3) it is a suprathreshold test so that stimulus shapes are easily visible and the test performance is less sensitive to the changes in other stimulus parameters such as contrast27,28 and viewing distance.28 It is expected that the hSDH test would have these features found in previous studies because of the close agreement between the hSDH and dSDH tests demonstrated in this study (Fig. 2).
For instance, the results shown in Figure 2, where hSDH measured at a range of viewing distances (mean ± SD, 400 ± 64 mm) was compared with dSDH measured at a 1000-mm fixed test distance, demonstrated close agreement between hSDH and dSDH estimates, suggesting that variations in the viewing distance did not significantly affect SDH measurements among patients with VA of 20/100 or better. This result is consistent with previous findings that SDH is insensitive to viewing distance change.28
It is also evident in this study that hSDH is less sensitive to other variables that are unrelated to disease conditions. Figure 5 shows that VA (but not hSDH) in grade 2 AMD was slightly worse than that of the visually normal subjects. Because there was no retinal pathology in the fovea area for patients with grade 2 (early) AMD, it is less likely that the worsening of VA in grade 2 AMD was caused by the disease condition. Rather, this VA difference can be explained by the fact that for the visually normal subjects the eyes with better VA were selected for the data analysis, while for the diseased eyes the eyes with worse VA were selected for the data analysis. Indeed, for the visually normal subjects, the mean ± SD VA of the eyes with worse acuity was 0.11 ± 0.09 logMAR, which was significantly different from that of the normal eyes with better VA but was comparable to the eyes with grade 2 AMD (mean ± SD VA, 0.11 ± 0.20 logMAR). On the other hand, the mean ± SD hSDH of the weaker normal eyes was −0.68 ± 0.12 logMAR, which is not significantly different from the mean ± SD hSDH of the better normal eyes (−0.69 ± 0.10 logMAR) and is consistent with the finding shown in Figure 3 that there is no significant correlation between hSDH and VA for the visually normal subjects. The decreased sensitivity of hSDH to the various conditions of normal eyes also suggests that hSDH could represent a more stable baseline for comparison with visual function loss caused by eye diseases.
Furthermore, the results shown in Figure 3 suggest that hSDH reveals additional visual function deficits that VA or CS could not demonstrate for patients still having good VA. This is consistent with previous findings in high-risk early AMD27 and provides additional evidence to support the hypothesis that patients with maculopathy have more difficulty performing visual tasks that require global integration of visual stimuli over a large retinal area than performing a localized task such as VA. For a group of AMD eyes with VA of 20/50 or better, preliminary results of receiver operating characteristic (ROC) curve analyses demonstrated that the area under the ROC curve for differentiating exudative AMD from moderate AMD by SDH was significantly greater than that of VA and CS, and SDH showed high sensitivity and specificity compared with VA and CS (Wang Y-Z, et al. IOVS 2011;52:ARVO E-Abstract 100).
One of the limitations of the current version of the hSDH test is that it is only validated for patients with VA of 20/100 or better. It is unknown if the hSDH test would work appropriately for patients with VA worse than 20/100. For instance, a patient with VA of 20/200 has a resolution limit of 3 cyc/deg. Given the designed peak spatial frequency of 3 cyc/deg and the one-octave bandwidth of the stimulus pattern,28 at the default test distance of 16 in (406 mm) some of the spatial frequency components are beyond 3 cyc/deg and are not resolvable to the patient with VA of 20/200. Hence, unlike patients with VA of 20/100 or better who can see complete stimulus patterns and perform the hSDH test at the default test distance, the patient with VA of 20/200 has to bring the hSDH test device closer to see the complete stimulus patterns as observed in laboratory testing. This change in the viewing distance may lead to undefined measurement of SDH. Hence, for patients with VA worse than 20/100, an estimate of the viewing distance during self-testing may be required to obtain meaningful measurement of SDH.
Using the front-facing camera that is now available on many mobile devices, it is possible to implement a method to estimate the viewing distance through the detection of a known calibration target during vision function self-testing so that changes in the viewing distance from the default can be compensated for when estimating SDH. In addition, other features can be implemented to identify if the same patient is taking the test, to detect if the correct eye is patched, and so forth. Detection of the patient's level of attentiveness should also be possible. The patient will only need to be observed every few seconds to ensure that he or she is facing the device screen and is not distracted. With these supervisory features, the patient's identity can be verified, and patients can be alerted to change their eye patch if the incorrect eye has been patched before the tests. Future studies are needed to evaluate the feasibility of using front-facing camera in self-testing.
Another potential limitation of the current version of the hSDH test is that because the test requires touch input subjects with eye, head, and hand coordination problems or subjects who have issues with dexterity, arthritis, hand tremor, and so forth may have difficulty performing the test, although none of our subjects had such difficulties. An hSDH test with additional voice control features may help subjects with such issues to use the test.
