Abstract
Objectives
When Lactobacillus spp. dominate the vaginal microbiota of women of reproductive age they acidify the vagina to pH <4.0 by producing ∼1% lactic acid in a nearly racemic mixture of d- and l-isomers. We determined the HIV virucidal activity of racemic lactic acid, and its d- and l-isomers, compared with acetic acid and acidity alone (by the addition of HCl).
Methods
HIV-1 and HIV-2 were transiently treated with acids in the absence or presence of human genital secretions at 37°C for different time intervals, then immediately neutralized and residual infectivity determined in the TZM-bl reporter cell line.
Results
l-lactic acid at 0.3% (w/w) was 17-fold more potent than d-lactic acid in inactivating HIVBa-L. Complete inactivation of different HIV-1 subtypes and HIV-2 was achieved with ≥0.4% (w/w) l-lactic acid. At a typical vaginal pH of 3.8, l-lactic acid at 1% (w/w) more potently and rapidly inactivated HIVBa-L and HIV-1 transmitter/founder strains compared with 1% (w/w) acetic acid and with acidity alone, all adjusted to pH 3.8. A final concentration of 1% (w/w) l-lactic acid maximally inactivated HIVBa-L in the presence of cervicovaginal secretions and seminal plasma. The anti-HIV activity of l-lactic acid was pH dependent, being abrogated at neutral pH, indicating that its virucidal activity is mediated by protonated lactic acid and not the lactate anion.
Conclusions
l-lactic acid at physiological concentrations demonstrates potent HIV virucidal activity distinct from acidity alone and greater than acetic acid, suggesting a protective role in the sexual transmission of HIV.
Keywords: vaginal lactobacilli, carboxylic acids, virucidal, female reproductive tract
Introduction
The majority of HIV infections worldwide are transmitted sexually from infected females to uninfected males, and vice versa. The probability of HIV acquisition via the female reproductive tract is lower compared with rectal or parenteral transmission,1 probably due to antimicrobial defence mechanisms at the endocervix and the vaginal/ectocervix lumenal surface (vagina), including physical barriers such as the squamous epithelium and mucous secretions, innate defence peptides produced by epithelial and immune cells, and agents produced by commensal bacteria, including organic acids and bacteriocins.2–6 In addition, factors present in the vagina that decrease the amount of active HIV virions shed by infected women may potentially reduce HIV transmission to men.7–10
Studies of the vaginal microbiome of asymptomatic women of reproductive age reveal five distinct bacterial communities, the majority of which are dominated by lactobacilli.11,12 However, about one in three women of reproductive age in the USA13 and about one in two in sub-Saharan Africa14,15 do not have a lactobacillus-dominated vaginal microbiota that acidifies the vagina with lactic acid. Thus, many women worldwide with bacterial vaginosis (BV) and an ‘intermediate’ vaginal microbiota, as defined by Nugent score,16 have microbial communities that increase vaginal pH to >4 and are associated with a significantly increased risk of acquiring sexually transmitted infections (STIs),13,17 including HIV,18,19 or of transmitting HIV to their male partners.20 Altogether, the evidence suggests that lactobacilli appear to reduce susceptibility to infections, while BV appears to increase it.
