Abstract
The high vocal center (HVC) of adult male canaries, Serinus canaria, is necessary for the production of learned song. New neurons are added to HVC every day, where they replace older neurons that have died, but the length of their survival depends on the time of year when they are born. A great number of HVC neurons born in the fall, when adult canaries learn a new song, are still present 8 mo later, when this song is used during the breeding season. By contrast, most of the neurons born in HVC in the spring, when little song learning takes place, disappear much sooner. Here we show that infusion of brain-derived neurotrophic factor into HVC during days 14-20 after new HVC neurons are born in the spring confers on them a life expectancy comparable to that of fall-born neurons; this extension on life is not seen when infusion occurs 10 days earlier or later. We suggest that there is, in the adult HVC, a subset of neurons whose life expectancy is determined by brain-derived neurotrophic factor during a sensitive period soon after these neurons reach destination and start forming connections.
New neurons are born in the adult canary brain in the ventricular zone lining the wall of the lateral ventricles (1, 2). They migrate into the high vocal center (HVC) and assume a sedentary phenotype 8-15 days after their birth. The number of these new neurons is much reduced during their third week of life (3). By day 30, the axons of many of the surviving new HVC neurons have reached their target, and many of the new cells have become a functional part of existing circuits (3-5). Thereafter, the life expectancy of the neurons depends on the time of year when they are born. Whereas the number of fall-born neurons is virtually the same 30 and 120 days after their birth, this number drops by one-half at the later survival time in spring-born neurons (6). However, the mechanism that regulates this season-dependent survival/attrition has not been worked out in detail.
In addition to season, the presence of new neurons in HVC has been shown to depend on variables such as amount of singing (7, 8), auditory experience (9), blood testosterone levels (8, 10), and the presence of brain-derived neurotrophic factor (BDNF) in HVC (11). Some of these factors are related. For example, both amount of singing and blood testosterone levels change seasonally (12) and both influence the amount of BDNF present in HVC (8). There are at least two major sources of BDNF in HVC: neurons that project to the robust nucleus of the archistriatum (7) and endothelial cells lining HVC capillaries (13); in addition, a small percentage of HVC neurons that project to area X also express BDNF (7). Knowing that BDNF can influence neuron survival and that there is a dramatic decline in new neuron numbers 15-22 days after their birth (3), we decided to test whether BDNF infusion during this period could enhance the survival of new neurons born in the spring. We found that, whereas BDNF infusion into HVC during days 14-20 after new neurons are born markedly extends their survival, the same effect does not occur if BDNF is infused into HVC 10 days earlier or 10 days later.
Materials and Methods
Subjects. We used adult male Waterslager canaries from our colony at the Rockefeller University Field Research Center. Their ages at the start of the experiments were between 13 and 18 mo. Canaries were housed singly under New York state photoperiod with food and water available ad libitum. The care and experimental manipulation of the animals was carried out in accordance with the guidelines of the National Institutes of Health and was reviewed and approved by the Rockefeller University's Institutional Animal Care and Use Committee. Our experiments started in the last week of May for survival of spring-born cells and in mid-October for survival of fall-born cells.
Surgery. Canaries were anesthetized by an i.m. injection of Nembutal (pentobarbital, 25 mg/kg body weight, Abbott) and placed in a stereotaxic apparatus. The skin above the skull was cut, and a small hole was made through the bone above the estimated position of the lateral edge of either the right or left HVC, revealing the brain surface. A 23-gauge guide cannula was then implanted so that it barely touched the dura overlying the brain and fixed to the skull with dental cement after which the birds were allowed to recover for a period of 2 weeks. The cannula was sealed with a wire that fit snugly into its bore. Infusion started on the day of pump implantation, when the birds were once again anesthetized, and a 30-gauge needle of the length required to reach HVC was inserted into the guide cannula and fixed in place with dental cement. The other end of the needle was connected to plastic tubing, which led over the back and under the wing into an Alzet microosmotic pump implanted in the peritoneal cavity (Fig. 1). The pumps were filled with 100 μl of a 30 μg/ml solution of BDNF dissolved in carrier or carrier alone (Amgen). This concentration of BDNF was within the range of previous studies (11, 14). Because the TrkB receptors that bind to BDNF are highly expressed in HVC and sparser in the surrounding tissue (11), we assume that much of the infused BDNF was bound within HVC. The pumps were disconnected after 6 days of infusion. These protocols are shown graphically in Fig. 1.
