Abstract
Activation of factor XI (FXI) by thrombin in vivo plays a role in coagulation by providing an important positive feedback mechanism for additional thrombin generation. FXI is activated in vitro by thrombin, or FXIIa in the presence of dextran sulfate. In this report, we investigated the effect of β2-glycoprotein I (β2GPI) on the activation of FXI. β2GPI bound FXI in vitro and inhibited its activation to FXIa by thrombin and FXIIa. The affinity of the interaction between β2GPI and FXI was equivalent to the interaction between FXI and high molecular weight kininogen. Inhibition of FXI activation occurred with lower concentrations of β2GPI than found in human plasma. Proteolytic clipping of β2GPI by plasmin abolished its inhibition of FXI activation. The results suggest a mechanism of regulation whereby physiological concentrations of β2GPI may attenuate thrombin generation in vivo by inhibition of FXI activation. Plasmin cleavage of β2GPI provides a negative feedback that counteracts its inhibition of FXI activation.
β2 -Glycoprotein I (β2GPI) (also known as Apolipoprotein H, Apo H) is a plasma glycoprotein of 50 kDa that circulates in plasma at ≈4 μM. β2GPI has 326 amino acids and consists of repeated sequences in a form typical of the complement control protein (CCP) module. Individual modules are also known as short consensus repeats (SCR), a key feature of which is disulphide bridges joining the first to third and second to fourth cysteine residues (1-4). The first four domains have four cysteines and ≈60 aa each. The fifth domain contains an extra disulphide bond and C-terminal extension of 20 aa where the terminating cysteine forms a disulphide bridge (5). β2GPI has affinity for negative charged macromolecules such as anionic phospholipids and proteoglycans. Within domain 5 the region Cys-281-Cys-288 is critical for phospholipid and heparin binding and is highly conserved (6-10). The C-terminal extension in the fifth domain is surface exposed and susceptible to proteolytic cleavage (9). We have previously reported that β2GPI is proteolytically clipped between Lys-317 and Thr-318 in the fifth domain abolishing binding to anionic phospholipids (7). The cleavage at Lys-317-Thr-318 is generated in vitro by plasmin and at low efficiency by activated factor X (FXa) and in vivo in pathological states of increased fibrinolysis (11-13).
β2GPI is the primary target antigen recognized by autoantibodies in patients with the antiphospholipid syndrome (APS) (14, 15). APS is characterized by recurrent thrombotic events, miscarriages, thrombocytopenia, and the presence of antiphospholipid antibodies (6, 16, 17). Binding of autoantibodies to β2GPI is now generally accepted as an important feature of APS, and a number of studies have shown there is a significant correlation between thrombotic manifestations and the presence of anti-β2GPI antibodies (17). Although the physiological function of β2GPI in normal individuals remains to be elucidated, plasma from β2GPI knockout mice exhibits impaired thrombin generation in vitro (18). β2GPI has both pro- and anticoagulant properties when examined in vitro. It inhibits the generation of FXa in the presence of activated platelets (19), prothrombinase activity (20), and the ADP-mediated aggregation of platelets (21). The in vitro anticoagulant activity of activated protein C (APC) is also inhibited by β2GPI (22). Furthermore, it has been proposed that β2GPI can influence the activity of lipoprotein lipase (23) and apoptosis (24). The association of β2GPI with multiple procoagulant and anticoagulant effects may result from its ability to bind anionic phospholipids on activated platelets and endothelial cells. β2GPI may act by competing with coagulation factors for phospholipid surfaces (17).
The contact pathway of coagulation is initiated with activation of FXII by contact with negatively charged surfaces (25). FXIIa in turn cleaves FXI to FXIa in the presence of high molecular weight kininogen (HK) and prekallikreine (PK). Although previous studies showed that β2GPI inhibits the phospholipid mediated autoactivation of FXII (26) and the contact activation pathway of coagulation (27), the mechanism by which β2GPI acts on this pathway has not been determined. We now report that β2GPI binds FXI in vitro and inhibits activation to FXIa by thrombin and FXIIa. In vivo activation of FXI by thrombin is thought to be an important mechanism by which coagulation is accelerated via components of the contact activation pathway. Thus, β2GPI may attenuate the contact activation pathway by inhibiting activation of FXI by thrombin. Moreover, because β2GPI is the dominant autoantigen in patients with APS, dysregulation of this pathway by autoantibodies may be an important mechanism for thrombosis in patients with APS.
Experimental Procedures
Proteins. Plasma-derived FXI, FXIa, HK (single chain), and FXIIa were purchased from Calbiochem-Novabiochem. Substrates S2366 and S2302 were obtained from Chromogenix Instrumentation Laboratory (Milan, Italy). Spectrozyme TH was acquired from American Diagnostica (Greenwich, CT). Kaolin was purchased from Gradipore (Sydney). Hirudin, thrombin, polybrene (Hexadimethrine Bromide), human serum albumin (HSA), BSA, plasmin, phosphatidylserine (PS), and dextran sulfate (DS) (500,000 Da) were purchased from Sigma. β2GPI-deficient plasma was purchased from Affinity Biologicals (Ancaster, ON, Canada). Plasma-derived native β2GPI (nβ2GPI) was purchased from Haematologic Technologies (Essex Junction, VT) or purified in our laboratory by using cardiolipin affinity chromatography as described (14) or by a sequential protocol with perchloric acid precipitation (28), cation exchange chromatography (14), heparin affinity chromatography (28), and gel filtration with Sephacryl S-300. A preparation of nβ2GPI proteolytically clipped by plasmin at Lys-317-Thr-318 was generated as described (10). Recombinant human β2GPI (rhβ2GPI) was produced as described (9, 29).
