Background: The chondroitin sulfate (CS) chain of bikunin has both sulfated and unsulfated regions.
Results: Bikunin CS contains heterogeneous sulfation in the chain and linkage region with CS-D containing chains facilitating HA·HC formation.
Conclusion: Sulfation of the bikunin CS chain regulates HC transfer to HA.
Significance: Bikunin CS chain sulfation may modulate HA tissue stabilization in disease.
Keywords: Chondroitin Sulfate, Extracellular Matrix Proteins, Glycosaminoglycan, Proteoglycan, Proteoglycan Structure, Bikunin, Hyaluronan, Inter-α-trypsin Inhibitor
Abstract
Inter-α-trypsin inhibitor (IαI) is a complex comprising two heavy chains (HCs) that are covalently bound by an ester bond to chondroitin sulfate (CS), which itself is attached to Ser-10 of bikunin. IαI is essential for the trans-esterification of HCs onto hyaluronan (HA). This process is important for the stabilization of HA-rich matrices during ovulation and some inflammatory processes. Bikunin has been isolated previously by anion exchange chromatography with a salt gradient up to 0.5 m NaCl and found to contain unsulfated and 4-sulfated CS disaccharides. In this study, bikunin-containing fractions in plasma and urine were separated by anion exchange chromatography with a salt gradient of 0.1–1.0 m NaCl, and fractions were analyzed for their reactivity with the 4-sulfated CS linkage region antibody (2B6). The fractions that reacted with the 2B6 antibody (0.5–0.8 m NaCl) were found to predominantly contain sulfated CS disaccharides, including disulfated disaccharides, whereas the fractions that did not react with this antibody (0.1–0.5 m NaCl) contained unsulfated and 4-sulfated CS disaccharides. IαI in the 0.5–0.8 m NaCl plasma fraction was able to promote the trans-esterification of HCs to HA in the presence of TSG-6, whereas the 0.1–0.5 m NaCl fraction had a much reduced ability to transfer HC proteins to HA, suggesting that the CS containing 4-sulfated linkage region structures and disulfated disaccharides are involved in the HC transfer. Furthermore, these data highlight that the structure of the CS attached to bikunin is important for the transfer of HC onto HA and emphasize a specific role of CS chain sulfation.
Introduction
Bikunin is a CS2 proteoglycan that was initially purified from urine and was first called urinary trypsin inhibitor (1). Bikunin is also found in plasma both alone and covalently linked via the CS chain to one or two of three HC proteins designated HC1, HC2, and HC3 (2). IαI contains bikunin, HC1, and HC2, whereas pre-α-inhibitor (PαI) contains bikunin and HC3 (3). HC1-bikunin and HC2-bikunin have also been reported (4). IαI and PαI/HC-bikunin are also present in synovial fluid and tissues during inflammation where they have an important role in the stabilization of hyaluronan (HA)-rich matrices (5–7). The level of IαI and PαI/HC-bikunin present in synovial fluid has been correlated with disease severity (8).
The CS chain is covalently attached to the bikunin protein core by an O-glycosidic link between the serine residue on the protein core and a xylopyranose residue on the CS chain. HCs are covalently bound to the CS chain via an ester bond between the α-carbon of the C-terminal Asp and carbon-6 of an internal N-acetylgalactosamine on the CS chain (3, 9).
HCs are also known as serum-derived hyaluronan-associated proteins and were first discovered in the culture medium of fibroblasts (10); however, it was known prior to this that IαI could become covalently associated with HA in synovial fluid of arthritis patients (11). HCs covalently attached to the bikunin CS chain present in IαI and PαI/HC-bikunin can be transferred to HA via a trans-esterification process in which HA substitutes for the CS chain on bikunin to bind to the HCs via an ester bond. This trans-esterification process requires the presence of tumor necrosis factor α-stimulated gene-6 (TSG-6) and cations (4, 12, 13). Although bikunin and its CS chain are not present in HA·HC complexes, they are essential for HA·HC complexes to form (12).
Modulation of the concentration of HA may be a mechanism by which an organism can control the mechanical properties and hydration state of tissues, thereby creating differences in the surrounding environment where inflammation, tumor growth, and tissue repair take place (14). Formation of HA·HC through the covalent attachment of HC proteins to HA is essential for the stabilization of the HA-rich matrix that is generated around oocytes of placental mammals prior to ovulation and for fertilization (12, 13). HA·HC complexes are also found in inflammatory processes such as in the synovial fluid of rheumatoid arthritis patients (8, 15) where is it speculated to restore the hydrodynamic/viscoelastic properties of the fragmented HA (17). The formation of HA·HC has recently been shown to be a reversible process where bikunin acts as both an HC donor and acceptor (18). HA·HC has been reported to provide protection against oxygen-derived free radical degradation of HA (19) and has been shown to enhance CD44-mediated leukocyte adhesion to HA, thereby possibly enhancing inflammation (20). Interestingly, binding of bikunin to the surface of inflammatory cells has an anti-inflammatory effect (21). In addition, it has been shown that HA·HC may provide antifibrotic properties (22).
The CS chains of urinary bikunin and plasma IαI have been found to contain 15 ± 3 disaccharides of which ∼5 disaccharides are 4-sulfated on GalNAc and clustered near the reducing end (23–27). The CS chain linkage region of bikunin in urine and plasma has been shown to contain a disulfated hexasaccharide (24, 25) on its non-reducing end, whereas other groups have suggested that the non-reducing end is monosulfated on the GalNAc residue (9, 28). HC1 and HC2 are located relatively close to each other on the bikunin CS chain in IαI with HC2 located toward the reducing end of the CS chain and HC1 located further toward the non-reducing end (26). HCs are covalently linked to the unsulfated region of the CS chain on bikunin (26). The CS chain attached to plasma and urinary bikunin has been shown to increase in length and to become undersulfated in patients with inflammatory syndromes (29).
This study investigated the CS chain attached to bikunin in plasma and urine from pooled samples with the aim of investigating differences in the CS chain obtained from different biological fluids. This study has demonstrated that the bikunin CS chain can display different sulfation patterns and that the sulfation pattern determines its role in the extent of trans-esterification of HCs from the CS chain on bikunin in plasma IαI to HA.
EXPERIMENTAL PROCEDURES
Materials
Chondroitinase ABC, chondro-4- and -6-sulfatase, and mouse monoclonal anti-CS chain type D (clone MO-225) antibody were purchased from Seikagaku Corp. (Tokyo, Japan). Mouse monoclonal antibodies (mAbs) reactive to the linkage regions of CS chains following chondroitinase ABC digestion (clones 1B5, 2B6, and 3B3) were provided by Professor Bruce Caterson (Cardiff University, UK). Rabbit polyclonal anti-bikunin antibody (ab43073) was purchased from Abcam (Cambridge, MA). Polyclonal anti-N-terminal HC2 (clone N-17) antibodies were purchased from Santa Cruz Biotechnology. The rabbit polyclonal anti-TSG-6 (RAH-1) (30), polyclonal anti-N-terminal HC1 (CP7), and purified TSG-6 (31) were provided by Professor Tony Day (Manchester University, UK). Biotinylated secondary anti-mouse and anti-rabbit antibodies were purchased from Merck-Millipore (Sydney, Australia). Secondary horseradish peroxidase (HRP)-conjugated antibodies were purchased from Dako (Sydney, Australia). HA (Arthrease; 3-MDa average molecular mass; prepared by bacterial fermentation) was purchased from Biotechnology General Ltd. (Israel), and a highly purified pharmaceutical preparation of HA derived from cockerel combs (170,000 average molecular weight) was kindly provided by Dr. James Melrose (Kolling Institute, The University of Sydney) and obtained from Fidia (Abano Terme, Italy) (32). All other chemicals were purchased from Sigma-Aldrich.
Human Plasma and Urine
Plasma and urine samples were obtained under ethics approval by The University of New South Wales Human Research Ethics Committee and The University of New South Wales Human Ethics Advisory Panel, respectively. Data from healthy volunteers with no known medical conditions are presented. Samples were collected and used fresh or stored at −80 °C until analyzed.
Proteoglycan Enrichment
Anion exchange chromatography using a diethylaminoethyl column (1-ml DEAE-Sepharose Fast Flow, GE Healthcare) attached to an FPLC system (ÄKTA purifier, GE Healthcare) was used to purify proteoglycans from plasma and urine. Briefly, the DEAE column was equilibrated at 1 ml/min with running buffer (250 mm NaCl, 20 mm Tris, 10 mm EDTA, pH 7.5) before the addition of urine (20 ml) or plasma (1 ml), and base-line absorbance was re-established with running buffer. Molecules of interest were eluted using an eluting buffer (1 m NaCl, 20 mm Tris, 10 mm EDTA, pH 7.5) and concentrated.
Additionally, some samples were reloaded onto the DEAE column and eluted with a linear gradient of 0.1–1.0 m NaCl, 20 mm Tris, 10 mm EDTA, pH 7.5 over 36 column volumes at 0.2 ml/min and collected in 1-ml fractions. Proteoglycan-enriched fractions were subsequently concentrated and analyzed for protein concentration using a Coomassie Blue protein assay (Thermo Scientific, Scoresby, Australia).
