Background: Pyruvate-isocitrate cycling is involved in control of glucose-stimulated insulin secretion (GSIS), but the underlying mechanisms are unknown.
Results: Pyruvate-isocitrate cycling controls expression of the voltage-gated potassium channel family member Kv2.2 in islet cells.
Conclusion: Pyruvate-isocitrate cycling maintains Kv2.2 expression, allowing it to serve as a negative regulator of Kv channel activity.
Significance: Kv2.2 is a potential new target for reversing β-cell dysfunction of type 2 diabetes.
Keywords: Diabetes, Glucose Metabolism, Insulin Secretion, Ion Channels, Pancreatic Islets
Abstract
Recent studies have shown that the pyruvate-isocitrate cycling pathway, involving the mitochondrial citrate/isocitrate carrier and the cytosolic NADP-dependent isocitrate dehydrogenase (ICDc), is involved in control of glucose-stimulated insulin secretion (GSIS). Here we demonstrate that pyruvate-isocitrate cycling regulates expression of the voltage-gated potassium channel family member Kv2.2 in islet β-cells. siRNA-mediated suppression of ICDc, citrate/isocitrate carrier, or Kv2.2 expression impaired GSIS, and the effect of ICDc knockdown was rescued by re-expression of Kv2.2. Moreover, chronic exposure of β-cells to elevated fatty acids, which impairs GSIS, resulted in decreased expression of Kv2.2. Surprisingly, knockdown of ICDc or Kv2.2 increased rather than decreased outward K+ current in the 832/13 β-cell line. Immunoprecipitation studies demonstrated interaction of Kv2.1 and Kv2.2, and co-overexpression of the two channels reduced outward K+ current compared with overexpression of Kv2.1 alone. Also, siRNA-mediated knockdown of ICDc enhanced the suppressive effect of the Kv2.1-selective inhibitor stromatoxin1 on K+ currents. Our data support a model in which a key function of the pyruvate-isocitrate cycle is to maintain levels of Kv2.2 expression sufficient to allow it to serve as a negative regulator of Kv channel activity.
Introduction
Glucose-stimulated insulin secretion (GSIS)2 is essential for control of metabolic fuel homeostasis, and its impairment is a key element of β-cell failure in type 2 diabetes. The canonical mechanistic model of GSIS holds that glucose metabolism in β-cells leads to ATP-mediated closure of KATP channels, which in turn activates voltage-gated L-type Ca2+ channels, resulting in Ca2+ influx to trigger insulin secretion (for reviews, see Refs. 1 and 2).
It has become increasingly clear that KATP channel-independent pathways also contribute to GSIS (3–5). Our laboratory and others have implicated pathways of pyruvate exchange with tricarboxylic acid cycle intermediates (“pyruvate cycling”) in GSIS (6–23), and work from our group has demonstrated that the pyruvate-isocitrate cycle has a particularly prominent role both in both primary islets and β-cell lines (8, 10, 11, 23). The pyruvate-isocitrate cycle begins with anaplerotic entry of pyruvate into the mitochondria via pyruvate carboxylase. The product of the pyruvate carboxylase reaction, oxaloacetate, undergoes mitochondrial metabolism to produce citrate and isocitrate, which are subsequently exported out of the mitochondria via the citrate-isocitrate carrier (CIC). Isocitrate is then converted to α-ketoglutarate in a reaction catalyzed by cytosolic NADP-dependent isocitrate dehydrogenase (ICDc). Consistent with this model, silencing of either CIC or ICDc causes marked impairment of GSIS in β-cell lines and primary rat islets (11, 23). Knockdown of the mitochondrial α-ketoglutarate/malate antiporter also inhibits GSIS (10), suggesting that completion of the pyruvate-isocitrate cycle may involve re-entry of α-ketoglutarate into the mitochondria (8). The molecular mechanisms linking pyruvate-isocitrate cycling activity to GSIS remain unresolved.
Glucose metabolism-induced closure of KATP channels and membrane depolarization are counteracted by repolarization mediated by voltage-gated potassium (Kv) channels. Kv channels belong to the six-transmembrane family of K+ channels, and to date, members of five subfamilies (Kv1, -2, -3, -6, and -9) have been identified in primary β-cells (for reviews, see Refs. 2 and 24). Of these, members of the Kv1, Kv2, and Kv3 subfamilies form tetrameric functional channels as homo- and heteromultimers. In contrast, members of the Kv6 and Kv9 subfamilies encode “silent subunits” that do not form functional homomultimers but modulate Kv2 and Kv3 channel activities when expressed in heterologous systems (25, 26). In addition to these interactions, Kv channel activities can be modified by association with regulatory β-subunits (27–29) or by post-translational modifications such as phosphorylation, ubiquitination, SUMOylation, and palmitoylation (30–33).
The specific roles of the various subtypes of Kv subunits in control of insulin secretion are not fully understood. Kv2.1 is considered the dominant Kv channel species in rodent β-cells, and pharmacologic inhibition of Kv2.1 enhances GSIS (34–37). Furthermore, Kv2.1-null mice show improved glucose tolerance with reduced fasting glucose as a consequence of enhanced insulin secretion (38). However, β-cells from Kv2.1-null mice have residual Kv current (38), demonstrating that channels other than Kv2.1 are active in these cells. Furthermore, although abundant Kv2.1 protein and channel activity are observed in human islets (37, 39), Kv2 inhibitors do not affect human β-cell electrical function or insulin secretion (40), an observation concordant with mathematical modeling studies (41). Immunolocalization studies suggest that Kv2.2 is expressed primarily in islet δ-cells (42), but these studies do not rule out expression in other islet cell types.
NADPH produced in the ICDc-catalyzed reaction is a potential coupling factor derived from pyruvate-isocitrate cycling activity (8, 22). Consistent with this idea, a linear correlation between NADPH:NADP ratio and GSIS has been observed, and suppression of pyruvate-isocitrate cycling activity and GSIS by siRNA-mediated silencing of CIC, ICDc, or α-ketoglutarate/malate antiporter decreases the NADPH:NADP ratio (10, 11, 23). Also, NADPH stimulates insulin granule exocytosis when added to permeabilized β-cells (11, 43). NADPH has been suggested to be a direct regulator of plasma membrane electrical potential by interaction with the NADPH-dependent oxidoreductase-like motif of Kv channel β-subunits, ostensibly resulting in inhibition of Kv2.1 channel activity and enhanced GSIS (44).
In this study, we investigated the possible linkages between pyruvate-isocitrate cycling and Kv channel expression and activity. Surprisingly, although Kv2.1 expression was unchanged, Kv2.2 channel expression was reduced rather than increased in multiple models of impairment of pyruvate-isocitrate cycling and GSIS. siRNA-mediated suppression of Kv2.2 expression impaired GSIS in 832/13 cells, whereas restoration of Kv2.2 in the background of reduced ICDc expression rescued impaired GSIS in both insulinoma cells and primary rat islets. Co-overexpression of Kv2.1 and Kv2.2 in 832/13 cells impaired the increase in outward K+ current observed with Kv2.1 overexpression alone, and co-immunoprecipitation studies demonstrated a physical interaction between the two proteins. Finally, chronic exposure of 832/13 cells to elevated levels of fatty acids, a maneuver that causes impairment of GSIS and loss of glucose regulation of pyruvate cycling activity (6), resulted in suppression of Kv2.2 but not Kv2.1 expression. Taken together, our data support a model in which a key function of the pyruvate-isocitrate cycle is to maintain expression of Kv2.2 at levels required for it to serve as a negative regulator of Kv2.1 channel activity in β-cells. Our findings point to Kv2.2 as a potential new target for reversing the β-cell dysfunction observed in type 2 diabetes.
EXPERIMENTAL PROCEDURES
Reagents
All reagents and solutions were obtained from Sigma-Aldrich unless otherwise indicated.
Cell Lines and Primary Islets
The highly glucose-responsive insulinoma cell line 832/13 was derived from INS-1 cells (45) via a transfection-selection strategy and cultured as described previously (46). Rat primary islets were harvested from male Sprague-Dawley rats weighing ∼300 g under a protocol approved by the Duke University Institutional Animal Care and Use Committee and cultured as described previously (12). We used both MIP-GFP (47) and MIP-Cherry3 mice to study gene expression in purified primary mouse β-cells. A mouse δ-cell reporter (B6(Cg)-Ssttm1(cre/ERT2)Zjh/J) was obtained from The Jackson Laboratory (Sacramento, CA), and nuclear translocation of the Cre/ERT2 fusion was achieved by continuous tamoxifen administration in chow (250 mg/kg; custom formulation; Harlan Teklad, Madison WI). Glucagon-Cre mice (49) were obtained from Mutant Mouse Regional Resource Centers (Chapel Hill, NC). Both Cre lines were crossed to tdTomato reporter mice (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; The Jackson Laboratory) to visualize Cre recombinase activity. All mouse protocols were approved by the Salk Institute for Biological Studies Institutional Animal Care and Use Committee, and mouse islets were isolated and cultured as described (50).
Glucose-stimulated Insulin Secretion
Insulin secretion from 832/13 cells and rat primary islets (∼20 islets per incubation) was measured as described previously (7).
Lipotoxicity Studies
For studies of lipid-induced impairment of GSIS in 832/13 cells, a 10 mm oleate/palmitate (O/P; 2:1 molar ratio) stock solution complexed to 10% fatty acid-free bovine serum albumin (BSA) was prepared as described (6). The O/P solution was added to cells at a final concentration of 1 mm in complete medium for 24 or 48 h as indicated. In parallel, control cells were treated with 1% BSA. Subsequent GSIS analyses were performed in the absence of O/P.
Adenovirus Design and Construction
A human Kv2.2 cDNA/pENTR223 clone was obtained from Open Biosystems (Lafayette, CO), and human Kv2.1/pENTR223 was obtained from the Dana-Farber/Harvard Cancer Center DNA Resource Core at Harvard Medical School, Boston, MA (clone HsCD00399288). Both constructs were inserted into the pAd/CMV/V5-DEST gateway vector (Invitrogen) using recombination-based cloning according to the manufacturer's recommendations. The viral vector was subsequently sequenced, digested with PacI, and transfected into the 293A cell line (Invitrogen) to generate viral stocks. Adenoviruses containing siRNA sequences specific for Kv2.2 and Kv2.1 (sequences described below) were constructed using vectors EH006 and pJM17 as described previously (51). After amplification in 293 cells, adenoviruses were purified by CsCl2 gradient, and titers of pure viral preparations were determined by measurement of A280 (51, 52).