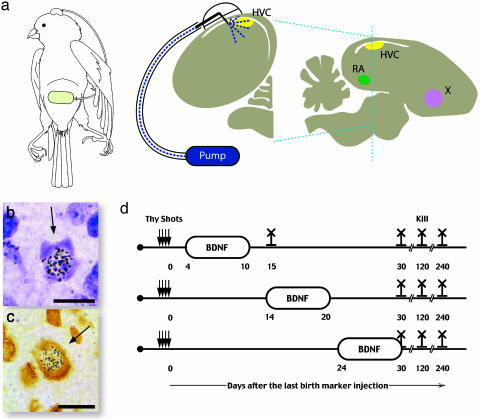
Fig. 1.
BDNF infusion into HVC. (a) Schematic diagram of the infusion system that delivered BDNF into HVC (yellow) as seen in a transverse section of canary brain; the plane of section is shown as a dotted blue line in the saggital representation of the brain, that also shows the position of two other song nuclei, the nucleus Robustus of the Archistriatum (RA) (green) and area X (pink) that receive projections from HVC. The bubble overlying the infusion needle represents the dental cement bonding the needle to the cannula and the cannula to the skull. The tubing used for drug infusion connects the needle to the Alzet pump. The cartoon shows the placement of the pump in the peritoneal cavity and its connection to the skull. Cresyl-stained (b) and Hu-stained (c) sections showing new neurons labeled with [3H]thymidine. (d) Protocol for infusion of BDNF into HVC during days 4-10, 14-20, or 24-30 and for the different survival times after the last [3H]thymidine injection.
Treatment. The i.m. injections of [3H]thymidine were given at 12-h intervals for 4 days. Birds in the infusion experiments received these injections 15 days after guide cannula placement. These birds then received BDNF or carrier infusion into HVC 4-10, 14-20, or 24-30 days after the last [3H]thymidine injection. Fall and spring birds that received no infusion as well as birds from all infusion groups were allowed to survive for 1, 4, or 8 mo after the last [3H]thymidine injection; we also had a 15-day survival for uninfused spring and fall birds and for the birds that received BDNF infusion during days 4-10 after the last [3H]thymidine injection. All birds were killed by injection of a lethal dose of Nembutal followed by saline perfusion (11), and their brains were prepared for histological analysis.
Analysis and Quantification. Most of the results presented here were obtained during year 1 of this study. Brains were embedded in polyethylene glycol and sectioned sagittally at 6-μm intervals. A set of six sections, obtained from the middle of HVC at a spacing of 60 μm, was mounted on slides and stained with Cresyl violet, which, other studies have shown, can be used to reliably identify neuronal nuclei (3, 6, 15). Sections were then treated for autoradiography with Kodak NTB2 nuclear track emulsion, incubated for 4 weeks in the dark, and developed with D19 (Kodak) as described (7, 11). During year 2, we replicated part of the work done on year 1, using the same histological methods but this time using, for each HVC, two sets of adjacent sections: one stained, as before, with Cresyl violet, and the other one stained with the neuron-specific marker Hu (18). Examples of [3H]thymidine-labeled cells stained by the two methods are shown in Fig. 1. The total volume of HVC was estimated by using a third set of sections, also obtained at 60-μm intervals and stained with Cresyl violet; this set subtended the entire HVC from its most medial to its most lateral edges; the area of HVC in each of these sections was obtained by tracing its perimeter; areas derived in this manner were then multiplied by the sampling interval to estimate the volume of HVC. Volume estimates were then used to calculate the total number of HVC neurons and [3H]thymidine-labeled neurons. The diameter of subsets of 50 randomly chosen HVC neurons was measured in each bird, as well as the diameter of 25 HVC neurons labeled with [3H]thymidine. We counted as new neurons cells that, in addition to their neuronal staining, had a number of exposed silver grains over their nucleus that was at least ×20 above background. The very stringent criterion for positive identification of a labeled neuron we used tended to ignore nuclear diameters of <5 μm. For this reason, and taking into account the range of diameters observed and thickness of sections, we did not correct new neuron counts for cell splitting (16). Had these corrections been used, they would have been small and would not have changed the results reported here.