Many pathogens are well recognized as being acid susceptible, but only recently has lactic acid, at physiological concentrations, been shown to be highly potent at killing BV-associated bacteria – all 17 of the most abundant BV-associated species are potently inactivated at pH 4.5 with 55–111 mM racemic dl-lactic acid.3,21–23 As typically measured aerobically in the clinic, lactobacilli acidify the vagina to pH ∼4.2,24 but the vagina is hypoxic and is partially acidified by 5% systemic CO2 compared with 0% CO2 in air. The most reliable observation of vaginal pH in vivo, based on radio telemetry from intravaginal capsules, revealed that lactobacilli lower vaginal pH to ∼3.7,25 a significantly more microbicidal pH. Lactobacillus-dominated microbiota are associated with high levels of lactic acid compared with other metabolites26 and acidify the vagina to pH <4.0 by producing, and maintaining, a vaginal concentration of ∼1% (w/v) lactic acid (D. O'Hanlon and R. Cone, Johns Hopkins University, and T. Moench, ReProtect Inc., unpublished data) with a nearly racemic mixture of d- and l-isomers.27 Human metabolism produces only the l-isomer, and <15% of vaginal lactic acid appears to be produced by anaerobic metabolism of the vaginal epithelium.23,27,28
Lactic acid is a lipid-soluble membrane-permeant carboxylic acid (pKa = 3.9) and exists predominantly as the neutral protonated form under acidic conditions and the charged unprotonated lactate anion under neutral conditions.23 Glycogen is thought to be the primary energy source metabolized by lactobacilli in producing lactic acid, and during the reproductive years human vaginal epithelial cells provide high concentrations of glycogen to bacteria in the vaginal lumen.29,30 Intriguingly, women with lactobacillus-dominated vaginas are unique among mammals in having vaginas strongly acidified with lactic acid; other species, including non-human primates, have much less acidic vaginas and low levels of lactobacilli and lactic acid.31,32
The antimicrobial and HIV virucidal activity of lactobacilli has often been ascribed to the ability of some species to produce hydrogen peroxide (H2O2).33 However, more recent studies indicate that H2O2 is unlikely to act as an intravaginal protective factor: Lactobacilli make only trace amounts of H2O2 in cervicovaginal secretions (CVS) under the hypoxic conditions normally found in the vagina;34 the maximum concentrations of H2O2 found in vaginal fluids, even when potentiated with myeloperoxidase, failed to inactivate BV organisms, Neisseria gonorrhoeae, or HSV-2.34 Vaginal secretions diluted 100-fold completely blocked the microbicidal activity of H2O2, even at 50 000 times its vaginal concentration.23 Lactobacilli are themselves more susceptible to inactivation by H2O2 than BV organisms,23 and inhibition of N. gonorrhoeae by lactobacilli under anaerobic growth conditions is mainly due to acidification rather than H2O2.23,34,35
Several previous reports have described the acid sensitivity of HIV.36–40 These studies used HCl, acetic acid, phosphate/citrate buffers or lactic acid to acidify virus-containing media. However, a direct comparison of the HIV virucidal activity of lactic acid compared with other acids found in the vagina has not been performed. To investigate the potential role of lactic acid in modulating heterosexual transmission of HIV, we determined the relative virucidal activities of dl-, l- and d-lactic acid, and compared the activity of l-lactic against acetic acid and low pH without an organic acid (acidified with HCl). We also examined the anti-HIV activity of l-lactic acid against a panel of HIV strains, including different subtypes and transmitter/founder strains,41 evaluated the activity in the presence of genital secretions and determined whether protonated lactic acid or the lactate anion mediates HIV virucidal activity.
Materials and methods
Cells
The TZM-bl indicator cell line expressing the CD4, CXCR4 and CCR5 receptors and stably integrated with the Escherichia coli β-galactosidase and firefly luciferase genes under the control of the HIV promoter were obtained through the NIH AIDS Research and Reference Reagent Program. TZM-bl cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS; Sigma-Aldrich), 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM l-glutamine (DMEM-10).42 293T cells (obtained from Richard Axel, Columbia University) were cultured in DMEM-10 (Invitrogen). Phytohaemagglutinin-stimulated human peripheral blood mononuclear cells (PBMCs) from uninfected donors were prepared as previously described43 with the following modifications: PBMCs were isolated from blood bank packs supplied by the Australian Red Cross (South Melbourne) and were resuspended at 2 × 106 cells/mL in Roswell Park Memorial Institute medium (1640) supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 20 U/mL recombinant human interleukin 2 (IL-2 medium, Roche). PBMCs were stimulated in the presence of 10 μg/mL phytohaemagglutinin (Sigma-Aldrich) and incubated for 3 days at 37°C/5% CO2 in either silicone-coated Teflon pots (Savillex) or 75 cm2 tissue culture flasks (Falcon) prior to infection with HIV.