The visual stimulus patterns used in the hSDH test have a mean radius of 1° of visual angle, which covers a 2.5 to 3° area on the retina. Patients with maculopathy often show structural abnormalities outside the fovea in the early stage of the disease. The functional deficits associated with such paracentral abnormalities may not be detected by hSDH or other functional tests for foveal vision. Visual function tests that target the paracentral area can be potentially useful for early detection of maculopathy outside the fovea. A macular perimetry test implemented on a touch-screen mobile device could complement the hSDH test for remote monitoring of visual function in patients with maculopathy (Wang Y-Z, et al. IOVS 2013;54:ARVO E-Abstract 5019).
Acknowledgments
Supported by National Institutes of Health National Eye Institute Grant 1R43EY020016-01.
Disclosure: Y.-Z. Wang, Vital Art and Science, Inc. (F, I, C, S), P; Y.-G. He, Vital Art and Science, Inc. (I, C, S); G. Mitzel, None; S. Zhang, None; M. Bartlett, Vital Art and Science, Inc. (I, E, S), P
References
- 1. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355: 1432–1444 [DOI] [PubMed] [Google Scholar]
- 2. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364: 1897–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355: 1419–1431 [DOI] [PubMed] [Google Scholar]
- 4. Photocoagulation treatment of proliferative diabetic retinopathy: relationship of adverse treatment effects to retinopathy severity: Diabetic Retinopathy Study report No 5. Dev Ophthalmol. 1981; 2: 248–261 [PubMed] [Google Scholar]
- 5. Early Treatment Diabetic Retinopathy Study Research Group Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Ophthalmology. 1991; 98: 766–785 [PubMed] [Google Scholar]
- 6. Nguyen QD, Shah SM, Heier JS, et al. Primary end point (six months) results of the Ranibizumab for Edema of the Macula in Diabetes (READ-2) study. Ophthalmology. 2009; 116: 2175–2181.e1 [DOI] [PubMed] [Google Scholar]
- 7. Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the Ranibizumab for Edema of the Macula in Diabetes (READ-2) study. Ophthalmology. 2010; 117: 2146–2151 [DOI] [PubMed] [Google Scholar]
- 8. Loewenstein A; Richard & Hinda Rosenthal Foundation. The significance of early detection of age-related macular degeneration: Richard & Hinda Rosenthal Foundation Lecture, The Macula Society 29th Annual Meeting. Retina. 2007; 27: 873–878 [DOI] [PubMed] [Google Scholar]
- 9. Early Treatment Diabetic Retinopathy Study Research Group Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema: Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology. 1987; 94: 761–774 [DOI] [PubMed] [Google Scholar]
- 10. Javitt JC, Aiello LP. Cost-effectiveness of detecting and treating diabetic retinopathy. Ann Intern Med. 1996; 124: 164–169 [DOI] [PubMed] [Google Scholar]
- 11. Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000; 283: 889–896 [DOI] [PubMed] [Google Scholar]
- 12. Lim JH, Wickremasinghe SS, Xie J, et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol. 2012; 153: 678–686.e1 –2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003; 121: 785–792 [DOI] [PubMed] [Google Scholar]
- 14. Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004; 111: 1280–1287 [DOI] [PubMed] [Google Scholar]
- 15. Klein ML, Francis PJ, Ferris FL III, Hamon SC, Clemons TE. Risk assessment model for development of advanced age-related macular degeneration. Arch Ophthalmol. 2011; 129: 1543–1550 [DOI] [PubMed] [Google Scholar]
- 16. Javitt JC, Zhou Z, Maguire MG, Fine SL, Willke RJ. Incidence of exudative age-related macular degeneration among elderly Americans. Ophthalmology. 2003; 110: 1534–1539 [DOI] [PubMed] [Google Scholar]
- 17. Hartnett ME, Key IJ, Loyacano NM, Horswell RL, Desalvo KB. Perceived barriers to diabetic eye care: qualitative study of patients and physicians. Arch Ophthalmol. 2005; 123: 387–391 [DOI] [PubMed] [Google Scholar]
- 18. Nagi DK, Gosden C, Walton C, et al. A national survey of the current state of screening services for diabetic retinopathy: ABCD-diabetes UK survey of specialist diabetes services 2006. Diabet Med. 2009; 26: 1301–1305 [DOI] [PubMed] [Google Scholar]
- 19. Hazin R, Barazi MK, Summerfield M. Challenges to establishing nationwide diabetic retinopathy screening programs. Curr Opin Ophthalmol. 2011; 22: 174–179 [DOI] [PubMed] [Google Scholar]