Methods. Radiolabeling of FXI or rhβ2GPI with 125iodine. FXI and rhβ2GPI were radiolabeled with 125iodine by using the IODOGENE method (IODO-Beads, Pierce) according to the manufacturer's instructions.
Protein determination. Concentrations of 125I-FXI and 125I-rhβ2GPI were determined by using a Micro BCA protein assay kit (Pierce) according to the instructions provided by the manufacturer.
Specific radioactivity. Specific radioactivity was determined as described by Baird and Walsh (30). The specific activity of 125I-FXI was 2.12 × 1018 cpm/mol and that of 125I-rhβ2GPI was 1.36 × 1018cpm/mol. SDS/PAGE analysis of the iodinated proteins revealed only one radioactive band, indicating that there was no contamination of FXI with FXIa.
Binding of 125I-FXI to native and recombinant β2GPI. Binding of 125I-FXI to immobilized rhβ2GPI was performed by using Lockwell microtiter plates (Nunc) as described (31). The microtiter wells were coated with 100 μl of nβ2GPI, rhβ2GPI, or HSA (3.12-200 nM) by incubation overnight at 4°C. Plates were washed five times with PBS/0.1%Tween-20 (PBST) by using an automated microplate washer (Beckman Coulter). The wells were blocked with 2% BSA/PBST for 2 h at 25°C and then washed five times with PBST and five times with PBS. One hundred microliters of 125I-FXI (280 pM) in 0.5%BSA/PBS was added to individual wells and incubated for 4-5 h at 25°C. The wells were washed five times with 0.5% BSA/PBS, air dried, and counted in a γ counter. The number of cpm bound were measured and converted to fmol of FXI bound.
Saturation binding of 125I-FXI to β2GPI and HK. Saturation binding of 125I-FXI to immobilized β2GPI or HK (single chain) was performed by using Lockwell microtiter plates. The wells were coated with 100 μl of rhβ2GPI, nβ2GPI, HK, or BSA (100 nM) by incubation overnight at 4°C. The plate was washed as described above, then 100 μl of various concentrations (0.08-40 nM) of 125I-FXI was added and processed as above. A dissociation constant (Kd) was calculated by nonlinear regression (graphpad prism 3.03, GraphPad, San Diego).
Competitive inhibition of 125I-FXI binding to immobilized rhβ2GPI by using fluid phase β2GPI. Effects of nβ2GPI and rhβ2GPI on the binding of 125I-FXI to rhβ2GPI were studied by using Lockwell microtiter plates, the wells of which were coated with 100 μl of rhβ2GPI (50 nM) by incubation overnight at 4°C. The wells were washed and blocked as described above, then 50 μl of either β2GPI or BSA (1.3 nM-20 μM in 0.5% BSA/PBS) was added with 50 μl of 125I-FXI (560 pM) and the wells were incubated 4-5 h at 25°C. The wells were thoroughly washed with 0.5% BSA/PBS, air dried, and counted in a γ counter. The number of cpm bound were measured and converted to percentage of total binding by dividing by the cpm bound in the absence of β2GPI competitor. The IC50 was calculated by nonlinear regression (one site binding model, graphpad prism 3.03).
Amidolytic assay of thrombin, FXIa, and FXIIa in the presence of β2GPI. To determine whether β2GPI affects the amidolytic assay of thrombin, FXIIa, or FXIa, incubations were carried out by using the chromogenic substrates Spectrozyme TH, S2302, and S2366 in the presence of thrombin, FXIIa, or FXIa, respectively. Briefly, thrombin (2 nM), FXIIa (20 nM), or FXIa (0.83 nM) was incubated with β2GPI or BSA (10 μM) for 5 min at 37°C. Incubation mixtures were then diluted 1:5 with TBSA (50 mM Tris·HCl/150 mM NaCl/0.1% BSA, pH 7.6) and 100 μl of each substrate (Spectrozyme TH, 0.50 mM; S2302, 0.24 mM; and S2366, 1.2 mM) was added to 100 μl of the incubation mixture. The reactions were incubated for 1 h at 25°C, and the optical density was measured at 405 nm by using a Microplate Scanning Spectrophotometer (Bio-Tek Instruments, Winooski, VT).
Activation of FXI by thrombin or FXIIa in the presence of DS. Activation of FXI by thrombin or FXIIa was carried out in TBSA with various concentrations of DS (500,000 Da) as described (32, 33), with some modifications. Briefly, FXI (60 nM) was mixed with rhβ2GPI or BSA (1 μM) and various concentrations of DS (0-5 μg/ml) in TBSA. The mixtures were then incubated for 5 min at 37°C, followed by addition of thrombin (2 nM) or FXIIa (2 nM) and incubation for 5-10 min at 37°C. Reactions were stopped by chilling on ice and diluting 1:5 with TBS. For the reactions using thrombin as activator, hirudin (final concentration, 25 units/ml) was added to quench thrombin activity. For the reactions using FXIIa as activator, polybrene (final concentration, 200 μg/ml) was added to neutralize the DS. One hundred-microliter aliquots of each reaction mixture were dispensed into individual microtiter wells and mixed with 100 μl of S2366 (1.2 mM). The optical density was measured at 405 nm by using a Microplate Scanning Spectrophotometer. The amount of FXIa generated was derived from a standard curve constructed with known concentrations of FXIa. There was a linear correlation between OD405 and the amount of FXIa that was 0.06-0.32 pmol (r2 = 0.9984) with thrombin as activator and 0.02-0.1 pmol (r2 = 0.9950) with FXIIa as activator.
