Background: Methionine adenosyltransferase 2B protein (MATβ) binds to resveratrol, but it exerts the opposite effects on growth and apoptosis.
Results: Resveratrol induces HuR, SIRT1, and MATβ expression. These proteins interact, which stabilizes them. MATβ induction blunts the resveratrol effect on growth and apoptosis.
Conclusion: MATβ-HuR-SIRT1 interaction impacts resveratrol actions.
Significance: This is the first demonstration of MATβ in SIRT1 signaling.
Keywords: Apoptosis, Cell Growth, Liver Cancer, Resveratrol, Sirt1, HuR, Methionine Adenosyltransferase 2B
Abstract
Resveratrol is growth-suppressive and pro-apoptotic in liver cancer cells. Methionine adenosyltransferase 2B (MAT2B) encodes for two dominant variants V1 and V2 that positively regulate growth, and V1 is anti-apoptotic when overexpressed. Interestingly, crystal structure analysis of MAT2B protein (MATβ) protomer revealed two resveratrol binding pockets, which raises the question of the role of MAT2B in resveratrol biological activities. We found that resveratrol induced the expression of MAT2BV1 and V2 in a time- and dose-dependent manner by increasing transcription, mRNA, and protein stabilization. Following resveratrol treatment, HuR expression increased first, followed by SIRT1 and MAT2B. SIRT1 induction contributes to increased MAT2B transcription whereas HuR induction increased MAT2B mRNA stability. MATβ interacts with HuR and SIRT1, and resveratrol treatment enhanced these interactions while reducing the interaction between MATβ and MATα2. Because MATβ lowers the Ki of MATα2 for S-adenosylmethionine (AdoMet), this allowed steady-state AdoMet level to rise. Interaction among MATβ, SIRT1, and HuR increased stability of these proteins. Induction of MAT2B is a compensatory response to resveratrol as knocking down MAT2BV1 potentiated the resveratrol pro-apoptotic and growth-suppressive effects, whereas the opposite occurred with V1 overexpression. The same effect on growth occurred with MAT2BV2. In conclusion, resveratrol induces HuR, SIRT1, and MAT2B expression; the last may represent a compensatory response against apoptosis and growth inhibition. However, MATβ induction also facilitates SIRT1 activation, as the interaction stabilizes SIRT1. This complex interplay among MATβ, HuR, and SIRT1 has not been previously reported and suggests that these proteins may regulate each other's signaling.
Introduction
Resveratrol (3,4,5-trihydroxy-trans-stilbene, RSV),3 a naturally occurring polyphenol that exists in grapes, berries, peanuts, and other plant sources, is reported to possess both chemopreventive and chemotherapeutic activities in several cancers including liver cancer (1). Despite the identification of numerous molecular targets, the underlying mechanisms involved in the anti-cancer activities of resveratrol are not completely understood. RSV activates sirtuin 1 (SIRT1), which is a major mediator of RSV effects (2, 3).
Methionine adenosyltransferase (MAT) is an essential enzyme that catalyzes the formation of S-adenosylmethionine (AdoMet) from methionine and ATP (4). Three distinct forms of MAT (MATI, MATII, and MATIII), encoded by two distinct genes (MAT1A and MAT2A), have been identified in mammals (4). MAT1A encodes for α1, which forms a tetramer (MATI) and dimer (MATIII) that are largely expressed in normal hepatocytes (4). MATII is a polymeric complex composed of catalytic subunit MATα2, encoded by MAT2A, and regulatory MATβ subunit, encoded by MAT2B (4). The MATβ regulatory subunit lowers the Km of MATII for methionine and the Ki for AdoMet (5). MAT2B has multiple splice forms; two dominant forms are V1 and V2 that are differentially expressed in normal human tissues (6).
In hepatocellular carcinoma (HCC), MAT1A is often silenced whereas MAT2A and MAT2B are induced (4, 7, 8). Both MAT2BV1 and V2 offer the cancer cell a growth advantage, and V1 also regulates apoptosis (6). Although MATβ is best known for regulation of MATII enzymatic activity, our recent works revealed a much broader role for MAT2B protein in cancer biology. We reported that both MATβ variants (MATβV1 and MATβV2) are found in the nucleus, and they interact with human antigen R (HuR), a RNA-binding protein that stabilizes its target mRNAs, which include several cyclins (9). Overexpression of MAT2B variants resulted in higher cytoplasmic HuR content, higher expression of its target genes such as cyclin D1 and cyclin A, and growth (9). More recently we also found that MATβ variants act as a scaffold that is essential in MEK/ERK signaling (10). Interestingly, the crystal structure of MATβ reveals two RSV binding pockets per MATβ protomer (11). Because RSV is growth-suppressive and pro-apoptotic but MATβ variants exert the opposite effect in liver cancer cells, we undertook this study to examine how RSV affects MAT2B biology and how MAT2B in turn affects RSV actions. In the course of this study we uncovered a novel cross-talk among MAT2B, HuR, and SIRT1 and the interdependence they have on each other. Our results also suggest that these proteins may regulate each other's signaling pathways.
EXPERIMENTAL PROCEDURES
Materials
RSV was obtained from Sigma. [α-32P]dCTP (3,000 Ci/mmol) was purchased from PerkinElmer Life Sciences. All other reagents were of analytical grade and obtained from commercial sources.
Cell Culture and Transfection
HepG2 and Huh7 HCC cell lines were obtained from the Cell Culture Core facility at the University of Southern California Research Center for Liver Diseases. HepG2 and Huh7 cells were maintained in DMEM with 10% fetal bovine serum.
The expression plasmids of full-length human MAT2BV1 and V2 (pcDNA3.1-V5-His/MAT2BV1 and pcDNA3.1-V5-His/MAT2BV2) were described previously (6). For gene overexpression experiments, 1.5 × 105 HepG2 or 1 × 105 Huh7 cells per well of a 6-well plate were transiently transfected with V1 and V2 expression plasmids or empty vector using Superfect (Qiagen) according to the manufacturer's protocol. For gene knockdown studies, 10 nm siRNA against MAT2B (sc-7553) ELAVL1 (gene name for HuR, sc-35619), SIRT1 (sc-40986) and equivalent scramble control (sc-37007) were obtained from Santa Cruz Biotechnology and were delivered into HepG2 or Huh7 cells by Lipofectamine RNAiMAX (Invitrogen) following the manufacturer's protocol. Twenty-four hours after transfection, RSV (15 μm) was added to cells for different durations, depending on the experiment.