RNAi-mediated Gene Silencing and Kv2.2 Overexpression in Insulinoma Cells
832/13 cells cultured in 12-well plates (40% confluence) were transfected with siRNA duplexes (Integrated DNA Technologies, Coralville, IA) for 24 h using Dharmafect Transfection Reagent 1 (Dharmacon, Lafayette, CO) at a final concentration of 50 nm. After an additional 48 h in culture, cells were used for insulin secretion, gene expression analysis (qRT-PCR), or patch clamp studies. The siICDc target sequence was GTA TGA TGG ACG CTT CAA AGA (11), and the siCIC target sequence was GACCGAATACGTGAAGACT (23). Kv2.2 siRNA targets were GTA CCA TTC TTC TAG AAG A and CCA ACA AGT CTT ACG AGA A. The Kv2.1 siRNA target sequences were CAG ATG AAC GAG GAG CTG A and GTA AGA TGG CCA AGA CAC A. A scrambled duplex (siControl), GAG ACC CTA TCC GTG ATT, with no biological effect relative to untreated β-cells was used as a control (7). For experiments involving Kv2.2 overexpression, cells were treated with either 0.1 or 0.2 μl of AdCMV-hKV2.2 (equivalent to ∼0.6 × 1011 or ∼1.2 × 1011 viral particles/ml, respectively) or AdCMV-βgal (∼1.2 × 1011 viral particles/ml) for 16 h followed by culture for 24 h before analyses.
Adenovirus-mediated siICDc Silencing and Kv2.2 Overexpression in Primary Rat Islets
Freshly isolated islets were left untreated or incubated with AdsiRNA viruses for 20 h. Viral titers applied were 2.4 × 1012 particles/125–200 islets in 2 ml of medium. Islets were subsequently cultured for 72 h with medium changes daily. For Kv2.2 rescue experiments, AdCMV-hKV2.2 or AdCMV-βgal (4 × 1011 viral particles per 125 islets in 2 ml of medium) was added to the AdsiRNA-treated islets on day 3 of culture. After 16 h of transduction, medium was changed, and the islets were incubated for an additional 24 h prior to GSIS.
Fluorescence-activated Cell Sorting
For FACS analyses of rat islets, Ad-siControl- or Ad-siICDc-treated islets were transduced with AdRIP-GFP for the last 48 h of culture and subsequently dissociated in 0.025% trypsin and EDTA solution for 10 min at 37 °C aided by gentle vortexing every 2 min. Dissociated primary islet cells were washed in PBS containing 0.5% BSA and sorted on a FACS Vantage sorter (BD Biosciences). GFP-, Cherry-, or tdTomato-positive and GFP-negative fractions were subsequently collected in lysis buffer prior to RNA isolation and qRT-PCR analyses as described previously (50).
Electrophysiological Measurements
832/13 cells were transfected with siControl, siICDc, siKv2.1, or siKv2.2 duplexes as described above together with a plasmid encoding GFP under control of the CMV promoter and cultured for 72 h (same time point as used for GSIS analyses). For Kv2.1 and Kv2.2 overexpression experiments, 832/13 cells were transduced with AdCMV-βgal, AdCMV-hKV2.2, AdCMV-hKV2.1, or combinations thereof and subsequently cultured for 24 h. Cells were seeded on small pieces of coverslips (for RNAi experiments, only GFP-positive cells were picked) and transferred to a Warner chamber perfused with a solution consisting of 140 mm NaCl, 2 mm MgCl2, 2.5 mm CaCl2, 2 mm glucose, and 10 mm HEPES, pH 7.4. Pipettes were pulled from borosilicate glass (Sutter P-87) with a resistance between 2.5 and 4 megaohms when filled with pipette solution containing 30 mm KCl, 95 mm potassium gluconate, 5 mm Na2ATP, 10 mm HEPES, 11 mm EGTA, 1 mm CaCl2, and 2 mm MgSO4, pH 7.2. The cells were held at −80 mV, and the K+ currents were activated by depolarizing steps from −80 to 60 mV with an interval of 10 mV for a 2-s duration. For siRNA experiments, measurements were repeated after addition of glucose to a final concentration of 12 mm. Whole cell currents were recorded using an Axopatch 200B amplifier with a sample rate of 5 kHz. The junction potential was nulled before the pipette contacted the cell, and no further correction was done. The whole cell current recordings from those cells having a seal resistance larger than 1 gigaohm were used for final analysis. Normalized current-voltage relationships were determined by plotting currents at the end of the test as pulse versus voltage.
For stromatoxin1 (ScTx1) sensitivity studies, a range of ScTx1 doses (0–100 nm) was included in patch clamping experiments performed on 832/13 cells in which Kv2.1 or Kv2.2 was overexpressed by adenovirus-mediated gene transfer. Based on these experiments, a dose of ScTx that preferentially suppresses Kv2.1 relative to Kv2.2 activity (25 nm) was chosen for further study.
Immunoblot Analyses
832/13 cells were seeded in P150 plates at 80% confluence and either left untreated or transduced with AdCMV-hKv2.2 for 16 h followed by medium change and an additional 24-h incubation. The viral dose applied was 35 × 1012 viral particles/plate in 20 ml of medium, which is 6.3 and 3.2 times higher per cell than the doses used for siICDc rescue experiments. Cell surface proteins were biotinylated using the Scientific Cell Surface Protein Isolation kit from Thermo Scientific (Rockford, IL). After cell lysis, labeled surface proteins were affinity-purified using agarose-linked avidin and subsequently eluted in 300 μl of SDS-PAGE sample buffer (Invitrogen) containing 50 mm DTT for 60 min at room temperature. Eluted cell surface proteins (30 μl) were separated on 4–12% NuPAGE SDS-polyacrylamide gels (Invitrogen) and transferred to polyvinylidene difluoride membranes (Invitrogen). Blots were blocked with 5% BSA and 1% TBS with Tween 20 for 45 min and incubated overnight with either anti-Kv2.2 antibody (Alomone Labs, Jerusalem, Israel; diluted 1,000× in 1% polyvinylpyrrolidone) or anti-V5 antibody (Invitrogen; diluted 5,000× in 1% polyvinylpyrrolidone). Donkey horseradish peroxidase-conjugated anti-rabbit IgG (Kv2.2; 1:10,000) or sheep horseradish peroxidase-conjugated anti-mouse IgG (for V5; 1:10,000) was used as secondary antibody (GE Healthcare) prior to ECL detection.
Co-immunoprecipitation of Kv2.1 and Kv2.2
For immunoprecipitation, 5 × 106 832/13 cells were lysed in 0.5 ml of cold lysis buffer containing HALT protease inhibitors (Thermo Scientific), 150 mm NaCl, 50 nm Tris-HCl, pH 7.5, 5 mm EDTA, 1 mm EGTA, and 1% Triton X-100. Lysates were incubated on ice for 30 min and centrifuged at 10,000 × g for 15 min at 4 °C. The supernatant was immunoprecipitated overnight with 2 μg of anti-Kv2.2 (Alomone Labs) or anti-Kv2.1 (QED Bioscience, San Diego, CA) antibody and either rabbit (Kv2.2) or mouse (Kv2.1) IgG-TrueBlot IP beads (eBioscience, San Diego, CA). Precipitated proteins were eluted according to the manufacturer's protocol and detected by immunoblot as described above. Blots were incubated with primary antibodies against Kv2.2 (Alomone) and Kv2.1 (QED Bioscience; 3 μg/ml in 1% polyvinylpyrrolidone) followed by incubation with Alexa Fluor 680 anti-rabbit (Invitrogen) and IRDye 800CW anti-mouse (LI-COR Biosciences, Lincoln, NE) fluorescent secondary antibodies. Blots were then imaged using the LI-COR Odyssey CLx.
Real Time PCR Analyses (qRT-PCR)
RNA was purified from 832/13 cells and islets using the RNAeasy Mini and Micro kits (Qiagen, Valencia, CA), respectively, and reverse transcribed with the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturers' recommendations. Real time analyses were performed on a ViiA 7 Real Time PCR System (Invitrogen) using iTaq SYBR Supermix with ROX (Bio-Rad) with primers exhibiting at least 95% efficiency. Primer sequences are available on request.
Statistical Analysis
Statistical significance was determined by two-sample, unpaired equal variance Student's t test. A p value of <0.05 was considered significant. All data are expressed as mean ± S.E. of at least three independent experiments unless otherwise noted.
RESULTS
Kv2.2 mRNA Levels Are Lowered in 832/13 Cells with Suppressed Pyruvate-Isocitrate Cycling Activity
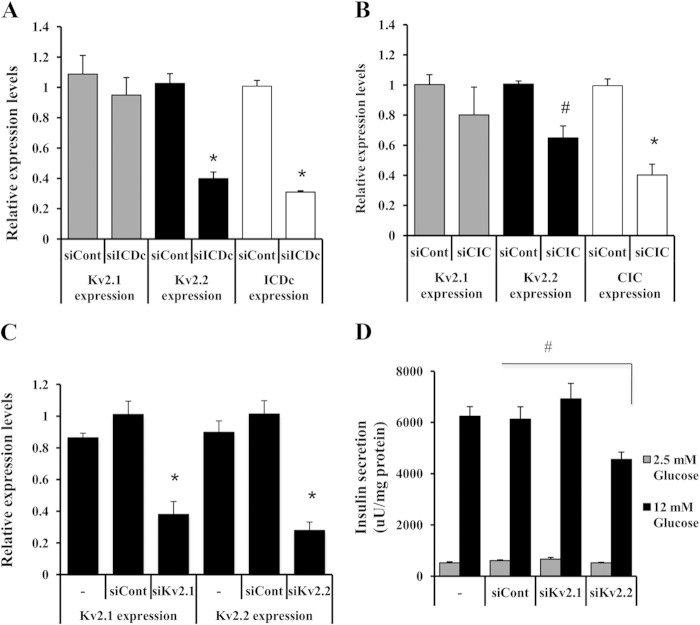
Clear correlations between pyruvate cycling and NADPH production (7, 11, 23) and between NADPH production and insulin secretion (8, 11, 43) have been established in recent years. There are also data suggesting that NADPH regulates Kv channel activity in β-cells purportedly by interaction with the NADPH-dependent oxidoreductase-like motif of Kv channel β-subunits (44). To further investigate possible cause and effect relationships among these variables, we began to assess the impact of manipulation of pyruvate-isocitrate cycling activity on Kv channel expression and function. Our prior studies demonstrating a role for pyruvate-isocitrate cycling in regulation of GSIS used siRNAs specific for ICDc or CIC to suppress pyruvate-isocitrate cycling activity, resulting in clear impairment in GSIS (11, 23). Here, treatment of INS-1-derived 832/13 cells with siICDc and siCIC duplexes resulted in 69 ± 1 and 60 ± 7% decreases in ICDc and CIC mRNA levels, respectively, with no effect on Kv2.1 mRNA levels (Fig. 1, A and B). In contrast, siRNA-mediated suppression of either ICDc or CIC caused a significant decrease in Kv2.2 expression (60 ± 4 and 36 ± 7% reduction, respectively; p < 0.01; Fig. 1, A and B).
