Abstract
Background/Aims
We evaluated the potential preventive effect of Nephrology On-Site (i.e. nephrologists integrated into the postoperative cardiac intensive care unit, ICU, team) versus Nephrology On-Demand (i.e. nephrology consultation depending on intensivist criteria) in the ICU on in-hospital outcomes.
Methods
This was a retrospective cohort study comparing outcomes during 2 consecutive time periods: from March 1, 2009 to February 28, 2010 with Nephrology On-Demand, and from March 1, 2010 to February 28, 2011 with Nephrology On-Site. Adult patients admitted to the postoperative cardiac ICU in an academic hospital in Mexico City were eligible. Patients with chronic kidney disease stage 5 or minimally invasive procedures were excluded.
Results
We analyzed 1,096 patients, 558 and 538 in the respective periods. The patients were 52.4 ± 16.2 years old, 56.1% were males, 17.2% had diabetes and 37.6% had hypertension. Further, the patients' median Euroscore was 5 (3-5) and their median Thakar score was 3 (2-4). With Nephrology On-Site, we observed a lower incidence of acute kidney injury [AKI; 25.7 vs. 31.9%, p = 0.02; adjusted OR 0.71 (0.53-0.95), p = 0.02], lower in-hospital mortality among patients with severe AKI [34.1 vs. 55.9%, p = 0.06; adjusted OR 0.33 (0.12-0.95), p = 0.04] and higher renal recovery [61.0 vs. 35.3%, p = 0.03; adjusted OR 3.57 (1.27-10.11), p = 0.02]. No differences were found in the length of stay at the ICU and mechanical ventilation.
Conclusion
Integrating nephrologists into the postoperative cardiac ICU team was associated with a lower incidence of AKI. Patients who developed severe AKI had lower in-hospital mortality and higher renal recovery.
Key Words : Acute kidney injury, Intensive care unit, Early nephrology
Introduction
The incidence of acute kidney injury (AKI) after cardiac surgery defined using the internationally accepted RIFLE [1] and AKIN [2] criteria has been reported to be around 30%, contributing to a mortality rate of 30-50% as the AKI stage increases [3,4,5,6,7].
Delayed recognition leads to delayed implementation of therapeutic strategies, reducing their chance to be effective [8]. Moreover, nephrologists are usually not involved until severe AKI is established. The impact of early nephrology consultation has been evaluated either by the time since admission to the intensive care unit (ICU) [9], by the increase in serum creatinine (SCr) at consultation [10] or by standardized interventions once AKI has been recognized through an automatic detector [11], showing general better outcomes. However, the preventive role of nephrologists in routinely screening postoperative cardiac patients even prior to the development of AKI has not been explored yet.
The aim of our study was to compare in-hospital outcomes before and after the integration of nephrologists into the postoperative cardiac ICU team.
Methods
Study Design and Population
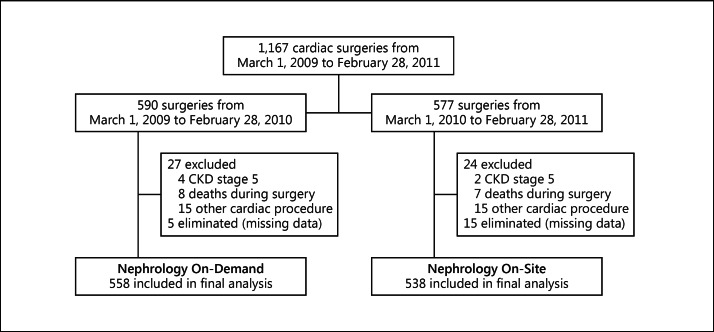
We conducted a retrospective cohort study comparing outcomes during 2 consecutive time periods in a single center – the Instituto Nacional de Cardiologia Ignacio Chavez, a tertiary referral public academic hospital in Mexico City. We included all adult patients who underwent open cardiac or thoracic large vessel surgery from March 1, 2009 to February 28, 2011. Patients were excluded in case of preexisting chronic kidney disease stage 5, preoperative AKI, minimally invasive procedures, and death in the operating room or during the first 24 h after ICU admission. Patients were eliminated if information about in-hospital outcomes was missing.
