Background: Angiogenesis is primarily driven by the VEGF-induced activation of VEGFR-2.
Results: We have identified PDCL3 as a novel chaperone protein involved in angiogenesis by regulating expression of VEGFR-2.
Conclusion: PDCL3 activity is required for angiogenesis.
Significance: Targeting PDCL3 represents an attractive therapeutic strategy to block angiogenesis and tumor growth.
Keywords: Angiogenesis, Cell Biology, Cell Signaling, Receptor Regulation, Ubiquitination, Vascular Endothelial Growth Factor (VEGF)
Abstract
Angiogenesis, a hallmark step in tumor metastasis and ocular neovascularization, is driven primarily by the function of VEGF ligand on one of its receptors, VEGF receptor 2 (VEGFR-2). Central to the proliferation and ensuing angiogenesis of endothelial cells, the abundance of VEGFR-2 on the surface of endothelial cells is essential for VEGF to recognize and activate VEGFR-2. We have identified phosducin-like 3 (PDCL3, also known as PhLP2A), through a yeast two-hybrid system, as a novel protein involved in the stabilization of VEGFR-2 by serving as a chaperone. PDCL3 binds to the juxtamembrane domain of VEGFR-2 and controls the abundance of VEGFR-2 by inhibiting its ubiquitination and degradation. PDCL3 increases VEGF-induced tyrosine phosphorylation and is required for VEGFR-2-dependent endothelial capillary tube formation and proliferation. Taken together, our data provide strong evidence for the role of PDCL3 in angiogenesis and establishes the molecular mechanism by which it regulates VEGFR-2 expression and function.
Introduction
Angiogenesis is one of the hallmarks of cancer progression and is associated with various other human diseases, ranging from neovascular age-related macular degeneration to inflammation (1–3). The vast majority of tumor growth depends highly on the recruitment of functional blood vessels. Tumors unable to induce successful angiogenesis remain dormant. The transition of tumor cells from a prevascular to a highly growing and vascularized mass is determined by a shift in the balance of expression of proangiogenic factors, favoring endothelial cell growth (4).
Receptor tyrosine kinases constitute critical machinery of the signaling system that controls key cellular functions, ranging from cell proliferation, migration, and differentiation to gene expression. By catalyzing phosphorylation of their target protein substrates, receptor tyrosine kinases retain unparalleled levels of control over the delicately balanced signaling system of the cell. VEGFR-22 is a receptor tyrosine kinase whose activity is paramount for vascular development during embryogenesis and pathological angiogenesis in diseases such as cancer and wet age-related macular degeneration (5, 6). Upon binding to the VEGF family of ligands, VEGFR-2 forms a dimer, resulting in its increased tyrosine kinase activity and phosphorylation of multiple cytoplasmic tyrosine residues by serving docking sites for downstream effectors of VEGFR-2 (3, 6, 7). Given the critical role of VEGFR-2 signaling in diverse pathological conditions (1, 8), regulation of VEGFR-2 expression and function represent important rate-limiting mechanisms for angiogenesis.
After stimulation by the VEGF family of ligands, VEGFR-2 is removed from the cell surface through endocytosis and down-regulation (9, 10). The ligand-mediated clearance of VEGFR-2 from the plasma membrane is established through βTrcp1, an ubiquitin E3 ligase that recognizes VEGF-stimulated VEGFR-2 through a unique phosphodegron motif present in the carboxyl tail of VEGFR-2, leading to its ubiquitination and degradation (11). Despite significant biological and clinical interest in VEGFR-2, the molecular mechanisms governing its abundance are poorly understood. In well characterized angiogenesis-associated diseases such as cancer, retinopathy of prematurity, and age-related macular degeneration, emphasis has been placed on the expression of VEGF ligands, but the molecular regulation of expression of VEGFR-2 is largely ignored (8).
In general, newly synthesized cell surface receptors are transported through the endoplasmic reticulum membrane in an unfolded form, where molecular chaperones and other enzymes facilitate their folding and assembly into their native conformation (12). Proteins that cannot reach their proper conformation are often kept in the endoplasmic reticulum and degraded rapidly (13, 14). Phosducin-like proteins (PhLPs) are a conserved family of proteins with thioredoxin-like domains that were initially identified as modulators of G protein signaling (15, 16). The PhLP gene family proteins include PhLP1, PhLP2A (also called PDCL3), PhLP2B, and PhLP3. All PhLP gene products share an N-terminal helical domain, a central thioredoxin-like domain, and a charged carboxyl terminus (16). In this study, we identified PDCL3 as a novel chaperone protein that regulates VEGFR-2 stability and function. Given the critical role of PDCL3 in the regulation of VEGFR-2, targeting PDCL3 may represent an attractive therapeutic strategy to block angiogenesis and tumor growth.
MATERIALS AND METHODS
Reagents and Antibodies
Recombinant VEGF was purchased from R&D Systems. Rabbit anti-VEGFR-2 antibody was raised against amino acids corresponding to the kinase insert of VEGFR-2 (17). The following antibodies were purchased from Santa Cruz Biotechnology Inc.: preadsorbed goat-rabbit IgG (catalog no. sc-2054), goat anti-mouse IgG (catalog no. sc-2055), and goat anti-rat IgG (catalog no. sc-2006) secondary antibodies conjugated to horseradish peroxidase(HRP); anti-c-myc (9E10) (catalog no. sc-40); anti-HSP 70 (W27) (catalog no. sc-24), anti-Hsp90α/β (F-8) (catalog no. sc-13119); anti-PLCγ1) (catalog no. sc-81); and monoclonal anti-VEGFR-2 (A-3) (catalog no. sc-6251). Mouse monoclonal anti-phosphotyrosine antibodies, 4G10 and PY-20, were purchased from Millipore and Transduction Laboratories (Lexington, KY), respectively. Anti-phospho-VEGFR-2 (pY1054) was purchased from Cell Signaling Technology (Beverly, MA). Anti-PDCL3 antibody was purchased from Novus Biological. Protein A-agarose Fast Flow and Protein G-agarose Fast Flow was purchased from Amersham Biosciences. MG-132 and cycloheximide were purchased from Calbiochem (La Jolla, CA).
Yeast Two-hybrid System
Mouse cDNA encoding the entire cytoplasmic domain of VEGFR-2, including the juxtamembrane domain, was cloned into the pGBKT7 vector in-frame with the GAL4 DNA-binding domain. The GAL4-based MATCHMAKER Yeast Two-Hybrid System 3 (Clontech) was used to screen for protein-protein interactions. This system utilized the vector pGBKT7, yeast Saccharomyces cerevisiae strains AH109 and Y187, and pretransformed Y187 S. cerevisiae with the pGADT7 vector/17-day mouse embryo cDNA libraries. The screening was performed according to the recommendations of the manufacturer.
Cell Lines
Porcine aortic endothelial (PAE) cells and HEK-293 cells were grown in DMEM supplemented with 10% FBS plus antibiotics. PAE and HEK-293 cells were used to express VEGFR-2 constructs. Human umbilical vascular endothelial cells (HUVECs) were grown in endothelial cell medium. The pMSCV puro retroviral vector was used to clone Myc-tagged PDCL3. Viruses were produced in 293GPG cells as described (17). HEK-293 cells expressing truncated VEGFR-2 receptors were established by a retroviral system as described previously (17).