FIGURE 1.
Regulation of Kv2.2 by pyruvate-isocitrate cycling and regulation of GSIS by Kv2.2. 832/13 cells were treated with siControl (siCont), siCIC, or siICDc for 72 h, and total RNA was isolated for qRT-PCR analyses of CIC, ICDc, Kv2.1, and Kv2.2 expression levels. Expression levels for siICDc- (A) and siCIC-treated (B) 832/13 cells are shown. Data represent four and three independent experiments, respectively, each performed in duplicate. *, p < 0.001; #, p < 0.01. 832/13 cells were either left untreated or transduced with siKV2.1, siKv2.2, or a control (siControl) duplex and cultured for 3 days before qRT-PCR (C) or GSIS (D) analyses. Data represent three independent experiments, each performed in triplicate. *, p < 0.001; #, p < 0.01. Error bars represent S.E. uU, microunits.
Suppression of Kv2.2 but Not Kv2.1 Expression in 832/13 Cells Impairs GSIS
We next studied the direct effects of silencing of Kv2.1 or Kv2.2 expression on insulin secretion using siKv2.1, siKv2.2, or siControl duplexes in 832/13 cells. Silencing of Kv2.1 by 61 ± 7% had no significant effect on GSIS (p > 0.3), whereas a 72 ± 5% knockdown of Kv2.2 mRNA levels caused a clear impairment (p < 0.01) (Fig. 1, C and D). Results were confirmed with independent Kv2.1- and Kv2.2-specific siRNA duplexes (data not shown).
Kv2.2 Overexpression Rescues the Inhibitory Effects of siICDc on GSIS in 832/13 Cells
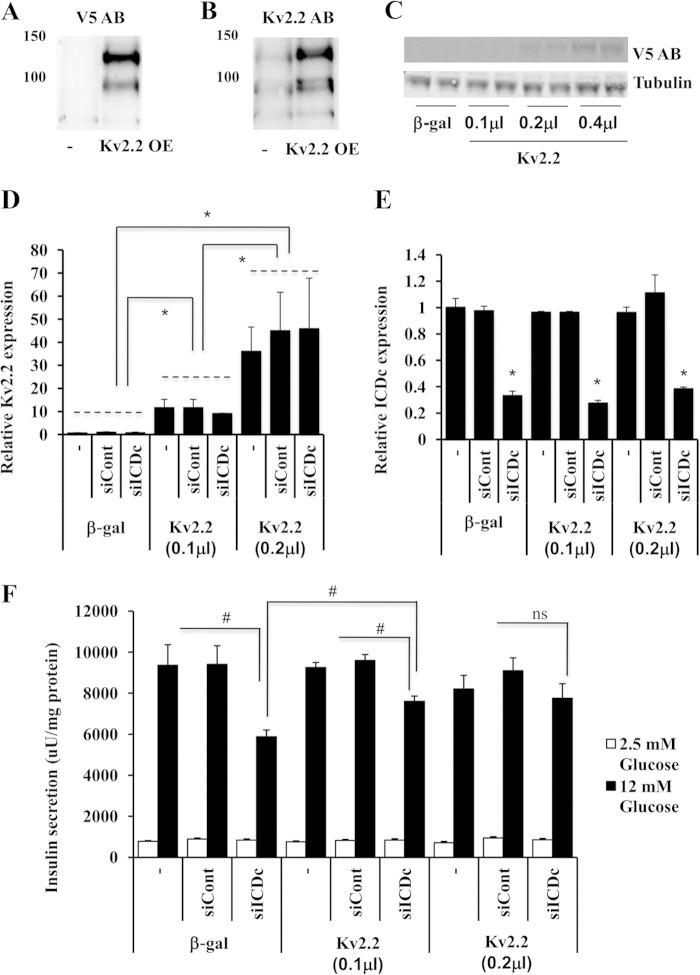
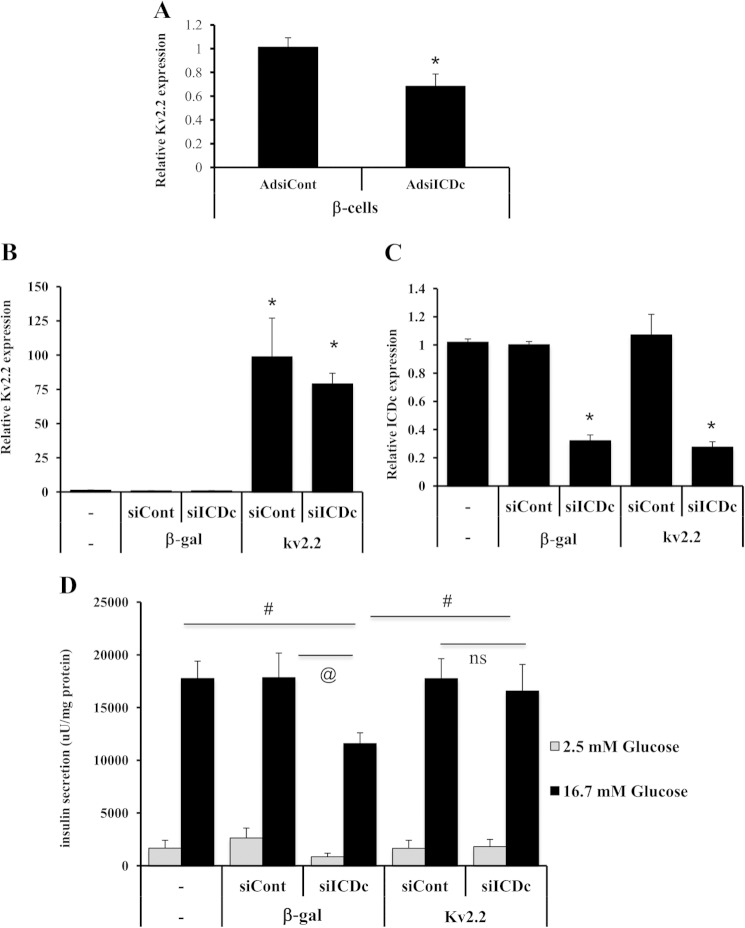
As a tool for manipulating Kv2.2 expression, we constructed an adenovirus containing the human Kv2.2 cDNA fused to a C-terminal V5 epitope tag (AdCMV-hKv2.2). Immunoblot analyses with an antibody to the V5 epitope detected two major bands of ∼100 and 130 kb in cell membrane fractions from AdCMV-hKv2.2-treated 832/13 cells but not from untreated cells (Fig. 2A). These results were confirmed by blotting with an anti-Kv2.2 antibody (Fig. 2B). The deduced size of Kv2.2 is 102.4 kDa (V5-tagged Kv2.2 is ∼106 kDa), and we hypothesize that the lower of the two bands is the native Kv2.2 protein, whereas the higher band may represent a post-translationally modified form of the protein. Consistent with this interpretation, a similar, major band at 140 kDa has been shown to represent phosphorylated Kv2.2 protein in brain membrane fractions (30). Our data show that treatment of 832/13 cells with AdCMV-hKv2.2 results in expression of proteins of physiologically relevant sizes that are recognized by the Kv2.2 antibody and localized to the cell membrane.
FIGURE 2.
Inhibitory effects of siICDc on GSIS are rescued by Kv2.2 overexpression in 832/13 cells. Immunoblot analyses of membrane protein fractions isolated from untreated (−) and from AdCMV-hKv2.2 (Kv2.2 OE)-treated 832/13 cells are shown. The AdCMV-hKv2.2 adenovirus includes a C-terminal V5 epitope tag. Immunoblots were probed with antibodies against V5 epitope (A) and Kv2.2 (B). C, immunoblot analyses of V5 epitope in whole cell extracts from 832/13 cells treated with increasing doses of AdCMV-hKv2.2. For D, E, and F, 832/13 cells were either left untreated or transfected with siICDc or siControl (siCont) duplexes for 72 h. Then, for the last 40 h of the experiment, cells were either transduced with AdCMV-βgal or one of two doses of Ad-CMW-hKv2.2 also used in the dose-response experiment shown in C (0.1 or 0.2 μl). qRT-PCR analyses of Kv2.2 expression (D) and ICDc expression (E) are shown. F, insulin secretion in response to basal and stimulatory glucose concentrations. Data in C–E represent mean ± S.E. (error bars) of three independent experiments performed in triplicate. *, p < 0.001; #, p < 0.01. uU, microunits; AB, antibody; ns, not significant.