Alternatively, pooled samples were ethanol-precipitated to enrich for proteins. Ice-cold 100% (v/v) ethanol was added to the urine or plasma samples in a volume ratio of 4:1 and incubated at 4 °C for 5 min before centrifuging at 4000 × g for 3 min. The supernatant was discarded and replaced with an equal volume of ice-cold 100% (v/v) ethanol, incubated at 4 °C for 5 min, and centrifuged at 4000 × g for 3 min. The supernatant was discarded and replaced with SDS-PAGE running buffer.
Endoglycosidase Digestion
Samples of proteoglycan-enriched material were digested with 50 milliunits/ml chondroitinase ABC in 0.1 m Tris acetate, pH 8 at 37 °C for 16 h to confirm the presence and structure of the CS. Selected samples were further digested with a final concentration of 500 milliunits/ml chondro-4-sulfatase or chondro-6-sulfatase at 37 °C for 16 h. Alternatively, selected samples were treated with 100 mm NaOH for 10 min at 25 °C and then neutralized with 100 mm HCl.
ELISA
Proteoglycan-enriched samples (50 μg/ml based on Coomassie Blue protein assay) with and without endoglycosidase digestion were coated onto high binding 96-well ELISA plates (Greiner, Australia) for 2 h at 25 °C. Wells were rinsed twice with Dulbecco's phosphate-buffered saline, pH 7.4 (DPBS) followed by blocking with 0.1% (w/v) casein in DPBS for 1 h at 25 °C. Wells were rinsed twice with DPBS with 1% (v/v) Tween 20 (PBST) followed by incubation with primary antibodies diluted in 0.1% (w/v) casein in DPBS for 2 h at 25 °C. Wells were rinsed twice with PBST followed by incubation with biotinylated secondary antibodies (1:1000) diluted in 0.1% (w/v) casein in DPBS for 1 h at 25 °C, rinsed again twice with PBST, and then incubated with streptavidin-HRP (1:500) for 30 min at 25 °C. Binding of the antibodies to the samples was detected using the colorimetric substrate, 2,2′-azinodi-(3-ethylbenzthiazoline sulfonic acid), and absorbance was measured at 405 nm.
A sandwich ELISA was performed by coating ELISA plates with a capture antibody, ab43073, in 0.1 m sodium carbonate buffer, pH 9.6 for 16 h at 4 °C. Wells were rinsed twice with DPBS followed by blocking with 0.1% (w/v) casein in DPBS for 2 h at 25 °C. Wells were rinsed with PBST followed by incubation with the proteoglycan-enriched samples (200 μg/ml based on Coomassie Blue protein assay) for 2 h at 25 °C and subsequent detected with primary and secondary antibodies as for the standard ELISA. Data for both the ELISA and sandwich ELISA were corrected for background absorbance.
Western Blot Analysis
Proteoglycan-enriched samples (200 μg/ml based on Coomassie protein assay) with and without endoglycosidase digestion were electrophoresed in 4–12% (w/v) BisTris gels (Invitrogen) under non-reducing conditions using MES buffer (50 mm MES, 50 mm Tris, 0.1% (w/v) SDS, 1 mm EDTA, pH 7.3) at 200 V for 45 min. A series of molecular mass markers (Precision Plus All Blue, Bio-Rad) was electrophoresed on each gel. Samples were then transferred to polyvinylidene difluoride (PVDF) membrane using transfer buffer (5 mm Bicine, 5 mm BisTris, 0.2 mm EDTA, 50 μg/ml SDS, 10% (v/v) methanol, pH 7.2) in a semidry blotter at 300 mA and 20 V for 60 min. The membrane was blocked with 1% (w/v) bovine serum albumin (BSA) in Tris-buffered saline (TBS) (20 mm Tris base, 136 mm NaCl, pH 7.6) with 0.1% (v/v) Tween 20 (TBST) for 2 h at 25 °C followed by incubation with primary antibody diluted in 1% (w/v) BSA in TBST for 2 h at 25 °C. Membranes were subsequently rinsed with TBST, incubated with secondary HRP-conjugated antibodies (1:50,000) for 45 min at 25 °C, and rinsed with TBST and TBS before being imaged using chemiluminescence reagent (Femto reagent kit, Pierce) and x-ray film.
Mass Spectrometry
DEAE-enriched samples (50 μg/ml) were reduced (10 mm DTT for 10 min at 95 °C), alkylated (25 mm iodoacetamide for 20 min at 25 °C), and electrophoresed on 4–12% (w/v) BisTris gels as described above. The gels were stained with 0.1% (w/v) Coomassie Blue G-250 in 2% (v/v) phosphoric acid, 10% (w/v) ammonium sulfate, 20% (v/v) methanol at 25 °C for 16 h followed by destaining with 50% (v/v) methanol. Gels were imaged prior to excising bands of interest for liquid chromatography-tandem mass spectrometry (LC-MS2) analysis. Excised bands were destained with 50% (v/v) acetonitrile in 50 mm NH4HCO3, washed with 100% (v/v) acetonitrile, and dried in an oven at 50 °C. Trypsin (sequencing grade; Promega, Sydney, Australia) at a final concentration of 20 μg/ml in 50 mm NH4HCO3 was added to the dried bands and incubated at 30 °C for 16 h. Ten microliters of 50 mm NH4HCO3 was then added to each band prior to sonication for 5 min. Twenty microliters of each sample was subjected to peptide analysis by LC-MS2.
Alternatively, samples were prepared for LC-MS2 analysis by in-solution digestion, which involved reduction with 10 mm DTT for 10 min at 95 °C and alkylation with 25 mm iodoacetamide for 20 min at 25 °C. Samples were then incubated with 20 μg/ml sequencing grade trypsin in 50 mm NH4HCO3 at 30 °C for 16 h and subjected to peptide analysis by LC-MS2.
Samples were analyzed by LC-MS2 using an LTQ mass spectrometer (Thermo Fisher Scientific). The results were analyzed with XcaliburTM software (Bioworks version 3.1, Thermo Fisher Scientific) and the Mascot database with a National Center for Biotechnology Information protein (Homo sapiens) database.
Fluorophore-assisted Carbohydrate Electrophoresis
Fluorophore-assisted carbohydrate electrophoresis was performed as described previously (33–35) with slight modification. Standard Δ-disaccharides created by bacterial endoglycosidases were prepared at 250 nm in Milli-Q water and labeled with 5 μl of 100 mm 2-aminoacridone hydrochloride in DMSO with 15% (v/v) glacial acetic acid followed by 5 μl of 1 m sodium cyanoborohydride in DMSO. The solution was mixed gently and incubated for 16 h at 37 °C before the addition of 500 μl of 50% (v/v) filter-sterilized (0.22 μm) glycerol. Equal volumes of samples were combined with the glycerol solution containing 0.001% (w/v) bromphenol blue immediately prior to loading the gel for electrophoresis. Samples were electrophoresed on acrylamide gels consisting of a resolving gel (23.1% (w/v) acrylamide, 1.9% (w/v) bisacrylamide, 187.5 mm Tris borate, pH 8.8, 187.5 mm Tris, pH 8.8, 2.5% (w/w) glycerol) and a stacking gel (4.25% (w/v) acrylamide, 0.75% (w/v) bisacrylamide, 0.36 m Tris, pH 6.3–6.8, 4.4% (w/v) PEG 8000). All equipment and buffers were maintained at 4 °C throughout the experiment. Pre-electrophoresis was performed at 300 V per gel for ∼30 min. Samples and standards were loaded and electrophoresed for 3 h at 330 V per gel until the dye front was ∼10–15 mm from the bottom of the gel. Gels were imaged promptly by UV light using the Gel Doc system (DKSH, Australia). Analysis of the bands was performed by comparing pixel intensity with those of the standard CS ΔDi-0S, ΔDi-4S, and ΔDi-6S disaccharides that were run on each gel.
In-solution Formation of HA·HC Complexes
Hyaluronan (4 μg) was incubated with proteoglycan-enriched fractions of plasma (4 μg) and purified recombinant TSG-6 (2 μg) for 24 h at 37 °C. Prior to incubation with HA, selected plasma samples were incubated with a final concentration of 0.05 units/ml chondroitinase ABC for 16 h at 37 °C. After incubation with HA, selected samples were incubated with 20 units/ml Streptomyces hyaluronidase for 2 h at 37 °C prior to analysis by Western blotting for the presence of bikunin, TSG-6, and HC2 as described above.
HC Transfer Assay Using Immobilized HA
High binding 96-well ELISA plates were coated with 10 μg/ml hyaluronan-binding protein (36) for 1 h at 37 °C, rinsed with DPBS, and then coated with 200 μg/ml 170,000 HA for 2 h at 25 °C. Wells were then blocked with 0.1% (w/v) casein for 1 h at 37 °C, rinsed with PBST, and then incubated with proteoglycan-enriched plasma (5 μg/ml) and/or 2–10 nm TSG-6 for 2 h at 37 °C. Wells were then rinsed with PBST and incubated with primary antibodies against bikunin, HC1, HC2, or TSG-6 and developed as described above for the standard ELISA.