Our reason for using both Cresyl staining and Hu immunoreactivity on year 2 of the study was to have two independent ways of establishing neuronal identity. We counted the labeled and unlabeled neurons per unit area by using computer-assisted microscopy (15) and used these counts to estimate total number of neurons per HVC. We report results as percentage of total HVC neurons that are [3H]thymidine-labeled ± SD. The person doing the neuron counts did not know the treatment group to which each bird belonged.
Results
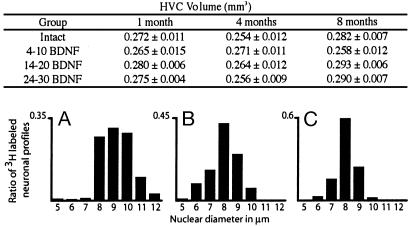
BDNF Did Not Affect the Volume of HVC or Nuclear Diameter of New Neurons. The volume of HVC and the number of HVC neurons/mm3 did not differ systematically between treatment groups and between the various survival groups. As a result, our estimate of number of HVC neurons, corrected for cell splitting, was rather similar for all these groups, with a mean of 47,743 neurons. There were, however, differences in the nuclear diameter of the 3H-labeled neurons. As in previous studies, the nuclear diameters of 3H-labeled neurons were larger at 1 mo than at 4 mo (9.21 vs. 7.97 μm, Fig. 2). For reasons explained in Materials and Methods, we did not correct for these differences when calculating the percentage of new neurons surviving 1, 4, and 8 mo after [3H]thymidine injection.
Fig. 2.
The table shows the mean volume (±SD) of HVC in each of the experimental/survival groups. The underlying histograms show the distribution of 3H-labeled nuclear diameters of HVC neurons at 1-mo (A), 4-mo (B), and 8-mo (C) survival times after label injections.
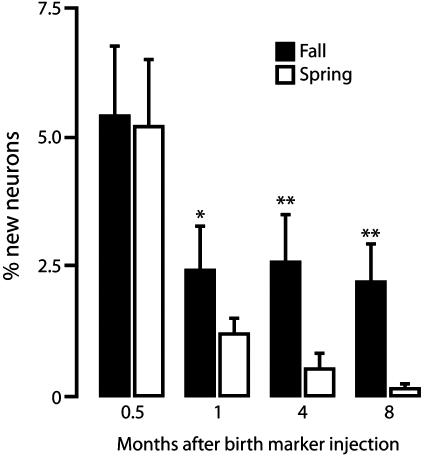
Effect of Season on New Neuron Survival Occurs After Day 15. There were no significant seasonal differences in the percentage of new HVC neurons present in HVC 15 days after their birth (5.44 ± 1.36 in fall vs. 5.26 ± 1.28 in spring). However, the percentage labeled 1, 4, or 8 mo after [3H]thymidine injection was significantly higher in the fall birds than in the spring birds (Fig. 3). This observation is in line with results reported in an earlier publication. In that publication, too, one-half of spring-born HVC neurons present 1 mo after their birth had disappeared 3 mo later, but there was no such loss in fall-born HVC neurons (6).
Fig. 3.
Survival of new HVC neurons born in fall or spring; birds in these two groups received [3H]thymidine injections in October (black bars) or April (white bars), respectively, when ≈12- or 18-mo-old and were killed 0.5, 1, 4, or 8 mo after the last injection. Each group includes four to six birds, and the bars represent SD; *, P < 0.05; **, P < 0.01 for comparisons within a same survival time.