Using the AdCMV-hKv2.2 adenovirus, we next investigated whether overexpression of Kv2.2 can rescue the impairment of GSIS caused by reduced ICDc expression. For this purpose, 832/13 cells were transfected with siICDc or siControl duplexes or left untreated for 72 h. For the last 40 h of the experiment, cells were either transduced with AdCMV-βgal or AdCMV-hKv2.2 (Fig. 2, C–F). To limit the extent of overexpression of Kv2.2 to reasonable levels, we tested the impact of different doses of the AdCMV-Kv2.2 adenovirus on Kv2.2 protein expression in total cell extracts as shown in Fig. 2C. The lowest doses of AdCMV-Kv2.2 that caused detectable increases in Kv2.2-V5 protein were 0.1 and 0.2 μl of purified virus (Fig. 2C). These doses of AdCMV-hKv2.2 were 6.3 and 3.2 times lower than the doses used for the cell membrane immunoblot studies in Fig. 2, A and B, and gave rise to 10 ± 2- and 45 ± 7-fold increases in Kv2.2 mRNA levels relative to AdCMV-βgal-treated cells (Fig. 2D). siICDc treatment resulted in a 66 ± 2% decrease in ICDc mRNA levels, and the degree of knockdown was unaffected by the presence of AdCMV-hKv2.2 virus (Fig. 2E). ICDc knockdown alone resulted in 38% impairment in insulin secretion at 12 mm glucose relative to non-transfected and siControl-treated cells (p = 0.006 and p = 0.004; Fig. 2F). This impairment was partially reversed in cells treated with the low dose (0.1 μl) of AdCMV-hKv2.2 (p = 0.004 compared with β-gal/siICDc) and completely rescued to the level of control cells by the higher dose of AdCMV-hKv2.2 (0.2 μl). These experiments demonstrate that impaired GSIS in siICDc-treated 832/13 cells can be fully rescued by expression of Kv2.2. In contrast, overexpression of hKv2.1 did not rescue the inhibitory effects of Ad-siICDc on GSIS.
Kv2.2 Expression Is Reduced by Chronic Exposure to Elevated Fatty Acid Levels
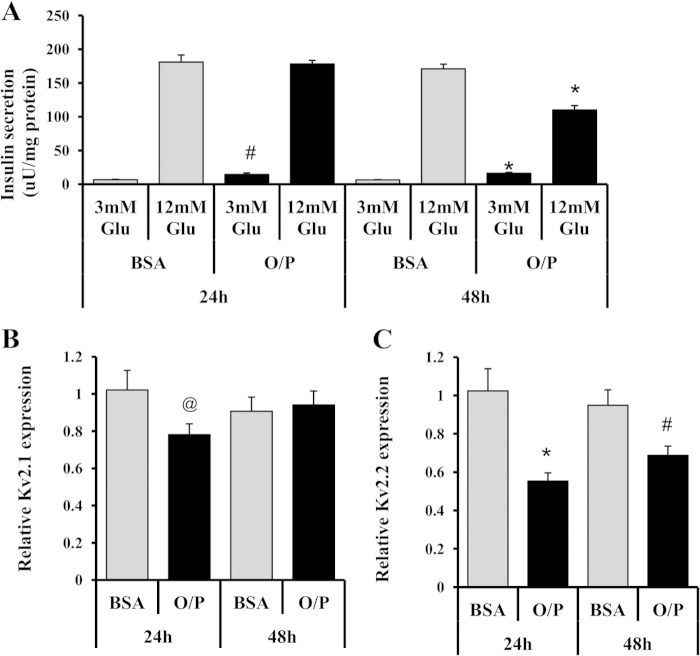
We and others have demonstrated that chronic exposure of insulinoma cells or primary islets to elevated concentrations of fatty acids causes impairment of GSIS (6, 53–56). We have also shown that chronic lipid exposure impairs normal regulation of pyruvate cycling activity by glucose (6). We therefore measured the impact of excess fatty acids on Kv2.1 and Kv2.2 expression. Exposure of 832/13 cells to 1 mm O/P (2:1) complexed to albumin for 24 or 48 h caused 45 ± 4 and 31 ± 5% decreases in Kv2.2 mRNA levels, respectively, compared with BSA-cultured controls (p = 0.0009 and p = 0.007; Fig. 3A). In contrast, the same lipid exposure caused a small decrease in Kv2.1 at 24 h (22 ± 5%, p = 0.04) but had no effect at 48 h (p = 0.7) (Fig. 3B). Consistent with our prior work (6), 24 h of exposure to O/P increased basal insulin secretion compared with BSA-treated control cells (p < 0.01), whereas 48 h of O/P exposure led to both an increase in secretion at basal glucose (p < 0.001) and a decrease in insulin secretion at stimulatory glucose (p < 0.001; Fig. 3C). The lack of effect of fatty acids on insulin secretion at stimulatory glucose at 24 h despite a decrease in Kv2.2 mRNA at this time point may reflect a delay in turnover of the Kv2.2 protein. We were unable to test this possibility directly due to limitations in sensitivity of our antibody for detection of endogenous Kv2.2 protein.
FIGURE 3.
Kv2.2 mRNA expression is reduced by chronic exposure to elevated fatty acids. 832/13 cells were cultured for a total of 3 days and exposed to a mixture of 1 mm O/P complexed to BSA in a 2:1 ratio or to BSA alone for the final 24 or 48 h as indicated. qRT-PCR measurement of Kv2.1 expression (A) and Kv2.2 expression (B) is shown. C, GSIS analysis. Data for all panels represent six independent experiments, each performed in duplicate. *, p < 0.001; #, p < 0.01; @, p < 0.05. Error bars represent S.E. uU, microunits.
Kv2.2 Is Expressed in α-, β-, and δ-Cells of the Intact Islet
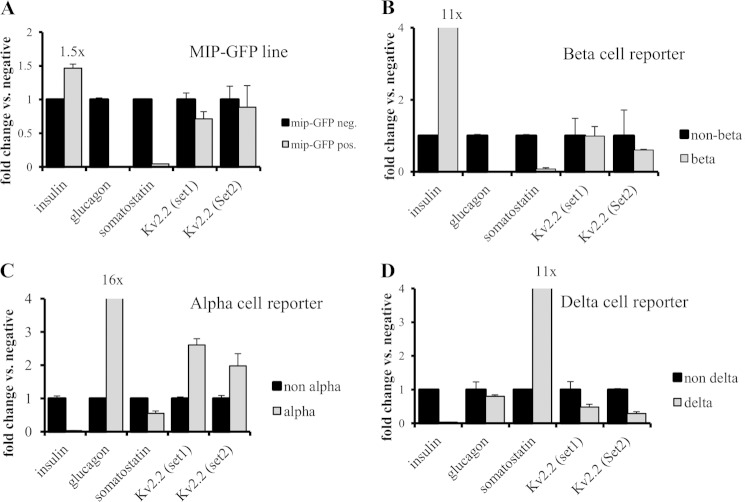
Two reports have suggested that Kv.2.2 is mainly expressed in δ-cells of the intact islet and that expression may either be lacking or very low in β-cells (42, 57). We note first that Kv2.2 is readily expressed in our well differentiated β-cell line 832/13 (Figs. 1–3), and manipulation of its expression affects GSIS, suggesting an intrinsic role for Kv2.2 in the β-cell. Nevertheless, to address this issue more completely, we investigated expression of Kv2.2 in FACS-sorted α-, β-, and δ-cell populations of the mouse islet (Fig. 4). In Fig. 4, A and B, we used transgenic β-cell reporter mouse models that express GFP and mCherry, respectively, under control of the mouse insulin promoter (47).3 In agreement with earlier studies (50, 58), we observed that only ∼50% of the β-cells of the MIP-GFP line express GFP, explaining the relatively modest enrichment (∼1.5-fold) of insulin expression in the GFP-positive fraction relative to the GFP-negative pool. The MIP-mCherry β-cell reporter line exhibited higher insulin enrichment (∼11-fold over control cells (Fig. 4B)). FACS-purified β-cells from both transgenic lines demonstrated markedly reduced expression of the non-β-cell markers glucagon and somatostatin, indicating successful purification, while retaining expression of Kv2.2 as measured by two independent qRT-PCR primer sets (Fig. 4, A and B). To study Kv2.2 expression in mouse α- and δ-cells, we used a glucagon-Cre reporter line (49) and an SST-CreER line, respectively, that were both crossed to a floxed tdTomato reporter line (Fig. 4, C and D). As expected, FACS-sorted tdTomato-positive α-cells were enriched for glucagon (16×) with low levels of somatostatin and insulin expression. FACS-sorted cells of the SST-CreER line were enriched for somatostatin (11-fold). Although the insulin signal was absent in these cells, glucagon expression was comparable with that of the corresponding non-δ-cell fraction. Kv2.2 was detectable in the α/δ-enriched fractions but also in the non-α/δ-cell pool of cells sorted from these islets. These data suggest that Kv2.2 is not restricted to δ-cells but is expressed in all major endocrine cell types of the mouse islet, including β-cells. Studies of Kv2.2 expression within islets from other species have relied mainly on immunofluorescence (42, 57) rather than the cell sorting approach taken here, so it remains to be determined how our current findings from mouse islets translate to non-human primate and human islets.
FIGURE 4.
Kv2.2 is expressed in α-, β- and δ-cells of the intact rodent islet. A–D, dissociated mouse islet cells were sorted based on expression of fluorescent proteins and tested for Kv2.2 expression by qRT-PCR analyses. A, transgenic reporter mouse model that expresses GFP under control of the insulin promoter (MIP-GFP). B, transgenic reporter mouse model that expresses mCherry under control of the insulin promoter. C, the α-cell reporter line, glucagon-Cre. D, the δ-cell reporter line, SST-CreER. Error bars represent S.E. pos., positive; neg., negative.
Kv2.2 Is Regulated by Pyruvate-Isocitrate Cycling and Rescues the Inhibitory Effects of siICDc on GSIS in Primary Islet β-Cells
We next investigated whether Kv2.2 expression is reduced in primary β-cells in response to suppression of ICDc as already established for 832/13 insulinoma cells in Fig. 1. For this purpose, we treated rat islets with adenoviruses containing shRNAs specific for ICDc (Ad-siICDc) or a sequence with no known homology (Ad-siControl) (11) followed by transduction with an adenovirus containing the cDNA encoding GFP under control of the rat insulin promoter (AdRIP-GFP). Subsequently, islet cells were FACS-sorted, and qRT-PCR analyses were performed. We observed a ∼2-fold enrichment in insulin expression in GFP-positive cells relative to GFP-negative cells, and Ad-siICDc treatment resulted in an 86 ± 2% reduction in ICDc mRNA levels in the GFP-positive β-cell fraction. This was accompanied by a 32 ± 10% (p < 0.001) reduction in Kv2.2 expression relative to Ad-siControl-treated β-cells (Fig. 5A). These results demonstrate that ICDc knockdown reduces Kv2.2 expression in primary β-cells, consistent with our findings in 832/13 insulinoma cells.