RESULTS
Bikunin Expression and Glycosylation in Different Biological Fluids
Proteoglycan-enriched plasma and urine samples were prepared by a step elution from an anion exchange column using 1 m NaCl and analyzed for the presence of bikunin and CS linkage regions after chondroitinase ABC digestion. Proteoglycan-enriched urine and plasma samples were found to be equally reactive with the bikunin antibodies when analyzed at equal protein concentration (Fig. 1A). Samples were also tested for the presence of sulfated CS linkage regions (un-, 4-, and 6-sulfated) after digestion with chondroitinase ABC. Samples were found to contain the 4-sulfated CS linkage region (Fig. 1B), whereas un- and 6-sulfated CS linkage regions could not be detected (data not shown). The proteoglycan-enriched plasma had low reactivity with the 4-sulfated CS linkage region antibody compared with the proteoglycan-enriched urine. To confirm that the 4-sulfated CS linkage region was attached to bikunin in urine and plasma, a sandwich ELISA was performed after treating the proteoglycan-enriched samples with chondroitinase ABC to remove the CS chains (Fig. 1C). The bikunin antibody was used as a capture antibody, and the presence of the 4-sulfated CS linkage region was detected using the 2B6 antibody. More reactivity was observed with the proteoglycan-enriched urine compared with the proteoglycan-enriched plasma (Fig. 1C), which is in agreement with the ELISA data shown in Fig. 1B.
FIGURE 1.
The presence of bikunin (A) and 4-sulfated CS linkage regions (B) in proteoglycan-enriched fractions of urine and plasma after digestion with chondroitinase ABC as determined by ELISA is shown. C, the presence of 4-sulfated CS linkage regions attached to bikunin in proteoglycan-enriched urine as determined by sandwich ELISA using a bikunin antibody (ab43073) capture and detected with the 4-sulfated CS linkage region antibody (2B6). Data are presented as mean ± S.D. (error bars) (n = 4). Western blot analyses of bikunin and 4-sulfated CS linkage regions in proteoglycan-enriched urine (D) and analyses of bikunin, 4-sulfated CS linkage regions, HC2, and HC1 in proteoglycan-enriched plasma (E) are shown. Samples were analyzed without and with chondroitinase ABC digestion (C'ase ABC) or NaOH treatment.
Bikunin Complexes in Urine and Plasma
The presence of bikunin and the 4-sulfated CS linkage region in proteoglycan-enriched urine was investigated by Western blotting (Fig. 1D). Bikunin was found to contain a protein core with a relative mass (Mr) of 20,000 and was decorated with CS (15,000–25,000). Treatment of proteoglycan-enriched urine with chondroitinase ABC resulted in 4-sulfated CS linkage region reactivity at 20,000, further indicating that the bikunin protein core was likely to be decorated with this linkage region (Fig. 1D). Analysis of bikunin in proteoglycan-enriched plasma indicated that bikunin was predominantly present at Mr 100,000–230,000 (Fig. 1E), indicating that it was present as IαI and PαI/HC-bikunin. IαI and PαI/HC-bikunin were held together by the CS chain as shown by the loss of reactivity between Mr 100,000 and 230,000 after digestion with chondroitinase ABC and the generation of the bikunin protein core (20,000). There were also detectable levels of intermediate bikunin-reactive species (40,000–70,000) in the undigested sample that were sensitive to chondroitinase ABC treatment. Treatment of the proteoglycan-enriched plasma with chondroitinase ABC resulted in the detection of the 4-sulfated CS linkage region at 20,000, indicating that the protein core of bikunin was decorated with the 4-sulfated CS linkage region in plasma (Fig. 1E).
The proteoglycan-enriched plasma was also analyzed for the presence of HC1 and HC2 (Fig. 1E). HC1 was detected at Mr consistent with it being present in IαI as well as HC-bikunin. Treatment of the proteoglycan-enriched plasma with chondroitinase ABC resulted in the loss of IαI and generation of bands at Mr 120,000 and 160,000 consistent with the presence of HC·HC complexes. Treatment of proteoglycan-enriched plasma with NaOH resulted in the loss of IαI and the release of free HC1. HC2 was detected at Mr consistent with it being present in IαI as well as 160,000, which represents HC·HC complexes. Treatment of the proteoglycan-enriched plasma with chondroitinase ABC resulted in the loss of IαI; however, the band at 160,000 remained unchanged. HC2 was detected at Mr 160,000 as well as Mr 85,000, which represents HC released from the complex by NaOH. Together these data suggest that it is difficult to completely digest the CS chain between the HC proteins to release the individual HC proteins (Fig. 1E).
Characterization of Bikunin Isoforms in Urine and Plasma
Analysis of ethanol-precipitated pooled urine by Western blotting demonstrated that bikunin was present as a single band that aligned with the proteoglycan form of bikunin, whereas the protein core of bikunin was not present at detectable levels (Fig. 2A, lane 1). Similarly, the bikunin protein core was not detected in ethanol-precipitated plasma (Fig. 2A, lane 2). Proteoglycan-enriched urine and plasma samples were eluted from the anion exchange column using a linear elution gradient over 36 column volumes and collected in 1-ml fractions to separate molecules based on their acidity. Fractions were analyzed for the presence of bikunin as well as the 4-sulfated CS linkage region by ELISA (Fig. 2, B and C). The fractions that did not react with the 2B6 antibody eluted at low salt concentrations between 0.1 and 0.5 m NaCl were pooled and termed fraction 1. The presence of sulfate groups on the CS chain increases the acidity of molecules, which resulted in the 4-sulfated CS linkage region-reactive species predominantly eluting in the second half of the elution gradient (0.50–0.8 m NaCl) in the urine and plasma samples (Fig. 2, B and C). These were pooled and termed fraction 2. Area under the curve analysis of bikunin reactivity in urine and plasma in fractions 1 and 2 indicated that fraction 2 constituted ∼20 and 40% of the bikunin-containing fractions in urine and plasma, respectively.
FIGURE 2.
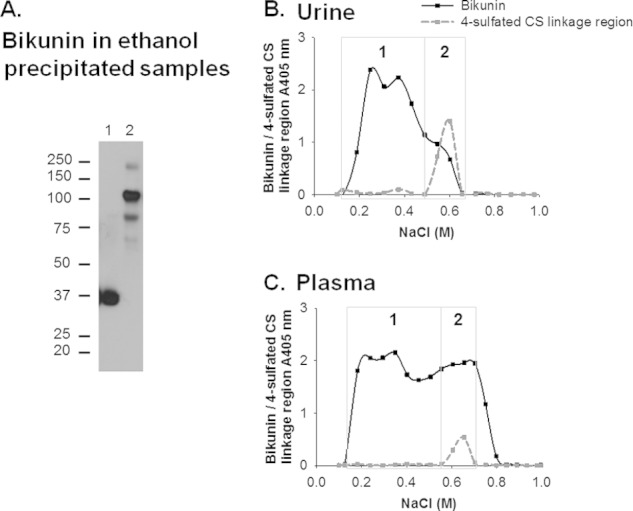
A, Western blot analysis of bikunin in ethanol-precipitated urine (lane 1) and plasma (lane 2). The presence of bikunin and 4-sulfated CS linkage regions in proteoglycan-enriched fractions of urine (B) and plasma (C) separated by anion exchange chromatography with a linear elution gradient of 0.1–1.0 m NaCl is shown. The fractions that were analyzed are denoted by the dots on the curves representing the absorbance of fractions analyzed for the presence of bikunin and 4-sulfated CS linkage regions and assigned their average NaCl concentration. The fractions in the boxed region denoted “1” were pooled and subsequently termed fraction 1, and similarly, the fractions in the boxed region denoted “2” were pooled and subsequently termed fraction 2.
Characterization of the Bikunin CS Chains in Urine
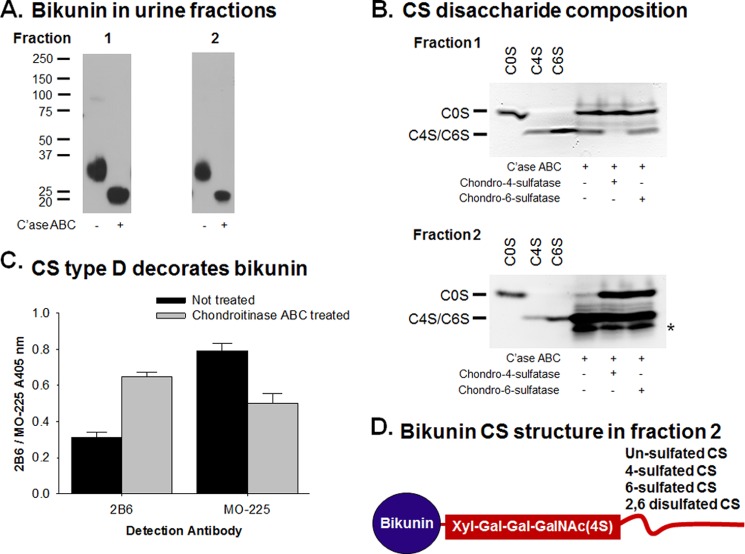
Fractions 1 and 2 of proteoglycan-enriched urine were analyzed for the presence of bikunin before and after chondroitinase ABC digestion by Western blotting (Fig. 3A). Both fractions were found to contain bikunin in the Mr range of 30,000–37,000 that was reduced after digestion with chondroitinase ABC to the bikunin protein core (∼20,000). This indicated that both fractions contained CS attached to bikunin.