Virus
HIVBa-L, obtained from the NIH AIDS Research and Reference Reagent Program, is a CCR5 (R5)-using laboratory strain of HIV type 1 (HIV-1) propagated in human PBMCs and macrophages. HIV-1 clinical isolates MACS3-LN (subtype B, R5 strain), MACS1-spln (subtype B, dual tropic) and CB1-br [subtype B, CXCR4 (X4)-using strain] were isolated from HIV-1 infected individuals44 and provided by Dana Gabuzda (Dana-Farber Cancer Institute). HIV-1 strains 92RW016 (subtype A, R5 strain), 92BR025 (subtype C, R5 strain), CMU02 (subtype EA, X4 strain), 93BR020 (subtype F, dual tropic), HIV type 2 (HIV-2, CDC310319, X4 strain), and the molecular clones pRHPA.c/2635 and pCH058.c/2960 of transmitter/founder strains RHPA (subtype B, R5 strain isolated from a female subject acquired heterosexually) and CH058 (subtype B, R5 strain isolated from a male subject)41,45 were obtained from the NIH AIDS Research and Reference Reagent Program. Infectious RHPA and CH058 virus were generated from pRHPA.c/2635 and pCH058.c/2960, respectively, by calcium phosphate transfection of 239T cells, as described previously,46 followed by propagation in human PBMCs.43
Acids
A 30% (w/w) solution of dl-lactic acid was prepared from an 85% (w/w) stock (Sigma-Aldrich); a 30% (w/w) d-(−)-lactic acid solution was prepared from solid powder (Sigma-Aldrich); a 30% (w/w) sodium l-lactate solution was prepared from solid powder (Sigma-Aldrich); and 30% (w/w) l-(+)-lactic acid solution (Sigma-Aldrich) was used as purchased. A 30% (w/w) acetic acid solution was prepared by dilution of glacial acetic acid (17.4 M, 99.5% w/w; Merck); 1 M and 0.1 M HCl were prepared from a 12 M stock of HCl (Sigma-Aldrich); and 1 M and 0.1 M NaOH were prepared from solid NaOH (Sigma-Aldrich). Lactic acid stereoisomer stock concentrations were confirmed using the d-lactic acid/l-lactic acid ultraviolet method according to the manufacturer's instructions (Boehringer Mannheim/R-BioPharm), which is based on conversion of d-lactate or l-lactate by d-lactate dehydrogenase and l-lactate dehydrogenase, respectively, to pyruvate and NADH. The concentrations of lactic acid and acetic acid are presented in the text as w/w unless otherwise specified.
Collection and processing of CVS and seminal plasma
Human CVS and seminal plasma (SP) were purchased from Lee Biosolutions (St Louis, MO, USA). CVS was supplied undiluted and pooled from six asymptomatic donors aged between 20 and 40 years. The collection procedure involved arousal of female donors followed by collection of, typically, 2 mL of fluid using a spoon-like device, with samples immediately frozen at −80°C. Donors were negative for HIV-1, HIV-2, syphilis, hepatitis C and hepatitis B; however, Nugent scores were not determined. The pH of the CVS was ∼5.4. SP was prepared from semen obtained from four donors by masturbation after a minimum of 48 h abstinence from ejaculation. The pooled semen was liquefied by incubating the sample containers in a water bath at 37°C for 1 h. Liquefied semen was transferred to a 10 mL centrifuge tube and clarified by centrifugation at 1000 g for 10 min. The SP fraction was aspirated while trapping the pellet with a plunger at the bottom of the centrifuge tube and frozen at −80°C.
HIV-1 inactivation studies with no pH adjustment
l-, d- and dl-lactic acid from 30% stocks was applied to PBMC-grown HIV stocks in IL-2 medium to achieve the desired final acid concentration. The pH of the medium was recorded at the beginning and end of the incubation using the Aqua-pH pH-mV-Temperature meter v2.2 (TPS Pty Ltd, Brisbane, Australia) and MI-411-S Micro-combination pH electrode (Microelectrodes Inc., NH, USA) calibrated according to manufacturers' instructions. HIV was incubated for 30 min at 37°C with continuous gentle stirring using the Jenway 1103 hotplate stirrer (Bibby Scientific Ltd, Staffordshire, UK) to avoid exposure of the virus to localized pH changes. Following incubation the mixture was immediately neutralized by 10-fold dilution in DMEM-10 containing 25 mM HEPES (DMEM-HEPES) and the pH adjusted to neutral, if required, with NaOH. The mixture was then subjected to additional dilutions in DMEM-10 containing 10 μg/mL DEAE-dextran (GE Healthcare) and the viral infectivity determined in TZM-bl cells.