The 2 study periods were from March 1, 2009 to February 28, 2010 when nephrology consultation depended on intensivist criteria (Nephrology On-Demand) and from March 1, 2010 to February 28, 2011 when nephrologists were integrated into the postoperative cardiac ICU team (Nephrology On-Site). The Nephrology On-Site scheme required the daily presence of nephrology fellows actively participating in clinical rounds with intensivists, followed by a clinical round with the attending nephrologists. The main tasks among others were to identify and control risk factors, assess fluid balances, adjust drug dosing and nutritional support, and monitor the RIFLE/AKIN criteria. Decisions on extracorporeal renal support therapy (RST) were taken by consensus within the ICU team on an individual basis without standardized criteria.
Data Collection and Outcomes
Pre-, trans- and postoperative data were systematically collected from the patients' medical records. Data on urine output, fluid balance and medications were not available. No specific medical record was generated that could delimit nephrology interventions from the rest of the postoperative cardiac ICU interventions. The main outcome was the incidence of AKI. Secondary outcomes were in-hospital mortality, length of stay at the ICU, length of mechanical ventilation and renal recovery at hospital discharge.
Definitions
AKI was defined by the RIFLE/AKIN criteria based only on SCr within 7 days after cardiac surgery. The baseline SCr level was the lowest value within the previous 3 months before surgery or at hospitalization. Severe AKI was defined as RIFLE-I/AKIN-2 or RIFLE-F/AKIN-3. Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 ml/min/1.73 m2 calculated by the CKD-EPI equation [12].
Renal recovery at hospital discharge was defined as follows: (1) no recovery if patients continued on RST or if the AKI stage was equal to the maximum AKI; (2) complete recovery if SCr was less than 26.5 µmol/l above the baseline value, and (3) partial recovery if the AKI stage was lower than the maximum AKI but the SCr level was at least 26.5 µmol/l higher than the baseline value.
Statistical Analysis
Quantitative variables were expressed as means ± standard deviations or medians with 25th-75th percentiles and compared between the 2 time periods using Student's t test or the Mann-Whitney U test. Categorical variables were expressed as proportions and compared using the χ2 or Fisher's exact test. A p value <0.05 was considered statistically significant. Survival analysis was done by Kaplan-Meier curves and log-rank test to compare the 2 study periods. Multivariate regression models were performed using the ‘stepwise forward’ method. Patients with partial and complete recoveries were taken as one group in the multivariate analysis, which was repeated with and without death as a no-recovery group. An event/parameter ratio of ≥10 was targeted. Subgroup analysis was performed for AKI by preoperative risk, and for mortality and renal recovery by AKI stage. SPSS v16 was used for calculations.
Results
During the study, 1,167 adult patients were admitted to the postoperative cardiac ICU. The final cohort included 1,096 patients, 558 in the first and 538 in the second period (fig. 1). Baseline and surgery-related characteristics are shown in table 1.
Fig. 1.
Flow chart of study participants.
Table 1.