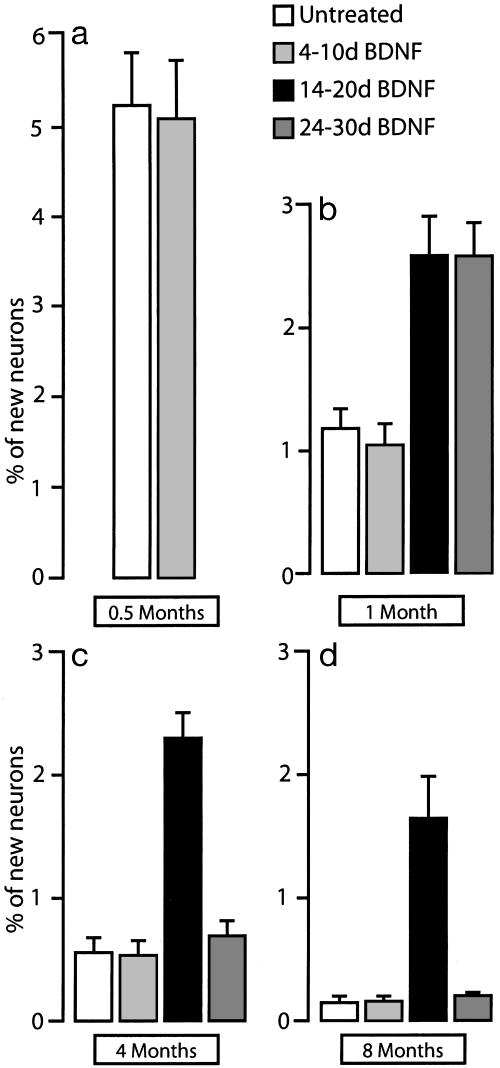
Time of BDNF Infusion Reveals a Critical Window for the Promotion of Survival. We then tested whether new neurons that would otherwise die could be rescued by 6 days of BDNF infusion into HVC starting 4, 14, or 24 days after the end of [3H]thymidine treatment. Birds that received BDNF at these three different times were killed 1, 4, or 8 mo after [3H]thymidine treatment. Additionally, we included a group of birds that received infusion of carrier starting 14 days after [3H]thymidine treatment. At the 1-mo survival point, we found a positive effect of BDNF treatment in birds that had received it on days 14-20 and 24-30; the mean percentage of 3H-labeled HVC neurons in these birds was 2.59 ± 0.71 and 2.58 ± 0.60, respectively; treatment with BDNF on days 4-10 (1.043 ± 0.358) or with carrier on days 14-20 (1.183 ± 0.370) did not affect the percentage of new HVC neurons compared to untreated controls (Figs. 4 and 5).
Fig. 4.
Effects of BDNF infusion on the longevity of new HVC neurons. (a) Percentage of new HVC neurons that survive up to 15 days after the last [3H]thymidine injection. There was no difference between untreated birds and birds that received BDNF during days 4-10. (b-d) Percentage of new HVC neurons at 1-, 4-, and 8-mo survivals, with BDNF infused on days 4-10, 14-20, or 24-30 after last [3H]thymidine injection. Notice that the life expectancy of the new neurons that received BDNF during days 14-20 is considerably higher than that of any of the other groups (error bars represent the SD).
Fig. 5.
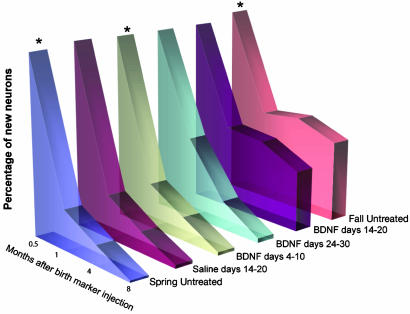
Summary of results. Comparison of the timeline of survival of new HVC neurons born in untreated spring and fall birds and in birds that were treated at various times after [3H]thymidine injection with BDNF or saline. There is a striking resemblance in the survival profile of the spring neurons treated with BDNF 14-20 days after their birth and the new neurons of the untreated fall birds. The percentage of new neurons at 15-day survivals was established only for the three groups (asterisk) and assumed to be the same for the three others.
At the 4-mo survival point, the percentage of 3H-labeled HVC neurons in the birds that received BDNF on days 14-20 was significantly higher than that seen in birds that received BDNF during days 4-10 or 24-30 (2.288 ± 0.513 vs. 0.535 ± 0.273, P < 0.01 and vs. 0.696 ± 0.281, P < 0.01, respectively). This difference was even more marked at the end of the 8-mo survival time (14-20: 1.652 ± 0.666; 4-10: 0.165 ± 0.090; and 24-30: 0.197 ± 0.036, Fig. 4).