FIGURE 5.
Kv2.2 overexpression rescues the inhibitory effects of siICDc on GSIS in primary islets. Freshly isolated rat islets were transduced with an equal dose of Ad-siControl (siCont) or Ad-siICDc (siICDc) and subsequently incubated for a total of 72 h. A, for the last 48 h of culture, islets were treated with RIP-GFP. After trypsinization and FACS sorting, GFP-positive β-cells were analyzed for Kv2.2 expression. For B, C, and D, the transduced islets were exposed to equal doses of AdCMV-βgal (β-gal) or AdCMV-hKv2.2 (kv2.2) for the last 40 h of culture. qRT-PCR analyses of Kv2.2 (B) and ICDc (C) mRNA levels are shown. D, glucose-stimulated insulin secretion. Data represent average ± S.E. (error bars) of three independent experiments performed in triplicate. *, p < 0.001; #, p < 0.01; @, p < 0.05. uU, microunits; ns, not significant.
Next, we investigated whether Kv2.2 overexpression can rescue the inhibitory effect of reduced ICDc expression on GSIS from intact rat islets. Ad-siICDc- and Ad-siControl-treated rat islets were co-treated with AdCMV-hKv2.2 or AdCMV-βgal (Fig. 5B). The dose of AdCMV-hKv2.2 used in these experiments in primary rat islets was 0.35 μl. Although this dose caused a large increase in Kv2.2 mRNA levels (Fig. 5B), the dose was similar to one (0.4 μl) that caused a large increase in Kv2.2 mRNA but a moderate increase in protein overexpression in 832/13 cells (Fig. 2C). In the background of AdCMV-βgal treatment, a 68 ± 4% reduction in ICDc mRNA levels (Fig. 5C) resulted in a ∼35% impairment of insulin secretion at 16.7 mm glucose relative to untreated (p = 0.004) or AdCMV-βgal/Ad-siControl-treated (p = 0.02) islets (Fig. 5D). Restoration of Kv2.2 expression in Ad-siICDc-treated cells (Fig. 5B) resulted in a significant increase in GSIS relative to AdCMV-βgal/Ad-siICDc-treated islets (p = 0.009) indistinguishable from that of untreated, AdCMV-βgal/Ad-siControl, and AdCMV-hKv2.2/Ad-siControl islets (p = 0.7) (Fig. 5D). These data demonstrate that Kv2.2 overexpression fully reverses impaired GSIS caused by silencing of ICDc expression in rat islets.
Outward K+ Currents Are Increased in 832/13 Cells with Reduced ICDc and Kv2.2 Expression at Stimulatory Glucose
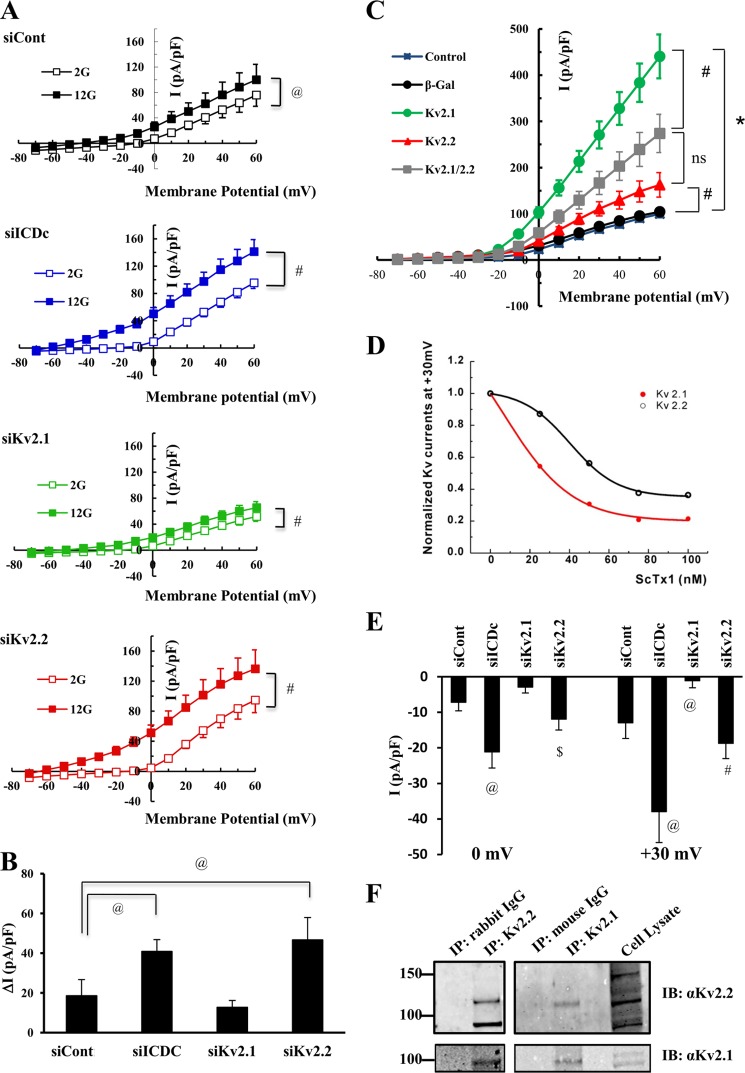
To determine whether our findings of a role for β-cell Kv2.2 in regulation of GSIS are related to changes in electrical activity, we performed patch clamp studies on 832/13 cells treated with siControl, siICDc, siKv2.1, or siKv2.2 siRNA duplexes. The experiments were performed in the presence of internal CaCl2, resulting in an estimated [Ca2+]free of 15 nm, allowing for activation of other K+ channels in addition to Kv channels. In the presence of 2 mm glucose, siControl-treated cells exhibited current activities of 7.2 ± 2.6 pA/pF at a membrane potential of 0 mV indistinguishable from currents observed for siICDc-, siKv2.1-, and siKv2.2-treated cells (9.2 ± 3.7, 6.6 ± 1.3, and 4.5 ± 2.0 pA/pF, respectively; p > 0.2; Fig. 6A, open symbols). At stimulatory glucose, a significant increase in current activities was seen for all conditions (closed symbols; p < 0.05). Interestingly, this glucose-stimulated increase in current activities was significantly larger for cells treated with siICDc or siKv2.2 duplexes (ΔI = 41 ± 6 and 46.7 ± 11 pA/pF at 0mV, respectively) compared with siControl-treated cells (19 ± 8 pA/pF; p = 0.05; Fig. 6, A and B). In contrast, ΔI was unaffected by siKv2.1 treatment (13 ± 4 pA/pF; p = 0.27). Combined, these data demonstrate that at stimulatory glucose siICDc- and siKv2.2-treated cells have increased outward K+ current suggestive of altered membrane excitability and consistent with the impairment of GSIS observed for both of these treatments.
FIGURE 6.
Interactions of Kv2.2 and Kv2.1 regulate outward K+ current in β-cells. 832/13 cells were transfected with siControl (siCont), siICDc, siKv2.1, or siKv2.2 duplexes in combination with a plasmid encoding GFP and cultured for 72 h prior to patch clamping in a Warner chamber. A, normalized current-voltage relationships at 2 mm glucose (open symbols) and 12 mm glucose (closed symbols). B, average glucose-stimulated K+ current at 0 mV. Data shown are average ± S.E. (error bars) of 5–12 cells patched per condition. #, p < 0.01, @, p < 0.05. For C and D, 832/13 cells were either left untreated or treated with adenoviruses expressing β-gal, Kv2.1, Kv2.2, or combinations thereof and cultured for 24 h prior to patch clamp analyses. C, data shown are average ± S.E. (error bars) of 7–21 cells patched per condition. *, p < 0.001; #, p < 0.01 at a membrane potential of 0 mV. D, ScTx1 dose-response curve in 832/13 cells overexpressing Kv2.1 or Kv2.2 by adenovirus-mediated gene transfer. E, inhibitory effects of ScTx (25 nm) on outward K+ current at 0 and +30 mV in 832/13 cells treated with siICDc, siKv2.1, siKv2.2, or siControl. Data shown are average ± S.E. (error bars) of 5–7 cells patched per condition. @, p < 0.05 compared with siControl; $, p < 0.05 compared with siKv2.1; #, p < 0.001 compared with siKv2.1. F, 832/13 cell lysates (native cells; no viral treatment) were immunoprecipitated (IP) by either anti-Kv2.2, anti-Kv2.1, or control IgG antibody. Immunoblots (IB) were probed with antibody specific for Kv2.2 or Kv2.1. Results are representative of three independent experiments.
Effect of Kv2.1 and Kv2.2 Overexpression on Outward Kv Currents in 832/13 Cells
Given the unanticipated increase in Kv current in response to Kv2.2 suppression, we investigated the effects of Kv2.1 and Kv.2.2 overexpression on Kv currents in 832/13 cells. Overexpression of Kv2.1 (28 ± 3-fold induction in Kv2.1 mRNA levels) resulted in a robust induction of Kv currents (green circles; 103.3 ± 11.3 pA/pF at a membrane potential of 0 mV) relative to AdCMV-βgal-treated (black circles; 30.7 ± 3.3 pA/pF; p < 0.001) or untreated (blue squares; 22.8 ± 2.1 pA/pF; p < 0.001) cells. In contrast, overexpression of Kv2.2 (0.2-μl dose, which caused a 59 ± 10-fold induction of mRNA levels) caused only a modest increase in Kv currents (red triangles; 41.0 ± 3.9 pA/pF at 0 mV; p < 0.01) relative to control cells (Fig. 6C). The relatively low intrinsic K+ channel activity attained with Kv2.2 overexpression is consistent with the low residual currents reported in islets from Kv2.1 knock-out mice (38). We also studied the effects of overexpressing Kv2.1 and Kv2.2 simultaneously. Interestingly, a 32 ± 7-fold induction in Kv2.1 mRNA expression combined with a 45 ± 9-fold induction in Kv2.2 mRNA expression resulted in a Kv current that was reduced by half compared with that observed with Kv2.1 overexpression alone (gray diamonds; 58.6 ± 9.8 pA/pF at 0 mV; p = 0.003; Fig. 6C). These findings suggest that Kv2.2 has relatively low K+ conductance compared with Kv2.1 and that it may also function as a negative modulator of Kv2.1 channel activity.