Activation of FXI by thrombin or FXIIa: Effect of rhβ2GPI. Activation of FXI by thrombin or FXIIa was carried out in TBSA as described by Baglia and Walsh (32) with some modifications. Briefly, FXI (60 nM), DS (1 μg/ml), and various concentrations of rhβ2GPI or BSA (0.016-10 μM) in TBSA were mixed together and incubated for 5 min at 37°C, followed by the addition of thrombin (2 nM) or FXIIa (2 nM) and incubation for a further 5-10 min at 37°C. Reaction mixtures and results were treated as described above. Data fitted a one site binding model, and IC50 was calculated by nonlinear regression (graphpad prism 3.03).
Activation of FXI by thrombin: Effect of nβ2GPI and clipped (c) nβ2GPI. Because β2GPI proteolytically cleaved between Lys-317 and Thr-318 abolishes its anionic phospholipid binding, we assessed the binding of cnβ2GPI to FXI and its effect on thrombin induced activation of FXI. Binding of 125I-FXI (25 nM) to immobilized intact and cnβ2GPI (100 nM) was performed as indicated above. Activation of FXI by thrombin in the presence of nβ2GPI or cnβ2GPI (1 μM) and quantitation of generated FXIa was performed as described above.
Activation of 125I-FXI in β2GPI-deficient human plasma. Activation of FXI was assessed in human plasma deficient in β2GPI in the presence of kaolin, which activates FXII. Activation of FXI was assessed by a semiquantitative analysis of 125I-FXIa according to the protocol described by Brunnee et al. (34). FXI was radiolabeled with 125Iodine as described above to a specific activity of 5.5 × 1016 cpm/mol. β2GPI-deficient human plasma was reconstituted by the addition of rhβ2GPI or BSA to a final concentration of 1.5 μM, then 125I-FXI was added to the reconstituted plasma at a final concentration of 2 nM. The mixtures were incubated for 1 min at 37°C followed by adding an equal volume of Kaolin diluted 2-fold in Tris·HCl buffer (50 mM Tris·HCl/150 mM NaCl, pH 7.6). The mixtures were incubated at 37°C, and at defined time points, 5-μl aliquots were removed and the reaction was stopped by snap freezing the samples. Aliquots at each time point were snap thawed into SDS/PAGE (4-12%) and run under reducing conditions to resolve 125I-FXI and 125I-FXIa heavy and light chains (NuPAGE, Invitrogen). The gel was dried under vacuum for 45-60 min at 70°C (543 Gel Dryer, Bio-Rad), and autoradiography was performed with Kodak BioMax MS film (Amersham Pharmacia) with a Kodak regular X-OMATIC intensifying screen. The film was developed, and densitometry was performed with a Bio-Rad Gel Doc 2000 analysis system. The relative intensity of the bands was calculated from the areas under the peaks on plots of optical density versus migration distance. The amount of 125I-FXIa (heavy plus light chains) was derived from a standard curve constructed with known concentrations of 125I-FXI (r2 = 0.976).
Saturation binding of 125I-rhβ2GPI to DS and an anti-β2GPI antibody. Saturation binding of 125I-rhβ2GPI to immobilized DS and anti-β2GPI antibody (moAb1) (35) was performed by using Lockwell microtiter plates, the wells of which were coated with 100 μl of moAb1, DS, or BSA (100 nM) by incubation overnight at 4°C. The plate was washed five times with PBS and residual binding sites in the wells were blocked with 2% BSA/PBS for 2 h at 25°C. The plate was washed five times with PBS. One hundred microliters of 125I-rhβ2GPI (1.56-200 nM) in 0.5% BSA/PBS was added to individual wells and incubated for 4 h at 25°C. The wells were thoroughly washed with 0.5% BSA/PBS, air dried, and counted in a γ counter. The number of cpm were measured and converted to pmol of the rhβ2GPI bound. The Kd was calculated by nonlinear regression (graphpad prism 3.03).
Binding of 125I-rhβ2GPI to PS or DS. 125I-rhβ2GPI (20 nM) binding to immobilized PS, BSA, or DS was performed by using Lockwell microtiter plates, the wells of which were coated with 100 μl of PS (5 μg/ml), DS (5 μg/ml), or BSA (5 μg/ml) by incubation overnight at 4°C. The plate was then processed as indicated above for 125I-rhβ2GPI binding to DS. The number of cpm bound were measured and converted to pmol of β2GPI bound.
Statistical analysis. Data are expressed as mean ± SD. Differences between groups were evaluated by using Student's t test.
Results
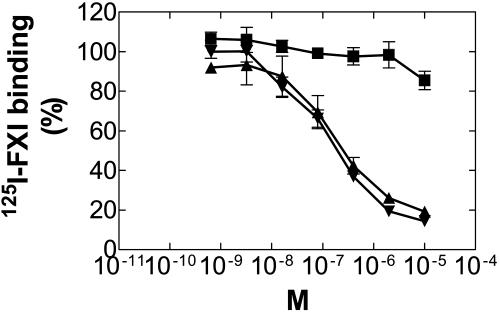
FXI Binds Immobilized Native and Recombinant β2GPI. 125I-FXI bound to rhβ2GPI, nβ2GPI, and HK (data not shown) in a dose-dependent manner, reaching a plateau at 50-100 nM of protein (Fig. 1).