BDNF Infusion at the 14- to 20-Day Window Gives Spring-Born Neurons Fall-Like Longevity. The effects of BDNF infusion in the 14- to 20-day group were compared to two groups of birds that received [3H]thymidine injections either in the spring or in the fall but were otherwise untreated. At the 1-mo survival point, there were significantly more 3H-labeled neurons in HVC in the above treatment group than in the spring group that received no BDNF (2.59 ± 0.711 vs. 1.13 ± 0.278, respectively); however, there were no significant differences with the fall group that received no BDNF (P > 0.05). For the 4- and 8-mo survival points, the percentage of 3H-labeled new neurons in the birds that received BDNF during days 14-20 remained significantly higher than in the spring birds that received no BDNF (Fig. 5).
Furthermore, we found no significant difference between the percentage of new neurons in the HVC contralateral to the injection site of the 14- to 20-day BDNF infusion group and the untreated spring controls at 1-mo survival; the percentage of new neurons in the HVC contralateral to the injection was 1.205 ± 0.334. This result was similar to the percentage seen in birds treated with carrier on days 14-20 (1.183 ± 0.370). There were no significant differences among these three conditions at longer survivals (4 and 8 mo, data not shown).
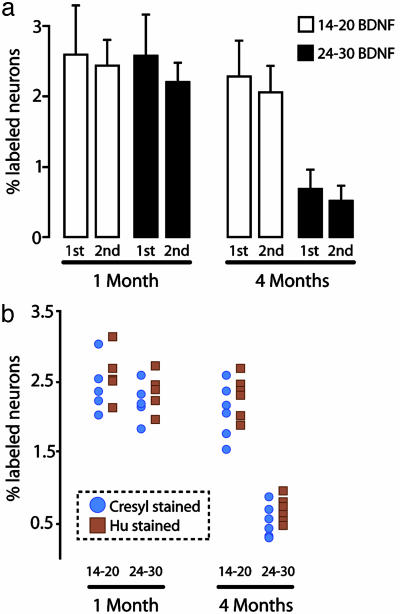
Cresyl Violet and Hu Immunostaining Provide Comparable Neuron Counts in Replication of Main Result. We repeated the BDNF infusion experiment on year 2 and included the use of the neuronal-specific Hu protein as an alternative to Cresyl staining for the identification of total neurons and new neurons in HVC. As in year 1, BDNF infusion 14-20 days after [3H]thymidine injection resulted in a higher percentage of 3H-labeled neurons at the 4-mo survival point than infusion at days 24-30 (Fig. 6a). These were the only two groups and survival times replicated on year 2, and both Hu and Cresyl staining gave similar results (Fig. 6b).
Fig. 6.
(a) Replicate in a different year of the 14-20 and 24-30 BDNF infusion groups at 1 mo and 4 mo after [3H]thymidine administration with Cresyl-violet staining. (b) Comparison of results obtained for new neuron percentage estimates with Cresyl-stained and Hu-stained material.
Discussion
Our results show that spring vs. fall differences in the survival of new HVC neurons in adult, untreated male canaries, become manifest some time between 16 and 30 days after the cells are born; by 30 days, the survival of the fall-born cells was twice as high as that of the spring-born ones. Thereafter, the number of the fall-born cells remained little changed up to the last sampling time, 7 mo later (5), but the number of the spring-born ones continued to drop until very few were left at the last survival time we checked (8 mo).
We do not know whether the levels of BDNF protein or of its TrkB receptor in HVC differed between our May and October birds. Unpublished observations suggest that the proportion of HVC neurons that expresses BDNF is higher in the spring than in the fall (17). We did not quantify amount of singing in any of our birds, nor did we measure their blood testosterone levels. However, blood testosterone levels tend to be comparable in May and in October (12), and male canaries sing a lot at these times. Both testosterone and singing are known to up-regulate BDNF expression in HVC (8). Thus, it is not clear why BDNF supplementation in the spring would result in a survival pattern typical of fall-born neurons. The one known difference between the spring and fall HVC is that there is less neuronal death in the months leading up to May, and so a seasonal difference in the number of vacancies could explain why the survival of neurons born in May is relatively poor compared to that of neurons born in October (18). Because previous neuronal death is known to affect new neuron survival (19), the BDNF treatment in the spring may have overcome a dearth of vacancies. We do not know whether BDNF infusion resulted, at that time or later, in a change in behavior. It is worth noting, although, that these infusions were unilateral and that the effect on neuron survival was restricted to the side of infusion and, presumably, restricted to just the few days of treatment. Survival of new neurons on the side contralateral to the infused HVC was no different from that seen in untreated spring birds.