Northern and Western Blot Analysis
Northern blotting probes for V1 and V2 were described previously (6). Total cellular RNA was extracted by using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Equal amounts of total RNA (15 μg) were denatured, fractionated by electrophoresis on a 15% polyacrylamide-8 m urea gel, electroblotted, and cross-linked onto a nylon membrane. Northern blot analysis was performed as described using Ultrahyb-Oligo (Invitrogen). As a control for normalization of RNA expression levels, blots were hybridized with an oligonucleotide probe complementary to the β-actin. Nuclear and cytosolic protein extracts from HepG2 and Huh7 cells after different treatments were isolated as described (12) and subjected to Western blot analysis. The modified radioimmuneprecipitation assay buffer recipe was 150 mm NaCl, 0.5% deoxycholate, 0.1% SDS, 1% Nonidet P-40, 50 mm Tris-HCl, pH 7.5. The protease and phosphatase inhibitor mixture was 1 mm EDTA, 1 mm EGTA, 25 mm NaF, 0.1 mm sodium orthovanadate, 0.2 mm PMSF, 5 μg/ml leupeptin. Fifteen micrograms of total protein extract was resolved on 12.5% SDS-polyacrylamide gels. Membranes were probed with antibodies to SIRT1, HuR, MATβ (recognize both variants), and forkhead box O3α (Abcam). To ensure equal loading, membranes were stripped and reprobed with anti-β-actin or histone H3 antibodies. Semiquantitative analysis was performed for both Northern and Western blots using Quantity One (Bio-Rad).
Quantitative PCR
TRIzol RNA solution (Invitrogen) was used for RNA isolation. cDNA synthesis and quantitative real-time PCR were done as described previously (6). Primers and probes for quantitative PCR for MAT2B, ELAVL1, and SIRT1 were purchased from Invitrogen. Hypoxanthine phosphoribosyltransferase 1 (HPRT1) was used as housekeeping gene. The thermal profile consisted of initial denaturation at 95 °C for 3 min followed by 45 cycles at 95 °C for 3 s and at 60 °C for 30 s. The cycle threshold (Ct value) of the target genes was normalized to that of HPRT1 to obtain the ΔCt. The ΔCt was used to find the relative expression of target genes according to the formula: relative expression = 2−ΔΔCt, where ΔΔCt = ΔCt of target genes in treatment condition −ΔCt of the target gene under a control condition.
Immunoprecipitation (IP) Studies
IP studies for cell cytoplasm were carried out as described previously with minor modifications (10). Briefly, cells were lysed in a modified radioimmuneprecipitation assay buffer with protease and phosphatase inhibitors. A total of 107 cells/100-mm dish was used to co-IP with antibodies against HuR, MATβ, and SIRT1. Western blotting was carried out to detect HuR, MATβ, SIRT1, and β-actin. Clean Blot IP Detector Reagent (Thermo Scientific) was used to reduce background. Normal IgG (Santa Cruz Biotechnology) was used as a control. Also, a total of 107 cells/100-mm dish was used for cell nuclear extraction. The nuclear extraction kit was purchased from Sigma. Co-IP with antibodies against HuR, MATβ, and SIRT1 and Western blotting were carried out to detect HuR, MATβ, SIRT1, and histone H3.
Pulldown Assay
To determine whether MATβ and SIRT1 can interact directly, 1 μg of recombinant SIRT1 protein (rSIRT1; Active Motif, Carlsbad, CA) was immobilized to the protein A/G plus agarose by incubating the protein with anti-SIRT1 antibody and subsequently the beads. The SIRT1 bound beads were washed and mixed with recombinant MATβ (rMAT2B; Novus) at 4 °C for 4 h. After extensive washing, the proteins bound to the beads were eluted with SDS sample buffer and immunoblotted using antibodies to MATβ and SIRT1.
Luciferase Assays
MAT2BV1 promoter constructs were described previously (6). To clone the promoter region of the human MAT2BV2, a primer corresponding to −2289 to −2316 and a primer corresponding to −2947 to −2925 of the human MAT2BV2 promoter region (GenBank accession no. AY223864) was used for PCR amplification (Advantage 2 PCR Enzyme system; BD Biosciences Clontech) of a human genomic library (Genome Walker; BD Biosciences Clontech) prepared by DraI, EcoRV, PvuII, or SspI restriction digests. A specific 660-bp fragment from the DraI library was isolated by gel electrophoresis and cloned into TOPO pCR2.1 (Invitrogen) and subsequently used to transform Top10F′ competent cells (Invitrogen). This 0.66-kb 5′-flanking region of the human MAT2BV2 was subcloned into the KpnI/XhoI site of promoterless pGL3-basic vector. Transient transfection of V1 and V2 promoter construct and a control Renilla luciferase expression vector was carried out in Huh7 and HepG2 cells with Superfect (Qiagen) for 24 h followed by RSV treatment for another 12 h. Luciferase assays were performed using the Dual Luciferase Reporter Assay System (Promega) as directed by the manufacturer's suggested protocol. Firefly luciferase activity was normalized to Renilla luciferase activity.
Measurement of mRNA and Protein Stability
After 5 h of RSV treatment, HepG2 cells were treated with 5 μg/ml actinomycin D (Sigma) to stop gene transcription, or 10 μg/ml cycloheximide (Sigma) to stop protein translation. At 0, 1, 2, 3, and 4 h after actinomycin D or cycloheximide treatment, real-time PCR was used to determine mRNA expression, and Western blotting was done to determine protein expression. The change in mRNA or protein levels over time after actinomycin D or cycloheximide treatment was used to determine the mRNA and protein half-lives, respectively, using linear regression analysis.
Ribonucleoprotein Immunoprecipitation (RNP-IP)
For immunoprecipitation of endogenous ribonucleoprotein (RNP) complexes, whole cell extracts were incubated (1 h, 4 °C) with a 50% (v/v) suspension of protein A-Sepharose beads (Sigma) precoated overnight with 30 mg of either IgG1 (BD Biosciences) or anti-HuR (Santa Cruz Biotechnology) as described (13). For the isolation of RNA in the IP material, beads were incubated with 100 μl of NT2 buffer containing 20 units of RNase-free DNase I (Invitrogen) for 15 min at 37 °C, washed with wash buffer, and further incubated in 100 μl of wash buffer containing 0.1% SDS and 0.5 mg/ml proteinase K (Invitrogen) for 15 min at 55 °C. RNA was extracted with acid-phenol-CHCl3 and precipitated overnight in the presence of 5 μl of glycoblue (Invitrogen), 25 μl of sodium acetate, pH 5.2, and 625 μl of 100% ethanol. Finally, bound MAT2B mRNA was measured by real-time PCR and normalized to GAPDH and to the unspecific bound of IgG1 as described (14).
Growth and Apoptosis Assays
Growth and apoptosis were measured as we described (6).
AdoMet Level Measurements
AdoMet levels were measured as described (15) in Huh7 cells after RSV treatment for 16, 20, and 24 h.