Fig. 1.
Binding of 125I-FXI to rhβ2GPI and nβ2GPI. A constant concentration of 125I-FXI was incubated in microtiter wells that were coated with serial dilutions of rhβ2GPI (▴), nβ2GPI (▾), or BSA (▪). The amount of 125I-FXI binding to immobilized proteins was determined as described in Methods.
FXI circulates in plasma as a noncovalent complex with HK (36). Our preliminary experiments showed that the amount of 125I-FXI binding to HK was higher than to β2GPI, so we carried out saturation binding experiments to compare the capacity of 125I-FXI binding to HK or β2GPI. 125I-FXI bound each protein coated onto the plate in a saturable manner. The Kd of 125I-FXI binding to HK was 15.43 ± 1.00 × 10-9 (M) and Bmax 0.73 ± 0.043 × 10-12 (mol) similar to that of rhβ2GPI and nβ2GPI 12.93 ± 4.78 × 10-9 (M) and Bmax 0.24 ± 0.089 × 10-12 (mol) and 11.65 ± 4.87 × 10-9 (M) and Bmax 0.22 ± 0.096 × 10-12 (mol), respectively (P = 0.5 and 0.39, respectively).
β2GPI Inhibits Binding of FXI to Immobilized β2GPI. Binding of 125I-FXI to rhβ2GPI was competitively inhibited in a dose-dependent manner by rhβ2GPI and nβ2GPI with IC50 values of 0.167 and 0.115 μM, respectively (Fig. 2).
Fig. 2.
Inhibitory effects of rhβ2GPI and nβ2GPI on binding of 125I-FXI to rhβ2GPI. A constant concentration of 125I-FXI was incubated with serial dilutions of rhβ2GPI (▴), nβ2GPI (▾), or BSA (▪) in wells of microtiter plates coated with rhβ2GPI. The number of cpm bound were converted to percentage of total binding by dividing by the cpm bound in the absence of β2GPI competitors.
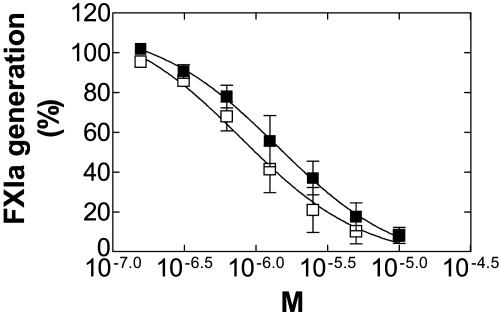
rhβ2GPI Inhibits Activation of FXI in the Presence of DS. The observation that FXI bound to β2GPI led us to evaluate the effects of β2GPI on activation of FXI by thrombin and FXIIa. In the presence of DS, FXI can be activated by FXIIa as well as by thrombin (32,33). The optimal concentration of DS for activation of FXI was 1-2 μg/ml (data not shown).
β2GPI inhibited FXI activation by thrombin and FXIIa (Fig. 3) with an IC50 of 1.39 and 0.79 μM, respectively. In the absence of inhibitor, 0.170 and 0.058 pmol of FXIa was generated with thrombin and FXIIa, respectively.
Fig. 3.
β2GPI dose-dependent inhibitory effect on the activation of FXI by thrombin and FXIIa in the presence of DS. Data are expressed as percentage of FXIa generated in the absence of inhibitor. ▪, Thrombin; □, FXIIa as activator.
β2GPI did not influence the enzymatic activity of FXIIa, thrombin, or FXIa in the amidolytic assay with their respective specific chromogenic substrates (data not shown).
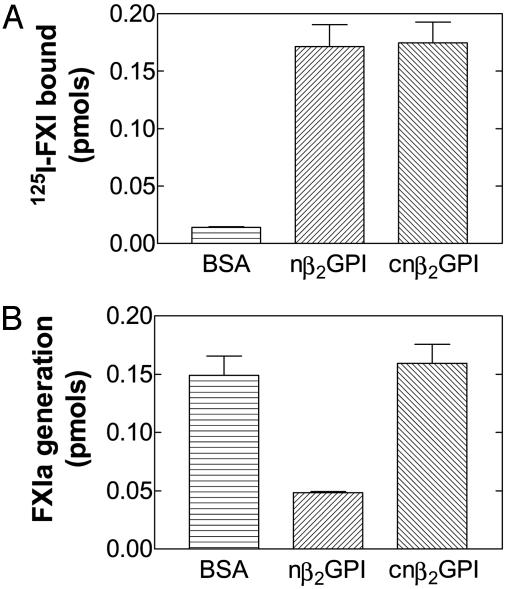
β2GPI Proteolytically Clipped at Lys-317-Thr-318 Binds FXI but Does Not Inhibit Its Activation by Thrombin. To determine whether the cnβ2GPI bound FXI as efficiently as nβ2GPI, we used binding experiments with a constant concentration of 125I-FXI (25 nM) added to wells coated with cnβ2GPI or nβ2GPI. FXI bound to nβ2GPI as efficiently as it did to cnβ2GPI (P = 0.9; n = 6) (Fig. 4A). The observation that cnβ2GPI bound FXI led us to assess the activation of FXI by thrombin in the presence of cnβ2GPI. The clipped preparation did not cause any inhibition, whereas nβ2GPI inhibited thrombin activation of FXI by 75% (P = 0.001; n = 4) (Fig. 4B).