FIGURE 3.
A, Western blot analysis of bikunin in proteoglycan-enriched fractions 1 and 2 of urine. Samples were analyzed without and with chondroitinase ABC digestion (C'ase ABC). B, disaccharide compositional analysis of the CS chains in urine fractions 1 and 2 after digestion with chondroitinase ABC and/or chondro-4-sulfatase or chondro-6-sulfatase. Standard unsaturated disaccharides, ΔDi-0S (C0S), ΔDi-4S (C4S), and ΔDi-6S (C6S), were electrophoresed on each gel. Arrows indicate the potential migration of sulfated GalNAc and disulfated disaccharides. * indicates the likely migration of disulfated disaccharides. C, the presence of 4-sulfated CS linkage regions and CS type D attached to bikunin in urine fraction 2 as determined by sandwich ELISA using a bikunin antibody (ab43073) capture and detected with the 4-sulfated CS linkage region antibody (2B6) and the CS type D antibody (MO-225). Data are presented as mean ± S.D. (error bars) (n = 4). D, schematic of bikunin in fraction 2 indicating the 4-sulfated CS linkage region (mAb 2B6 epitope) and the CS chain that contains un-, 4-, 6-, and disulfated CS disaccharides.
The disaccharide composition of the CS in the urine fractions was analyzed using fluorophore-assisted carbohydrate electrophoresis (Fig. 3B). Chondroitinase ABC digestion of each of the samples confirmed the presence of CS as shown by the appearance of disaccharides migrating in a similar way to ΔDi-0S, ΔDi-4S, and ΔDi-6S disaccharide standards. Treatment of fraction 1 with chondro-4-sulfatase resulted in the loss of disaccharides that ran to the same positions as the ΔDi-4S and ΔDi-6S, whereas treatment of this fraction with chondro-6-sulfatase had no effect on the disaccharide migration patterns. This confirmed that fraction 1 contained approximately two-thirds ΔDi-0S and one-third ΔDi-4S disaccharides. The presence of disulfated CS disaccharides was noted in fraction 2 as shown by the band that migrated farther than the ΔDi-6S and ΔDi-4S standards. This disulfated disaccharide was sensitive to both chondro-4- and -6-sulfatase as shown by the reduction in intensity of this band. Fraction 2 was also found to contain ΔDi-4S and ΔDi-6S disaccharides by the use of chondro-4- and -6-sulfatases, which resulted in the generation of ΔDi-0S disaccharides. As fraction 2 contained both mono- and disulfated disaccharides, treatment with either chondro-4- or -6-sulfatase resulted in the generation of ΔDi-0S from the monosulfated disaccharides and of monosulfated disaccharides from the disulfated disaccharides. Hence, there was little overall change in the ΔDi-4S/ΔDi-6S band intensity after treatment with either chondro-4- or -6-sulfatase. The bands that migrated between ΔDi-0S and ΔDi-4S/ΔDi-6S were likely to be 4S-GalNAc and 6S-GalNAc (34). The intensity of bands in each lane was quantified to determine the relative proportions of disaccharides in each of the samples. Fraction 2 contained ∼10% ΔDi-0S, 30% ΔDi-4S, and 20% ΔDi-6S disaccharides and 5% 4S-GalNAc and 6S-GalNAc with the remainder (35%) being disulfated disaccharides that were a mixture of ΔDi-2,4S, ΔDi-2,6S, and ΔDi-4,6S. Although the fractions were not purified for bikunin before disaccharide compositional analysis, peptide identification of proteoglycan-enriched urine by mass spectrometry indicated that bikunin was the most abundant CS proteoglycan present in this sample (Table 1).
TABLE 1.
Proteoglycans present in proteoglycan-enriched urine detected by peptide LC-MS2 from an in-solution tryptic digest and ranked by molecular mass search score (MOWSE score)
| Peptide identified | Protein molecular mass | MOWSE scorea | Peptides matchedb | Sequence coveragec | Accession numberd |
|---|---|---|---|---|---|
| % | |||||
| Chondroitin sulfate proteoglycans | |||||
| Inter-α-trypsin inhibitor light chain bikunin | 38,999 | 301 | 7 | 21.6 | P02760 |
| Bone marrow proteoglycan precursor (proteoglycan 2) | 25,232 | 129 | 4 | 19.8 | P13727 |
| Chondroitin sulfate proteoglycan 5 | 60,058 | 72 | 1 | 2.7 | O95196 |
| Aggrecan core protein precursor | 250,193 | 49 | 1 | 0.7 | P16112 |
| Heparan sulfate proteoglycans | |||||
| Syndecan 4 (amphiglycan) | 21,642 | 471 | 9 | 27.3 | P31431 |
| Heparan sulfate proteoglycan 2 | 468,825 | 174 | 4 | 1.25 | P98160 |
| Syndecan 1 | 32,477 | 57 | 5 | 6.1 | P18827 |
| Glypican | 61,650 | 46 | 1 | 2.9 | P35052 |
a Molecular mass search score as determined by Mascot query. This is the value (p) that is a measure of the probability that the match is a random event expressed as −10log(p). The higher the score, the more confidence that the match is not due to a random event.
b The number of peptide sequences that matched to theoretical sequence in the NCBI database.
c The number of amino acids detected as a proportion of the total number of amino acids in the protein listed in Mascot.
d Obtained from Swiss-Prot.
The CS substructure in fraction 2 was also analyzed by a sandwich ELISA. The bikunin antibody was used as a capture antibody, and the presence of 4-sulfated CS linkage regions and CS type D (2,6-sulfated CS) was detected using mAb MO-225. Fraction 2 was found to contain bikunin decorated with CS type D and a 4-sulfated CS linkage region (Fig. 3C). These data together with the fluorophore-assisted carbohydrate electrophoresis analysis confirmed that bikunin in fraction 2 was decorated with un-, 4-, 6-, and disulfated CS disaccharides with a 4-sulfated CS linkage region. A schematic of bikunin in fraction 2 indicating these CS structural features is shown in Fig. 3D.
Formation of HA·HC Using Bikunin Complexes Is CS Microstructure-dependent
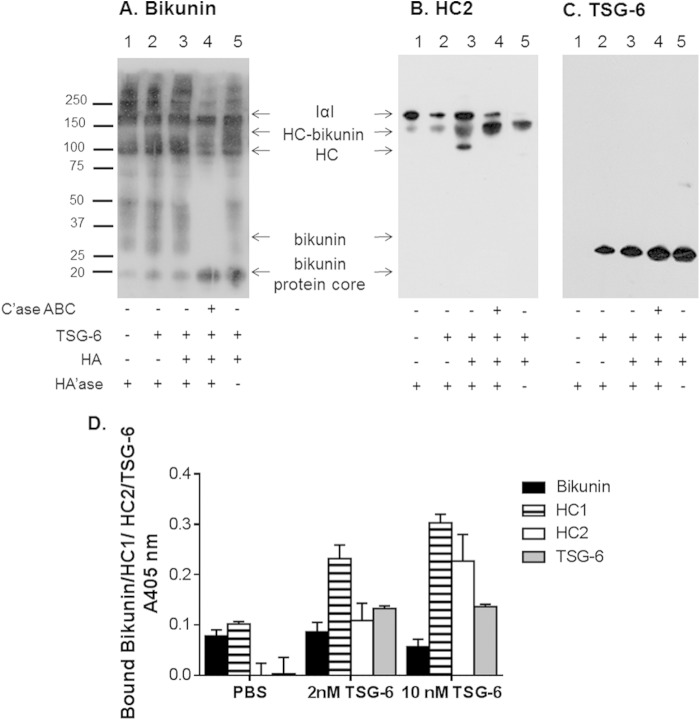
The interaction of IαI and PαI/HC-bikunin present in proteoglycan-enriched plasma with HA was analyzed by Western blotting to determine whether HA·HC could be formed (Fig. 4, A–C). Verification of the formation of HA·HC complexes was performed by monitoring the transfer of HCs from IαI and PαI/HC-bikunin to HA in the presence of TSG-6 (37). It was confirmed that the HA preparations used lacked HCs and TSG-6 (data not shown). Selected samples were treated with hyaluronidase prior to being analyzed by Western blotting to enable HC proteins that had been transferred to HA to enter the SDS-polyacrylamide gel. Hence, the presence of free HC2 was used as a surrogate marker for the formation of HC·HA complexes. Proteoglycan-enriched plasma treated with hyaluronidase or both TSG-6 and hyaluronidase contained IαI and PαI/HC-bikunin (Fig. 4A, lanes 1 and 2), whereas HC2 in both samples remained in IαI and HC2-bikunin (Fig. 4B, lanes 1 and 2). Incubation of proteoglycan-enriched plasma with TSG-6 and HA followed by hyaluronidase digestion did not change the intensity or Mr reactivity of bikunin compared with the Mr reactivity of bikunin in proteoglycan-enriched plasma incubated with TSG-6 and HA only (Fig. 4A, lanes 3 and 5). However, an HC2-reactive band was observed at ∼100,000 consistent with the generation of free HC2 in the proteoglycan-enriched plasma incubated with TSG-6 and HA followed by hyaluronidase treatment, indicating that HC2 was released (Fig. 4B, lane 3). This was not observed in the absence of hyaluronidase treatment (Fig. 4B, lane 5) and demonstrated that HC proteins could be transferred from IαI and HC-bikunin to HA in proteoglycan-enriched plasma in the presence of TSG-6. Proteoglycan-enriched plasma that had been treated with chondroitinase ABC prior to incubation with TSG-6 and HA followed by treatment with hyaluronidase resulted in the generation of the bikunin protein core (Fig. 4A, lane 4) but no change in HC2 reactivity compared with proteoglycan-enriched plasma alone (Fig. 4B, lanes 1 and 4). This demonstrated that HA·HC had not been formed in the absence of CS. TSG-6 was detected at 35,000 in each of the treatments in which it was exogenously added (Fig. 4C).