Table 1. Baseline and surgery-related characteristics
| Total (n = 1,096) | Nephrology On-Demand (n = 558) | Nephrology On-Site (n = 538) | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| General characteristics | |||||||||
| Age, years | 52.4±16.2 | 52.1±16.1 | 52.7±16.3 | 0.53 | |||||
| Weight, kg | 68.4±12.7 | 69.3±12.4 | 67.6±13.0 | 0.03 | |||||
| Male gender | 615 (56.1) | 313 (56.1) | 302 (56.1) | 0.99 | |||||
| Comorbidities | |||||||||
| Diabetes mellitus | 189 (17.2) | 92 (15.5) | 97 (18.0) | 0.50 | |||||
| Hypertension | 412 (37.6) | 210 (37.6) | 202 (37.5) | 0.98 | |||||
| Chronic pulmonary disease | 21(1.9) | 16 (2.9) | 5 (0.9) | 0.02 | |||||
| Cerebrovascular disease | 50 (4.6) | 24 (4.3) | 26 (4.8) | 0.67 | |||||
| Previous myocardial infarction | 268 (24.5) | 140 (25.1) | 128 (23.8) | 0.62 | |||||
| Previous cardiac surgery | 135(12.3) | 59 (10.6) | 76 (14.1) | 0.07 | |||||
| Endocarditis | 62 (5.7) | 30 (5.4) | 32 (5.9) | 0.68 | |||||
| New York Heart Association III—IV | 123 (11.2) | 58 (10.4) | 65 (12.1) | 0.38 | |||||
| Critical preoperative state | 120 (10.9) | 63 (11.3) | 57 (10.6) | 0.71 | |||||
| Left ventricular ejection fraction, % | 56.9±11.8 | 57.2±11.3 | 56.7±12.5 | 0.47 | |||||
| Pulmonary arterial pressure, mm Hg | 35 (27–47) | 36.50 (29–50) | 35 (25–45) | <0.001 | |||||
| Glomerular filtration rate, ml/min/1.73 m2 | 91.7±22.7 | 88.8±23.6 | 94.7±21.5 | <0.001 | |||||
| Chronic kidney disease | 83 (7.6) | 54 (9.7) | 29 (5.0) | 0.007 | |||||
| Baseline SCr, µmol/l | 79.6 (61.9–97.2) | 88.4(70.7–106.1) | 70.7 (61.9–88.4) | <0.001 | |||||
| Scores | |||||||||
| Euroscore | 5 (3–5) | 5(3–6) | 5 (3–7) | 0.14 | |||||
| Thakar score | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.77 | |||||
| Surgical variables | |||||||||
| Emergency surgery | 151(13.8) | 80 (14.0) | 71(13.2) | 0.58 | |||||
| Type of surgery | 0.22 | ||||||||
| Valvular | 623 (56.8) | 310 (55.6) | 313 (58.2) | ||||||
| Coronary bypass | 234(21.4) | 118 (21.1) | 116 (21.6) | ||||||
| Coronary bypass + valvular | 49 (4.5) | 25 (4.5) | 24 (4.5) | ||||||
| Congenital heart disease | 42 (3.8) | 17 (3.0) | 25 (4.6) | ||||||
| Surgery on the aortaa | 67 (6.1) | 30 (5.4) | 37 (6.9) | ||||||
| Othersb | 148 (13.5) | 88 (15.7) | 60 (11.1) | ||||||
| Extracorporeal circulation on-pump | 973 (88.8) | 480 (86.0) | 493 (91.6) | 0.003 | |||||
| Pump time, min | 102 (75–136) | 98 (73–129) | 105(76–140) | 0.01 | |||||
| Aortic clamp time, min | 70 (50–97) | 68 (50–95.7) | 72 (51–98) | 0.48 | |||||
| Bleeding, ml | 740(500–1,140) | 730 (500–1,132.5) | 740(506.5–1,147.5) | 0.48 | |||||
| Complications | |||||||||
| Infection | 121(11.0) | 70 (12.5) | 51 (9.5) | 0.11 | |||||
| Severe infectionc | 65 (5.9) | 32 (5.7) | 33 (6.1) | 0.08 | |||||
| Reoperation | 204 (18.6) | 114 (20.4) | 90 (16.8) | 0.12 | |||||
Data are shown as n (%), mean ± standard deviation or median (25th-75th percentiles).
Could be in addition to other surgery.
Including surgery on the pericardium, isolated atrial septum defect and myxoma.
Including pneumonia, mediastinitis and catheter-associated bacteremia.