Fig. 4.
Binding of 125I-FXI to nβ2GPI, cnβ2GPI, and BSA (A), and activation of FXI (B) by thrombin in the presence of nβ2GPI and cnβ2GPI. The amount of 125I-FXI bound and FXIa generated are expressed in pmol.
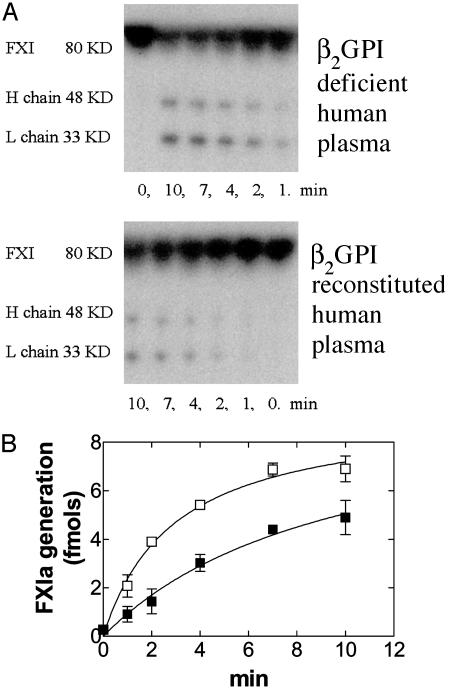
β2GPI Inhibits Activation of FXI in Human Plasma. 125I-FXI was rapidly activated in β2GPI-deficient human plasma and 27.1 ± 0.01% (mean ± SD; n = 3) of total 125I-FXI added converted to FXIa in the first 4 min. However, in β2GPI-deficient plasma reconstituted with β2GPI, 125I-FXIa generation was significantly slower (15.1 ± 1.74% of total 125I-FXI; mean ± SD; n = 3) than in β2GPI-deficient plasma (Fig. 5 A and B) (P = 0.01).
Fig. 5.
Activation of 125I-FXI in β2GPI-deficient human plasma. β2GPI-deficient human plasma was reconstituted by the addition of rhβ2GPI (▪) or BSA (□), and 125I-FXI was added. (A) Autoradiograph. This is a representative example of three different experiments. (B) FXIa generated expressed in fmol.
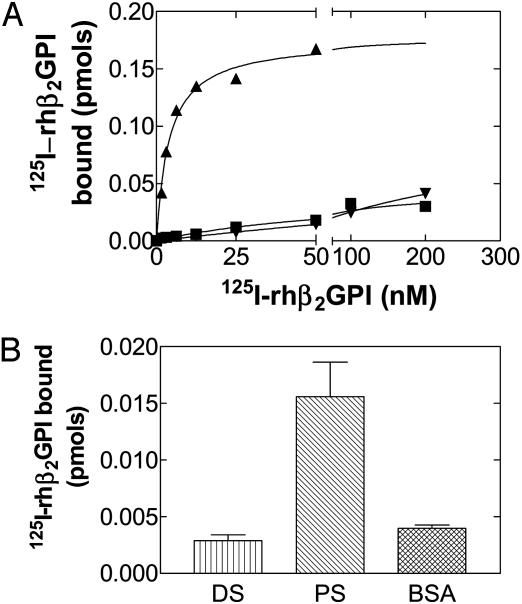
β2GPI Inhibits Activation of FXI by Thrombin and FXIIa but Does Not Bind to DS. The ability of β2GPI to inhibit activation of FXI by thrombin or FXIIa in the presence of DS was not overcome by increasing the DS concentration (data not shown). This result suggested that β2GPI does not inhibit FXI activation by competing for binding to DS. β2GPI bound to the anti-β2GPI antibody moAb1 but not DS in saturation binding experiments and in direct binding experiments bound PS but not DS or BSA (Fig. 6 A and B).
Fig. 6.
(A) Saturation binding of 125I-rhβ2GPI to DS and moAb1 to β2GPI. Saturation binding of 125I-rhβ2GPI (1.56-200 nM) to immobilized moAb1 (▴), DS (▾), and BSA (▪) was performed as indicated in Methods. (B) Direct Binding of 125I-rhβ2GPI to microtiter wells coated with PS, DS, or BSA. The amount of 125I-rhβ2GPI bound was determined as described in Methods.
Discussion
Although the physiological function of β2GPI has not been deduced, plasma from β2GPI null mice exhibits significantly diminished in vitro thrombin generation compared with plasma from β2GPI wild-type mice (18), implying that β2GPI may have an important in vivo role in the generation of thrombin. We have examined the interaction between β2GPI and FXI in the context of previous studies showing that β2GPI inhibits the contact activation pathway and other downstream coagulation reactions including the generation of FXa. We report that β2GPI binds FXI and inhibits its activation to FXIa by thrombin and FXIIa. Inhibition of FXI activation occurred in the presence of DS and was demonstrated with concentrations of β2GPI much lower than normally found in human plasma. The inhibition was dose-dependent and reached 100% at 10 μM.