FIGURE 4.
Western blot analyses of bikunin (A), HC2 (B), and TSG-6 (C) in proteoglycan-enriched plasma after incubation with HA and TSG-6 are shown. Plasma samples were incubated with a combination of HA and TSG-6 for 24 h at 37 °C, and then selected samples were treated with Streptomyces hyaluronidase (HA'ase) for 2 h at 37 °C before being electrophoresed on 4–12% BisTris gels and transferred to PVDF membranes. Selected samples were treated with chondroitinase ABC (C'ase ABC) for 16 h at 37 °C prior to incubation with HA and TSG-6. D, effect of TSG-6 on HC transfer to HA. 5 μg/ml proteoglycan-enriched plasma was incubated alone or with TSG-6 (2 or 10 nm) for 2 h at 37 °C on immobilized HA. Wells were then probed for bikunin, HC1, HC2, or TSG-6 bound to immobilized HA by ELISA. Data are presented as mean ± S.D. (error bars) (n = 3).
The transfer of HCs from IαI and HC-bikunin in proteoglycan-enriched plasma to HA was also analyzed in an ELISA using surface-immobilized HA (Fig. 4D). There was no transfer of HCs from IαI and HC-bikunin to HA in the absence of TSG-6, whereas the addition of TSG-6 dose-dependently promoted the transfer of HCs from IαI and HC-bikunin to HA. The possibility that IαI and/or HC-bikunin was bound to HA was excluded by analyzing the level of HA-bound bikunin that did not rise above background levels for each of the test conditions. Thus, the ELISA confirmed that HCs were transferred from IαI and HC-bikunin in proteoglycan-enriched plasma to HA in the presence of TSG-6.
Interactions among fractions 1 and 2 of proteoglycan-enriched plasma, TSG-6, and HA were also analyzed by Western blotting after treatment with hyaluronidase (Fig. 5). Fraction 1 showed a limited ability to transfer HCs from IαI and HC-bikunin to HA in the presence of TSG-6 (Fig. 5A) even though it was loaded into the gel at the same protein concentration as fraction 2 that was confirmed by densitometric analysis of bikunin in the blots presented in Fig. 5, A and B, lane 1. It is interesting to note that fraction 1 alone contained bikunin at Mr 37,000–50,000, whereas the formation of HA·HC in this fraction did not increase its intensity (Fig. 5A, lanes 1 and 3). Incubation of fraction 1 with HA and TSG-6 also reduced the intensity of HC2 at the Mr for IαI with a concomitant increase in lower HC2 Mr reactivity (Fig. 5A, lane 6), indicating that the trans-esterification process had occurred.
FIGURE 5.
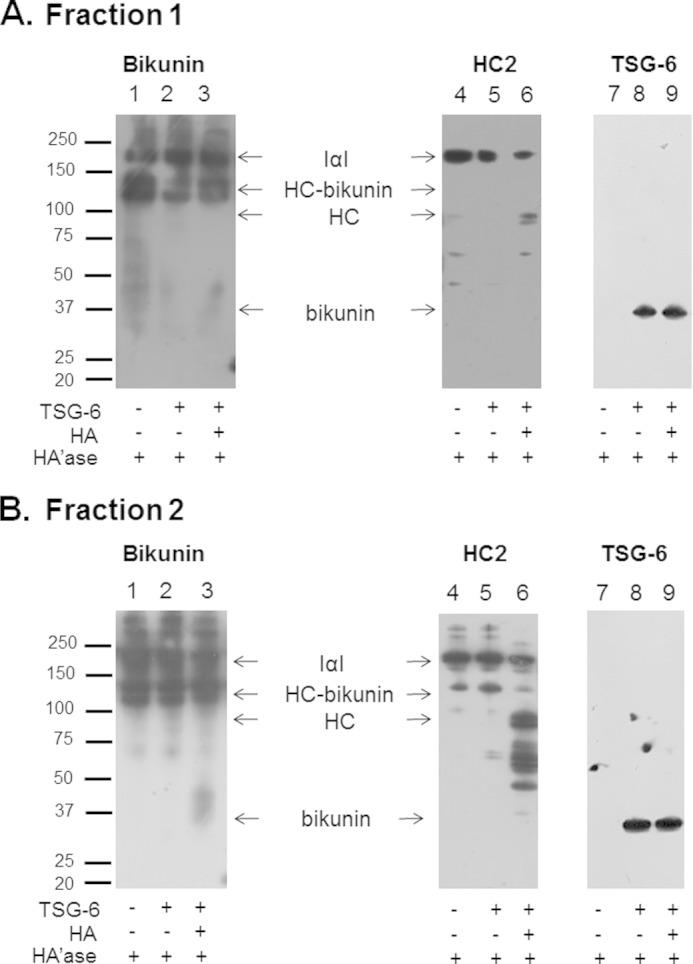
Western blot analyses of bikunin (lanes 1–3), HC2 (lanes 4–6), and TSG-6 (lanes 7–9) reactivity in fractions 1 (A) and 2 (B) of proteoglycan-enriched plasma incubated with TSG-6 and HA. Plasma samples were incubated with TSG-6 or a combination of HA and TSG-6 for 24 h at 37 °C, and then samples were treated with Streptomyces hyaluronidase (HA'ase) for 2 h at 37 °C before being electrophoresed on 4–12% BisTris gels and transferred to PVDF membranes.
Bikunin complexes were present at Mr indicative of IαI and HC-bikunin in fraction 2 and when this fraction had been incubated with TSG-6 (Fig. 5B, lanes 1 and 2). HC2 was predominantly located at the Mr for IαI and HC-bikunin in these same samples (Fig. 5B, lanes 4 and 5). Incubation of fraction 2 with HA and TSG-6 resulted in the release of bikunin observed at Mr ∼37,000 that was not present in fraction 2 after incubation with TSG-6 (Fig. 5B, lanes 2 and 3). HC2 was detected at the Mr for IαI, HC-bikunin, and HC2 alone and at ∼50,000 (Fig. 5B, lane 6) in this same fraction. These changes in Mr of the HC2 bands could be attributed to complexes formed between HC2 and TSG-6; however, analysis of the reactivity of TSG-6 indicated that it was predominantly present at ∼35,000 (Fig. 5B, lane 9), suggesting that the HC2 bands detected at Mr less than bikunin-HC were released due to the trans-esterification of HC from IαI and HC-bikunin to HA and subsequent treatment with hyaluronidase. This indicated that fraction 2 contains a factor involved in the transfer of HCs to HA.
The transfer of HCs from IαI and HC-bikunin in plasma fractions 1 and 2 to surface-immobilized HA was analyzed in the presence or absence of TSG-6 (Fig. 6). Fraction 1 was found to support the transfer of HC1 and HC2 from IαI and HC-bikunin to HA in the presence of TSG-6, whereas fraction 2 when analyzed at the same concentration was able to transfer HC1 and HC2 from IαI and HC-bikunin to HA to a higher extent. Additionally, neither fraction supported the binding of bikunin to HA, indicating that the trans-esterification process of HCs from IαI and HC-bikunin to HA had taken place.
FIGURE 6.
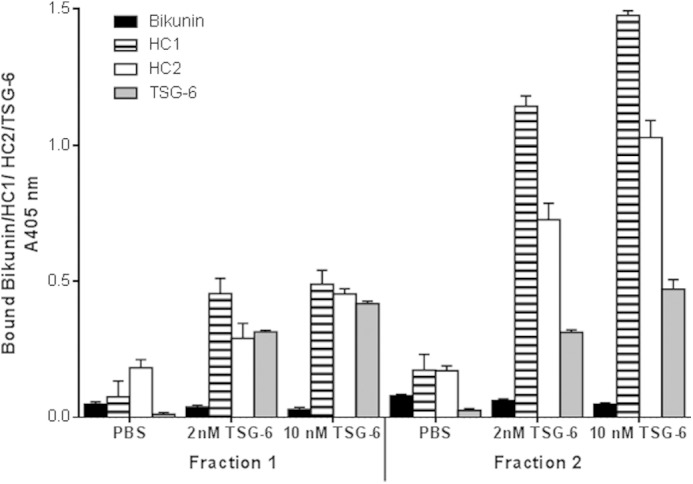
Effect of CS sulfation on HC transfer to immobilized HA. 5 μg/ml proteoglycan-enriched fractions 1 and 2 were incubated alone or with TSG-6 (2 or 10 nm) for 2 h at 37 °C on immobilized HA. Wells were then probed for bikunin, HC1, HC2, or TSG-6 bound to immobilized HA by ELISA. Data are presented as mean ± S.D. (error bars) (n = 3).