Outcomes
Acute Kidney Injury
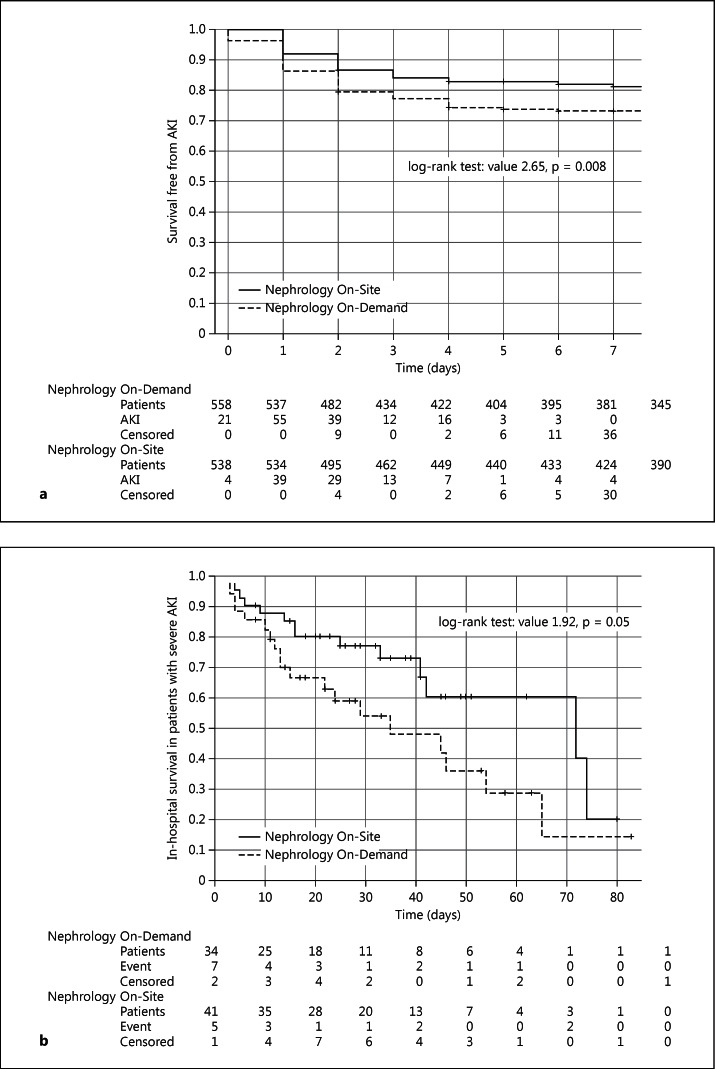
A significantly lower incidence of AKI was observed with Nephrology On-Site, mainly due to a reduction in patients with RIFLE-R/AKIN-1 (table 2). The number needed to treat was 16 patients. Survival analysis demonstrated a significant increase in survival free from AKI (fig. 2a), and multivariate analysis confirmed a lower adjusted OR for AKI (table 3). In the subgroup analysis, the benefit was higher in patients with intermediate risk (Thakar score 3-4; table 3). The onset of AKI was delayed from 1.69 ± 1.34 to 2.06 ± 1.53 days (p = 0.024).
Table 2.