Plasmids and siRNA
Human phosducin-like protein 3 (PDCL3, also called PHLP2A) (clone no. 3344703, accession no. BC001021) was purchased from Open Biosystems and was further cloned into pcDNA3.1/Myc-His(-)A via XhoI and KpnI and into pGEX2T via BamHI and XhoI for making GST fusion protein in Escherichia coli and into the retroviral vector, pMSCV, via XhoI and HpaI for expression in mammalian cells. The human PDCL3 cDNA was used as a template in the PCR reaction to generate N terminus PDCL3 (1–72 amino acids) and the thioredoxin domain (73–241 amino acids). The resultant constructs were further cloned into pcDNA3.1/Myc-His(-)A via XhoI and KpnI and pGEX2T via BamHI and XhoI for making GST fusion protein in E. coli. VEGFR-2/FLK-1(clone no. 4238984) was purchased from Open Biosystems and used for transient transfections in the HEK-293 cells. All VEGFR-2 and chimeric VEGFR-2 (CKR, where the extracellular domain of VEGFR-2 was replaced with the extracellular domain of human colony-stimulating factor-1 receptor) constructs were generated by PCR, and all were sequenced before use.
Immunoprecipitation and Western Blotting
Cells were prepared and lysed as described (11). Briefly, cells were washed twice with H/S buffer (25 mm HEPES (pH 7.4), 150 mm NaCl, and 2 mm Na3VO4) and lysed in EB lysis buffer (10 mm Tris-HCl,10% glycerol (pH 7.4), 5 mm EDTA, 50 mm NaCl, 50 mm NaF, 1% Triton X-100, 1 mm PMSF, 2 mm Na3VO4, and 20 μg/ml aprotinin). The normalized cell lysates were immunoprecipitated by using the appropriate antibodies and were subsequently subjected to Western blotting using anti-VEGFR-2 antibody the appropriate antibody as indicated in the figure legends. In some instances, the membranes were stripped by incubating them in a buffer containing 6.25 mm Tris-HCl (pH 6.8), 2% SDS, and 100 mm β-mercaptoethanol in 50 °C for 30 min and reprobed with the desired antibody. Cell surface biotinylation was performed as described (40) and according to the recommendations of the manufacturer.
In Vitro GST Pull-down Assay
The assay was performed as described (20). Briefly, HEK-293 cells expressing VEGFR-2 were grown in 25-cm plates. The plates were washed with H/S buffer and lysed in EB lysis buffer. The normalized cell lysates were incubated with equal amounts of immobilized GST fusion proteins for 3 h at 4 °C. The beads were washed with phosphate-buffered saline solution with protease inhibitors and sodium vanadate. The eluted proteins were boiled in sample buffer and analyzed by Western blotting using the appropriate antibody.
Proliferation Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT) assay was used to measure proliferation. In brief, cells (1 × 104) were seeded in 96-well plates after 24 h of transfection (quadruple wells/group). Cells were washed with 1× PBS and replaced with growth medium without FBS before 16 h of stimulation. On the day of stimulation, cells were stimulated with VEGF (10 ng/ml) and incubated for 24 h. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye was added at the end of 24 h, incubated for 4 h at 37 °C, and processed as recommended by the manufacturer.
Matrigel Tube Formation Assay
Endothelial cells were seeded on the Matrigel with endothelial cell growth medium (Clonetics Co., San Diego, CA) in the absence or presence of VEGF and photographed after 16 h as described (11). Experiments were repeated three times, and representative data are shown.
Statistical Analysis
Images were quantified with ImageJ software. Data are presented as mean ± S.E. from at least three independent experiments. p values were calculated by two-tailed Student's t test.
RESULTS
Identification of PDCL3 as a Novel VEGFR-2 Binding Protein
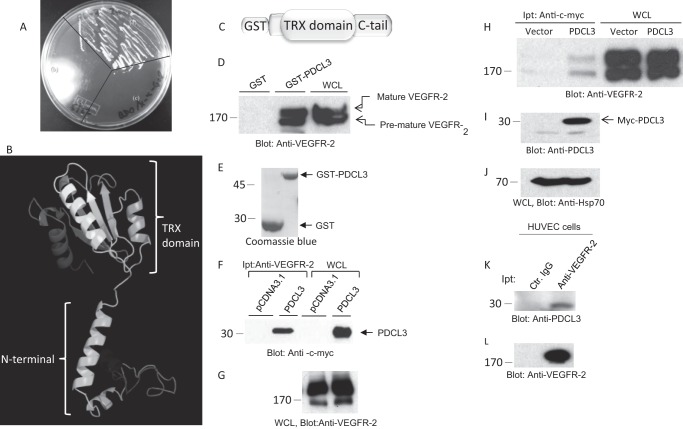
To identify putative VEGFR-2 binding proteins, we screened a 17-day-old mouse embryo cDNA library that could interact with the cytoplasmic domain of mouse VEGFR-2 in a yeast-two hybrid system. The library of proteins that interacted with the cytoplasmic domain of VEGFR-2 was selected and tested further for their binding specificity with VEGFR-2. Of the numerous unique sequences that identified one unique sequence was identified as phosducin-like 3 (PDCL3, also known as PhLP2A). As shown, when Y187 yeast cells containing pGADT7-PDCL3 were mated with AH109 cells containing PGBKT7-VEGFR-2 and plated on QDO/X-α-Gal medium together with their haploid constituents, only pGADT7 PDCL3/pGBKT7-VEGFR-2 diploids were able to produce colonies, indicating the binding of PDCL3 with VEGFR-2 in the yeast cells (Fig. 1A).
FIGURE 1.
Identification of PDCL3 as a VEGFR-2 binding protein. pGADT7-PDCL3 was transformed into Y187 yeast cells, and PGBKT7-VEGFR-2 was transformed into AH109 yeast cells. The two lines were mated and plated on QDO/X-α-Gal medium along with their haploid constituents. A, pGADT7-PDCL3/pGBKT7-VEGFR-2 diploids (a), pGBKT7-VEGFR-2 AH109 (b), and pGADT7-PDCL3/Y187 (c). B, the predicted structure of PDCL3. TRX, thioredoxin. C, schematic of the GST-PDCL3 fusion protein. D, cell lysates derived from PAE cells expressing VEGFR-2 were subjected to in vitro GST pull-down assay. WCL, whole cell lysate. E, Coomassie Blue stain of GST and GST-PDCL3. F, HEK-293 cells cotransfected with VEGFR-2 and an empty vector or PDCL3 were lysed, immunoprecipitated (Ipt) with anti-VEGFR-2 antibody, and blotted with anti-c-myc antibody. G, whole cell lysates from the same group were probed with anti-VEGFR-2 antibody. H, HEK-293 cells cotransfected with VEGFR-2 and an empty vector or PDCL3. Cells were lysed, immunoprecipitated with anti-c-myc antibody, and blotted with anti-VEGFR-2 antibody. Whole cell lysates from the same group were probed with anti-PDCL3 antibody (I) and anti-HSP70 antibody (J). K, cell lysates derived from HUVECs immunoprecipitated with control IgG or anti-VEGFR-2 antibody and blotted with an anti-PDCL3 antibody. L, the same membrane was reblotted with anti-VEGFR-2 antibody. All Western blot analyses and GST-pull down experiments were repeated at least three times.