The C-terminal loop region of β2GPI is surface exposed and is susceptible to cleavage by the proteases plasmin and FXa. Cleavage at Lys-317-Thr-318 of the polypeptide chain exists with the two cleaved segments linked by a disulphide bonded complex (7). This cleavage alters the spatial array of the three critical Lys residues and abolishes the ability of β2GPI to bind anionic phospholipid surfaces (7). It has previously been reported that cleavage of β2GPI at Lys-317-Thr-318 disturbs the nearby electrostatic environment (37). It is believed that the integrity of the 317/318 peptide bond is important in tethering the cluster of positively charged and hydrophobic residues in this region. Although the hydrophobic C-terminal loop is important in phospholipid binding, a preparation of nβ2GPI cleaved at Lys-317-Thr-318 by plasmin bound to FXI just as well as nβ2GPI but did not inhibit the activation of FXI by thrombin (Fig. 4 A and B).
β2GPI is often clipped at Lys-317-Thr-318 in plasma of patients with lupus anticoagulant and in disseminated intravascular coagulation (DIC) (12,13). The clipped form of β2GPI correlated with in vitro markers of fibrinolytic activity. Thus, in vivo the clipped form is most likely generated by plasmin. The C-terminal hydrophobic loop of cβ2GPI has been confirmed by heteronuclear magnetic resonance to be tightly fixed by electrostatic interaction with the lysine cluster at the phospholipid binding site while at the same time enhancing stability and neutralizing the positive charge in this region (37). Thus, the C-terminal loop of the intact β2GPI is more mobile than that of the clipped molecule, allowing a better interaction with FXI/thrombin complexes, which may explain why cβ2GPI binds FXI but does not inhibit its activation by thrombin.
Both β2GPI and FXI contain protein modules. In FXI these consist of ≈90 amino acids each, are located at the N terminus, and are commonly termed apple domains. The apple domains mediate binding with other proteins and proteoglycans. Although this study did not investigate domain-specific interactions between FXI and β2GPI, we noted that the interaction was not affected by HK (data not shown), suggesting that HK and β2GPI bind to different sites on FXI. There was no binding of HK to β2GPI (data not shown).
Platelet-bound FXIa converts FIX by proteolytic cleavage to its active form, FIXa. FIX is a zymogen form of a protease critical to regulation of blood coagulation (38) that may also be activated by the complex of tissue factor with FVIIa (39,40). FXI circulates in human plasma as a covalent dimer of two identical 80-kDa molecules and lacks the Gla domain that is characteristic of plasma coagulation proteases (41). It has been proposed that FXI functions by binding the catalytic surface of platelets with one polypeptide, whereas the other polypeptide interacts with FIX (42). Clotting factors that are protease zymogens such as FIX are typically activated in the presence of a protein cofactor plus divalent cations and an appropriate procoagulant phospholipid surface. Interestingly, no protein cofactor has been identified that is required for FIX activation, and its activation is not influenced by the addition of phospholipid.
β2GPI at physiological concentrations has been demonstrated to inhibit the generation of FXa in the presence of activated gel filtered platelets (19). Activated platelets provide an appropriate in vivo procoagulant surface for assembly of surface-bound protease substrate complexes. Moreover, activated platelets rather than endothelial cells are the preferred procoagulant surface for binding and activating FXI, which is critical for the initiation of the consolidation phase of coagulation (41). FXI/FXIa binds activated platelets in a saturable and reversible manner in the presence of either HK and zinc ions (43, 44) or prothrombin and calcium ions (45). Platelet-bound FXI can be activated by FXIa, thrombin, or FXIIa, although the preferred in vivo activator is most likely thrombin (41). Thus, activation of FXI on activated platelet surfaces by thrombin accelerates coagulation by the sequential activation of FIXa and FXa.
The effect of β2GPI on FXI activation by thrombin and FXIIa has major implications for understanding the role of β2GPI in coagulation and the prothrombotic diathesis seen in patients with APS. Whereas β2GPI inhibits generation of FXa on activated platelets, antiphospholipid antibodies are known to interfere with this inhibition, thereby increasing FXa generation (19). Inhibition of FXI activation by β2GPI using a system with purified coagulation factors and in human β2GPI-deficient plasma provides a regulatory mechanism for FXa and thrombin generation in vivo. The demonstration that β2GPI binds FXI and inhibits its activation by thrombin and FXIIa provides a possible alternative explanation for the thrombosis in patients with APS. Specifically, antiphospholipid antibodies that generally have low affinity for antigen may efficiently bind platelet-associated β2GPI, thereby blocking its attenuating effect on FXI activation.
Although FXI is not essential for haemostasis, unlike coagulation factors of the extrinsic and common pathways, FXI activity is essential for certain in vivo thrombosis models in rabbits (46) and mice (47), as well as in the early postnatal mortality observed in protein C-deficient mice (48). In a recent report, FXI was demonstrated to be essential for thrombus propagation in a primate arterial thrombosis model (49). In that study, the authors concluded that extension of thrombi in vivo was dependent on the activation of FXI. Interestingly, elevated levels of FXI have been reported to be an independent risk factor for both venous and arterial thrombosis in humans (45,46).
We have demonstrated that β2GPI can bind FXI and inhibit the activation of FXI by thrombin and FXIIa. Secondly, we have demonstrated that cnβ2GPI can bind FXI but does not inhibit its activation by thrombin. We propose that β2GPI is a physiological inhibitor of FXI activation in vivo and that cnβ2GPI is part of the positive feedback loop in FXI activation.
We propose that this interaction may be important in vivo and that the inhibition of this effect by antiphospholipid antibodies may be an important mechanism for thrombosis in patients with APS.