To further investigate the potential regulatory effect of Kv2.2 on Kv2.1, we next sought to exploit a reported preferential effect of ScTx1 as an inhibitor of Kv2.1 relative to Kv2.2 (59). We first performed a ScTx1 dose-response study in 832/13 cells with equal levels of overexpression of Kv2.1 or Kv2.2 and identified a dose of ScTx1 (25 nm) at which Kv2.1 was inhibited by ∼50%, whereas Kv2.2 was not significantly inhibited at this same dose (Fig. 6D). Using this dose of ScTx1, we found that K+ currents recorded from 832/13 cells treated with siICDc were more sensitive to ScTx1 than siControl- or siKv2.1-treated cells studied at membrane potentials of either 0 or +30 mV (Fig. 6E). A similar trend for enhanced inhibition was noted in siKv2.2-treated cells that did not reach statistical significance relative to the siControl group. However, cells treated with siKv2.2 were significantly more susceptible to inhibition of K+ current by ScTx1 compared with cells treated with siKv2.1 at either 0 or 30 mV of current (Fig. 6E). Taken together, these data demonstrate that conditions that reduce Kv2.2 expression (either direct Kv2.2 silencing or in response to suppression of ICDc) remove an inhibitory effect of Kv2.2 channels on K+ currents in 832/13 cells, rendering the cells more sensitive to the Kv2.1-selective inhibitor.
To investigate whether the foregoing results could be related to an ability of Kv2.1 and Kv2.2 to form heterodimers in β-cells as has been shown in neuronal cells (60), we performed co-immunoprecipitation studies. We prepared extracts of 832/13 cells and immunoprecipitated with an antibody specific for Kv2.2 followed by immunoblotting with antibody for Kv2.2 or Kv2.1. Note that this experiment was performed with non-virus-treated 832/13 cells to study interactions of their endogenous Kv2.1 and Kv2.2 proteins. Both Kv2.2 and Kv2.1 were clearly detected in samples immunoprecipitated with the Kv2.2 antibody but not in samples treated with a control IgG (Fig. 6F). We also performed the converse experiment of immunoprecipitation with an antibody for Kv2.1 followed by immunoblotting for Kv2.1 and Kv2.2. Again, both Kv2.2 and Kv2.1 were clearly present in samples immunoprecipitated with the Kv2.1-specific antibody (Fig. 6F). In aggregate, the data shown in Fig. 6 are consistent with a model in which Kv2.2 engages in a physical interaction with Kv2.1 in β-cells, and that engagement of Kv2.1 in such complexes suppresses its ability to create Kv current. The model further holds that this suppressive function of Kv2.2 is controlled in part by pyruvate-isocitrate cycling activity.
DISCUSSION
Both type 1 and type 2 diabetes are syndromes of insulin deficiency, although the origins of the diseases are different. In type 1 diabetes, β-cells are destroyed by the host immune system (61). Type 2 diabetes is associated with impaired control of insulin secretion in response to nutrients coupled with gradual depletion of β-cell mass mainly due to increased apoptosis and inadequate β-cell regeneration (62). A clearer understanding of basic β-cell function is critical to develop surrogate cells for insulin replacement therapies for type 1 diabetes and for expansion of therapeutic options for treatment of type 2 diabetes.
Previously, we have demonstrated that maintaining flux though the pyruvate-isocitrate pathway is essential for normal GSIS (8, 10, 11, 23), but the molecular mediators of this effect were not identified. NADPH is a potential second messenger generated by the pyruvate-isocitrate cycle, and one study has suggested that NADPH can inactivate Kv channels (44), possibly serving to slow membrane repolarization, thereby allowing the effects of suppression of KATP channels to be sustained. To test a possible relationship among pyruvate-isocitrate cycling, Kv channels, and insulin secretion, we measured expression levels of Kv2 channels in cells exhibiting reduced pyruvate-isocitrate activity and impaired GSIS. Surprisingly, we found that cells with reduced ICDc and CIC expression have decreased rather than increased Kv2.2 mRNA expression with no effect on expression of Kv2.1. Impairment of GSIS caused by suppression of ICDc expression was rescued by re-expression of Kv2.2 but not Kv2.1. Importantly, these responses to suppression of pyruvate-isocitrate cycling were observed in primary rat islets as well as in the 832/13 insulinoma cell line. Exposure of 832/13 cells to chronic elevations of fatty acids, another model of impaired GSIS and dysregulated pyruvate cycling (6), also caused a decrease in Kv2.2 mRNA levels. Moreover, targeted knockdown of Kv2.2 impaired GSIS. When we measured Kv currents using patch clamp techniques in 832/13 cells with reduced ICDc or Kv2.2 expression, we observed increases in outward Kv currents at stimulatory glucose concentrations relative to control cells. In contrast, Kv2.1 silencing caused a trend to increase GSIS with no measureable effect on Kv current.
We interpret our findings to suggest a novel modulatory role of pyruvate-isocitrate cycling and Kv2.2 in control of electrical activity and GSIS in islet β-cells. In this model, pyruvate-isocitrate cycling serves to maintain levels of Kv2.2 expression that are sufficient to provide tonic suppression of Kv2.1 function. In support of such a model, co-overexpression of Kv2.1 and Kv2.2 in β-cells resulted in reduced Kv current compared with overexpression of Kv2.1 alone. Moreover, suppression of Kv2.2 expression either by direct knockdown or in response to knockdown of ICDc resulted in greater sensitivity of 832/13 cells to inhibition of K+ currents by the Kv2.1-selective inhibitor ScTx1, supporting the proposed suppressor function of endogenous Kv2.2 channels. Also consistent with the model, co-immunoprecipitation experiments demonstrated an interaction of the endogenous Kv2.1 and Kv2.2 channels. This interaction could reflect formation of a Kv2.1-Kv2.2 channel complex as supported by our functional data and by studies in oocytes and pyramidal neurons (60, 63). In both of the prior studies, co-expression of a dominant negative Kv2.2 subunit with wild-type Kv2.1 or Kv2.2 channels in oocytes resulted in reduced Kv channel activity, fully supporting a model of formation of heteromultimeric complexes (60, 63). However, in one of those studies (60), co-overexpression of native Kv2.1 and Kv2.2 channels in oocytes resulted in increased K+ current relative to expression of either channel alone. We have no immediate explanation for this apparent discrepancy but note that our study was performed in the relevant cell type, the islet β-cell, rather than in oocytes and that our model of a suppressive effect of Kv2.2 is supported by the four distinct lines of evidence summarized above.
It is important to note that other models are also possible. Kv2.1 and Kv2.2 are highly homologous proteins in their N-terminal regions but diverge substantially at their C termini. The different C termini of the two proteins allow them to interact differentially with other proteins. For example, Kv2.2 appears to be guided to the plasma membrane in part by the β-subunit Kvβ4, whereas this mechanism is not involved in transport of Kv2.1 (64). Thus, it is possible that the negative impact of Kv2.2 suppression on GSIS is indirect, regulated by increases in the free pool of β-subunits that can now interact with other proteins, including other ion channels.
MacDonald and co-workers (65, 66) have recently described novel biological roles of Kv2.1 in islet cells. They demonstrate that overexpression of Kv2.1 augments insulin exocytosis as measured by membrane capacitance measurements in patch-clamped islet cells. Because these experiments are performed under depolarized conditions, this effect of Kv2.1 is independent of its effects on electrical function. The potentiating effect of Kv2.1 appears to be mediated by its interaction with the SNARE protein syntaxin-1 via a specific motif in the Kv2.1 C-terminal region. Deletion of that interaction domain or interference with the interaction by delivery of a “decoy” peptide reverses the activating effect of Kv2.1 on exocytosis, and the interfering peptide also impairs GSIS in intact islets. Other recent studies have demonstrated that Kv channels in islets are modified by SUMOylation, and this modification may participate in regulating the Kv2.1/syntaxin-1 interaction (66, 67). Our finding of impairment in GSIS in response to knockdown of Kv2.2 does not seem to fit with the syntaxin-1 model of Kv2.1 function because one might anticipate that a decrease in Kv2.2 would create a larger pool of free Kv2.1 for interaction with syntaxin-1. Further studies will be required to fully understand the impact of Kv2.2 suppression on functional Kv channel assembly and stability, interaction of channels with syntaxin-1 and potentially other SNARE proteins, and modification of the channels by SUMOylation and other post-translational events.
Our study provides new information about expression of Kv2.2 in the various endocrine cell types of the pancreatic islet. We were led to investigate this issue based on reports that Kv2.2 is expressed primarily in δ-cells (42, 57) and another recent study showing that global knock-out of Kv2.1 in transgenic mice results in enhanced GSIS in isolated islets and that Ad-siRNA-mediated knockdown of Kv2.2 in mouse islets enhances somatostatin but not insulin secretion (68). The latter findings should be interpreted with caution as mouse islets are reported to be refractory to penetration of recombinant adenoviruses to the β-cell core relative to rat islets (48, 52), possibly explaining the absence of an effect of Kv2.2 knockdown on insulin secretion in the setting of the mouse islet. The current study used FACS-sorted mouse islet cells to demonstrate clear expression of Kv2.2 in β-cell-, α-cell-, and δ-cell-enriched cell pools. Moreover, sorting of β-cells from rat islets with a RIP-GFP adenovirus allowed us to demonstrate that suppression of ICDc in β-cells causes a decrease in β-cell Kv2.2 expression, resulting in impaired GSIS, and that this defect is rescued by re-expression of Kv2.2. Taken together, our findings demonstrate significant expression of Kv2.2 in β-cells as well as a β-cell-autonomous effect of pyruvate-isocitrate cycling to regulate Kv2.2 and GSIS. Consistent with these findings, Kv2.2 is clearly expressed in our well differentiated INS-1-derived β-cell line, 832/13.
siRNA-mediated suppression of either of two enzymes involved in the pyruvate-isocitrate cycle, CIC or ICDc, resulted in reduced expression of Kv2.2 but not Kv2.1 at the RNA level; a similar response is observed upon chronic culture of islet cells with elevated free fatty acids. The mechanisms involved in the control of Kv2.2 transcript levels remain to be resolved. The temporal scale of these effects (measured over several days of siRNA treatment or fatty acid culture) and the known temporal dynamics of changes in gene expression (generally measured in hours rather than minutes) suggest that regulation of Kv2.2 expression by pyruvate-isocitrate cycling is an adaptive mechanism rather than an acute event in control of GSIS. The possibility remains that other signals generated by pyruvate-isocitrate cycling activity, including NADPH production via the cytosolic ICDc reaction, could play a direct role in acute regulation of GSIS, and this is a subject of ongoing investigation.