One of the unique characteristics of PDCL3 is the presence of the thioredoxin domain, the so-called “thioredoxin fold.” Unlike the classical thioredoxin domain containing proteins, PDCL3 does not have thioltransferase activity because of the absence of the sequence CXXC in the active site. Generally, the thioredoxin fold consists of a fold of five-stranded β-sheet flanked by four helices (β/α/β/α/β/α/β/β/α) and comprises ∼100 residues (18, 19). The predicted structure of PDCL3 shows a helix N-terminal domain and a C-terminal thioredoxin fold domain (Fig. 1B), which is similar to the known crystal structure of phosducin (21).
To establish the direct binding of PDCL3 with VEGFR-2, we generated recombinant GST-PDCL3 protein and performed a GST pull-down assay using previously established PAE cells expressing VEGFR-2 (17). The result showed that the GST-PDCL3 fusion protein binds to both mature (fully glycosylated and likely localized to the plasma membrane) and premature (newly synthesized, unglycosylated/partially glycosylated) forms of VEGFR-2 (Fig. 1D).
VEGFR-2 is a glycosylated cell surface receptor protein. The apparent molecular weight of mature and fully glycosylated VEGFR-2 is 180 kDa, and the apparent molecular weight of premature and partially glycosylated VEGFR-2 is about 160 kDa (9, 21).
The binding of PDCL3 to VEGFR-2 was independent of ligand stimulation of VEGFR-2, and treatment of cells with ligand did not increase the binding of PDCL3 to VEGFR-2 (data not shown), suggesting that ligand-mediated phosphorylation of VEGFR-2 is not required for association of PDCL3 with VEGFR-2. Further analysis showed that PDCL3 binds to VEGFR-2 in HEK-293 cells cotransfected with VEGFR-2 and c-Myc-tagged PDCL3. A coimmunoprecipitation assay was performed using anti-VEGFR-2 antibody, followed by immunoblotting with anti-c-Myc antibody (Fig. 1F). Reverse coimmunoprecipitation, where PDCL3 was immunoprecipitated with an anti-c-Myc antibody followed by immunoblotting with an anti-VEGFR-2 antibody, also showed a similar result (Fig. 1H). In addition, PDCL3 was coprecipitated with chimeric VEGFR-2 (CKR, where the extracellular domain of VEGFR-2 was replaced with human CSF-1R) in PAE cells (17), further supporting the specific binding of PDCL3 with VEGFR-2 (data not shown).
To establish the binding of endogenous PDCL3 with VEGFR-2, particularly in the background of primary endothelial cells, we determined the binding of PDCL3 with VEGFR-2 in primary HUVECs. PDCL3 was coimmunoprecipitated with VEGFR-2 in HUVECs (Fig. 1K), further underscoring the data obtained from the yeast two-hybrid system and overexpression of PDCL3 in HEK-293 and PAE cells. Taken together, the data demonstrate that PDCL3 binds to the cytoplasmic domain of VEGFR-2.
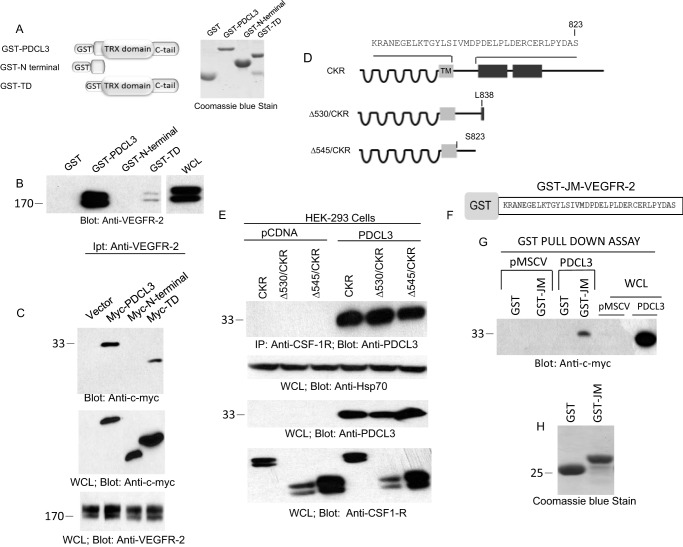
To determine the molecular mechanisms of recognition of VEGFR-2 by PDCL3, we first tested the ability of N terminus-truncated GST-PDCL3 constructs, as shown, to bind VEGFR-2 (Fig. 2A). The GST-N terminus PDCL3, in the absence of the thioredoxin domain, did not bind to VEGFR-2 (Fig. 2B). However, the GST-Thx domain plus the C terminus were able to interact with VEGFR-2, although significantly less than the full-length PDCL3 (Fig. 2B), suggesting that N terminus is required for the optimal recognition of VEGFR-2 by PDCL3. To further examine the association of PDCL3 with VEGFR-2, the same PDCL3 constructs were engineered to express in the mammalian cells and analyzed for their potentials to interact with VEGFR-2. Consistent with the in vitro GST pull-down assay, the N terminus alone failed to interact with VEGFR-2. Also, in the absence of the N terminus, the binding with VEGFR-2 was significantly less compared with the wild-type PDCL3 binding to VEGFR-2 (Fig. 2C, top panel). Taken together, the data demonstrate that PDCL3 recognizes VEGFR-2 primarily through the thioredoxin domain plus its C terminus but that the presence of N terminus is required for the optimal recognition of VEGFR-2.
FIGURE 2.
Juxtamembrane of VEGFR-2 is required for interaction with PDCL3. A, schematic of GST-PDCL3, GST-N terminus PDCL3, and GST-thioredoxin domain (TD) and the Coomassie Blue stain of recombinant proteins purified from E. coli. TRX, thioredoxin. B, cell lysates derived from HEK-293 expressing VEGFR-2 subjected to an in vitro GST-pull down assay using GST alone or GST-PDCL3, GST-N terminus PDCL3, and GST-TD. WCL, whole cell lysate. C, top panel, HEK-293 cells cotransfected with an empty vector, PDCL3, or truncated PDCL3 constructs with VEGFR-2 were lysed, immunoprecipitated (Ipt) with anti-VEGFR-2 antibody, and blotted with anti-c-myc antibody. Whole cell lysates derived from the same group were blotted with anti-c-myc antibody and anti-VEGFR-2 antibody. D, schematic of carboxyl domain-deleted chimeric VEGFR-2 (CKR). HEK-293 cells were engineered to express wild-type CKR and truncated CKR constructs alone or coexpress with PDCL3. E, top panel, cells were lysed and subjected to coimmunoprecipitation (IP) using anti-CSF-1R antibody that specifically recognizes the extracellular domain, followed by blotting with anti-PDCL3 antibody. Whole cell lysates were blotted for protein loading control, anti-Hsp70 antibody, anti-PDCL3 antibody, and anti-VEGFR-2 antibody. F, schematic of the GST-JM (juxtamembrane) domain of VEGFR-2. G, the GST-JM domain of VEGFR-2 was incubated with PAE cell lysates expressing GST or GST-PDCL3, subjected to GST-pull down assay, and blotted with anti-c-myc antibody. H, Coomassie Blue stain of GST and the GST-JM domain of VEGFR-2. The blots are representative of at least three independent experiments.