Statistical Analysis
Data are given as means ± S.E. Statistical analysis was performed using analysis of variance followed by Fisher's test for multiple comparisons. For changes in mRNA and protein levels, ratios of genes or proteins to housekeeping genes or proteins densitometric values were compared. All p values were derived from at least three independent experiments. Significance was defined by p < 0.05.
RESULTS
RSV Induces MAT2B Expression
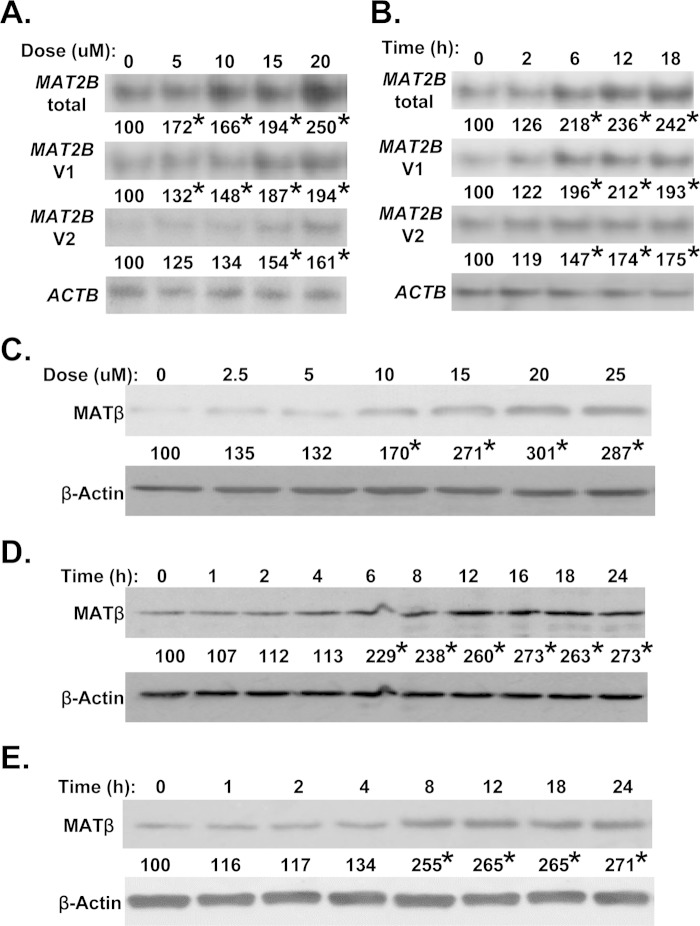
RSV treatment in Huh7 cells increased the mRNA levels of both MAT2B variants in a dose- (Fig. 1A) and time- (Fig. 1B) dependent manner. Maximum induction occurred with 15 μm RSV after 6 h. A similar change in MATβ levels was also observed (Fig. 1, C and D). The same occurred in HepG2 cells (Fig. 1E). For all subsequent studies, the RSV dose was 15 μm, and treatment duration was 12 h unless specified otherwise.
FIGURE 1.
Effect of RSV on MAT2B expression. Huh7 and HepG2 cells were treated with RSV, and mRNA levels of MAT2B variants were measured by Northern blotting, and total MAT2B protein (MATβ) levels were measured by Western blotting as described under “Experimental Procedures.” The dose-dependent effect of RSV (for 12 h) in Huh7 cells at the mRNA (A) and protein (C) levels shows maximal response at 15 μm. The time-course response of RSV (at 15 μm) at the mRNA (B) and protein (D) levels shows maximal response at 12 h. A similar response to 15 μm RSV in time dependence also occurred in HepG2 cells (E). Numbers below the blots represent the densitometric values expressed as percentage of their respective controls from three independent experiments. *, p < 0.05 versus untreated control (A and C) or time 0 (B, D, and E).
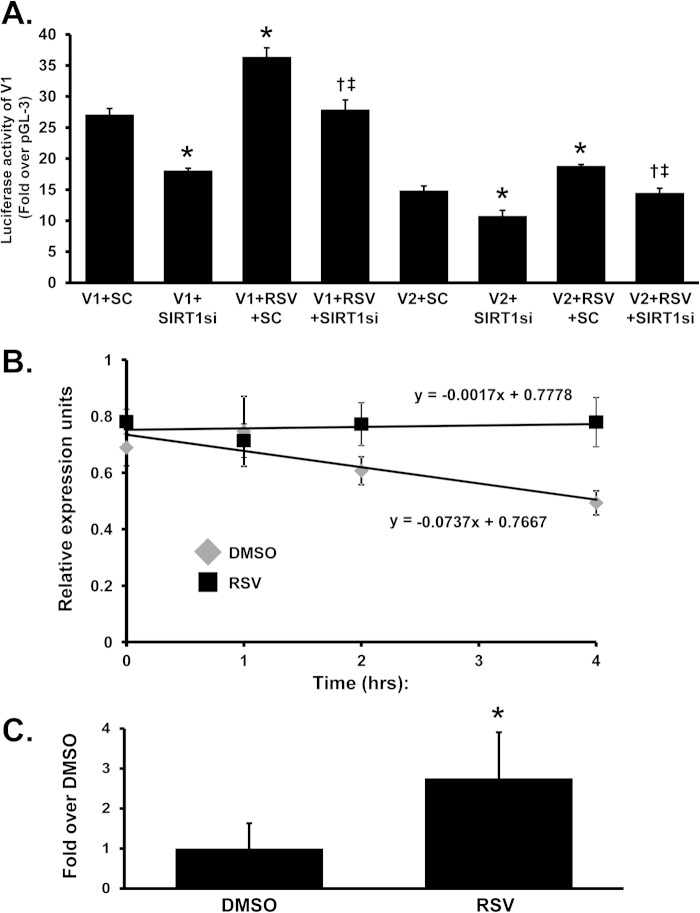
To determine the mechanism for the increase in MAT2B mRNA level, V1 and V2 promoter activities were measured following transient transfection. Fig. 2A shows that RSV treatment increased the promoter activity of both variants, but only minimally (V1 promoter increased by 34%, V2 by 26%) in HepG2 cells (RSV increased their activity by approximately 50% in Huh7 cells; data not shown). Because SIRT1 is known to be a major mediator of the RSV effect on gene expression (3), we examined whether the induction required SIRT1. Knockdown of SIRT1 lowered the promoter activity of both variants; and although RSV was still able to induce their activity in the presence of SIRT1 siRNA, it was to a lower level than scramble siRNA control.
FIGURE 2.