In conclusion, the current study identifies a novel link between a β-cell metabolic pathway and Kv channels. Importantly, this pathway becomes impaired in response to chronic exposure of islets to high fat, a model often used as a surrogate for the “nutritional overload” experienced on the path to type 2 diabetes. Our findings suggest that interventions that reactivate pyruvate-isocitrate cycling or that maintain Kv2.2 expression may be of benefit for reversing islet dysfunction of diabetes.
This work was supported, in whole or in part, by National Institutes of Health Grants DK42583 (to C. B. N.) and DK026741 (to W. W. V. and M. O. H.). This work was also supported by the Juvenile Diabetes Research Foundation (to M. O. H.).
M. O. Huising, manuscript in preparation.
- GSIS
- glucose-stimulated insulin secretion
- ICDc
- cytosolic NADP-dependent isocitrate dehydrogenase
- KATP
- ATP-sensitive potassium
- Kv
- voltage-gated potassium
- MIP
- mouse insulin promoter
- O/P
- oleate/palmitate
- ScTx1
- stromatoxin1
- SUMO
- small ubiquitin-like modifier
- qRT-PCR
- quantitative RT-PCR
- Ad
- adenovirus
- pF
- picofarad.
REFERENCES
- 1. Ashcroft F. M. (2005) ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Investig. 115, 2047–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drews G., Krippeit-Drews P., Düfer M. (2010) Electrophysiology of islet cells. Adv Exp. Med. Biol. 654, 115–163 [DOI] [PubMed] [Google Scholar]
- 3. Komatsu M., Sato Y., Aizawa T., Hashizume K. (2001) KATP channel-independent glucose action: an elusive pathway in stimulus-secretion coupling of pancreatic β-cell. Endocr. J. 48, 275–288 [DOI] [PubMed] [Google Scholar]
- 4. Sato Y., Anello M., Henquin J. C. (1999) Glucose regulation of insulin secretion independent of the opening or closure of adenosine triphosphate-sensitive K+ channels in β cells. Endocrinology 140, 2252–2257 [DOI] [PubMed] [Google Scholar]
- 5. Gembal M., Gilon P., Henquin J. C. (1992) Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J. Clin. Investig. 89, 1288–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boucher A., Lu D., Burgess S. C., Telemaque-Potts S., Jensen M. V., Mulder H., Wang M. Y., Unger R. H., Sherry A. D., Newgard C. B. (2004) Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J. Biol. Chem. 279, 27263–27271 [DOI] [PubMed] [Google Scholar]
- 7. Jensen M. V., Joseph J. W., Ilkayeva O., Burgess S., Lu D., Ronnebaum S. M., Odegaard M., Becker T. C., Sherry A. D., Newgard C. B. (2006) Compensatory responses to pyruvate carboxylase suppression in islet β-cells. Preservation of glucose-stimulated insulin secretion. J. Biol. Chem. 281, 22342–22351 [DOI] [PubMed] [Google Scholar]
- 8. Jensen M. V., Joseph J. W., Ronnebaum S. M., Burgess S. C., Sherry A. D., Newgard C. B. (2008) Metabolic cycling in control of glucose-stimulated insulin secretion. Am. J. Physiol. Endocrinol. Metab. 295, E1287–E1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu D., Mulder H., Zhao P., Burgess S. C., Jensen M. V., Kamzolova S., Newgard C. B., Sherry A. D. (2002) 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc. Natl. Acad. Sci. U.S.A. 99, 2708–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Odegaard M. L., Joseph J. W., Jensen M. V., Lu D., Ilkayeva O., Ronnebaum S. M., Becker T. C., Newgard C. B. (2010) The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J. Biol. Chem. 285, 16530–16537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ronnebaum S. M., Ilkayeva O., Burgess S. C., Joseph J. W., Lu D., Stevens R. D., Becker T. C., Sherry A. D., Newgard C. B., Jensen M. V. (2006) A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J. Biol. Chem. 281, 30593–30602 [DOI] [PubMed] [Google Scholar]
- 12. Ronnebaum S. M., Jensen M. V., Hohmeier H. E., Burgess S. C., Zhou Y. P., Qian S., MacNeil D., Howard A., Thornberry N., Ilkayeva O., Lu D., Sherry A. D., Newgard C. B. (2008) Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J. Biol. Chem. 283, 28909–28917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joseph J. W., Odegaard M. L., Ronnebaum S. M., Burgess S. C., Muehlbauer J., Sherry A. D., Newgard C. B. (2007) Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J. Biol. Chem. 282, 31592–31600 [DOI] [PubMed] [Google Scholar]
- 14. Huypens P., Pillai R., Sheinin T., Schaefer S., Huang M., Odegaard M. L., Ronnebaum S. M., Wettig S. D., Joseph J. W. (2011) The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic β cells. Diabetologia 54, 135–145 [DOI] [PubMed] [Google Scholar]
- 15. Farfari S., Schulz V., Corkey B., Prentki M. (2000) Glucose-regulated anaplerosis and cataplerosis in pancreatic β-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49, 718–726 [DOI] [PubMed] [Google Scholar]
- 16. Pongratz R. L., Kibbey R. G., Shulman G. I., Cline G. W. (2007) Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J. Biol. Chem. 282, 200–207 [DOI] [PubMed] [Google Scholar]
- 17. Cline G. W., Lepine R. L., Papas K. K., Kibbey R. G., Shulman G. I. (2004) 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J. Biol. Chem. 279, 44370–44375 [DOI] [PubMed] [Google Scholar]
- 18. Guay C., Madiraju S. R., Aumais A., Joly E., Prentki M. (2007) A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J. Biol. Chem. 282, 35657–35665 [DOI] [PubMed] [Google Scholar]
- 19. Fransson U., Rosengren A. H., Schuit F. C., Renström E., Mulder H. (2006) Anaplerosis via pyruvate carboxylase is required for the fuel-induced rise in the ATP:ADP ratio in rat pancreatic islets. Diabetologia 49, 1578–1586 [DOI] [PubMed] [Google Scholar]
- 20. Schuit F., De Vos A., Farfari S., Moens K., Pipeleers D., Brun T., Prentki M. (1997) Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in β cells. J. Biol. Chem. 272, 18572–18579 [DOI] [PubMed] [Google Scholar]
- 21. Brun T., Roche E., Assimacopoulos-Jeannet F., Corkey B. E., Kim K. H., Prentki M. (1996) Evidence for an anaplerotic/malonyl-CoA pathway in pancreatic β-cell nutrient signaling. Diabetes 45, 190–198 [DOI] [PubMed] [Google Scholar]
- 22. MacDonald M. J., Fahien L. A., Brown L. J., Hasan N. M., Buss J. D., Kendrick M. A. (2005) Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am. J. Physiol. Endocrinol. Metab. 288, E1–E15 [DOI] [PubMed] [Google Scholar]
- 23. Joseph J. W., Jensen M. V., Ilkayeva O., Palmieri F., Alárcon C., Rhodes C. J., Newgard C. B. (2006) The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J. Biol. Chem. 281, 35624–35632 [DOI] [PubMed] [Google Scholar]
- 24. Jacobson D. A., Philipson L. H. (2007) Action potentials and insulin secretion: new insights into the role of Kv channels. Diabetes Obes. Metab. 9, Suppl. 2, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerschensteiner D., Stocker M. (1999) Heteromeric assembly of Kv2.1 with Kv9.3: effect on the state dependence of inactivation. Biophys. J. 77, 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sano Y., Mochizuki S., Miyake A., Kitada C., Inamura K., Yokoi H., Nozawa K., Matsushime H., Furuichi K. (2002) Molecular cloning and characterization of Kv6.3, a novel modulatory subunit for voltage-gated K+ channel Kv2.1. FEBS Lett. 512, 230–234 [DOI] [PubMed] [Google Scholar]
- 27. Rettig J., Heinemann S. H., Wunder F., Lorra C., Parcej D. N., Dolly J. O., Pongs O. (1994) Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature 369, 289–294 [DOI] [PubMed] [Google Scholar]
- 28. Chouinard S. W., Lu F., Ganetzky B., Macdonald M. J. (2000) Evidence for voltage-gated potassium channel β-subunits with oxidoreductase motifs in human and rodent pancreatic β cells. Receptors Channels 7, 237–243 [PubMed] [Google Scholar]
- 29. Pongs O., Leicher T., Berger M., Roeper J., Bähring R., Wray D., Giese K. P., Silva A. J., Storm J. F. (1999) Functional and molecular aspects of voltage-gated K+ channel β subunits. Ann. N.Y. Acad. Sci. 868, 344–355 [DOI] [PubMed] [Google Scholar]
- 30. Hermanstyne T. O., Kihira Y., Misono K., Deitchler A., Yanagawa Y., Misonou H. (2010) Immunolocalization of the voltage-gated potassium channel Kv2.2 in GABAergic neurons in the basal forebrain of rats and mice. J. Comp. Neurol. 518, 4298–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jindal H. K., Folco E. J., Liu G. X., Koren G. (2008) Posttranslational modification of voltage-dependent potassium channel Kv1.5: COOH-terminal palmitoylation modulates its biological properties. Am. J. Physiol. Heart Circ. Physiol. 294, H2012–H2021 [DOI] [PubMed] [Google Scholar]
- 32. Boehmer C., Laufer J., Jeyaraj S., Klaus F., Lindner R., Lang F., Palmada M. (2008) Modulation of the voltage-gated potassium channel Kv1.5 by the SGK1 protein kinase involves inhibition of channel ubiquitination. Cell. Physiol. Biochem. 22, 591–600 [DOI] [PubMed] [Google Scholar]