To determine the specific site of recognition on VEGFR-2 by PDCL3, we initially examined whether the C-terminal of VEGFR-2 is engaged in the recruitment of PDCL3 to VEGFR-2. To this end, we examined a panel of previously characterized C terminus-truncated chimeric VEGFR-2 (CKR), where the extracellular domain of VEGFR-2 was replaced with the extracellular domain of human CSF-1R to interact with PDCL3 (9, 17, 21). Progressive deletion of the C-terminal by 152, 157, 165, and 196 amino acids does not abolish the binding of PDCL3 with VEGFR-2 (data not shown), indicating that the presence of 196 amino acids distal to the kinase domain of VEGFR-2 is not required for PDCL3 interaction with VEGFR-2. To determine the PDCL3 recognition motif on VEGFR-2 more precisely, additional truncated VEGFR-2 constructs were created in the CKR background, where the cytoplasmic domain of VEGFR-2 was deleted by 530 and 545 amino acids proximal to the kinase domain (Fig. 2D), and their ability to interact with PDCL3 was measured in a coimmunoprecipitation assay. Deletion of the cytoplasmic domain of VEGFR-2 by 545 amino acids does not impair its ability to interact with PDCL3 (Fig. 2E), indicating that the juxtamembrane (JM) domain of VEGFR-2 is a likely PDCL3 binding site. We generated a GST fusion protein encompassing the JM domain and directly tested for its ability to interact with PDCL3 (Fig. 2F). The data show that the JM domain of VEGFR-2 alone is sufficient for mediating the binding of VEGFR-2 with PDCL3 (Fig. 2G).
PDCL3 Increases the Stability of VEGFR-2 through Inhibition of Ubiquitination
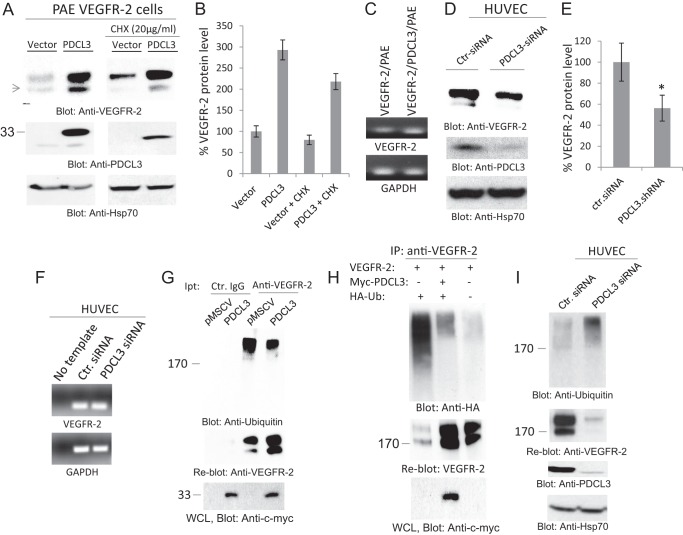
We noticed that coexpression of PDCL3 with VEGFR-2 in PAE cells increases expression of VEGFR-2 and that treatment of cells with the protein translation inhibitor cycloheximide (CHX) did not significantly inhibit the effect of PDCL3, suggesting that PDCL3 likely controls VEGFR-2 expression posttranslationally (Fig. 3A). Consistent with this idea, VEGFR-2 mRNA was not increased in PAE cells overexpressing PDCL3 (Fig. 3C). To further ascertain the role of PDCL3 in the regulation of VEGFR-2, we silenced expression of PDCL3 in HUVECs and assessed the expression of VEGFR-2 at both protein and mRNA levels. The data showed that depleting the expression of PDCL3 by siRNA significantly reduces VEGFR-2 protein (Fig. 3, D and E) but not its mRNA (F).
FIGURE 3.
PDCL3 regulates the abundance of VEGFR-2 protein through inhibition of VEGFR-2 ubiquitination. A, PAE cells coexpressing VEGFR-2 with an empty vector or PDCL3 were treated with vehicle or cycloheximide (CHX) (20 ng/ml, 90 min). Cells were washed and lysed. Whole cell lysates were subjected to Western blot analysis using anti-VEGFR-2 antibody, anti-PDCL3 antibody, and anti-HSP70 antibody. B, graph representing three independent experiments. C, the mRNA derived from PAE cells expressing VEGFR-2 alone and coexpressing VEGFR-2 with PDCL3 were subjected to RT-PCR analysis using PCR primers for VEGFR-2 or GAPDH. D, HUVECs were transfected with either control siRNA (Ctr-siRNA) or PDCL3 siRNA. After 48 h, cells were lysed, and whole cell lysates were blotted for anti-VEGFR-2 antibody, anti-PDCL3 antibody, and anti-Hsp70 antibody. E, graph representing three independent experiments. *, p < 0.031. F, the mRNA derived from HUVECs transfected with the control siRNA and PDCL3 siRNA was subjected RT-PCR analysis using PCR primers for human VEGFR-2 or GAPDH. G, top panel, PAE cells coexpressing VEGFR-2 with an empty vector (pMSCV) or PDCL3 were washed, lysed, immunoprecipitated (Ipt) with anti-VEGFR-2 antibody, and blotted with anti-ubiquitin antibody. The same membrane was reblotted with anti-VEGFR-2 antibody (center panel). Whole cell lysate (WCL) was subjected to Western blot analysis using anti-c-myc antibody for PDCL3 (bottom panel). H, HEK-293 cells were transfected with VEGFR-2 alone, VEGFR-2 with PDCL3, or VEGFR-2 with PDCL3 and HA-tagged ubiquitin (HA-Ub) (top panel). Cells were lysed, immunoprecipitated (IP) with anti-VEGFR-2 antibody, and blotted with anti-HA antibody. The same membrane was blotted with anti-VEGFR-2 antibody (center panel). Whole cell lysate from the same group was blotted for PDCL3 using anti-c-myc antibody (bottom panel). I, HUVECs were transfected with control or PDCL3 siRNA. Total cell lysates were subjected to immunoprecipitation using anti-ubiquitin antibody (top panel). The same membrane was reblotted with anti-VEGFR-2 antibody (center panel). Whole cell lysates were subjected to Western blot analysis using anti-PDCL3 antibody and anti-HSP70 antibody. All the Western blot analyses shown are the representative of at least three independent experiments.
How does PDCL3 regulate VEGFR-2 protein abundance? To address this question, we tested the hypothesis that PDCL3 exerts its effect on VEGFR-2 by preventing the ubiquitination of VEGFR-2. Ubiquitination plays a central role in protein homeostasis, including for VEGFR-2 (11, 22). Newly synthesized cell surface receptors are often guarded from degradation by molecular chaperones and other enzymes that facilitate cell surface receptors folding and assembly into their native conformation. Unprotected proteins are ultimately degraded through ubiquitylation and the 26 S proteasome pathway (22, 23). Interestingly, overexpression of PDCL3 significantly reduced ubiquitylation of VEGFR-2 (Fig. 3G), and the reduced ubiquitylation of VEGFR-2 coincided with the increased VEGFR-2 protein level (G, center panel). Moreover, cotransfection of HA-tagged ubiquitin with VEGFR-2 showed a significant ubiquitination of VEGFR-2 as detected by HA antibody and that ubiquitination of VEGFR-2 was markedly inhibited by PDCL3 (Fig. 3H).
To further examine the role of PDCL3 in the inhibition of VEGFR-2 ubiquitylation, we silenced expression of PDCL3 in HUVECs and analyzed ubiquitylation of VEGFR-2. Depletion of PDCL3 in HUVECs distinctively increased VEGFR-2 ubiquitination (Fig. 3I, top panel) and reduced VEGFR-2 protein (center panel). The reduced VEGFR-2 protein level was blocked by MG132 (data not shown). Taken together, the data demonstrate that PDCL3 increases VEGFR-2 protein abundance through inhibition of ubiquitination of VEGFR-2, thus reducing the rate of its degradation.