Effect of RSV on MAT2BV1 and V2 promoter activity and MAT2B mRNA stability. A, HepG2 cells were transfected with V1 or V2 promoter constructs and SIRT1 siRNA (SIRT1si) or scramble siRNA (SC) prior to treatment with RSV for 12 h as described under “Experimental Procedures.” SIRT1 knockdown lowered basal V1 and V2 promoter activity and blunted the RSV-mediated increase in V1 and V2 promoter activity. B, results of mRNA stability assays in HepG2 cells performed as described under “Experimental Procedures” are shown. DMSO, dimethyl sulfoxide. C, RNP-IP analysis was performed as described under “Experimental Procedures” to demonstrate HuR binding to the MAT2B 3′-UTR sequence in HepG2 cells. Results are mean ± S.E. (error bars) from three independent experiments. *, p < 0.05 versus respective controls; †, p < 0.05 versus either V1+SIRT1 siRNA or V2+SIRT1 siRNA; ‡, p < 0.05 versus either V1+RSV+SC or V2+RSV+SC treatment.
Because the increase in MAT2B variant mRNA levels is much higher than the transcriptional rate, we also examined whether the mRNA half-life might be affected. Fig. 2B shows that MAT2B mRNA half-life is markedly prolonged following RSV treatment. The MAT2B 3′-UTR region (ATTTA at 1948–1952) is a consensus binding site for HuR (AUUUA pentamer). To determine whether RSV treatment is able to modify HuR binding to MAT2B mRNA, we performed a RNP-IP assay by which we immunoprecipitated HuR bound to its target mRNAs and obtained a cDNA library that we can analyze by quantitative PCR. As shown in Fig. 2C, the quantitative real-time PCR analysis of the MAT2B mRNA bound to HuR reveals that RSV significantly increased the binding of HuR to MAT2B mRNA.
RSV Induces HuR and SIRT1 Expression in Huh7 and HepG2 Cells
RSV is known to activate SIRT1 activity (2, 16) as well as increase SIRT1 expression at the mRNA level (17). Consistent with this, the SIRT1 protein level is increased after RSV treatment in both Huh7 (Fig. 3A) and HepG2 (Fig. 3B) cells. Because we observed increased HuR binding to MAT2B mRNA, we also measured HuR expression following RSV and found the HuR protein level increased maximally by 2 h after RSV treatment in both liver cancer cells, prior to SIRT1 induction (by 4 h) and MATβ induction (by 6–8 h; Fig. 1, D and E). ELAVL1 (HuR) mRNA level was transiently increased following RSV (increased by 63% at 2 h but was back to base line by 4 h and remained at base line up to 24 h, data not shown).
FIGURE 3.
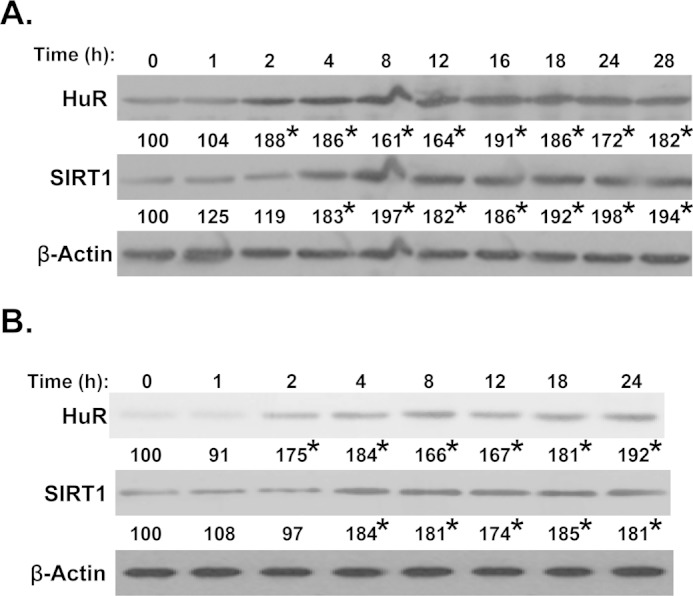
RSV-induced HuR and SIRT1 protein expression in Huh7 and HepG2 cells. Western blot analysis examined the effect of RSV over time on HuR and SIRT1 protein expression in Huh7 (A) and HepG2 (B) cells. β-Actin served as a loading control. The values under each blot represent the densitometric values expressed as percentage of time zero control for each time point from three independent experiments. *, p < 0.05 versus time 0 control.
Effect of RSV Treatment on MATβ Interaction with HuR, MATα2, and SIRT1
MATβ variants are known to interact with HuR and MATα2 and to localize in both the cytoplasm and nucleus (4, 9). RSV treatment increased the interaction between MATβ and HuR in both cytosol and nucleus, but decreased the interaction between MATβ and MATα2 (Fig. 4, A and B). RSV treatment did not affect MATα2 protein level in these compartments. Interestingly, MATβ also interacts with SIRT1 in the basal state, and this interaction was enhanced following RSV treatment in both cytosol and nucleus. Direct interaction between the two proteins was also demonstrated using recombinant MATβ and SIRT1 (Fig. 4C).
FIGURE 4.
Resveratrol increased MATβ interaction with SIRT1 and HuR while reducing its interaction with MATα2. A and B, co-IP experiments with cytoplasmic (A) and nuclear extracts (B) using an antibody to total MATβ in the presence or absence of RSV in Huh7 cells and Western blotting (WB) using antibodies to MATβ, SIRT1, HuR, and MATα2 were performed as described under “Experimental Procedures.” SIRT1 and HuR co-immunoprecipitated with MATβ and RSV enhanced these interactions with MATβ. RSV treatment reduced the interaction between MATβ and MATα2 despite no reduction in total MATα2 levels. β-Actin was used as a loading control for cytoplasmic extracts and histone H3 for nuclear extracts. C, in vitro pulldown binding assay using immobilized recombinant SIRT1 with recombinant MATβ, followed by Western blotting of both SIRT1 and MATβ is shown. 10 ng of rSIRT1 and rMATβ was loaded as an indicator for the migration of the proteins. Numbers below the blots represent the densitometric values expressed as percentage of their respective controls. Results are mean from three independent experiments. *, p < 0.05 versus dimethyl sulfoxide (DMSO) control.