Comparison of in-hospital outcomes
| Total (n = 1,096) | Nephrology On-Demand (n = 558) | Nephrology On-Site (n= 538) | p value | |
|---|---|---|---|---|
| AKI | 316 (28.8) | 178 (31.9) | 138 (25.7) | 0.02 |
| RIFLE-R/AKIN-1 | 241 (22.0) | 144 (25.8) | 97 (18.0) | 0.002 |
| Severe AKI | 75 (6.8) | 34(6.1) | 41 (7.6) | 0.32 |
| RST | 56 (5.1) | 23 (4.1) | 33 (6.1) | 0.13 |
| Renal recovery from AKIa | 216 (68.4) | 124 (69.7) | 92 (66.7) | 0.57 |
| RIFLE-R/AKIN-1 | 179 (74.3) | 112 (77.8) | 67 (69.1) | 0.13 |
| Severe AKI | 37 (49.3) | 12 (35.3) | 25 (61.0) | 0.03 |
| RST | 23 (41.1) | 6 (26.1) | 17(51.5) | 0.06 |
| Mortality | 76 (6.9) | 46 (8.2) | 30 (5.6) | 0.08 |
| AKI | 50 (15.8) | 29 (16.3) | 21(15.2) | 0.80 |
| RIFLE-R/AKIN-1 | 17 (7.1) | 10 (6.9) | 7 (7.2) | 0.94 |
| Severe AKI | 33 (44.0) | 19 (55.9) | 14(34.1) | 0.06 |
| RST | 30 (53.6) | 16 (69.6) | 14 (42.4) | 0.04 |
Data are shown as n (%).
Including complete and partial renal recovery.
Fig. 2.
Kaplan-Meier curves comparing the two study periods. a Survival free from AKI (censoring for deaths) and b in-hospital survival in patients with severe AKI.
Table 3.
Table 3. Multivariate logistic regression analysis for the effect of Nephrology On-Site on in-hospital outcomes, and subgroup analysis
| Outcome | Unadjusted OR | p value | Adjusted OR | p value |
|---|---|---|---|---|
| AKI | 0.80 (0.63–1.02) | 0.07 | 0.71 (0.53–0.95)c | 0.02 |
| Thakar score 0–2 | 0.62 (0.36–1.08)c | 0.09 | ||
| Thakar score 3–4 | 0.56 (0.36–0.88)c | 0.01 | ||
| Thakar score <5 | 1.18 (0.59–2.39)c | 0.64 | ||
| Renal recoveryab | 0.74(0.47–1.15) | 0.18 | 1.00 (0.61–1.65)d | 0.99 |
| Severe AKI | 3.57 (1.27–10.11)d | 0.02 | ||
| RST | 3.78 (1.01–14.21)d | 0.04 | ||
| Renal recoveryab | 0.72 (0.46–1.12) | 0.14 | 0.94 (0.57–1.54)d | 0.81 |
| Severe AKI | 3.27(1.16–9.17)d | 0.03 | ||
| RST | 3.48 (0.92–13.13)d | 0.07 | ||
| Mortality | 0.96 (0.76–1.21) | 0.72 | 0.49(0.29–0.83)e | 0.008 |
| AKI | 0.67 (0.34–1.32)e | 0.25 | ||
| Severe AKI | 0.33 (0.12–0.95)e | 0.04 | ||
| RST | 0.35 (0.10–1.18)e | 0.09 | ||
Partial or complete recovery.
Deaths included in the no-recovery group.
Adjusted for age, weight, reoperation, mechanical ventilation for more than 24 h, hypertension, previous myocardial infarction, baseline SCr and Thakar score; however, extracorporeal circulation time was tested but it did not affect the model.
Adjusted for severe infections, baseline SCr and Thakar score.
Adjusted for weight, severe infections and Euroscore.
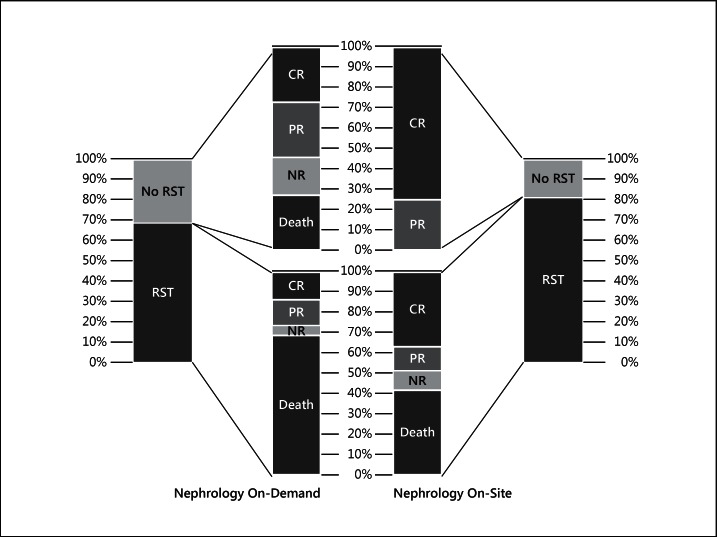
A nonsignificantly higher proportion of patients with severe AKI received RST with Nephrology On-Site (fig. 3). At the start of RST, a trend towards lower values for SCr [176.8 (159.1-221.0) vs. 212.2 (159.1-282.9) µmol/l, p = 0.08] and urea nitrogen [19.3 (14.6-24.6) vs. 26.4 (13.9-33.9) mmol/l, p = 0.07] was found with Nephrology On-Site.