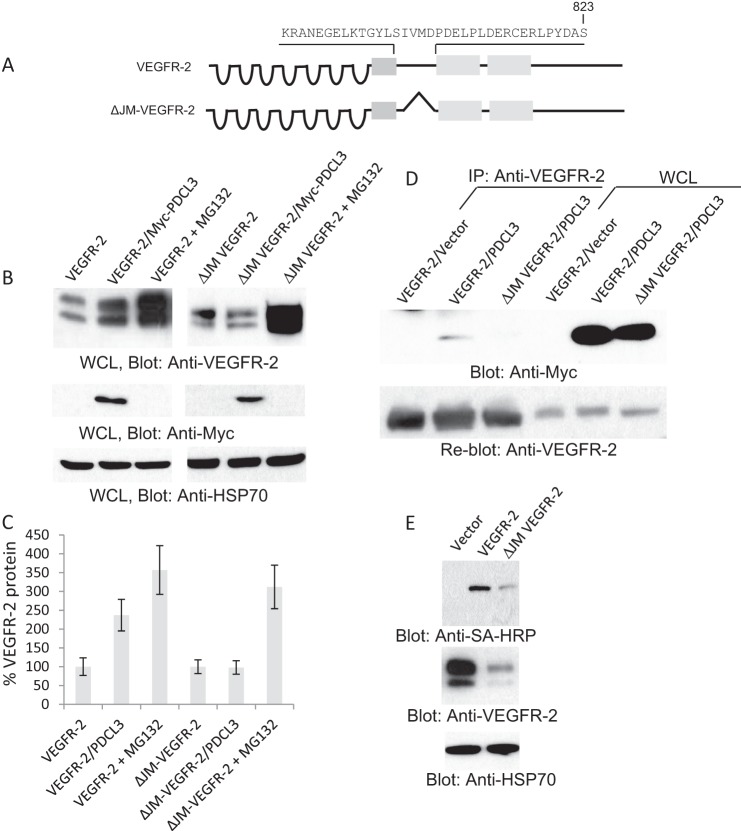
Because the JM domain of VEGFR-2 is the primary binding site for PDCL3 (Fig. 2K), we deleted the JM domain to examine its effect on the stability of VEGFR-2. The data show that VEGFR-2 lacking the JM domain (ΔJM-VEGFR-2) is refractory to the effect of PDCL3 (Fig. 4B) but that treatment with the proteasome inhibitor MG132 increased its expression. It was difficult to express ΔJM-VEGFR-2. It requires a high titer of virus and multiple infections (data not shown). As expected, the ΔJM-VEGFR-2 also did not bind to PDCL3 (Fig. 4D). Despite its low levels of expression and rapid degradation, ΔJM-VEGFR-2 is still present at the cell surface (Fig. 4D). Taken together, the data on the basis of the silencing of PDCL3 and truncation of VEGFR-2 demonstrate that preventing VEGFR-2 binding to PDCL3 renders VEGFR-2 an unstable protein.
FIGURE 4.
Deletion of the JM domain renders VEGFR-2 an unstable protein. A, schematic of VEGFR-2 and JM domain-deleted VEGFR-2 (ΔJM/VEGFR-2). B, whole cell lysates (WCL) from PAE cells expressing VEGFR-2 alone, ΔJM/VEGFR-2 alone, or coexpressing with PDCL3 or cells treated with MG132 were blotted with anti-VEGFR-2 antibody, anti-c-myc antibody for PDCL3, and HSp70 for protein loading control. C, graph representative of three independent experiments. D, cell lysates from PAE cells expressing VEGFR-2, ΔJM/VEGFR-2 alone, or coexpressing PDCL3 were immunoprecipitated (IP) with anti-VEGFR-2 antibody and blotted with anti-c-myc antibody for PDCL3. D, whole cell lysates (WCL) from the same groups. E, the same membrane was reblotted with anti-VEGFR-2. PAE cells expressing VEGFR-2 or ΔJM/VEGFR-2 were subjected to cell surface biotinylation and blotted with streptavidin-horseradish peroxidase (SA-HRP) (top panel), anti-VEGFR-2 antibody, and anti-Hsp70. All the Western blot analyses shown are the representative of at least three independent experiments.
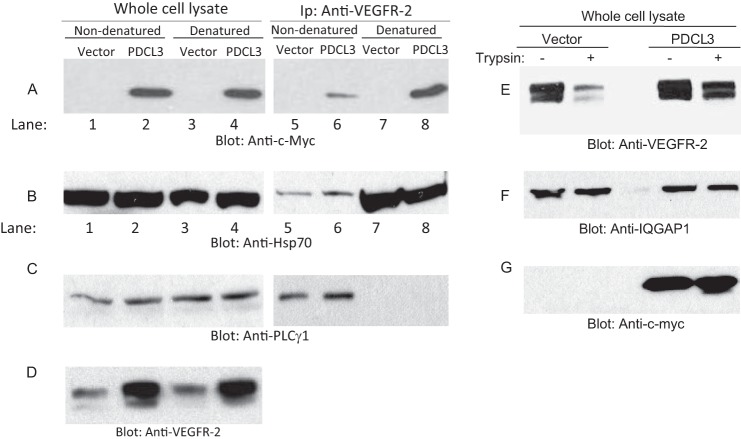
Proteins with chaperone activity are known to recognize their substrates with high affinity in their unfolded states and with a minimal or no affinity in their native state (24–26). This intrinsic property of chaperone proteins allows the release of folded proteins when they have reached their native conformation. To address whether PDCL3 interaction with VEGFR-2 is influenced by the state of VEGFR-2 structural folding, we heat-denatured VEGFR-2 protein and examined the binding of VEGFR-2 with PDCL3. As shown, although PDCL3 was coprecipitated with VEGFR-2 in its native non-denatured condition, the coprecipitation of PDCL3 with VEGFR-2 in the heat-denatured condition was significantly higher (Fig. 5A, lane 6 compared with lane 8). The binding of the chaperone protein Hsp70 to VEGFR-2 was also higher in the denaturing condition compared with the non-denaturing condition (Fig. 5B, lane 6 compared with lane 8). Unlike the PDCL3 and Hsp70 binding to heat-induced denatured VEGFR-2, the binding of PLCγ1 to VEGFR-2 was totally abolished (Fig. 5C). Altogether, the data demonstrate that PDCL3 binds preferentially to unfolded VEGFR-2. To further support the putative chaperone function of PDCL3, cell lysates derived from cells expressing VEGFR-2 alone or coexpressing VEGFR-2 with PDCL3 was subjected to a partial trypsin digest. In the cell lysates derived from expressing VEGFR-2 alone, the trypsin digestion decreased the VEGFR-2 protein level (Fig. 5E), whereas trypsin treatment of cell lysate derived from the cells coexpressing VEGFR-2 with PDCL3 had a minor effect on the VEGFR-2 protein level. This contrast further supports the importance of PDCL3 in the protection of VEGFR-2 from degradation by serving as a chaperone protein.
FIGURE 5.