Induction of MATβ, SIRT1, and HuR Requires Each Other following RSV
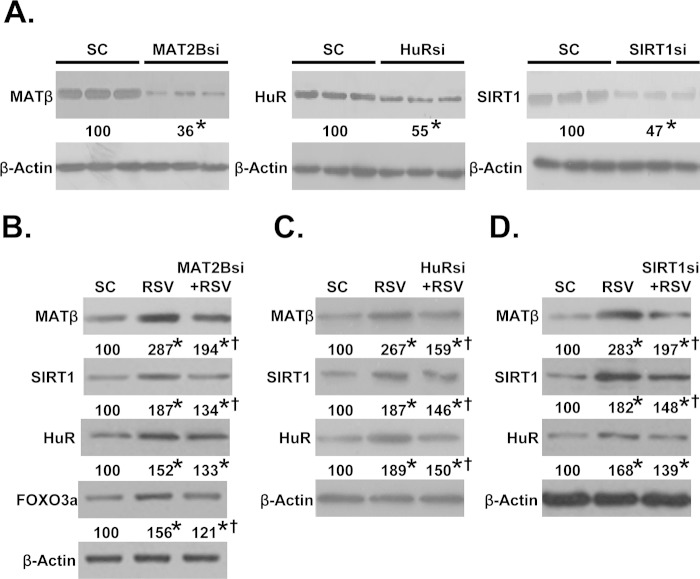
To see whether the increase in protein levels of MATβ, HuR, and SIRT1 requires each other, the expression of each was reduced by siRNA. Fig. 5A shows the knockdown efficiency of these siRNAs at the protein level, and they are 45% (HuR), 53% (SIRT1), and 64% (MATβ). At the mRNA level, MAT2B siRNA lowered it by 95%, SIRT1 siRNA by 87%, and ELAVL1 (HuR) siRNA by 82.5%. RSV treatment was not able to raise their mRNA levels in the presence of respective siRNA (data not shown). Knockdown of MAT2B blunted the effect of RSV on the increase in protein levels of MATβ, SIRT1, and HuR as well as SIRT1 downstream target forkhead box O3α (18) (Fig. 5B). Note however that despite 95% reduction in MAT2B mRNA level, the protein level was still nearly doubled following RSV, suggesting that MAT2B protein is being stabilized as well. Similarly, ELAVL1 (HuR) (Fig. 5C) and SIRT1 (Fig. 5D) knockdown blunted the RSV-induced increase in MATβ, SIRT1, and HuR protein levels. Like MAT2B siRNA, ELAVL1 (HuR) and SIRT1 siRNAs were also highly efficient, but their protein levels still remained significantly elevated after RSV. These results also suggest that SIRT1 and HuR were stabilized at the protein level following RSV.
FIGURE 5.
Knockdown of MATβ, HuR, or SIRT1 attenuated resveratrol-mediated induction of these proteins. A, Western blot analysis shows knockdown efficiency of MAT2B, HuR, or SIRT1 siRNA (si) at the protein level in Huh7 cells. B–D, cells were treated with siRNA against MAT2B (B), HuR (C), and SIRT1 or scramble siRNA (SC) (D) control with or without RSV for 12 h followed by Western blotting as described under “Experimental Procedures.” β-Actin served as loading control. Numbers below each blot represent the densitometric values expressed as percentage of SC. Results are the mean from three independent experiments. *, p < 0.05 versus SC; †, p < 0.05 versus RSV treatment.
To further examine whether RSV treatment affected protein stability of MATβ, SIRT1, and HuR, their respective protein levels were measured following inhibition of de novo protein synthesis by cycloheximide after an initial RSV treatment for 5 h. Fig. 6 shows that the protein half-life of all three increased following RSV treatment, nearly doubling for HuR and more than doubled for MATβ and SIRT1.
FIGURE 6.

Resveratrol increases MATβ, HuR, and SIRT1 protein stability. A, protein stability assays using cycloheximide followed by Western blot analysis were performed to assess the RSV effect on protein stability of MATβ, HuR, and SIRT1 in Huh7 cells. B, table shows MATβ, HuR, and SIRT1 protein half-lives as a result of RSV treatment compared with DMSO control. Results are mean ± S.E. of two (HuR and SIRT1) and three (MATβ) experiments.
MAT2B Induction Is a Compensatory Response to RSV Pro-apoptotic and Growth-suppressive Effect
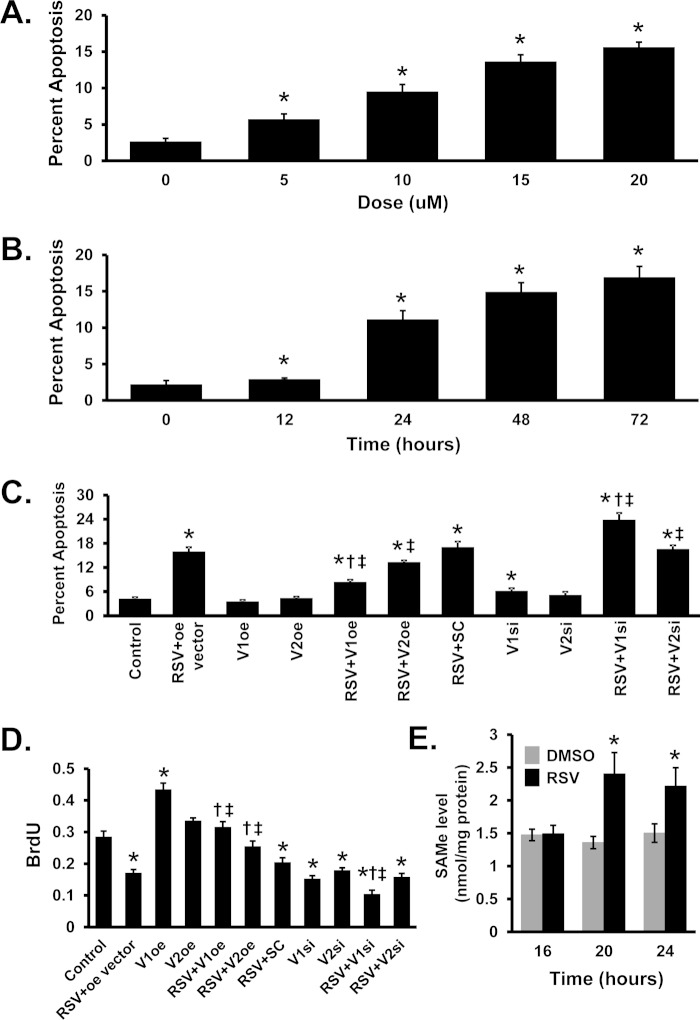
RSV induced apoptosis in a dose- and time-dependent fashion (Fig. 7, A and B). Overexpression of MAT2BV1 (but not V2) attenuated, whereas knockdown of MAT2BV1 (but not V2) worsened RSV-induced apoptosis (Fig. 7C). RSV treatment caused growth suppression, and this was attenuated by overexpression of either MAT2BV1 or V2 and worsened by knockdown of V1 (Fig. 7D).
FIGURE 7.