Acknowledgments
We thank Dr. Richard Smart for assistance with iodination and Ms. Alexandra de Nangle for expert secretarial assistance in preparation of the manuscript. This study was supported by a grant from the National Health and Medical Research Council of Australia.
Abbreviations: APS, antiphospholipid syndrome; cnβ2GPI, clipped native β2GPI; DS, dextran sulfate; FX, FXI, and FXII, factor X, XI, and XII; GPI, glycoprotein I; HK, high molecular weight kininogen; nβ2GPI, native β2GPI; PS, phosphatidylserine; rhβ2GPI, recombinant human β2GPI.
References
- 1.Polz, E. & Kostner, G. M. (1979) FEBS Lett. 102, 183-186. [DOI] [PubMed] [Google Scholar]
- 2.Steinkasserer, A., Estaller, C., Weiss, E. H., Sim, R. B. & Day, A. J. (1991) Biochem. J. 277, 387-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozier, J., Takahashi, N. & Putnam, F. W. (1984) Proc. Natl. Acad. Sci. USA 81, 3640-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid, K. B. & Day, A. J. (1989) Immunol. Today 10, 177-180. [DOI] [PubMed] [Google Scholar]
- 5.Steinkasserer, A., Barlow, P. N., Willis, A. C., Kertesz, Z., Campbell, I. D., Sim, R. B. & Norman, D. G. (1992) FEBS Lett. 313, 193-197. [DOI] [PubMed] [Google Scholar]
- 6.Kandiah, D. A., Sali, A., Sheng, Y., Victoria, E. J., Marquis, D. M., Coutts, S. M. & Krilis, S. A. (1998) Adv. Immunol. 70, 507-563. [DOI] [PubMed] [Google Scholar]
- 7.Hunt, J. E., Simpson, R. J. & Krilis, S. A. (1993) Proc. Natl. Acad. Sci. USA 90, 2141-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt, J. & Krilis, S. (1994) J. Immunol. 152, 653-659. [PubMed] [Google Scholar]
- 9.Sheng, Y., Sali, A., Herzog, H., Lahnstein, J. & Krilis, S. A. (1996) J. Immunol. 157, 3744-3751. [PubMed] [Google Scholar]
- 10.Guerin, J., Sheng, Y., Reddel, S., Iverson, G. M., Chapman, M. G. & Krilis, S. A. (2002) J. Biol. Chem. 277, 2644-2649. [DOI] [PubMed] [Google Scholar]
- 11.Ohkura, N., Hagihara, Y., Yoshimura, T., Goto, Y. & Kato, H. (1998) Blood 91, 4173-4179. [PubMed] [Google Scholar]
- 12.Horbach, D. A., van Oort, E. T., Lisman, T., Meijers, J. C., Derksen, R. H. & de Groot, P. G. (1999) Thromb. Haemostasis 81, 87-95. [PubMed] [Google Scholar]
- 13.Itoh, Y., Inuzuka, K., Kohno, I., Wada, H., Shiku, H., Ohkura, N. & Kato, H. (2000) J. Biochem. (Tokyo) 128, 1017-1024. [DOI] [PubMed] [Google Scholar]
- 14.McNeil, H. P., Simpson, R. J., Chesterman, C. N. & Krilis, S. A. (1990) Proc. Natl. Acad. Sci. USA 87, 4120-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galli, M., Comfurius, P., Maassen, C., Hemker, H. C., de Baets, M. H., van Breda-Vriesman, P. J., Barbui, T., Zwaal, R. F. & Bevers, E. M. (1990) Lancet 335, 1544-1547. [DOI] [PubMed] [Google Scholar]
- 16.McNeil, H. P., Chesterman, C. N. & Krilis, S. A. (1991) Adv. Immunol. 49, 193-280. [DOI] [PubMed] [Google Scholar]
- 17.Reddel, S. W. & Krilis, S. A. (2002) The Molecular Pathology of Autoimmune Diseases, eds. Theofilopoulos, A. & Bona, C. A. (Taylor and Francis, New York), 2nd Ed., pp. 325-352.
- 18.Sheng, Y., Reddel, S. W., Herzog, H., Wang, Y. X., Brighton, T., France, M. P., Robertson, S. A. & Krilis, S. A. (2001) J. Biol. Chem. 276, 13817-13821. [DOI] [PubMed] [Google Scholar]
- 19.Shi, W., Chong, B. H., Hogg, P. J. & Chesterman, C. N. (1993) Thromb. Haemostasis 70, 342-345. [PubMed] [Google Scholar]
- 20.Nimpf, J., Bevers, E. M., Bomans, P. H., Till, U., Wurm, H., Kostner, G. M. & Zwaal, R. F. (1986) Biochim. Biophys. Acta 884, 142-149. [DOI] [PubMed] [Google Scholar]
- 21.Nimpf, J., Wurm, H. & Kostner, G. M. (1987) Atherosclerosis 63, 109-114. [DOI] [PubMed] [Google Scholar]
- 22.Mori, T., Takeya, H., Nishioka, J., Gabazza, E. C. & Suzuki, K. (1996) Thromb. Haemostasis 75, 49-55. [PubMed] [Google Scholar]
- 23.Nakaya, Y., Schaefer, E. J. & Brewer, H. B., Jr. (1980) Biochem. Biophys. Res. Commun. 95, 1168-1172. [DOI] [PubMed] [Google Scholar]
- 24.Manfredi, A. A., Rovere, P., Heltai, S., Galati, G., Nebbia, G., Tincani, A., Balestrieri, G. & Sabbadini, M. G. (1998) Arthritis Rheum. 41, 215-223. [DOI] [PubMed] [Google Scholar]
- 25.Colman, R. W. (2000) Hemostasis and Thrombosis: Basic Principles and Clinical Practice, eds. Colman, R. W., Hirsh, J., Marder, V. J., Clowes, A. W. & George, G. N. (Lippincott Williams & Wilkins, Philadelphia), pp. 103-122.