PDCL3 protects VEGFR-2 from proteolytic degradation. PAE cells coexpressing VEGFR-2 with an empty vector or with PDCL3 were lysed, and whole cell lysates were divided into two groups. One of the lysate groups was denatured by boiling at 95 °C, and both groups were subjected to immunoprecipitation (IP) using anti-VEGFR-2 antibody. Immunoprecipitated proteins were analyzed by Western blot analysis using anti-c-myc antibody (A), anti-HSP70 antibody (B), and anti-PLCγ1 antibody (C). The whole cell lysates from the same groups were also blotted with anti-c-myc antibody, anti-Hsp70 antibody, and anti-PLCγ1 antibody. Cell lysates from PAE cells coexpressing VEGFR-2 with an empty vector or PDCL3 were treated with trypsin (25 ng/50 μl of cell lysate) or vehicle, followed by Western blot analysis using anti-VEGFR-2 antibody (E), anti-IQGAP1 antibody as a loading control (F), and anti-c-myc antibody for PDCL3 (G).
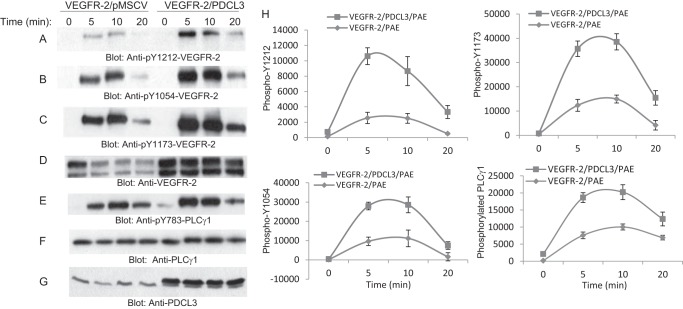
To further examine the biological consequence of PDCL3, we also analyzed phosphorylation of VEGFR-2 in the context of overexpression of PDCL3. Overexpression of PDCL3 in PAE cells significantly increased tyrosine phosphorylation of VEGFR-2, as demonstrated by phosphospecific VEGFR-2 antibodies, including phospho-Tyr-1212, phospho-Tyr-1054, and phospho-Tyr-1173 antibodies (Fig. 6A, B, and C). Consistent with the increased phosphorylation of VEGFR-2, phosphorylation of PLCγ1, a major substrate of VEGFR-2, was also elevated in cells overexpressing PDCL3 compared with PAE cells expressing VEGFR-2 alone (Fig. 6E). Quantification of phosphorylation of VEGFR-2 and PLCγ1 representing three independent experiments are shown (Fig. 6H). Overexpression of PDCL3 also increased VEGFR-2-dependent phosphorylation of p42/p44 MAPK (data not shown).
FIGURE 6.
PDCL3 potentiates VEGF-mediated activation and signaling of VEGFR-2. PAE cells coexpressing VEGFR-2 with an empty vector or with PDCL3 were serum-starved and stimulated or left unstimulated with VEGF for the indicated times. Whole cell lysates were subjected Western blot analysis using anti-phospho-Tyr-1212-VEGFR-2 antibody (A), anti-phospho-Tyr-1054-VEGFR-2 antibody (B), anti-phospho-Tyr-1173-VEGFR-2 antibody (C), anti-VEGFR-2 (D), anti-phospho-Tyr-783-PLCγ1 antibody (E), PLCγ1 antibody (F), and anti-PDCL3 antibody (G). H, representative of three independent experiments corresponding to phosphorylation of VEGFR-2 at the multiple sites and phosphorylation of PLCγ1.
PDCL3 Regulates VEGFR-2-dependent Endothelial Cell Proliferation and Angiogenesis
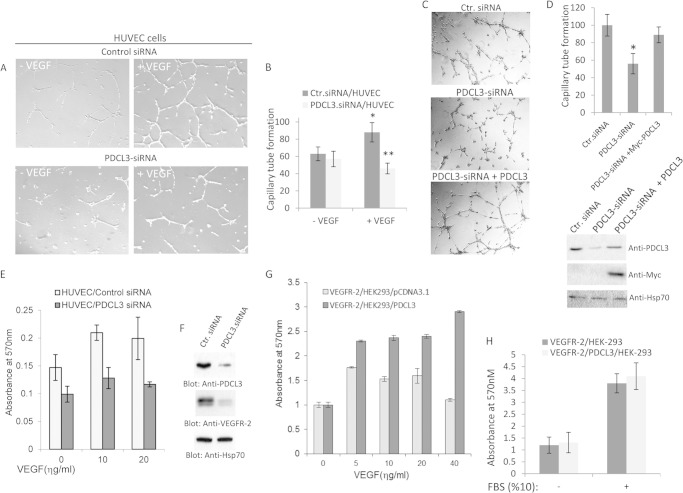
Overexpression of PDCL3 significantly increases tyrosine phosphorylation of VEGFR-2 and its substrates, suggesting that PDCL3 activity could also regulate VEGFR-2-dependent angiogenic events. Overexpression of PDCL3 in PAE cells also increased VEGF-induced capillary tube formation, and disabling PDCL3 binding with VEGFR-2 (i.e. N terminus deletion of PDCL3 as shown in Fig. 2) inhibited the effect of PDCL3 (data not shown). Moreover, we knocked down the expression of PDCL3 in primary endothelial cells (HUVECs) by siRNA and measured their capillary tube formation. Depletion of PDCL3 by siRNA significantly reduced the ability of VEGF to stimulate capillary tube formation of HUVECs (Fig. 7A). Quantification of the effect of PDCL3 knockdown on the capillary tube formation of HUVECs representing three independent experiments is shown (Fig. 7B). Re-expression of PDCL3 reversed the PDCL3-siRNA-dependent inhibition of tube formation (Fig. 7C), indicating that the PDCL3-siRNA specifically targets PDCL3. Quantification of the effect of expression of PDCL3 in the context of PDCL3-siRNA and expression of PDCL3 is shown (Fig. 7D). However, PDCL3-siRNA had no effect on the basic fibroblast growth factor-induced capillary tube formation (data not shown).
FIGURE 7.
PDCL3 activity is required for endothelial cell proliferation and angiogenesis. A, HUVECs were transfected with control (Ctr.) or PDCL3 siRNA, and after 16 h, cells were seeded onto Matrigel and stimulated with VEGF or vehicle. Cells were photographed after 16 h. B, the graph is the representative of four different fields and is presented as the mean ± S.D. *, p < 0.05; **, p < 0.021. C, HUVECs were transfected with control (Ctr.), PDCL3 siRNA alone or cotransfected with siRNA PDCL3 with c-Myc-PDCL3. Cells then were subjected to Matrigel tubulogenesis assay as in A. D, the graph is representative of four different fields and is presented as the mean ± S.D. *, p < 0.001. Cells lysates from the same group were blotted for PDCL3 and Hsp70 for protein loading control. E, HUVECs transfected with control siRNA or PDCL3 siRNA were subjected to a proliferation assay in the presence or absence of VEGF. F, whole cell lysates from the same siRNA-transfected HUVECs were subjected to Western blot analysis using anti-PDCL3 antibody and anti-Hsp70 antibody for protein levels. G, HEK-293 cells expressing VEGFR-2 were transfected with an empty vector or PDCL3. Cells were subjected to a proliferation assay and stimulated with varying amounts of VEGF. The proliferation of HEK-293 cells expressing VEGFR-2 alone or coexpressing VEGFR-2 with PDCL3 cells either kept in serum-free DMEM or stimulated with 10% FBS was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as in E. H, the graph represents the quadruple of the mean ± S.D.