HIV-1 inactivation time-course study at fixed pH
Carboxylic acids were prepared at twice the final concentration in DMEM-10 followed by adjustment of the media to pH 3.8 using HCl or NaOH. DMEM-10 acidified to pH 3.8 with HCl was also prepared to represent conditions of acidity alone. To avoid exposure of virus to local high concentrations of pH, acid was added to the side of the tube, which was sealed and immediately inverted to mix. An equal volume of HIV stock in IL-2 medium was added to the acid preparations and the pH rapidly adjusted back to pH 3.8 with HCl, without allowing the pH to drop below 3.8. Virus was incubated for different lengths of time at 37°C with continuous gentle stirring. At each timepoint 200 μL of treated virus was removed and immediately neutralized by 10-fold dilution in DMEM-HEPES, followed by serial dilution in DMEM-HEPES containing 10 μg/mL DEAE-dextran and viral infectivity determined in TZM-bl cells.
HIV-1 inactivation studies with pH adjustment at a fixed timepoint
Sodium l-lactate and l-, d- and dl-lactic acid were prepared at twice the final required concentration in DMEM-10 followed by adjustment to the target pH (3.8, 4.0 or 7.0) using HCl or NaOH. DMEM-10 acidified to pH 4.0 with HCl was also prepared to represent conditions of acidity alone. An equal volume of HIV-1Ba-L was added to the lactic acid or sodium lactate preparations and the pH adjusted, where necessary, back to the target pH. Virus was incubated for 30 min at 37°C with continuous gentle stirring. Dilution with DMEM-HEPES was performed to stop the treatment and neutralize samples, followed by serial dilution in DMEM-HEPES containing 10 μg/mL DEAE-dextran and viral infectivity determined in TZM-bl cells.
HIV-1 inactivation in the presence of CVS
Undiluted CVS (50 μL) was added to tubes, followed by the addition of l-lactic acid to achieve the desired final concentration, and made up to 60 μL with DMEM-10. To this, 40 μL of HIV-1Ba-L was added and mixed thoroughly, and the tubes were then incubated for 30 min at 37°C. In parallel, HIV-1Ba-L, in the absence of CVS, was prepared and treated with l-lactic acid in exactly the same manner as for the HIV-1Ba-L/CVS samples. After 30 min treatment the pH was measured and samples were diluted 1 : 100 in DMEM-HEPES to neutralize the acid and dilute out cytotoxic effects due to CVS. Samples were serially diluted 10-fold in DMEM-HEPES containing 10 μg/mL DEAE-dextran and viral infectivity determined in TZM-bl cells.
HIV-1 inactivation in the presence of SP
For studies performed in the presence of 75% SP, 75 μL of undiluted SP was added to tubes, followed by the addition of l-lactic acid to achieve the desired final concentration. To this, HIV-1Ba-L was added to achieve a final volume of 100 μL and the tubes were mixed thoroughly. Samples tested in the presence of 12.5% and 25% SP were diluted in OptiMEM medium (Invitrogen) to achieve the required concentration prior to the addition of l-lactic acid and virus. Samples were mixed thoroughly and the pH measured. The samples were incubated for 30 min at 37°C, after which the pH was measured again and the samples diluted 1 : 10 in DMEM-HEPES to stop the acid treatment and to dilute out cytotoxic effects due to SP. Samples were neutralized with NaOH where required. Each sample was subjected to 10-fold serial dilutions in DMEM-HEPES containing 10 μg/mL DEAE-dextran and viral infectivity determined in TZM-bl cells.
Determination of infectious HIV in TZM-bl cells
The infectious titre of HIV stocks prepared by propagation in PBMCs and the presence of infectious virus following acid treatment was determined by counting blue foci-forming cells in the TZM-bl reporter cell line as described previously.47 The cytotoxicity of diluted samples containing neutralized lactic acid, CVS or SP was assessed visually by microscopy and by using the MTS reagent (CellTitre 96 Aqueous One, Promega) as previously described.48 The lower limit of HIV detection in the infectivity assays was 100 infectious units per mL except where otherwise specified. The dynamic range of the assay is a function of the virus stock titre, where high viral titres (i.e. 105–106 infectious units/mL) can reveal a greater-fold decrease in infectivity compared with virus with lower titres (i.e. 103–104 infectious units/mL). Thus, the ‘maximum’ decrease in HIV virucidal activity observed for a given virus stock will differ depending on the initial virus titre. The apparent variation at the maximum virucidal concentration, as suggested by the presence of error bars in some of the graphs, is a consequence of the data being derived from independent assays using virus stocks with different titres. Decreases in HIV infectivity in the presence of acid were normalized to the corresponding untreated virus incubated in the absence of acid.
Statistical analysis
The statistical significance between two acid treatments was determined using the Wilcoxon rank-sum test and differences between more than two treatments were determined using the Kruskal–Wallis test.