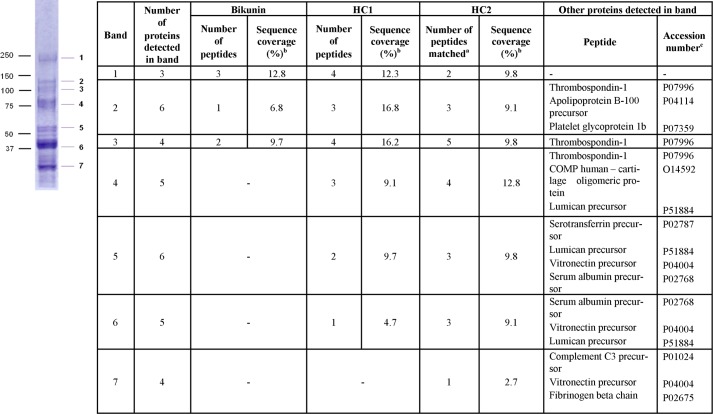
The presence of HC2 fragments in the plasma fractions observed in Fig. 5 was supported by peptide identification of this protein at several Mr analyzed by mass spectrometry. The proteins detected are shown in Fig. 7 and confirm the presence of HC2 as well as HC1 at several Mr between 30,000 and 230,000. Importantly, HC1 and HC2 were detected at Mr ∼50,000, which aligned with the reactivity of fragments of HC2 released upon the trans-esterification of HCs from IαI and HC-bikunin to HA (Fig. 5, A and B, lane 6).
FIGURE 7.
Bikunin (Swiss-Prot accession number P02760), HC1 (Swiss-Prot accession number P19827), and HC2 (Swiss-Prot accession number P19823) detected by peptide LC-MS2 in proteoglycan-enriched plasma excised at multiple Mr as well as other peptides detected in the excised bands. a, the number of peptide sequences that matched to theoretical sequence in the NCBI database; b, the number of amino acids detected as a proportion of the total number of amino acids in the protein listed in Mascot; c, obtained from Swiss-Prot. -, not detected.
DISCUSSION
Bikunin has been extensively studied in urine as it is present in high abundance (15). Urinary levels of bikunin have been reported to change with disease (38), whereas plasma levels have been reported to remain constant except in secondary disorders affecting liver and kidney functions (39), indicating physiological control over bikunin levels in vivo. Proteoglycan-enriched urine and plasma samples analyzed in this study were found to contain the same level of bikunin. IαI and HC-bikunin in plasma were found to contain HC1 and HC2 that could not be completely separated by chondroitinase ABC digestion, a phenomenon that has been reported previously (4, 18, 37, 40, 41). The absence of un- and 6-sulfated CS linkage region structures attached to bikunin has been reported previously (9) and was confirmed in this study. The 4-sulfated CS linkage region structure detected on bikunin in both proteoglycan-enriched urine and plasma analyzed in this study has been reported previously for bikunin in peritoneal fluid (42).
The data presented in this study suggest that bikunin in proteoglycan-enriched urine and plasma does not contain a uniform CS linkage region as not all fractions reacted with the 2B6 antibody, which detects 4-sulfated CS linkage regions. Previous reports of the bikunin CS linkage region structure are based on analysis of urine and plasma that eluted from an anion exchange column with a salt gradient up to 0.5 m NaCl (1, 2, 43). These fractions have been reported to contain the disulfated CS linkage region hexasaccharide structure ΔHexAα1–3GalNAc(4S)β1–4GlcAβ1–3Gal(4S)β1–3Galβ1–4Xyl-ol (24, 25, 44, 45). The 2B6 antibody is known to react with the linkage region hexasaccharide ΔHexAα1–3GalNAc(4S)β1–4GlcAβ1–3Galβ1–3Galβ1–4Xyl-ol (46). Therefore, it seems reasonable to suggest that fraction 1 analyzed in this study contains a disulfated CS linkage region as it eluted from the anion exchange column with a salt gradient up to 0.5 m NaCl, whereas fraction 2 was confirmed to contain the 4-sulfated CS linkage region structure. Interestingly, Yamada et al. (25) only analyzed the CS structure of one of the four IαI fractions separated by Mono Q chromatography. Hence, it is possible to speculate that the four fractions may have displayed different CS linkage region structures, which enabled their separation by this method. Other studies have reported that the CS linkage region hexasaccharide in plasma and urinary bikunin is monosulfated and of the form ΔHexAα1–3GalNAc(4S)β1-4GlcAβ1–3Galβ1–3Galβ1-4Xyl-ol (9, 28). These data agree with fraction 2 analyzed in this study that eluted at salt concentrations higher than 0.5 m NaCl.
Approximately 30 isomers of urinary bikunin have been isolated with differences in the degree of CS chain sulfation and length (23, 47). Separation of urinary bikunin in this study using anion exchange chromatography and subsequent analysis of the disaccharide composition of the CS chains supported the presence of isomers. Urine fraction 2 was found to contain un-, 4-, 6-, and disulfated CS disaccharides, whereas urine fraction 1 was found to contain un- and 4-sulfated CS disaccharides. The CS composition of purified IαI from human plasma has been reported to contain un- and 4-sulfated disaccharides in a ratio of 3:1 (26, 27). This is in agreement with fraction 1 analyzed in this study that was found to be the major population of bikunin in each of the fluids analyzed.
The biological significance of the CS chains that decorate bikunin was investigated in vitro by monitoring the formation of HC·HA. Experiments were performed in the presence or absence of TSG-6 and confirmed that it is required for the formation of HA·HC through intermediate TSG-6·HC complexes (4, 37, 48, 49). The in vitro assays utilized in this study to monitor HC·HA formation used excess purified recombinant TSG-6 to obtain maximal substitution of HA in each of the plasma fractions (18). The Western blot assay analyzed the Mr reactivity of bikunin, HC2, and TSG-6 as both HC2 and TSG-6 have been reported to be necessary for the trans-esterification reaction to occur (37). Analysis of the Mr reactivity of bikunin and HC2 with combinations of chondroitinase ABC and hyaluronidase digestions indicated that the CS chain that decorates bikunin is essential for the formation of HC·HA as reported previously (50). However, incomplete transfer of HCs to HA was demonstrated in this study and has been reported previously (4, 37, 51, 52).
The biological importance of the structural diversity in the CS chain of bikunin remains largely unexplored. The bikunin CS chain has been shown to be longer and less sulfated in inflammatory syndromes (29, 53), which has been hypothesized to facilitate the trans-esterification of the HCs onto HA (54–56). This study has demonstrated for the first time that fraction 2 contained CS structures involved in the formation of HC·HA. Although fraction 1 was also able to facilitate this trans-esterification process both in solution and to immobilized HA, the CS contained in fraction 2 was more effective. HC2 was detected at several Mr in hyaluronidase-digested plasma fraction 2 after incubation with HA, TSG-6, and hyaluronidase using an N-terminal HC2 antibody, indicating that C-terminal truncations were present. The presence of truncated forms of HC2 in plasma was confirmed using mass spectrometry, and truncated forms were also found to be present for HC1. C-terminally truncated forms of HC1 and HC2 of Mr reactivity similar to those observed in this study have been reported in plasma and cartilage (40). HC-bikunin generated by the formation of TSG-6-HC intermediates is reported to break down spontaneously to release bikunin (41). The detection of TSG-6 only at ∼35,000 in this study would indicate that the TSG-6-HC species are also transient.
Fraction 2 analyzed in this study was found to contain ∼35% disulfated CS disaccharides and was demonstrated to be decorated with CS type D, whereas fraction 1 only contained un- and 4-sulfated CS disaccharides. Plasma fraction 2 was more effective in mediating the formation of HA·HC complexes in the presence of TSG-6 than plasma fraction 1. Fraction 2 accounted for ∼40% of the bikunin present in plasma, indicating that this is a biologically significant fraction. This indicates a potential role for highly sulfated CS disaccharides in the formation of TSG-6·HC complexes as these complexes precede HA·HC formation. The role of disulfated CS disaccharides remain to be explored; however, heparin has been shown to interact with TSG-6 through the link module domain and compete with 4-sulfated CS for binding to TSG-6 (57). The binding site for heparin on the link module domain of TSG-6 has been shown to be distinct from the 4-sulfated CS disaccharide binding site on the bikunin CS chain, which overlaps but is not identical to the HA binding groove (16, 57). Interestingly, dermatan 4-sulfate and chondroitin 6-sulfate are not acceptors for TSG-6-mediated HC transfer (52), whereas HCs have been shown to bind to unsulfated CS disaccharides attached to bikunin (26). From the data presented in this study, it can be hypothesized that the disulfated CS disaccharides attached to bikunin are likely to play a role in binding TSG-6 in a conformation that allows transfer of HCs from the unsulfated CS disaccharides to TSG-6. Hence, the sulfation pattern of the CS chain that decorates bikunin determines the extent of TSG-6·HC complex formation, which controls the amount of HC·HA that is subsequently formed.