Fig. 3.
Renal outcomes and mortality in patients with severe AKI in both periods. CR = Complete renal recovery; PR = partial renal recovery; NR = no renal recovery.
Renal Recovery
Renal recovery was higher with Nephrology On-Site among patients with severe AKI or RST (table 2). Similar results were obtained when death was included in the no-recovery group (data not shown). These results were confirmed in the multivariate analysis (table 3).
Mortality
Nephrology On-Site was associated with a trend towards lower in-hospital mortality, mainly among patients with severe AKI or RST (table 2). The survival curve showed a noticeable difference at 30 and 60 days in patients with severe AKI (fig. 2b). Again, these observations were reinforced in the multivariate analysis (table 3).
Length of Stay and Mechanical Ventilation
The general length of stay at the ICU was 4 (3-6) days, which increased to 5 (3-7) days with RIFLE-R/AKIN-1, to 13 (8-23) days with severe AKI and to 15 (9.3-25.8) days with RST. There was no difference between the 2 periods. The length of mechanical ventilation was higher with Nephrology On-Site [2 (2-4) vs. 2 (1-3) days, p < 0.001], although it lost its significance in patients with AKI. The duration of mechanical ventilation in patients with RIFLE-R/AKIN-1 was 3 (2-4) versus 3 (2-5) days (p = 0.06), and in those with severe AKI it was 9 (5-20) versus 8 (3-13) days (p = 0.20), respectively. A shift from 1 day to 2 days of mechanical ventilation was noticed in a subgroup of patients with Nephrology On-Site who had a higher proportion of previous cardiac surgery and valvular, combined and on-pump techniques (data not shown). The extreme values prevented the transformation of these variables to fit a normal distribution, so multivariate linear regression analysis was not performed.
Discussion
We found a significant reduction in AKI with Nephrology On-Site and a baseline AKI incidence with Nephrology On-Demand of 31.9%, similar to previous studies [3,4]. As expected, a higher positive impact was achieved in patients with intermediate risk. Of note, the cutoff points for Thakar scores [13] were adapted to our population, RST in 1.54% corresponding to scores 0-2, in 3.52% to scores 3-4 and in 18.56% to scores ≥5.
With Nephrology On-Site, there was a trend towards a higher proportion of patients receiving RST, together with some parameters of earlier dialysis, even though the proportion of severe AKI was lower than previously reported [4]. Although no specific criterion was used to determine when RST should be started, in our center patients with RIFLE-I/AKIN-2 plus an unfavorable state (mechanical ventilation, hemodynamic instability, inputs higher than urine output or sepsis) are usually considered for RST, and with Nephrology On-Site this recognition process might be accelerated. While patients requiring RST usually are at higher risk, as an early intervention it could contribute to better outcomes [14]. Nevertheless, in our study, lower values of SCr and urea nitrogen at the start of RST were not associated with renal recovery or survival. It is clear that supporting patients with less severe AKI and those who are maybe less critically ill would produce a bias towards survival benefit; however, our outcomes among patients with severe AKI were better with Nephrology On-Site with or without RST (fig. 3). Without RST, 45.4% of patients either died or did not recover renal function with Nephrology On-Demand versus 0.0% with Nephrology On-Site (fig. 3). In this sense, daily evaluation not only consists of starting RST early, but also of preventing and choosing the right patient for RST.