Results
l-lactic acid is a more potent inactivator of HIVBa-L than d-lactic acid
We evaluated the relative capacity of racemic dl-, d- and l-lactic acid to inactivate HIVBa-L. All forms of lactic acid demonstrated maximum decreases in HIVBa-L infectivity at physiological concentrations [0.5%–1%; ∼56–111 mM] compared with the untreated control virus incubated in the absence of lactic acid (Figure 1a). No effect of the neutralized lactic acid was observed on TZM-bl cell viability (data not shown). l-lactic acid at 0.3% showed greater virucidal activity than d- and dl-lactic acid (Figure 1a). To exclude the possibility that the difference in virucidal activity of the lactic acid stereoisomers was due to small differences in the final pH of the medium, we performed experiments where dl-, l- and d-lactic acid were adjusted to pH 4.0 (the average pH observed for all three forms of lactic acid at 0.3%) prior to virus addition and the pH monitored continuously (and adjusted if necessary) during incubation at 37°C. In parallel, HIVBa-L was treated with media adjusted to pH 4.0 with HCl to evaluate the virucidal activity of acidity alone (HCl adjusted). l-lactic acid (n = 8) demonstrated HIVBa-L virucidal activity that was 17-fold more potent than d-lactic acid (P = 0.002, n = 6) while dl-lactic acid showed intermediate activity (Figure 1b). Furthermore, while l-lactic acid treatment resulted in a maximal 2.2 × 104-fold decrease in HIVBa-L infectivity compared with the untreated control, treatment with HCl resulted in only a 12-fold decrease in infectivity (P < 0.001, n = 8). These data demonstrate that l-lactic acid is a more potent inactivator of HIVBa-L than d-lactic acid. Since l-lactic acid is the most potent virucidal form of lactic acid at threshold concentrations, subsequent experiments were performed using l-lactic acid.
Figure 1.
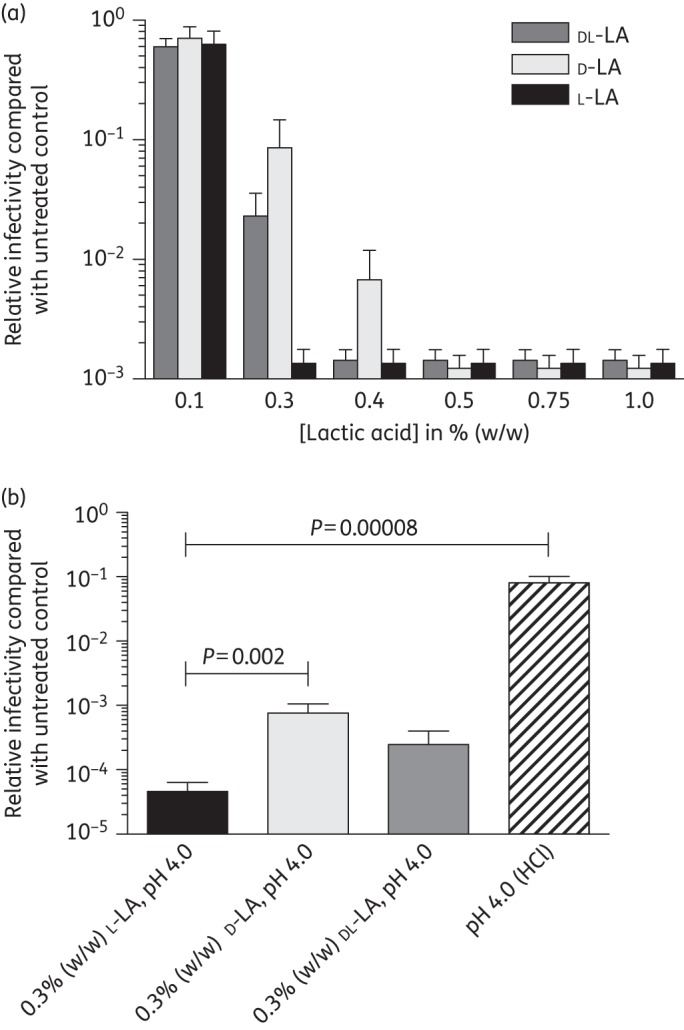
Inactivation of HIVBa-L by l-, d- and dl-lactic acid. (a) HIVBa-L was incubated with different forms of lactic acid at 37°C for 30 min without pH adjustment; data are the mean of four independent assays. (b) HIVBa-L was incubated in the presence of different forms of lactic acid adjusted to pH 4.0 prior to virus addition or with media adjusted to pH 4.0 with HCl and incubated at 37°C for 30 min followed by immediate neutralization. The lower limit of detection of virus in these assays was 30 infectious units/mL and the data are the mean of at least six independent assays. Viral infectivity was determined in the TZM-bl reporter cell line and expressed relative to virus incubated in the absence of acid. Error bars denote standard error of the mean. Statistically significant differences were determined using the Wilcoxon rank-sum test. LA, lactic acid.