Endothelial cell proliferation is an important aspect of angiogenesis and, therefore, we also analyzed proliferation of HUVECs in response to VEGF when PDCL3 expression was blocked by siRNA. Silencing the expression of PDCL3 markedly reduced the ability of VEGF to stimulate proliferation of HUVECs (Fig. 7E), indicating that expression of PDCL3 in endothelial cells is biologically important for the VEGF-induced angiogenic responses. To establish the direct role of PDCL3 in VEGFR-2-mediated cellular events, we created HEK-293 cells expressing VEGFR-2. Selective stimulation of HEK-293 cells expressing VEGFR-2 with VEGF stimulated proliferation. However, stimulation of HEK-293 cells expressing PDCL3 with VEGF alone did not stimulate proliferation (data not shown), indicating that VEGF stimulates growth of these cells only in the presence of VEGFR-2 and that expression of PDCL3 alone is not sufficient to stimulate proliferation. Moreover, VEGF stimulation of HEK-293 cells coexpressing PDCL3 with VEGFR-2 significantly increased proliferation, demonstrating that the increased proliferation of HEK-293 cells is due to the effect of PDCL3 on VEGFR-2 and not on the other putative PDCL3-interacting proteins (Fig. 7G). Consistent with this idea, proliferation of HEK-293 cells coexpressing VEGFR-2 with PDCL3 or alone were similar (Fig. 7H). Taken together, the data indicate that PDCL3 plays an important role in VEGF-mediated angiogenesis.
DISCUSSION
The normal function of most cellular proteins hinges upon the ability of the folding machinery system to properly process newly synthesized proteins, enabling their full functional activity. The majority of newly synthesized secreted and cell surface receptors are susceptible to degradation and often need protection against unwanted and harmful interactions. This is generally accomplished by the function of chaperone proteins until they have successfully completed their folding and maturation (22, 23). VEGFR-2 is one of the most important and most studied proangiogenic receptor tyrosine kinases whose expression is strongly associated with pathological angiogenesis. This, in turn, is linked to tumor growth, metastasis, and numerous other pathological conditions, ranging from age-related macular degeneration to retinopathy of prematurity (27).
In this study, we have demonstrated that PDCL3 plays an important role in stabilizing VEGFR-2 protein. In the absence of PDCL3, VEGFR-2 is partially protected and is targeted for ubiquitylation and degradation. In general, chaperone proteins recognize hydrophobic residues and/or unstructured regions in their substrates that are otherwise buried upon completion of folding (23, 28). Consistent with the general function of chaperone proteins, PDCL3 also interacts with in vitro-translated VEGFR-2 that is not fully folded (data not shown). PDCL3 association with VEGFR-2 also increases upon heat-induced denaturation of VEGFR-2, making VEGFR-2 less susceptible to proteolytic degradation.
The data presented in this manuscript suggest that, in the absence of PDCL3, VEGFR-2 is an unstable signal transducer and that PDCL3 keeps VEGFR-2 poised for activation by VEGF family ligands. Elevating the expression of PDCL3 significantly increases ligand-stimulated phosphorylation of VEGFR-2 and activation of a key VEGFR-2 substrate, PLCγ1. Activation of PLCγ1 in endothelial cells is directly linked to endothelial cell proliferation and angiogenesis in vivo (29, 30). Modulation of the angiogenic phenotype of endothelial cells by PDCL3 is likely linked, in part, to increased activation of PLCγ1 by VEGFR-2. Recent studies have shown that the molecular chaperone heat shock protein 90 (Hsp90) facilitates stabilization and activation of a variety of kinases, including EGF receptor, Eph receptor (31), and AKT (32). The data presented in this manuscript are the first demonstration of the chaperone function of PDCL3 in the stabilization of kinases, although this putative role of PDCL3 remains to be established beyond VEGFR-2.
PDCL3 was originally identified as a protein that participates in G protein signaling by binding to the Gβγ dimer with high affinity (33, 34) and was thought to facilitate G protein function by interacting with the chaperonin containing TCP-1 (CCT), which is critical to the folding of actin, tubulin, and other proteins into their native structures (35). Recent insights into the function of PDCL3, however, suggest that PDCL3 directly facilitates the function of the G protein by modulating the assembly of Gβγ dimers (36, 37). Consistent with these findings, our data show that PDCL3 associates with several CCT subunits,3 further supporting the assumption that PDCL3 could contribute to stability of VEGFR-2 through the CCT molecular chaperone complex. In the milieu of VEGFR-2 stabilization by PDCL3, our data suggest that PDCL3 distinctively contributes to the stabilization of VEGFR-2 by inhibiting ubiquitination of VEGFR-2 because depletion of PDCL3 increased ubiquitination of VEGFR-2, and its overexpression reduced ubiquitylation of VEGFR-2. On the basis of these observations, we propose that PDCL3 interaction with VEGFR-2 protects VEGFR-2 from ubiquitin-dependent degradation.
Homeostasis of VEGFR-2 protein in endothelial cells is critical for the fidelity of angiogenic signaling. Endothelial cells must maintain sufficient VEGFR-2 numbers to efficiently receive and transmit survival and proliferation signals while limiting receptor numbers to prevent hyperangiogenic signaling that can lead to pathological angiogenesis (9, 11, 38, 39). Although transcriptional regulation is known to contribute to VEGFR-2 homeostasis, accumulating evidence now suggests that posttranscriptional mechanisms such as ubiquitination also play central roles in determining the cellular levels of VEGFR-2 (11). The data presented in this manuscript demonstrate that PDCL3 chaperone function significantly contributes to VEGFR-2 expression and subsequent angiogenesis.
In sum, successful expression of functional VEGFR-2 in endothelial cells depends upon a myriad of events, including assistance from PDCL3 to prevent VEGFR-2 from degradation, rendering VEGFR-2 a more stable and functional angiogenic protein. Given the critical importance of PDCL3 in VEGFR-2 expression and function, targeting PDCL3 in the endothelial cells may provide a novel therapeutic strategy for treatment of angiogenesis-associated diseases where elevated VEGFR-2 function is considered a main culprit.
Acknowledgments
We thank Cheryl Chi for proofreading the manuscript.
This work was supported, in whole or in part, by grants from the NEI, National Institutes of Health (to N. R.). This work was also supported by a grant from the Department of Pathology, Boston University and by a grant from the Massachusetts Lions Foundation (to the Department of Ophthalmology, Boston University).
S. Srinivasan, R. D. Meyer, R. Lugo, and N. Rahimi, unpublished data.
- VEGFR
- VEGF receptor
- PhLP
- phosducin-like protein
- PAE
- porcine aortic endothelial
- HUVEC
- human umbilical vein endothelial cell
- JM
- juxtamembrane.