Our results suggest that there is a time window of a few days (but the duration could be shorter) when exposure of a spring-born HVC neuron to exogenous BDNF markedly prolongs its survival and makes its life expectancy very much like that of a fall-born neuron of the same kind. The robustness of this effect is surprising, because the 8 mo subsequent to spring BDNF infusion included a period from midsummer to early fall, when two factors that affect the early survival of the HVC's replaceable neurons, blood testosterone levels and amount of singing, are known to drop markedly (12). Interestingly, new neurons born in the fall also face a similar drop in testosterone levels in January and February (12) and also manage to survive for 8 mo. Apparently, exposure to BDNF during days 14-20 after a neuron is born modifies it in ways that promote its survival even under subsequent low testosterone levels. By contrast, the excess of new HVC neurons that were still present at the 1-mo survival time after BDNF exposure during days 24-30 may well have disappeared soon after the exogenous BDNF was no longer available. By 4 mo, the survival of cells in this latter cohort was not significantly different from that of cells exposed to saline during days 14-20.
Our results do not show how the effect of BDNF on long-term survival came about. It is known that BDNF strengthens connections (20-22), and this may be the primary phenomenon behind the survival effect we saw. New neurons recruited into HVC can be first back-filled from one of their targets, nucleus Robustus of the Archistriatum, ≈15 days after they are born. If the new HVC neurons also started to form synapses at about that time, then BDNF might exert its effect on the survival of these cells by stabilizing these synapses. BDNF could also promote survival by hastening the demise (23) of older neurons, thus creating vacancies. As mentioned above, there is a positive relation between the death of replaceable neurons in adult HVC and the number of new HVC neurons found in place thereafter (19). A link between cell death and the sensitive period for the effect of BDNF on survival would only occur if new cells aged 14-20 days benefited from the vacancies created. These two potential mechanisms (and others) need not be mutually exclusive. However, if the primary effect of BDNF were on cell death, then we might expect that it would more readily benefit younger (e.g., 4- to 10-day-old), rather than older (e.g., 14- to 20-day-old) new neurons. This would be the case, for example, if younger cells, some still in their migratory phase, could more readily appropriate vacancies than older, already sedentary cells. By days 14-20, most of the new HVC neurons are thought to have ended their migration (8).
The massive loss of new HVC neurons that we and others (8) observed during the third week of these cells' life has parallels in mammals. Similar timing for the winnowing of new neurons has been reported in the hippocampus and olfactory bulb of adult mice (24-26) and in the hippocampus of adult tree shrews (27). In all these instances, the big wave of new cell mortality occurs after the new cells have acquired a postmigratory phenotype and are in the process of establishing connections. It would be interesting to know whether, in these other systems, a neurotrophin such as BDNF could reset the time clock for survival.
The mechanism whereby BDNF promotes new neuron survival remains unknown, but a metaphor comes to mind. Might BDNF during a narrowly defined sensitive period wind up a molecular clock that then determines when that neuron will die? Could evidence for this clock be found in a changed profile of gene expression? It seems unlikely that neuronal replacement is a chance affair. We may have taken an initial step toward identifying a part of the mechanism that determines when vacancies, and therefore replacement, occur.
Acknowledgments
We thank the staff of the Rockefeller Field Research Center, in particular Daun Jackson, Sharon Sepe, and Helen Ecklund, for their assistance in handling our avian subjects, and Leopoldo Petreanu for valuable discussion in the preparation of this manuscript. Our work was supported by U.S. Public Health Service Grants MH18343 and MH63132, the Mary Flagler Cary Charitable Trust, the Herbert and Nell Singer Foundation, and the Phipps Family Foundation.
Abbreviations: BDNF, brain-derived neurotrophic factor; HVC, high vocal center.