- 33. Nitabach M. N., Llamas D. A., Thompson I. J., Collins K. A., Holmes T. C. (2002) Phosphorylation-dependent and phosphorylation-independent modes of modulation of shaker family voltage-gated potassium channels by SRC family protein tyrosine kinases. J. Neurosci. 22, 7913–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roe M. W., Worley J. F., 3rd, Mittal A. A., Kuznetsov A., DasGupta S., Mertz R. J., Witherspoon S. M., 3rd, Blair N., Lancaster M. E., McIntyre M. S., Shehee W. R., Dukes I. D., Philipson L. H. (1996) Expression and function of pancreatic β-cell delayed rectifier K+ channels. Role in stimulus-secretion coupling. J. Biol. Chem. 271, 32241–32246 [DOI] [PubMed] [Google Scholar]
- 35. MacDonald P. E., Sewing S., Wang J., Joseph J. W., Smukler S. R., Sakellaropoulos G., Wang J., Saleh M. C., Chan C. B., Tsushima R. G., Salapatek A. M., Wheeler M. B. (2002) Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic β-cells enhances glucose-dependent insulin secretion. J. Biol. Chem. 277, 44938–44945 [DOI] [PubMed] [Google Scholar]
- 36. Herrington J., Zhou Y. P., Bugianesi R. M., Dulski P. M., Feng Y., Warren V. A., Smith M. M., Kohler M. G., Garsky V. M., Sanchez M., Wagner M., Raphaelli K., Banerjee P., Ahaghotu C., Wunderler D., Priest B. T., Mehl J. T., Garcia M. L., McManus O. B., Kaczorowski G. J., Slaughter R. S. (2006) Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes 55, 1034–1042 [DOI] [PubMed] [Google Scholar]
- 37. Tamarina N. A., Kuznetsov A., Fridlyand L. E., Philipson L. H. (2005) Delayed-rectifier (KV2.1) regulation of pancreatic β-cell calcium responses to glucose: inhibitor specificity and modeling. Am. J. Physiol. Endocrinol. Metab. 289, E578–E585 [DOI] [PubMed] [Google Scholar]
- 38. Jacobson D. A., Kuznetsov A., Lopez J. P., Kash S., Ammälä C. E., Philipson L. H. (2007) Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 6, 229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herrington J., Sanchez M., Wunderler D., Yan L., Bugianesi R. M., Dick I. E., Clark S. A., Brochu R. M., Priest B. T., Kohler M. G., McManus O. B. (2005) Biophysical and pharmacological properties of the voltage-gated potassium current of human pancreatic β-cells. J. Physiol. 567, 159–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Braun M., Ramracheya R., Bengtsson M., Zhang Q., Karanauskaite J., Partridge C., Johnson P. R., Rorsman P. (2008) Voltage-gated ion channels in human pancreatic β-cells: electrophysiological characterization and role in insulin secretion. Diabetes 57, 1618–1628 [DOI] [PubMed] [Google Scholar]
- 41. Pedersen M. G. (2010) A biophysical model of electrical activity in human β-cells. Biophys. J. 99, 3200–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wolf-Goldberg T., Michaelevski I., Sheu L., Gaisano H. Y., Chikvashvili D., Lotan I. (2006) Target soluble N-ethylmaleimide-sensitive factor attachment protein receptors (t-SNAREs) differently regulate activation and inactivation gating of Kv2.2 and Kv2.1: Implications on pancreatic islet cell Kv channels. Mol. Pharmacol. 70, 818–828 [DOI] [PubMed] [Google Scholar]
- 43. Ivarsson R., Quintens R., Dejonghe S., Tsukamoto K., in 't Veld P., Renström E., Schuit F. C. (2005) Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54, 2132–2142 [DOI] [PubMed] [Google Scholar]
- 44. MacDonald P. E., Salapatek A. M., Wheeler M. B. (2003) Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic β-cells. J. Physiol. 546, 647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asfari M., Janjic D., Meda P., Li G., Halban P. A., Wollheim C. B. (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130, 167–178 [DOI] [PubMed] [Google Scholar]
- 46. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 47. Hara M., Wang X., Kawamura T., Bindokas V. P., Dizon R. F., Alcoser S. Y., Magnuson M. A., Bell G. I. (2003) Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 284, E177–E183 [DOI] [PubMed] [Google Scholar]
- 48. Sigalla J., David A., Anegon I., Fiche M., Huvelin J. M., Boeffard F., Cassard A., Soulillou J. P., Le Mauff B. (1997) Adenovirus-mediated gene transfer into isolated mouse adult pancreatic islets: normal β-cell function despite induction of an anti-adenovirus immune response. Hum. Gene Ther. 8, 1625–1634 [DOI] [PubMed] [Google Scholar]
- 49. Herrera P. L. (2000) Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127, 2317–2322 [DOI] [PubMed] [Google Scholar]
- 50. Huising M. O., van der Meulen T., Vaughan J. M., Matsumoto M., Donaldson C. J., Park H., Billestrup N., Vale W. W. (2010) CRFR1 is expressed on pancreatic β cells, promotes β cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 107, 912–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bain J. R., Schisler J. C., Takeuchi K., Newgard C. B., Becker T. C. (2004) An adenovirus vector for efficient RNA interference-mediated suppression of target genes in insulinoma cells and pancreatic islets of Langerhans. Diabetes 53, 2190–2194 [DOI] [PubMed] [Google Scholar]
- 52. Becker T. C., BeltrandelRio H., Noel R. J., Johnson J. H., Newgard C. B. (1994) Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J. Biol. Chem. 269, 21234–21238 [PubMed] [Google Scholar]
- 53. Milburn J. L., Jr., Hirose H., Lee Y. H., Nagasawa Y., Ogawa A., Ohneda M., BeltrandelRio H., Newgard C. B., Johnson J. H., Unger R. H. (1995) Pancreatic β-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J. Biol. Chem. 270, 1295–1299 [DOI] [PubMed] [Google Scholar]
- 54. Zhou Y. P., Grill V. E. (1994) Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J. Clin. Investig. 93, 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prentki M., Joly E., El-Assaad W., Roduit R. (2002) Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in β-cell adaptation and failure in the etiology of diabetes. Diabetes 51, Suppl. 3, S405–S413 [DOI] [PubMed] [Google Scholar]
- 56. Poitout V., Robertson R. P. (2002) Minireview: secondary β-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 143, 339–342 [DOI] [PubMed] [Google Scholar]
- 57. Yan L., Figueroa D. J., Austin C. P., Liu Y., Bugianesi R. M., Slaughter R. S., Kaczorowski G. J., Kohler M. G. (2004) Expression of voltage-gated potassium channels in human and rhesus pancreatic islets. Diabetes 53, 597–607 [DOI] [PubMed] [Google Scholar]
- 58. Rieck S., White P., Schug J., Fox A. J., Smirnova O., Gao N., Gupta R. K., Wang Z. V., Scherer P. E., Keller M. P., Attie A. D., Kaestner K. H. (2009) The transcriptional response of the islet to pregnancy in mice. Mol. Endocrinol. 23, 1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Escoubas P., Diochot S., Célérier M. L., Nakajima T., Lazdunski M. (2002) Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol. Pharmacol. 62, 48–57 [DOI] [PubMed] [Google Scholar]
- 60. Blaine J. T., Ribera A. B. (1998) Heteromultimeric potassium channels formed by members of the Kv2 subfamily. J. Neurosci. 18, 9585–9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Belle T. L., Coppieters K. T., von Herrath M. G. (2011) Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol. Rev. 91, 79–118 [DOI] [PubMed] [Google Scholar]
- 62. Muoio D. M., Newgard C. B. (2008) Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 193–205 [DOI] [PubMed] [Google Scholar]
- 63. Kihira Y., Hermanstyne T. O., Misonou H. (2010) Formation of heteromeric Kv2 channels in mammalian brain neurons. J. Biol. Chem. 285, 15048–15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fink M., Duprat F., Lesage F., Heurteaux C., Romey G., Barhanin J., Lazdunski M. (1996) A new K+ channel β subunit to specifically enhance Kv2.2 (CDRK) expression. J. Biol. Chem. 271, 26341–26348 [DOI] [PubMed] [Google Scholar]
- 65. Dai X. Q., Manning Fox J. E., Chikvashvili D., Casimir M., Plummer G., Hajmrle C., Spigelman A. F., Kin T., Singer-Lahat D., Kang Y., Shapiro A. M., Gaisano H. Y., Lotan I., Macdonald P. E. (2012) The voltage-dependent potassium channel subunit Kv2.1 regulates insulin secretion from rodent and human islets independently of its electrical function. Diabetologia 55, 1709–1720 [DOI] [PubMed] [Google Scholar]
- 66. Dai X. Q., Plummer G., Casimir M., Kang Y., Hajmrle C., Gaisano H. Y., Manning Fox J. E., MacDonald P. E. (2011) SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes 60, 838–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dai X. Q., Kolic J., Marchi P., Sipione S., Macdonald P. E. (2009) SUMOylation regulates Kv2.1 and modulates pancreatic β-cell excitability. J. Cell Sci. 122, 775–779 [DOI] [PubMed] [Google Scholar]
- 68. Li X. N., Herrington J., Petrov A., Ge L., Eiermann G., Xiong Y., Jensen M. V., Hohmeier H. E., Newgard C. B., Garcia M. L., Wagner M., Zhang B. B., Thornberry N. A., Howard A. D., Kaczorowski G. J., Zhou Y. P. (2013) The roles of voltage-gated potassium channels Kv2.1 and Kv2.2 in the regulation of insulin and somatostatin release from pancreatic islets. J. Pharmacol. Exp. Ther. 344, 407–416 [DOI] [PubMed] [Google Scholar]