Nephrology On-Site resulted in an increased renal recovery rate in patients with severe AKI or RST. In a study by Hobson et al. [15], 45% of patients undergoing cardiothoracic surgery were dialysis dependent at hospital discharge and 21% had complete recovery, while in our study 15.4% of patients were dialysis dependent in both periods; further, we found a nonsignificant increase in the complete recovery rate from 42.9 to 63.2%. It is remarkable that, even though in both study periods patients who required RST were treated by nephrologists, the collaborative work might have contributed to adjust the therapeutic strategies more efficiently for multiorgan benefits.
There was a reduction in mortality among patients with severe AKI from 55.9 to 34.1%, and among patients with RST from 69.6 to 42.4%, which is similar to results reported in the literature [3,6,16]. Of course, it is to be expected that high-quality centers with baseline mortality rates lower than ours might obtain a lower proportional benefit with the same approach.
Equivalent approaches in the literature have shown similar survival benefits. Higher adherence to evidence-based practice and lower rates of complications have been reported when intensivists are present in the ICU 24 h a day versus only on demand [17]. In systematic reviews, the constant presence of intensivists or obligatory consultation in the ICU has shown relative reduction rates for mortality of around 13.8-60% [18,19], while in our center this relative reduction was between 36.3 and 39.0% among the different AKI stages. This survival benefit seems plausible as it is associated with both a lower incidence of AKI and a higher renal recovery rate.
In our study, some variables associated with worse outcomes had an unbalanced distribution. Lower body weight, severe infections such as pneumonia as well as mechanical ventilation were more prevalent in patients with Nephrology On-Site, which could have played a major role reducing benefits in the length of stay at the ICU. Specifically, surgical techniques might explain the increase in mechanical ventilation.
The limitations of our study include that missing information on urine output and fluid balance could have influenced AKI diagnosis; however, as preventing and treating fluid overload were part of the tasks, we hypothesize that with Nephrology On-Demand the percentage of fluid overload could have been higher, and therefore also the real incidence of AKI. In this sense, 29.6% of patients with Nephrology On-Demand had a reduction in their SCr levels from baseline to day 1 versus 21.9% with Nephrology On-Site (p = 0.004), which could indicate a positive fluid balance. Other missing information such as medications including diuretics and severity scores would have allowed us to assess factors potentially associated with our results. As this was a retrospective/observational study, we could not control or assess the specific interventions with Nephrology On-Site; we recognize that these positive results could have been achieved by the original ICU team, simply by the effect of being observed and becoming sensitive towards renal care, but in our perspective this is still a positive achievement. Although not originally planned, we could have influenced the anesthesiology team towards avoiding the intraoperative use of NSAIDs and diuretics, which could explain the change in the early onset of AKI.
In the 90's, Ronco and Bellomo [20] emphasized that the complexity of AKI in ICUs demands nephrologists and intensivists to join forces in a new academic structure called Critical Care Nephrology, and an example of this new structure is the ‘Vicenza Model’ [21]. This multidisciplinary multitarget model with low costs by only optimizing human resources can have benefits beyond clinical outcomes through promoting cordial relationships among specialists, improving academic programs for fellows and potentiating research work.
Conclusion
Integrating nephrologists into the postoperative cardiac ICU team decreased the incidence of AKI and promoted renal recovery and survival among patients with severe AKI. This approach can have multiple benefits for academic centers.
Disclosure Statement
All authors declare no conflict of interest.
Acknowledgements
We thank Magdalena Madero, MD, and Gerardo Gamba, MD, PhD, for nonfinancial support, as well as Azyadet Parra, PN, Gabriel Soto, MD, Israel Campos, MD, and Catalina Santiago, RN, for facilitating data acquisition.