Effects of resveratrol, MAT2BV1, and V2 expression on apoptosis, growth, and AdoMet levels. A, Hoechst staining to assess apoptosis levels with increasing doses of RSV in Huh7 cells. B, apoptosis levels examined in Huh7 cells at 15 μm RSV in time-course experiments. C and D, apoptosis (C) and cellular growth (D) by bromodeoxyuridine (BrdU) incorporation in Huh7 cells either overexpressing (oe) or siRNA (si) knockdown of V1 or V2, in the presence or absence of RSV. E, AdoMet levels in Huh-7 cells treated with RSV from 16 to 24 h. DMSO, dimethyl sulfoxide. Results are mean ± S.E. (error bars) from three independent experiments. *, p < 0.05 versus control; †, p < 0.05 versus RSV+oe or RSV+SC; ‡, p < 0.05 versus respective V1 or V2 oe or si controls.
RSV Treatment Affects Steady-state AdoMet Level
Both MATβV1 and MATβV2 are known to lower the Ki of MATα2 for AdoMet (19); and because RSV treatment decreased the association of MATβ with MATα2, we measured the steady-state AdoMet level following RSV treatment. The AdoMet level increased significantly by 20 h after RSV treatment (Fig. 7E).
DISCUSSION
RSV, a naturally occurring polyphenol readily available in the diet, is reported to exert health benefit in a variety of conditions (20). RSV has been shown in experimental models to exert chemopreventive and therapeutic efficacy against HCC (21, 22). This may be related to its anti-proliferative and pro-apoptotic effects in liver cancer cells; however, the signaling mechanisms involved are still being defined (1, 21, 22). The effects of RSV in liver cancer cells appear to be the exact opposite of MATβ, which we have shown to be overexpressed in HCC and to be pro-growth and protect against apoptosis (6). The report that RSV binds avidly to MATβ (11) thus raised the question as to how this binding would affect each other's biological actions.
Our studies uncovered a complex and novel cross-talk among three proteins (MATβ, HuR, and SIRT1) whose expression is induced by RSV (Figs. 1 and 3). We first found that MAT2B expression is induced at both the mRNA and protein levels following RSV treatment in two different HCC lines. Although RSV treatment increased the promoter activity of both MAT2BV1 and V2, the degree of increase was not sufficient to explain the increase in the mRNA level. This led us to examine whether MAT2B mRNA stability was affected by RSV, and indeed, we found that RSV treatment increased HuR binding to the MAT2B 3′-UTR, leading to its stabilization (Fig. 2C). The reason for the increase in HuR binding was that RSV treatment also induced the expression of HuR. This to our knowledge has not been reported. We showed previously that MATβ variants interact with HuR, and increased expression of MATβ variants resulted in more cytoplasmic HuR content and higher expression of HuR target mRNAs such as cyclin D1 and cyclin A (9). Here we show a reciprocal regulation where increased HuR can further raise MAT2B expression by stabilizing MAT2B mRNA.
SIRT1 is one of the best-studied RSV targets. SIRT1 is an NAD+-dependent deacetylase that regulates diverse cellular functions largely via its ability to deacetylate key regulatory proteins to affect gene expression (23). RSV activates SIRT1 by lowering the Km of SIRT1 for its substrates (2), in addition to increasing SIRT1 expression at the mRNA level (17). Consistent with these reports, we confirmed that SIRT1 expression increased following RSV in the two liver cancer cell lines, but it occurred after the induction of HuR. Interestingly, HuR has also been shown to stabilize SIRT1 mRNA (24). Thus, it is possible that the increase in HuR expression contributed to the increase in SIRT1 expression following RSV treatment. SIRT1 in turn regulates MAT2B at the transcriptional level. Interestingly, the basal MAT2B transcriptional activity requires SIRT1 as knockdown of SIRT1 lowered the promoter activity of both V1 and V2 (Fig. 2A). However, SIRT1 is not the only mechanism for the increase in MAT2B promoter activity following RSV treatment as MAT2B promoter activity still increased despite SIRT1 knockdown (but not to the full extent).
An unexpected finding from our work is that MATβ interacts with SIRT1 at base line and this was increased following RSV treatment. Furthermore, direct interaction between the two proteins was shown using recombinant proteins (Fig. 4C). RSV treatment also enhanced interaction between MATβ and HuR. These occurred in both the cytosol and nucleus, and the increase in co-precipitated proteins (SIRT1 and HuR) with MATβ largely reflects the increase in their protein levels (Fig. 4). However, RSV treatment lowered the interaction between MATβ and MATα2 in both cytosolic and nuclear compartments. RSV did not alter the kinetic properties of MATII using pure recombinant proteins in vitro (data not shown). This suggests the possibility that interaction of MATβ with SIRT1 and HuR may interfere with the MATβ interaction with MATα2 in vivo. As expected, because the function of MATβ on MATII is to lower the Ki of MATII for AdoMet, reduced interaction between MATβ and MATα2 allowed steady-state AdoMet level to rise (Fig. 7E). We previously showed that raising the steady-state AdoMet level in liver cancer cells can inhibit growth and increase apoptosis (25). This suggests that this dissociation between MATβ and MATα2 may also contribute to the RSV-induced apoptosis and growth suppression.
Even more intriguing is the observation that full induction in protein levels of MATβ, HuR, and SIRT1 requires each other as knockdown of any one of these attenuated the ability of RSV to fully induce their protein expression (Fig. 5). This can only occur due to increased protein stability because the siRNA efficiency was very high, and it should have blocked expression from increased transcription and/or mRNA stability. Our hypothesis is that their interaction increased their protein stability. This is supported by the results showing increased MATβ, SIRT1, and HuR protein stability following RSV (Fig. 6) and that full induction requires each other (Fig. 5). Additional work will be required (such as identifying where the interactions take place and creating mutant constructs that cannot interact) to prove this hypothesis.
Our data showing that MATβ interacts with SIRT1 directly and regulates SIRT1 protein level are consistent with the known role of MATβ in HCC tumorigenesis (6, 9, 10). The role of SIRT1 in HCC is conflicting, as it has been reported to function as a tumor suppressor as well as an oncogenic protein (26). Transgenic mice overexpressing SIRT1 were protected against HCC induced by diethylnitrosamine/high fat diet, in part by blocking NFκB-mediated inflammation and hepatic cell malignant transformation (27). However, two recent reports found SIRT1 to be overexpressed in human HCC, and its overexpression contributed to tumorigenicity and resistance to chemotherapy (26, 28). In one of the reports, 95 of 172 HCCs overexpressed SIRT1, and this was associated with higher tumor grade and poor long term survival for patients with resected HCC (28). Interestingly, the increase in SIRT1 expression was thought to be from increased protein stability as the SIRT1 mRNA level was unchanged in HCC (28). The fact that MATβ is also overexpressed in HCC (6, 10) raises the possibility that the two proteins may stabilize each other to raise their expression.