l-lactic acid has broad-spectrum HIV-1 and HIV-2 virucidal activity
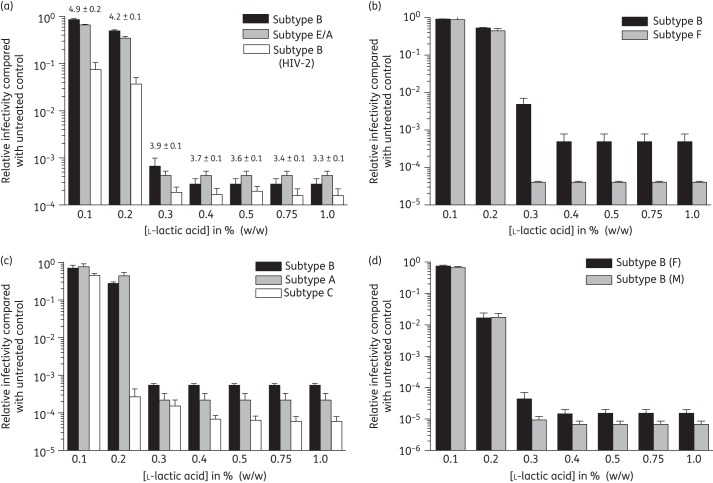
The previous experiments demonstrate that l-lactic acid inactivates the laboratory strain HIVBa-L. To determine whether l-lactic acid has potent broad-spectrum HIV virucidal activity we determined its activity against a panel of X4 (Figure 2a), dual tropic (Figure 2b) and R5 strains (Figure 2c and d). The panel includes several different HIV-1 subtypes as well as clinical isolates and transmitter/founder strains (Figure 2d), isolated immediately following HIV-1 transmission, and an X4 HIV-2 strain (Figure 2a). Maximum HIV virucidal activity was observed at concentrations of ≥0.4% l-lactic acid for all strains tested, with half of the isolates also being completely inactivated in the presence of 0.3% l-lactic acid (Figure 2a–c). Measurement of the final pH of virus-containing media treated with l-lactic acid indicated that virucidal activity under the experimental conditions of these assays was observed at pH <4.2 (Figure 2a). These data demonstrate that l-lactic acid at physiological concentrations has broad-spectrum HIV virucidal activity observed at acidic pH.
Figure 2.
HIV-1 and HIV-2 virucidal activity of l-lactic acid. HIV was incubated in the presence of l-lactic acid at 37°C for 30 min without pH adjustment, neutralized and viral infectivity determined in TZM-bl cells. (a) Inactivation of X4 HIV-1 strains CB1-br (subtype B) and CMU02 (subtype E/A), and the X4 HIV-2 strain CDC310319; (b) dual tropic HIV-1 strains MACS1-spln (subtype B) and 93BR020 (subtype F); (c) R5 HIV-1 strains MACS3-LN (subtype B), 92RW016 (subtype A) and 92BR025 (subtype C); and (d) subtype B transmitter/founder strains RHPA (F, isolated from a female subject) and CH058 (M, isolated from a male subject). The pH of the media following the addition of l-lactic acid and incubation is expressed as the mean ± standard error (a). Data were obtained from three independent assays and error bars denote the standard error of the mean.