References
- 1.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert AM, Kramer RS, Dacey LJ, Charlesworth DC, Leavitt BJ, Helm RE, Hernandez F, Sardella GL, Frumiento C, Likosky DS, Brown JR. Northern New England Cardiovascular Disease Study Group: Cardiac surgery-associated acute kidney injury: a comparison of two consensus criteria. Ann Thorac Surg. 2010;90:1939–1943. doi: 10.1016/j.athoracsur.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Che M, Li Y, Liang X, Xie B, Xue S, Qian J, Ni Z, Axelsson J, Yan Y. Prevalence of acute kidney injury following cardiac surgery and related risk factors in Chinese patients. Nephron Clin Pract. 2011;117:c305–c311. doi: 10.1159/000321171. [DOI] [PubMed] [Google Scholar]
- 5.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 6.Luckraz H, Gravenor MB, George R, Taylor S, Williams A, Ashraf S, Argano V, Youhana A. Long and short-term outcomes in patients requiring continuous renal replacement therapy post cardiopulmonary bypass. Eur J Cardiothorac Surg. 2005;27:906–909. doi: 10.1016/j.ejcts.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C, Lamy A, Legare JF, Mazer CD, McCluskey SA, Rubens FD, Sawchuk C, Beattie WS. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 8.Van Biesen W, Van Massenhove J, Hoste E, Vanholder R. Defining acute kidney injury: playing hide-and-seek with the unknown man? Nephrol Dial Transplant. 2011;26:399–401. doi: 10.1093/ndt/gfq653. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RL, McDonald B, Gabbai F, Pahl M, Farkas A, Pascual MT, Zhuang S, Kaplan RM, Chertow GM. Nephrology consultation in acute renal failure: does timing matter? Am J Med. 2002;113:456–461. doi: 10.1016/s0002-9343(02)01230-5. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Valdivieso JR, Bes-Rastrollo M, Monedero P, de Irala J, Lavilla FJ. Prognosis and serum creatinine levels in acute renal failure at the time of nephrology consultation: an observational cohort study. BMC Nephrol. 2007;8:14. doi: 10.1186/1471-2369-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balasubramanian G, Al-Aly Z, Moiz A, Rauchman M, Zhang Z, Gopalakrishnan R, Balasubramanian S, El-Achkar TM. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am J Kidney Dis. 2011;57:228–234. doi: 10.1053/j.ajkd.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI. Kusek JW. Eggers P. Van Lente F. Greene T. Coresh J. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 14.Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, Bagshaw SM. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72. doi: 10.1186/cc10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 16.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, Schaff HV. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16. doi: 10.1186/cc9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gajic O, Afessa B, Hanson AC, Krpata T, Yilmaz M, Mohamed SF, Rabatin JT, Evenson LK, Aksamit TR, Peters SG, Hubmayr RD, Wylam ME. Effect of 24-hour mandatory versus on-demand critical care specialist presence on quality of care and family and provider satisfaction in the intensive care unit of a teaching hospital. Crit Care Med. 2008;36:36–44. doi: 10.1097/01.CCM.0000297887.84347.85. [DOI] [PubMed] [Google Scholar]
- 18.Young MP, Birkmeyer JD. Potential reduction in mortality rates using an intensivist model to manage intensive care units. Eff Clin Pract. 2000;3:284–289. [PubMed] [Google Scholar]
- 19.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 20.Ronco C, Bellomo R. Critical care nephrology: the time has come. Nephrol Dial Transplant. 1998;13:264–267. doi: 10.1093/oxfordjournals.ndt.a027816. [DOI] [PubMed] [Google Scholar]
- 21.Ronco C. Critical care nephrology: can we clone the ‘Vicenza Model'? Int J Artif Organs. 2007;30:181–182. doi: 10.1177/039139880703000301. [DOI] [PubMed] [Google Scholar]