Increased MAT2B expression may represent a compensatory response to the RSV pro-apoptotic and growth-suppressive effects. Supporting this notion is the fact that blocking its induction further exacerbated the apoptosis and growth suppression whereas overexpressing MAT2B protected against these events induced by RSV (Fig. 7).
HuR is well known for its modulation of the stability and translational efficiency of target mRNAs by interacting with AU- or U-rich sequence elements frequently found within 3′-UTRs of many proto-oncogenes (29). Whereas many papers have reported on HuR functions, very few have examined regulation of HuR protein level. HuR protein expression is often increased in cancer, which may be in part due to reduced expression of several miRNAs (miR-16, miR-125, miR-519) known to regulate HuR mRNA (29). Recently Dai et al. showed that HuR autoregulates its own expression, through a negative feedback loop (triggered by elevated nuclear HuR level) that affects only nuclear HuR (29). This mechanism would maintain HuR homeostasis when the nucleocytoplasmic HuR distribution is constant, but would allow cytoplasmic HuR to accumulate if the distribution was shifted toward the cytoplasm, which occurs in cancer (29). Because higher MATβ expression raises cytoplasmic HuR content (9), this could help to maintain high HuR expression after RSV treatment.
The sequence of induction-HuR followed by SIRT1 and MAT2B suggests the following scenario. Following RSV treatment, HuR is induced transiently at the mRNA level and is rapidly translated to protein, which helps to raise MAT2B and SIRT1 expression by stabilizing their mRNAs. RSV also activates SIRT1, which can help to raise MAT2B expression at the transcriptional level. Higher MATβ expression helps to further increase cytoplasmic HuR content. These proteins also interact and further stabilize each other. However, RSV treatment reduced the interaction between MATβ and MATα2, allowing the steady-state AdoMet level to increase. Thus, although the induction in MAT2B expression may represent a compensatory response to defend against apoptosis and growth suppression, it also allows maximal SIRT1 signaling to occur, and the rise in AdoMet level can also contribute to the apoptosis and growth suppression. This scheme is summarized in Fig. 8.
FIGURE 8.
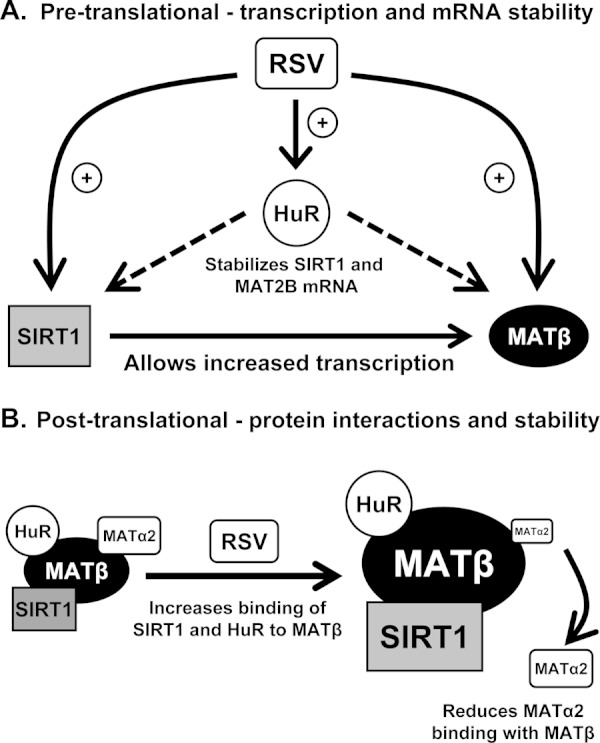
Model of MAT2B-HuR-SIRT1 interplay in resveratrol action. A, proposed pre-translational mechanism of RSV-mediated induction of ELAVL1 (HuR), MAT2B, and SIRT1 mRNA levels. Following RSV treatment the mRNA level of HuR, MAT2B, and SIRT1 all increased. HuR expression increased first, which can stabilize SIRT1 and MAT2B mRNAs. SIRT1 can also induce MAT2B transcription. B, post-translational effects of RSV on HuR, MATβ, and SIRT1. There is basal interaction between HuR and SIRT1 with MATβ, and this interaction is enhanced following RSV treatment. The interaction also stabilizes all three proteins. However, the basal interaction between MATα2 and MATβ is reduced after RSV treatment, which allows steady-state AdoMet level to rise. This complex interplay suggests that MATβ, HuR, and SIRT1 regulate each other's signaling pathways.
In summary, while studying the role of RSV in MAT2B biology we have uncovered a highly novel cross-talk among MATβ-HuR-SIRT1. RSV induces the expression of all three proteins by multiple interdependent mechanisms, including enhancing their interaction, which leads to protein stabilization. Our findings also suggest the possibility that MATβ, HuR, and SIRT1 regulate each other's signaling pathways. Although MAT2B dysregulation has only been recognized thus far in cancer biology (4), MAT2B is widely expressed in normal tissues (except adult normal hepatocytes), and these results suggest that it may have much broader functions such as those regulated by SIRT1 and HuR.
This work was supported, in whole or in part, by National Institutes of Health Grant R01DK51719 (to S. C. L., H. Y., and J. M. M.). This work was also supported by Plan Nacional of I+D SAF 2011-29851 (to J. M. M.), ETORTEK-2011, Sanidad Gobierno Vasco 2008, Educación Gobierno Vasco 2011 (PI2011/29), and Fondo de Investigacion Sanitaria (PI11/01588) (to M. L. M.-C.). Huh7 and HepG2 cells were provided by the Cell Culture Core of the University of Southern California Research Center for Liver Diseases under Grant P30DK48522.
- RSV
- resveratrol
- AdoMet
- S-adenosylmethionine
- co-IP
- co-immunoprecipitation
- HCC
- hepatocellular carcinoma
- HuR
- human antigen R
- IP
- immunoprecipitation
- MAT
- methionine adenosyltransferase
- RNP-IP
- ribonucleoprotein immunoprecipitation
- SIRT1
- sirtuin 1
- V1
- variant 1
- V2
- variant 2.