- 26.Schousboe, I. & Rasmussen, M. S. (1995) Thromb. Haemostasis 73, 798-804. [PubMed] [Google Scholar]
- 27.Schousboe, I. (1985) Blood 66, 1086-1091. [PubMed] [Google Scholar]
- 28.Wurm, H., Beubler, E., Polz, E., Holasek, A. & Kostner, G. (1982) Metabolism 31, 484-486. [DOI] [PubMed] [Google Scholar]
- 29.Iverson, G. M., Reddel, S. W., Victoria, E. J., Cockerill, K. A., Wang, Y. X., Marti-Renom, M. A., Sali, A., Marquis, D. M., Krilis, S. A. & Linnik, M. D. (2002) J. Immunol. 169, 7097-7103. [DOI] [PubMed] [Google Scholar]
- 30.Baird, T. R. & Walsh, P. N. (2002) J. Biol. Chem. 277, 28498-28503. [DOI] [PubMed] [Google Scholar]
- 31.Renne, T., Gailani, D., Meijers, J. C. & Muller-Esterl, W. (2002) J. Biol. Chem. 277, 4892-4899. [DOI] [PubMed] [Google Scholar]
- 32.Baglia, F. A. & Walsh, P. N. (2000) J. Biol. Chem. 275, 20514-20519. [DOI] [PubMed] [Google Scholar]
- 33.Scott, C. F. & Colman, R. W. (1992) Proc. Natl. Acad. Sci. USA 89, 11189-11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunnee, T., La Porta, C., Reddigari, S. R., Salerno, V. M., Kaplan, A. P. & Silverberg, M. (1993) Blood 81, 580-586. [PubMed] [Google Scholar]
- 35.Sheng, Y., Hanly, J. G., Reddel, S. W., Kouts, S., Guerin, J., Koike, T., Ichikawa, K., Sturgess, A. & Krilis, S. A. (2001) Clin. Exp. Immunol. 124, 502-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson, R. E., Mandle, R., Jr. & Kaplan, A. P. (1977) J. Clin. Invest. 60, 1376-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshino, M., Hagihara, Y., Nishii, I., Yamazaki, T., Kato, H. & Goto, Y. (2000) J. Mol. Biol. 304, 927-939. [DOI] [PubMed] [Google Scholar]
- 38.Davie, E. W., Fujikawa, K. & Kisiel, W. (1991) Biochemistry 30, 10363-10370. [DOI] [PubMed] [Google Scholar]
- 39.Di Scipio, R. G., Kurachi, K. & Davie, E. W. (1978) J. Clin. Invest. 61, 1528-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osterud, B. & Rapaport, S. I. (1977) Proc. Natl. Acad. Sci. USA 74, 5260-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh, P. N. (2001) Thromb. Haemostasis 86, 75-82. [PubMed] [Google Scholar]
- 42.Gailani, D., Ho, D., Sun, M. F., Cheng, Q. & Walsh, P. N. (2001) Blood 97, 3117-3122. [DOI] [PubMed] [Google Scholar]
- 43.Greengard, J. S., Heeb, M. J., Ersdal, E., Walsh, P. N. & Griffin, J. H. (1986) Biochemistry 25, 3884-3890. [DOI] [PubMed] [Google Scholar]
- 44.Sinha, D., Seaman, F. S., Koshy, A., Knight, L. C. & Walsh, P. N. (1984) J. Clin. Invest. 73, 1550-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baglia, F. A. & Walsh, P. N. (1998) Biochemistry 37, 2271-2281. [DOI] [PubMed] [Google Scholar]
- 46.Minnema, M. C., Friederich, P. W., Levi, M., von dem Born, P. A., Mosnier, L. O., Meijers, J. C., Biemond, B. J., Hack, C. E., Bouma, B. N. & ten Cate, H. (1998) J. Clin. Invest. 101, 10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen, E. D., Gailani, D. & Castellino, F. J. (2002) Thromb. Haemostasis 87, 774-776. [PubMed] [Google Scholar]
- 48.Chan, J. C., Ganopolsky, J. G., Cornelissen, I., Suckow, M. A., Sandoval-Cooper, M. J., Brown, E. C., Noria, F., Gailani, D., Rosen, E. D., et. al. (2001) Am. J. Pathol. 158, 469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruber, A. & Hanson, S. R. (2003) Blood 102, 953-955. [DOI] [PubMed] [Google Scholar]
- 50.Meijers, J. C., Tekelenburg, W. L., Bouma, B. N., Bertina, R. M. & Rosendaal, F. R. (2000) N. Engl. J. Med. 342, 696-701. [DOI] [PubMed] [Google Scholar]
- 51.Berliner, J. I., Rybicki, A. C., Kaplan, R. C., Monrad, E. S., Freeman, R. & Billett, H. H. (2002) Thromb. Res. 107, 55-60. [DOI] [PubMed] [Google Scholar]