REFERENCES
- 1. Carmeliet P. (2003) Angiogenesis in health and disease. Nat. Med. 9, 653–660 [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D., Weinberg R. A. (2011) Hallmarks of cancer. The next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 3. Rahimi N. (2012) The ubiquitin-proteasome system meets angiogenesis. Mol. Cancer Ther. 11, 538–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baeriswyl V., Christofori G. (2009) The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 19, 329–337 [DOI] [PubMed] [Google Scholar]
- 5. Carmeliet P., Jain R. K. (2000) Angiogenesis in cancer and other diseases. Nature 407, 249–257 [DOI] [PubMed] [Google Scholar]
- 6. Koch S., Tugues S., Li X., Gualandi L., Claesson-Welsh L. (2011) Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 437, 169–183 [DOI] [PubMed] [Google Scholar]
- 7. Rahimi N. (2006) Vascular endothelial growth factor receptors. Molecular mechanisms of activation and therapeutic potentials. Exp. Eye Res. 83, 1005–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Folkman J. (2006) Angiogenesis. Annu. Rev. Med. 57, 1–18 [DOI] [PubMed] [Google Scholar]
- 9. Singh A. J., Meyer R. D., Band H., Rahimi N. (2005) The carboxyl terminus of VEGFR-2 is required for PKC-mediated down-regulation. Mol. Biol. Cell 16, 2106–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruns A. F., Bao L., Walker J. H., Ponnambalam S. (2009) VEGF-A-stimulated signalling in endothelial cells via a dual receptor tyrosine kinase system is dependent on co-ordinated trafficking and proteolysis. Biochem. Soc. Trans. 37, 1193–1197 [DOI] [PubMed] [Google Scholar]
- 11. Meyer R. D., Srinivasan S., Singh A. J., Mahoney J. E., Gharahassanlou K. R., Rahimi N. (2011) PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Mol. Cell. Biol. 31, 2010–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bass J., Chiu G., Argon Y., Steiner D. F. (1998) Folding of insulin receptor monomers is facilitated by the molecular chaperones calnexin and calreticulin and impaired by rapid dimerization. J. Cell Biol. 141, 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hampton R. Y. (2002) ER-associated degradation in protein quality control and cellular regulation. Curr. Opin. Cell Biol. 14, 476–482 [DOI] [PubMed] [Google Scholar]
- 14. Kostova Z., Wolf D. H. (2003) For whom the bell tolls. Protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J. 22, 2309–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flanary P. L., DiBello P. R., Estrada P., Dohlman H. G. (2000) Functional analysis of Plp1 and Plp2, two homologues of phosducin in yeast. J. Biol. Chem. 275, 18462–18469 [DOI] [PubMed] [Google Scholar]
- 16. Blaauw M., Knol J. C., Kortholt A., Roelofs J., Ruchira, Postma M., Visser A. J., van Haastert P. J. (2003) Phosducin-like proteins in Dictyostelium discoideum. Implications for the phosducin family of proteins. EMBO J. 22, 5047–5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rahimi N., Dayanir V., Lashkari K. (2000) Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J. Biol. Chem. 275, 16986–16992 [DOI] [PubMed] [Google Scholar]
- 18. Carvalho A. P., Fernandes P. A., Ramos M. J. (2006) Similarities and differences in the thioredoxin superfamily. Prog. Biophys. Mol. Biol. 91, 229–248 [DOI] [PubMed] [Google Scholar]
- 19. Willardson B. M., Howlett A. C. (2007) Function of phosducin-like proteins in G protein signaling and chaperone-assisted protein folding. Cell. Signal. 19, 2417–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer R. D., Singh A. J., Rahimi N. (2004) The carboxyl terminus controls ligand-dependent activation of VEGFR-2 and its signaling. J. Biol. Chem. 279, 735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaudet R., Bohm A., Sigler P. B. (1996) Crystal structure at 2.4 angstroms resolution of the complex of transducin βγ and its regulator, phosducin. Cell 87, 577–588 [DOI] [PubMed] [Google Scholar]
- 22. Vembar S. S., Brodsky J. L. (2008) One step at a time. Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frydman J. (2001) Folding of newly translated proteins in vivo. The role of molecular chaperones. Annu. Rev. Biochem. 70, 603–647 [DOI] [PubMed] [Google Scholar]
- 24. Yaffe M. B., Beegen H., Eckert R. L. (1992) Biophysical characterization of involucrin reveals a molecule ideally suited to function as an intermolecular cross-bridge of the keratinocyte cornified envelope. J. Biol. Chem. 267, 12233–12238 [PubMed] [Google Scholar]
- 25. Farr G. W., Scharl E. C., Schumacher R. J., Sondek S., Horwich A. L. (1997) Chaperonin-mediated folding in the eukaryotic cytosol proceeds through rounds of release of native and nonnative forms. Cell 89, 927–937 [DOI] [PubMed] [Google Scholar]
- 26. Yang D., Mok Y. K., Forman-Kay J. D., Farrow N. A., Kay L. E. (1997) Contributions to protein entropy and heat capacity from bond vector motions measured by NMR spin relaxation. J. Mol. Biol. 272, 790–804 [DOI] [PubMed] [Google Scholar]
- 27. Carmeliet P., Jain R. K. (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dunn A. Y., Melville M. W., Frydman J. (2001) Review. Cellular substrates of the eukaryotic chaperonin TRiC/CCT. J. Struct. Biol. 135, 176–184 [DOI] [PubMed] [Google Scholar]
- 29. Husain D., Meyer R. D., Mehta M., Pfeifer W. M., Chou E., Navruzbekov G., Ahmed E., Rahimi N. (2010) Role of c-Cbl-dependent regulation of phospholipase Cγ1 activation in experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 51, 6803–6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer R. D., Husain D., Rahimi N. (2011) c-Cbl inhibits angiogenesis and tumor growth by suppressing activation of PLCγ1. Oncogene 30, 2198–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Annamalai B., Liu X., Gopal U., Isaacs J. S. (2009) Hsp90 is an essential regulator of EphA2 receptor stability and signaling. Implications for cancer cell migration and metastasis. Mol. Cancer Res. 7, 1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sato Y., Kanno S., Oda N., Abe M., Ito M., Shitara K., Shibuya M. (2000) Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann. N.Y. Acad. Sci. 902, 201–205; discussion 205–207 [DOI] [PubMed] [Google Scholar]
- 33. Savage J. R., McLaughlin J. N., Skiba N. P., Hamm H. E., Willardson B. M. (2000) Functional roles of the two domains of phosducin and phosducin-like protein. J. Biol. Chem. 275, 30399–30407 [DOI] [PubMed] [Google Scholar]
- 34. Schröder S., Lohse M. J. (1996) Inhibition of G-protein βγ-subunit functions by phosducin-like protein. Proc. Natl. Acad. Sci. U.S.A. 93, 2100–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McLaughlin J. N., Thulin C. D., Hart S. J., Resing K. A., Ahn N. G., Willardson B. M. (2002) Regulatory interaction of phosducin-like protein with the cytosolic chaperonin complex. Proc. Natl. Acad. Sci. U.S.A. 99, 7962–7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lukov G. L., Hu T., McLaughlin J. N., Hamm H. E., Willardson B. M. (2005) Phosducin-like protein acts as a molecular chaperone for G protein βγ dimer assembly. EMBO J. 24, 1965–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Humrich J., Bermel C., Grubel T., Quitterer U., Lohse M. J. (2003) Regulation of phosducin-like protein by casein kinase 2 and N-terminal splicing. J. Biol. Chem. 278, 4474–4481 [DOI] [PubMed] [Google Scholar]
- 38. Rahimi N. (2006) VEGFR-1 and VEGFR-2. Two non-identical twins with a unique physiognomy. Front. Biosci. 11, 818–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dayanir V., Meyer R. D., Lashkari K., Rahimi N. (2001) Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J. Biol. Chem. 276, 17686–17692 [DOI] [PubMed] [Google Scholar]
- 40. Rahimi N., Rezazadeh K., Mahoney J. E., Hartsough E., Meyer R. D. (2012) Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Mol. Biol. Cell 9, 1646–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]