HIV-1 inactivation by l-lactic acid at pH 3.8 is more rapid and potent than by acetic acid and acidity alone
We performed a time-course experiment to determine how quickly 1% l-lactic acid at pH 3.8 inactivates HIVBa-L (Figure 3) and the transmitter/founder strains RHPA and CH058 (Figure S1; available as Supplementary data at JAC Online). In parallel, we tested 1% acetic acid (pH 3.8) and acidity alone (pH 3.8, HCl adjusted). The pH of the medium was adjusted prior to virus addition and maintained at pH 3.8 throughout the experiment. A 130-fold decrease in HIVBa-L infectivity was observed following 1 min incubation with 1% l-lactic acid, with infectivity continuing to decrease over time resulting in a dramatic 105-fold drop in infectivity after 20 min compared with the untreated control. This decrease in HIVBa-L infectivity was ∼100-fold greater than found with 1% l-lactic acid treatment of HIVBa-L for 30 min (Figure 1a) since the titre of the virus stock used in the time-course study was ∼100-fold greater compared with the stock used in the fixed timepoint study, leading to a larger assay dynamic range in the time-course assay. After 1 min incubation only 4-fold and 1.7-fold decreases in HIVBa-L infectivity were observed in the presence of acetic acid and HCl (pH 3.8), respectively (Figure 3). Furthermore, after 20 min incubation a 3.6 × 104-fold decrease in HIVBa-L infectivity was observed for acetic acid while only a 29-fold reduction was observed with HCl (pH 3.8; Figure 3). The virucidal activity of the acid treatments was significantly different at 1 min (P = 0.015, n = 4), 2 min (P = 0.015), 5 min (P = 0.017), 10 min (P = 0.007) and 20 min (P = 0.012). Compared with HIVBa-L, similar acid inactivation patterns were observed with the transmitter/founder strains RHPA and CH058 (Figure S1). These data demonstrate that l-lactic acid is more potent and rapid in the inactivation of HIV-1 than acetic acid and acidity alone.
Figure 3.
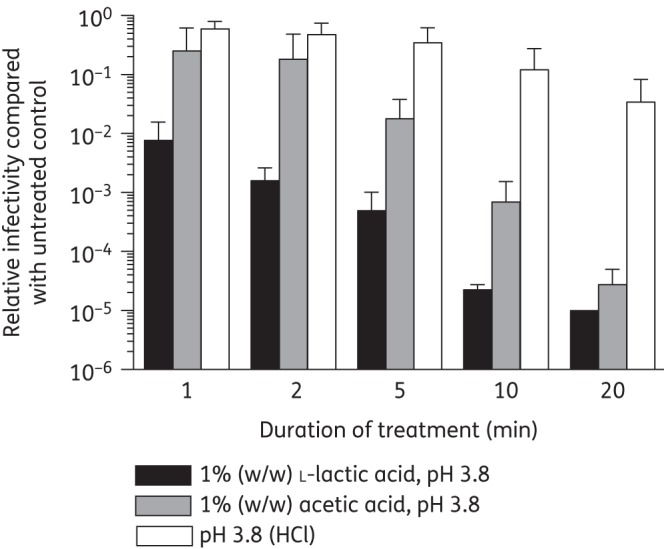
Acid inactivation time-course study of HIVBa-L at pH 3.8. Media acidified with l-lactic acid, acetic acid or HCl were adjusted to pH 3.8 prior to addition to virus; the pH was monitored and adjusted if required during incubation at 37°C. At indicated times an aliquot was removed, neutralized and viral infectivity determined in TZM-bl cells and expressed relative to virus incubated in the absence of acid. Data were obtained from four independent assays and error bars denote the standard error of the mean.
l-lactic acid does not enhance infectivity of HIV-1 strains at mildly acidic pH
A previous study reported that in the presence of a mildly acidic pH (4.5), the infectivity of non-subtype B HIV-1 isolates is enhanced.40 To investigate this possibility we evaluated the capacity of 0.3% l-lactic acid adjusted to pH 4.5 with HCl to inactivate HIV-1 subtype C (92BR025), subtype A (92RW016), subtype F (93BR020) and subtype B (RHPA). Experiments were performed as above, where the pH of the medium was monitored throughout the incubation. The infectivity of all strains tested was decreased relative to the corresponding untreated control, indicating no evidence of enhancement of HIV-1 infectivity under the highly controlled conditions of our assay (Figure S2; available as Supplementary data at JAC Online). These data demonstrate that lactic acid at mildly acidic pH does not enhance infectivity of the subtype B and non-subtype B HIV-1 strains tested.
l-lactic acid inactivates HIV-1 in the presence of genital secretions
To determine whether l-lactic acid inactivates HIV-1 in the presence of human CVS we compared the virucidal activity of l-lactic acid in the presence and absence of 50% CVS. The l-lactic acid HIVBa-L inactivation profiles in the absence and presence of CVS were similar (Figure 4a). Maximum HIVBa-L inactivation was observed in the presence of ≥0.5% l-lactic acid. In the presence of 0.3% l-lactic acid a 240-fold and 342-fold decrease in titre was observed compared with the control in the absence and presence of 50% CVS, which was not significantly different (P = 0.06, n = 3). These data demonstrate that l-lactic acid's HIV-1 virucidal activity is not inhibited in the presence of CVS.