In summary, these data suggest that there is diversity in the CS chain sulfation of bikunin, including the linkage region. The separation of urine and plasma fractions based on elution from an anion exchange column has enabled further insight into the structure of the bikunin CS chain, revealing heterogeneity in its sulfation. Sulfation of the CS chain attached to bikunin has implications for its biological role because IαI and HC-bikunin containing disulfated CS disaccharides in plasma were able to promote the trans-esterification of HCs to HA in an in vitro assay in the presence of TSG-6 to a larger extent than IαI and HC-bikunin containing only un- and 4-sulfated CS disaccharides. These findings provide further insights into the mechanism by which the HA-rich matrices are stabilized and the role that bikunin CS chain sulfation has in this process.
Acknowledgments
We acknowledge the technical assistance of Anne Simmons and Nicholas Truong.
This work was supported by Australian Research Council Linkage Project Grant LP0455407, Arthritis Research UK Grants 18472 and 19489, and Medical Research Council Grant G0701180.
- CS
- chondroitin sulfate
- IαI
- inter-α-trypsin inhibitor
- HC
- heavy chain
- HA
- hyaluronan
- TSG-6
- tumor necrosis factor α-stimulated gene-6
- PαI
- pre-α-inhibitor
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- Bicine
- N,N-bis(2-hydroxyethyl)glycine
- MS2
- tandem mass spectrometry
- HexA
- hexuronic acid
- GlcA
- glucuronic acid
- Xyl
- xylose
- ΔDi-0S
- unsulfated CS disaccharide
- ΔDi-4S
- 4-sulfated CS disaccharide
- ΔDi-6S
- 6-sulfated CS disaccharide
- ΔDi-2,4S
- 2,4-disulfated CS disaccharide
- ΔDi-2,6S
- 2,6-disulfated CS disaccharide
- ΔDi-4,6S
- 4,6-disulfated CS disaccharide.
REFERENCES
- 1. Proksch G. J., Routh J. I. (1972) The purification of the trypsin inhibitor from human pregnancy urine. J. Lab. Clin. Med. 79, 491–499 [PubMed] [Google Scholar]
- 2. Enghild J. J., Thøgersen I. B., Pizzo S. V., Salvesen G. (1989) Analysis of inter-α-trypsin inhibitor and a novel trypsin inhibitor, pre-α-trypsin inhibitor, from human plasma. Polypeptide chain stoichiometry and assembly by glycan. J. Biol. Chem. 264, 15975–15981 [PubMed] [Google Scholar]
- 3. Enghild J. J., Salvesen G., Thøgersen I. B., Valnickova Z., Pizzo S. V., Hefta S. A. (1993) Presence of the protein-glycosaminoglycan-protein covalent cross-link in the inter-α-inhibitor-related proteinase inhibitor heavy chain 2/bikunin. J. Biol. Chem. 268, 8711–8716 [PubMed] [Google Scholar]
- 4. Rugg M. S., Willis A. C., Mukhopadhyay D., Hascall V. C., Fries E., Fülöp C., Milner C. M., Day A. J. (2005) Characterization of complexes formed between TSG-6 and inter-α-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J. Biol. Chem. 280, 25674–25686 [DOI] [PubMed] [Google Scholar]
- 5. Blom A., Pertoft H., Fries E. (1995) Inter-α-inhibitor is required for the formation of the hyaluronan-containing coat on fibroblasts and mesothelial cells. J. Biol. Chem. 270, 9698–9701 [DOI] [PubMed] [Google Scholar]
- 6. Chen L., Mao S. J., Larsen W. J. (1992) Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. A role for a member of the inter-α-trypsin inhibitor family. J. Biol. Chem. 267, 12380–12386 [PubMed] [Google Scholar]
- 7. Yingsung W., Zhuo L., Morgelin M., Yoneda M., Kida D., Watanabe H., Ishiguro N., Iwata H., Kimata K. (2003) Molecular heterogeneity of the SHAP-hyaluronan complex. Isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J. Biol. Chem. 278, 32710–32718 [DOI] [PubMed] [Google Scholar]
- 8. Kida D., Yoneda M., Miyaura S., Ishimaru T., Yoshida Y., Ito T., Ishiguro N., Iwata H., Kimata K. (1999) The SHAP-HA complex in sera from patients with rheumatoid arthritis and osteoarthritis. J. Rheumatol. 26, 1230–1238 [PubMed] [Google Scholar]
- 9. Enghild J. J., Salvesen G., Hefta S. A., Thøgersen I. B., Rutherfurd S., Pizzo S. V. (1991) Chondroitin 4-sulfate covalently cross-links the chains of the human blood protein pre-α-inhibitor. J. Biol. Chem. 266, 747–751 [PubMed] [Google Scholar]
- 10. Yoneda M., Suzuki S., Kimata K. (1990) Hyaluronic acid associated with the surfaces of cultured fibroblasts is linked to a serum-derived 85-kDa protein. J. Biol. Chem. 265, 5247–5257 [PubMed] [Google Scholar]
- 11. Sandson J., Hamerman D., Schwick G. (1965) Altered properties of pathological hyaluronate due to a bound inter-α trypsin inhibitor. Trans. Assoc. Am. Physicians 78, 304–313 [PubMed] [Google Scholar]
- 12. Zhuo L., Yoneda M., Zhao M., Yingsung W., Yoshida N., Kitagawa Y., Kawamura K., Suzuki T., Kimata K. (2001) Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J. Biol. Chem. 276, 7693–7696 [DOI] [PubMed] [Google Scholar]
- 13. Fülöp C., Szántó S., Mukhopadhyay D., Bárdos T., Kamath R. V., Rugg M. S., Day A. J., Salustri A., Hascall V. C., Glant T. T., Mikecz K. (2003) Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 130, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 14. Gerdin B., Hällgren R. (1997) Dynamic role of hyaluronan (HYA) in connective tissue activation and inflammation. J. Intern. Med. 242, 49–55 [DOI] [PubMed] [Google Scholar]
- 15. Zhuo L., Salustri A., Kimata K. (2002) A physiological function of serum proteoglycan bikunin: the chondroitin sulfate moiety plays a central role. Glycoconj. J. 19, 241–247 [DOI] [PubMed] [Google Scholar]
- 16. Parkar A. A., Day A. J. (1997) Overlapping sites on the link module of human TSG-6 mediate binding to hyaluronan and chrondroitin-4-sulphate. FEBS Lett. 410, 413–417 [DOI] [PubMed] [Google Scholar]
- 17. Day A. J., de la Motte C. A. (2005) Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 26, 637–643 [DOI] [PubMed] [Google Scholar]
- 18. Sanggaard K. W., Scavenius C., Rasmussen A. J., Wisniewski H.-G., Thogersen I. B., Enghild J. J. (2010) The TSG-6/HC2-mediated transfer is a dynamic process shuffling heavy chains between glycosaminoglycans. J. Biol. Chem. 285, 21988–21993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hutadilok N., Ghosh P., Brooks P. M. (1988) Binding of haptoglobin, inter-α-trypsin inhibitor, and α1 proteinase inhibitor to synovial fluid hyaluronate and the influence of these proteins on its degradation by oxygen derived free radicals. Ann. Rheum. Dis. 47, 377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhuo L., Kanamori A., Kannagi R., Itano N., Wu J., Hamaguchi M., Ishiguro N., Kimata K. (2006) SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem. 281, 20303–20314 [DOI] [PubMed] [Google Scholar]
- 21. Kanayama S., Yamada Y., Onogi A., Shigetomi H., Ueda S., Tsuji Y., Haruta S., Kawaguchi R., Yoshida S., Sakata M., Sado T., Kitanaka T., Oi H., Yagyu T., Kobayashi H. (2008) Molecular structure and function analysis of bikunin on down-regulation of tumor necrosis factor-α expression in activated neutrophils. Cytokine 42, 191–197 [DOI] [PubMed] [Google Scholar]
- 22. He H., Li W., Tseng D. Y., Zhang S., Chen S.-Y., Day A. J., Tseng S. C. (2009) Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-α-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J. Biol. Chem. 284, 20136–20146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kakizaki I., Takahashi R., Ibori N., Kojima K., Takahashi T., Yamaguchi M., Kon A., Takagaki K. (2007) Diversity in the degree of sulfation and chain length of the glycosaminoglycan moiety of urinary trypsin inhibitor isomers. Biochim. Biophys. Acta 1770, 171–177 [DOI] [PubMed] [Google Scholar]
- 24. Yamada S., Oyama M., Yuki Y., Kato K., Sugahara K. (1995) The uniform galactose 4-sulfate structure in the carbohydrate-protein linkage region of human urinary trypsin inhibitor. Eur. J. Biochem. 233, 687–693 [DOI] [PubMed] [Google Scholar]
- 25. Yamada S., Oyama M., Kinugasa H., Nakagawa T., Kawasaki T., Nagasawa S., Khoo K. H., Morris H. R., Dell A., Sugahara K. (1995) The sulphated carbohydrate-protein linkage region isolated from chondroitin 4-sulphate chains of inter-α-trypsin inhibitor in human plasma. Glycobiology 5, 335–341 [DOI] [PubMed] [Google Scholar]