- 20. Dais RM. New paradigm aims for improved rates of diabetic retinal exams. Ophthalmol Times. 2006; 31: 6 [Google Scholar]
- 21. Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010; 117: 2134–2140 [DOI] [PubMed] [Google Scholar]
- 22. Zeimer R, Zou S, Meeder T, Quinn K, Vitale S. A fundus camera dedicated to the screening of diabetic retinopathy in the primary-care physician's office [ published correction appears in Invest Ophthalmol Vis Sci. 2002; 43:2066]. Invest Ophthalmol Vis Sci. 2002; 43: 1581–1587 [PubMed] [Google Scholar]
- 23. Yogesan K, Cuypers M, Barry CJ, Constable IJ, Jitskaia L. Tele-ophthalmology screening for retinal and anterior segment diseases. J Telemed Telecare. 2000; 6 (suppl 1): S96–S98 [DOI] [PubMed] [Google Scholar]
- 24. Romero-Aroca P, Sagarra-Alamo R, Basora-Gallisa J, Basora-Gallisa T, Baget-Bernaldiz M, Bautista-Perez A. Prospective comparison of two methods of screening for diabetic retinopathy by nonmydriatic fundus camera. Clin Ophthalmol. 2010; 4: 1481–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Germain N, Galusca B, Deb-Joardar N, et al. No loss of chance of diabetic retinopathy screening by endocrinologists with a digital fundus camera. Diabetes Care. 2011; 34: 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guyer DR, Yannuzzi LA, Chang S, Shields JA, Green WR. Retina-Vitreous-Macula. Philadelphia: Saunders; 1999. [Google Scholar]
- 27. Wang YZ, Wilson E, Locke KG, Edwards AO. Shape discrimination in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002; 43: 2055–2062 [PubMed] [Google Scholar]
- 28. Wilkinson F, Wilson HR, Habak C. Detection and recognition of radial frequency patterns. Vision Res. 1998; 38: 3555–3568 [DOI] [PubMed] [Google Scholar]
- 29. Hess RF, Wang YZ, Dakin SJ. Are judgments of circularity local or global? Vision Res. 1999; 39: 4354–4360 [DOI] [PubMed] [Google Scholar]
- 30. Jeffrey BG, Wang YZ, Birch EE. Circular contour frequency in shape discrimination. Vision Res. 2002; 42: 2773–2779 [DOI] [PubMed] [Google Scholar]
- 31. Loffler G, Wilson HR, Wilkinson F. Local and global contributions to shape discrimination. Vision Res. 2003; 43: 519–530 [DOI] [PubMed] [Google Scholar]
- 32. Wang YZ, Hess RF. Contributions of local orientation and position features to shape integration. Vision Res. 2005; 45: 1375–1383 [DOI] [PubMed] [Google Scholar]
- 33. Wang YZ. Effects of aging on shape discrimination. Optom Vis Sci. 2001; 78: 447–454 [DOI] [PubMed] [Google Scholar]
- 34. Wang YZ, Birch DG. Local and global visual function deficits in patients with Stargardt disease. In: Lakshminarayanan V. ed OSA Trends in Optics and Photonics. Washington: Optical Society of America; 2000: 77–84 [Google Scholar]
- 35. Chhetri AP, Wen FY, Wang YZ, Zhang K. Shape discrimination test on handheld devices for patient self-test. In: Proceedings of the First Annual ACM International Health Informatics Symposium (IHI 2010). Arlington, VA: ACM Press; 2010: 502–506 [Google Scholar]
- 36. Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997; 10: 433–446 [PubMed] [Google Scholar]
- 37. Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997; 10: 437–442 [PubMed] [Google Scholar]
- 38. Swanson WH, Birch EE. Extracting thresholds from noisy psychophysical data. Percept Psychophys. 1992; 51: 409–422 [DOI] [PubMed] [Google Scholar]
- 39. Nachmias J. On the psychometric function for contrast detection. Vision Res. 1981; 21: 215–223 [DOI] [PubMed] [Google Scholar]
- 40. Weibull W. A statistical distribution function of wide applicability. J Appl Mechanics. 1951; 18: 292–297 [Google Scholar]
- 41. Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003; 135: 194–205 [DOI] [PubMed] [Google Scholar]
- 42. Sloane ME, Owsley C, Jackson CA. Aging and luminance-adaptation effects on spatial contrast sensitivity. J Opt Soc Am A. 1988; 5: 2181–2190 [DOI] [PubMed] [Google Scholar]
- 43. Losada MA, Navarro R, Santamaria J. Relative contributions of optical and neural limitations to human contrast sensitivity at different luminance levels. Vision Res. 1993; 33: 2321–2336 [DOI] [PubMed] [Google Scholar]
- 44. Bittner AK, Ibrahim MA, Haythornthwaite JA, Diener-West M, Dagnelie G. Vision test variability in retinitis pigmentosa and psychosocial factors. Optom Vis Sci. 2011; 88: 1496–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]