REFERENCES
- 1. Mbimba T., Awale P., Bhatia D., Geldenhuys W. J., Darvesh A. S., Carroll R. T., Bishayee A. (2012) Alteration of hepatic proinflammatory cytokines is involved in the resveratrol-mediated chemoprevention of chemically induced hepatocarcinogenesis. Curr. Pharm. Biotechnol. 13, 229–234 [DOI] [PubMed] [Google Scholar]
- 2. Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 3. Vetterli L., Maechler P. (2011) Resveratrol-activated SIRT1 in liver and pancreatic β-cells: a Janus head looking to the same direction of metabolic homeostasis. Aging 3, 444–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu S. C., Mato J. M. (2012) S-Adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 92, 1515–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. LeGros H. L., Jr., Halim A. B., Geller A. M., Kotb M. (2000) Cloning, expression, and functional characterization of the β regulatory subunit of human methionine adenosyltransferase (MAT II). J. Biol. Chem. 275, 2359–2366 [DOI] [PubMed] [Google Scholar]
- 6. Yang H., Ara A. I., Magilnick N., Xia M., Ramani K., Chen H., Lee T. D., Mato J. M., Lu S. C. (2008) Expression pattern, regulation and function of methionine adenosyltransferase 2β alternative splicing variants in hepatoma cells. Gastroenterology 134, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang H., Cho M. E., Li T. W., Peng H., Ko K. S., Mato J. M., Lu S. C. (2013) MiRNAs regulate methionine adenosyltransferase 1A expression in hepatocellular carcinoma. J. Clin. Invest. 123, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramani K., Mato J. M., Lu S. C. (2011) Role of methionine adenosyltransferase genes in hepatocarcinogenesis. Cancers 3, 1480–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xia M., Chen Y., Wang L. C., Zandi E., Yang H., Bemanian S., Martínez-Chantar M. L., Mato J. M., Lu S. C. (2010) Novel function and intracellular localization of methionine adenosyltransferase 2β splicing variants. J. Biol. Chem. 285, 20015–20021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peng H., Dara L., Li T. W., Zheng Y., Yang H., Tomasi M. L., Tomasi I., Giordano P., Mato J. M., Lu S. C. (2013) Methionine adenosyltransferase 2B-GIT1 interplay activates MEK1-ERK1/2 to induce growth in human liver and colon cancer. Hepatology 57, 2299–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shafqat N., Muniz J. R.., Pilka E. S., Papagrigoriou E., von Delft F., Oppermann U., Yue W. W. (2013) Insight into S-adenosylmethionine biosynthesis from the crystal structures of the human methionine adenosyltransferase catalytic and regulatory subunits. Biochem. J. 452, 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang H., Ramani K., Xia M., Ko K. S., Li T. W., Oh P., Li J., Lu S. C. (2009) Dysregulation of glutathione synthesis during cholestasis in mice: molecular mechanisms and therapeutic implications. Hepatology 49, 1982–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antic D., Keene J. D. (1997) Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. Am. J. Hum. Genet. 61, 273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan J., Ishmael F. T., Fang X., Myers A., Cheadle C., Huang S. K., Atasoy U., Gorospe M., Stellato C. (2011) Chemokine transcripts as targets of the RNA-binding protein HuR in human airway epithelium. J. Immunol. 186, 2482–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu S. C., Ramani K., Ou X., Lin M., Yu V., Ko K., Park R., Bottiglieri T., Tsukamoto H., Kanel G., French S. W., Mato J. M., Moats R., Grant E. (2009) S-Adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology 50, 462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borra M. T., Smith B. C., Denu J. M. (2005) Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 280, 17187–17195 [DOI] [PubMed] [Google Scholar]
- 17. Costa Cdos S., Rohden F., Hammes T. O., Margis R., Bortolotto J. W., Padoin A. V., Mottin C. C., Guaragna R. M. (2011) Resveratrol up-regulated SIRT1, FOXO1, and adiponectin and down-regulated PPARγ1–3 mRNA expression in human visceral adipocytes. Obes. Surg. 21, 356–361 [DOI] [PubMed] [Google Scholar]
- 18. Hubbard B. P., Gomes A. P., Dai H., Li J., Case A. W., Considine T., Riera T. V., Lee J. E., E. S. Y., Lamming D. W., Pentelute B. L., Schuman E. R., Stevens L. A., Ling A. J., Armour S. M., Michan S., Zhao H., Jiang Y., Sweitzer S. M., Blum C. A., Disch J. S., Ng P. Y., Howitz K. T., Rolo A. P., Hamuro Y., Moss J., Perni R. B., Ellis J. L., Vlasuk G. P., Sinclair D. A. (2013) Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339, 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nordgren K. K., Peng Y., Pelleymounter L. L., Moon I., Abo R., Feng Q., Eckloff B., Yee V. C., Wieben E., Weinshilboum R. M. (2011) Methionine adenosyltransferase 2A/2B and methylation: gene sequence variation and functional genomics. Drug Metab. Dispos. 39, 2135–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakata R., Takahashi S., Inoue H. (2012) Recent advances in the study on resveratrol. Biol. Pharm. Bull. 35, 273–279 [DOI] [PubMed] [Google Scholar]
- 21. Bishayee A., Politis T., Darvesh A. S. (2010) Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat. Rev. 36, 43–53 [DOI] [PubMed] [Google Scholar]
- 22. Yeh C. B., Hsieh M. J., Lin C. W., Chiou H. L., Lin P. Y., Chen T. Y., Yang S. F. (2013) The antimetastatic effects of resveratrol on hepatocellular carcinoma through the down-regulation of a metastasis-associated protease by SP-1 modulation. PLoS ONE 8, e56661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knight J. R., Milner J. (2012) SIRT1, metabolism and cancer. Curr. Opin. Oncol. 24, 68–75 [DOI] [PubMed] [Google Scholar]
- 24. Abdelmohsen K., Pullmann R., Jr., Lal A., Kim H. H., Galban S., Yang X., Blethrow J. D., Walker M., Shubert J., Gillespie D. A., Furneaux H., Gorospe M. (2007) Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol. Cell 25, 543–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J., Ramani K., Sun Z., Zee C., Grant E. G., Yang H., Xia M., Oh P., Ko K., Mato J. M., Lu S. C. (2010) Forced expression of methionine adenosyltransferase 1A in human hepatoma cells suppresses in vivo tumorigenesis in mice. Am. J. Pathol. 176, 2456–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Portmann S., Fahrner R., Lechleiter A., Keogh A., Overney S., Laemmle A., Mikami K., Montani M., Tschan M. P., Candinas D., Stroka D. (2013) Antitumor effect of SIRT1 inhibition in human HCC tumor models in vitro and in vivo. Mol. Cancer Ther. 12, 499–508 [DOI] [PubMed] [Google Scholar]
- 27. Herranz D., Muñoz-Martin M., Cañamero M., Mulero F., Martinez-Pastor B., Fernandez-Capetillo O., Serrano M. (2010) Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen H. C., Jeng Y. M., Yuan R. H., Hsu H. C., Chen Y. L. (2012) SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann. Surg. Oncol. 19, 2011–2019 [DOI] [PubMed] [Google Scholar]
- 29. Dai W., Zhang G., Makeyev E. V. (2012) RNA-binding protein HuR autoregulates its expression by promoting alternative polyadenylation site usage. Nucleic Acids Res. 40, 787–800 [DOI] [PMC free article] [PubMed] [Google Scholar]