- 26. Enghild J. J., Thøgersen I. B., Cheng F., Fransson L. A., Roepstorff P., Rahbek-Nielsen H. (1999) Organization of the inter-α-inhibitor heavy chains on the chondroitin sulfate originating from Ser10 of bikunin: posttranslational modification of IαI-derived bikunin. Biochemistry 38, 11804–11813 [DOI] [PubMed] [Google Scholar]
- 27. Chi L., Wolff J. J., Laremore T. N., Restaino O. F., Xie J., Schiraldi C., Toida T., Amster I. J., Linhardt R. J. (2008) Structural analysis of bikunin glycosaminoglycan. J. Am. Chem. Soc. 130, 2617–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toyoda H., Kobayashi S., Sakamoto S., Toida T., Imanari T. (1993) Structural analysis of a low-sulfated chondroitin sulfate chain in human urinary trypsin inhibitor. Biol. Pharm. Bull. 16, 945–947 [DOI] [PubMed] [Google Scholar]
- 29. Mizon C., Mairie C., Balduyck M., Hachulla E., Mizon J. (2001) The chondroitin sulfate chain of bikunin-containing proteins in the inter-α-inhibitor family increases in size in inflammatory diseases. Eur. J. Biochem. 268, 2717–2724 [DOI] [PubMed] [Google Scholar]
- 30. Fujimoto T., Savani R. C., Watari M., Day A. J., Strauss J. F. (2002) Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-α and prostaglandin E2. Am. J. Pathol. 160, 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nentwich H. A., Mustafa Z., Rugg M. S., Marsden B. D., Cordell M. R., Mahoney D. J., Jenkins S. C., Dowling B., Fries E., Milner C. M., Loughlin J., Day A. J. (2002) A novel allelic variant of the human TSG-6 gene encoding an amino acid difference in the CUB module. Chromosomal localization, frequency analysis, modeling, and expression. J. Biol. Chem. 277, 15354–15362 [DOI] [PubMed] [Google Scholar]
- 32. Melrose J., Numata Y., Ghosh P. (1996) Biotinylated hyaluronan: a versatile and highly sensitive probe capable of detecting nanogram levels of hyaluronan binding proteins (hyaladherins) on electroblots by a novel affinity detection procedure. Electrophoresis 17, 205–212 [DOI] [PubMed] [Google Scholar]
- 33. Lehrman M. A., Gao N. (2003) Alternative and sources of reagents and supplies of fluorophore-assisted carbohydrate electrophoresis (FACE). Glycobiology 13, 1G–3G [DOI] [PubMed] [Google Scholar]
- 34. Calabro A., Benavides M., Tammi M., Hascall V. C., Midura R. J. (2000) Microanalysis of enzyme digests of hyaluronan and chondroitin/dermatan sulfate by fluorophore-assisted carbohydrate electrophoresis (FACE). Glycobiology 10, 273–281 [DOI] [PubMed] [Google Scholar]
- 35. Klock J. C., Starr C. M. (1998) Polyacrylamide gel electrophoresis of fluorophore-labeled carbohydrates from glycoproteins. Methods Mol. Biol. 76, 115–129 [DOI] [PubMed] [Google Scholar]
- 36. Melrose J. (2001) Cartilage and smooth muscle cell proteoglycans detected by affinity blotting using biotinylated hyaluronan. Methods Mol. Biol. 171, 53–66 [DOI] [PubMed] [Google Scholar]
- 37. Sanggaard K. W., Sonne-Schmidt C. S., Krogager T. P., Lorentzen K. A., Wisniewski H. G., Thøgersen I. B., Enghild J. J. (2008) The transfer of heavy chains from bikunin proteins to hyaluronan requires both TSG-6 and HC2. J. Biol. Chem. 283, 18530–18537 [DOI] [PubMed] [Google Scholar]
- 38. Pugia M. J., Lott J. A. (2005) Pathophysiology and diagnostic value of urinary trypsin inhibitors. Clin. Chem. Lab. Med. 43, 1–16 [DOI] [PubMed] [Google Scholar]
- 39. Itoh Y., Kawai T. (1990) Human α1-microglobulin: its measurement and clinical significance. J. Clin. Lab. Anal. 4, 376–384 [DOI] [PubMed] [Google Scholar]
- 40. Yoshihara Y., Plaas A., Osborn B., Margulis A., Nelson F., Stewart M., Rugg M. S., Milner C. M., Day A. J., Nemoto K., Sandy J. D. (2008) Superficial zone chondrocytes in normal and osteoarthritic human articular cartilages synthesize novel truncated forms of inter-α-trypsin inhibitor heavy chains which are attached to a chondroitin sulfate proteoglycan other than bikunin. Osteoarthritis Cartilage 16, 1343–1355 [DOI] [PubMed] [Google Scholar]
- 41. Forteza R., Casalino-Matsuda S. M., Monzon M. E., Fries E., Rugg M. S., Milner C. M., Day A. J. (2007) TSG-6 potentiates the antitissue kallikrein activity of inter-α-inhibitor through bikunin release. Am. J. Respir. Cell Mol. Biol. 36, 20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thomas G. J., Yung S., Davies M. (1998) Bikunin present in human peritoneal fluid is in part derived from the interaction of serum with peritoneal mesothelial cells. Am. J. Pathol. 153, 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ochiai H., Toyoda H., Onodera M. (1988) Analysis of chondroitin sulfates in human urinary trypsin inhibitor. Chem. Pharm. Bull. 36, 3726–3727 [DOI] [PubMed] [Google Scholar]
- 44. Ly M., Leach F. E., 3rd, Laremore T. N., Toida T., Amster I. J., Linhardt R. J. (2011) The proteoglycan bikunin has a defined sequence. Nat. Chem. Biol. 7, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laremore T. N., Leach F. E., 3rd, Amster I. J., Linhardt R. J. (2011) Electrospray ionization Fourier transform mass spectrometric analysis of intact bikunin glycosaminoglycan from normal human plasma. Int. J. Mass Spectrom. 305, 109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Couchman J. R., Caterson B., Christner J. E., Baker J. R. (1984) Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307, 650–652 [DOI] [PubMed] [Google Scholar]
- 47. Yuki Y., Nomura K., Kirihara M., Shimomura M., Hiratani H., Nishimura R., Kato K. (1993) Charge isomers of urinary bikunin (trypsin inhibitor). Biochim. Biophys. Acta 1203, 298–303 [DOI] [PubMed] [Google Scholar]
- 48. Lee T. H., Wisniewski H. G., Vilcek J. (1992) A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J. Cell Biol. 116, 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen L., Zhang H., Powers R. W., Russell P. T., Larsen W. J. (1996) Covalent linkage between proteins of the inter-α-inhibitor family and hyaluronic acid is mediated by a factor produced by granulosa cells. J. Biol. Chem. 271, 19409–19414 [DOI] [PubMed] [Google Scholar]
- 50. Sanggaard K. W., Sonne-Schmidt C. S., Jacobsen C., Thøgersen I. B., Valnickova Z., Wisniewski H. G., Enghild J. J. (2006) Evidence for a two-step mechanism involved in the formation of covalent HC x TSG-6 complexes. Biochemistry 45, 7661–7668 [DOI] [PubMed] [Google Scholar]
- 51. Colón E., Shytuhina A., Cowman M. K., Band P. A., Sanggaard K. W., Enghild J. J., Wisniewski H. G. (2009) Transfer of inter-α-inhibitor heavy chains to hyaluronan by surface-linked hyaluronan-TSG-6 complexes. J. Biol. Chem. 284, 2320–2331 [DOI] [PubMed] [Google Scholar]
- 52. Mukhopadhyay D., Asari A., Rugg M. S., Day A. J., Fülöp C. (2004) Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-α-trypsin inhibitor to hyaluronan: implications for the assembly of the cumulus extracellular matrix. J. Biol. Chem. 279, 11119–11128 [DOI] [PubMed] [Google Scholar]
- 53. Capon C., Mizon C., Lemoine J., Rodié-Talbère P., Mizon J. (2003) In acute inflammation, the chondroitin-4 sulphate carried by bikunin is not only longer, it is also undersulphated. Biochimie 85, 101–107 [DOI] [PubMed] [Google Scholar]
- 54. Sanggaard K. W., Karring H., Valnickova Z., Thøgersen I. B., Enghild J. J. (2005) The TSG-6 and IαI interaction promotes a transesterification cleaving the protein-glycosaminoglycan-protein (PGP) cross-link. J. Biol. Chem. 280, 11936–11942 [DOI] [PubMed] [Google Scholar]
- 55. Zhu L., Zhuo L., Watanabe H., Kimata K. (2008) Equivalent involvement of inter-α-trypsin inhibitor heavy chain isoforms in forming covalent complexes with hyaluronan. Connect. Tissue Res. 49, 48–55 [DOI] [PubMed] [Google Scholar]
- 56. Bost F., Diarra-Mehrpour M., Martin J. P. (1998) Inter-α-trypsin inhibitor proteoglycan family—a group of proteins binding and stabilizing the extracellular matrix. Eur. J. Biochem. 252, 339–346 [DOI] [PubMed] [Google Scholar]
- 57. Mahoney D. J., Mulloy B., Forster M. J., Blundell C. D., Fries E., Milner C. M., Day A. J. (2005) Characterization of the interaction between tumor necrosis factor-stimulated gene-6 and heparin: implications for the inhibition of plasmin in extracellular matrix microenvironments. J. Biol. Chem. 280, 27044–27055 [DOI] [PubMed] [Google Scholar]