References
- 1.Goldman, S. A. & Nottebohm, F. (1983) Proc. Natl. Acad. Sci. USA 80, 2390-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla, A., Garcia-Verdugo, J. M., Mateo, A. S. & Merchant-Larios, H. (1998) J. Neurosci. 18, 1020-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirn, J. R., Fishman, Y., Sasportas, K., Alvarez-Buylla, A. & Nottebohm, F. (1999) J. Comp. Neurol. 411, 487-494. [PubMed] [Google Scholar]
- 4.Paton, J. A. & Nottebohm, F. N. (1984) Science 225, 1046-1048. [DOI] [PubMed] [Google Scholar]
- 5.Kirn, J. R., Alvarez-Buylla, A. & Nottebohm, F. (1991) J. Neurosci. 11, 1756-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nottebohm, F., O'Loughlin, B., Gould, K., Yohay, K. & Alvarez-Buylla, A. (1994) Proc. Natl. Acad. Sci. USA 91, 7849-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li, X. C., Jarvis, E. D., Alvarez-Borda, B., Lim, D. A. & Nottebohm, F. (2000) Proc. Natl. Acad. Sci. USA 97, 8584-8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez-Borda, B. & Nottebohm, F. (2002) J. Neurosci. 22, 8684-8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang, N., Aviram, R. & Kirn, J. R. (1999) J. Neurosci. 19, 10554-10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasika, S., Nottebohm, F. & Alvarez-Buylla, A. (1994) Proc. Natl. Acad. Sci. USA 91, 7854-7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasika, S., Alvarez-Buylla, A. & Nottebohm, F. (1999) Neuron 22, 53-62. [DOI] [PubMed] [Google Scholar]
- 12.Nottebohm, F., Nottebohm, M. E., Crane, L. A. & Wingfield, J. C. (1987) Behav. Neural. Biol. 47, 197-211. [DOI] [PubMed] [Google Scholar]
- 13.Louissaint, A., Jr., Rao, S., Leventhal, C. & Goldman, S. A. (2002) Neuron 34, 945-960. [DOI] [PubMed] [Google Scholar]
- 14.Pencea, V., Bingaman, K. D., Wiegand, S. J. & Luskin, M. B. (2001) J. Neurosci. 21, 6706-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Buylla, A. & Vicario, D. S. (1988) J. Neurosci. Methods 25, 165-173. [DOI] [PubMed] [Google Scholar]
- 16.Clark, S. J., Cynx, J., Alvarez-Buylla, A., O'Loughlin, B. & Nottebohm, F. (1990) J. Comp. Neurol. 301, 114-122. [DOI] [PubMed] [Google Scholar]
- 17.Rasika, S. (1998) Ph.D. dissertation (The Rockefeller University, New York).
- 18.Kirn, J., O'Loughlin, B., Kasparian, S. & Nottebohm, F. (1994) Proc. Natl. Acad. Sci. USA 91, 7844-7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharff, C., Kirn, J. R., Grossman, M., Macklis, J. D. & Nottebohm, F. (2000) Neuron 25, 481-492. [DOI] [PubMed] [Google Scholar]
- 20.Thoenen, H. & Edgar, D. (1985) Science 229, 238-242. [DOI] [PubMed] [Google Scholar]
- 21.Thoenen, H. (1995) Science 270, 593-598. [DOI] [PubMed] [Google Scholar]
- 22.Altar, C. A., Cai, N., Bliven, T., Juhasz, M., Conner, J. M., Acheson, A. L., Lindsay, R. M. & Wiegand, S. J. (1997) Nature 389, 856-860. [DOI] [PubMed] [Google Scholar]
- 23.Hu, P. & Kalb, R. G. (2003) J. Neurochem. 84, 1421-1430. [DOI] [PubMed] [Google Scholar]
- 24.Seri, B., Garcia-Verdugo, J. M., McEwen, B. S. & Alvarez-Buylla, A. (2001) J. Neurosci. 21, 7153-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanapat, P., Hastings, N. B., Rydel, T. A., Galea, L. A. & Gould, E. (2001) J. Comp. Neurol. 437, 496-504. [DOI] [PubMed] [Google Scholar]
- 26.Petreanu, L. & Alvarez-Buylla, A. (2002) J. Neurosci. 22, 6106-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould, E., McEwen, B. S., Tanapat, P., Galea, L. A. & Fuchs, E. (1997) J. Neurosci. 17, 2492-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]