Figure 4.
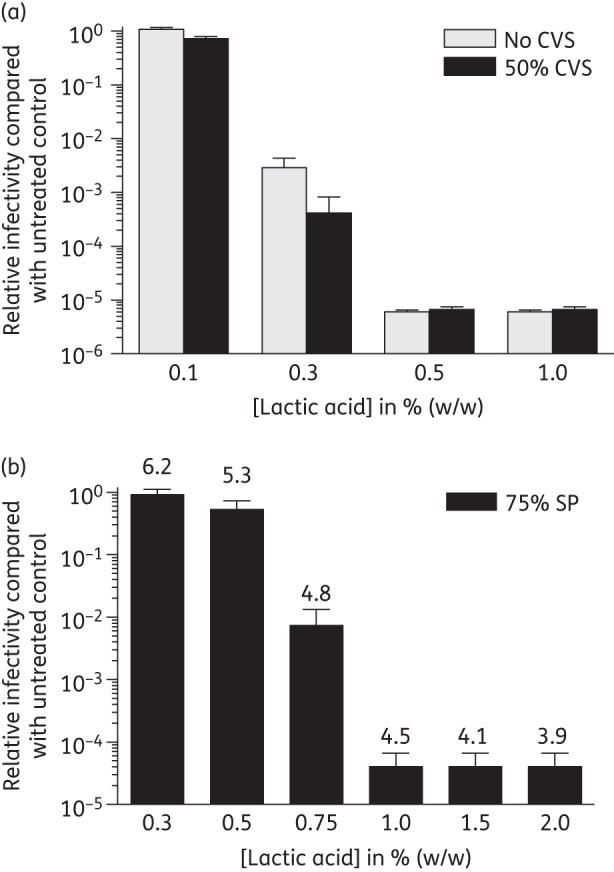
l-lactic acid inactivation of HIV-1 in the presence of genital secretions. HIVBa-L was incubated in the presence of l-lactic acid at 37°C for 30 min, without pH adjustment, in the absence and presence of 50% CVS (a) or 75% SP (b), neutralized and viral infectivity determined in TZM-bl cells relative to the appropriate control incubated in the absence of acid. The final pH of the media following mixing of SP, virus and lactic acid is shown above the bars (b). The data were obtained from three independent assays and error bars denote the standard error of the mean.
Semen is alkaline and neutralizes the vaginal lumen within seconds, providing a window of opportunity for acid-susceptible pathogens such as HIV to establish infection. To determine whether l-lactic acid inactivates HIV-1 in the context of semen we performed experiments in the presence of 75% SP as a surrogate for semen to simulate dilution of an estimated 1 mL of CVS in the vagina with an average 3 mL ejaculate.49 Under these conditions we found that a final concentration of 1% l-lactic acid was required to achieve a 2.4 × 104-fold decrease in HIVBa-L infectivity compared with the untreated control, while 0.75% l-lactic acid mediated a 135-fold reduction in HIVBa-L infectivity (Figure 4b). In addition, the virucidal activity of l-lactic acid was observed at pH ≤4.8 (Figure 4b). The pH in SP (Figure 4b) was higher for a given lactic acid concentration compared with treatment in the absence of SP (Figure 2a). Experiments performed in the presence of 12.5% and 25% SP demonstrated a dose-dependent attenuation of the virucidal activity of l-lactic acid (Figure S3; available as Supplementary data at JAC Online). Maximum HIVBa-L virucidal activity was observed at a final concentration of 0.5% l-lactic acid for both 12.5% and 25% SP, in contrast to 1% l-lactic acid for 75% SP, and the ability of 0.4% l-lactic acid to inactivate HIVBa-L was reduced with increasing SP concentrations (Figure S3 and Figure 4b). These data demonstrate that l-lactic acid inactivation of HIVBa-L in the presence of 75% SP is observed at final concentrations of ≥0.75%, in the context of our in vitro experiments.
Protonated lactic acid and not the lactate anion mediates HIV-1 virucidal activity
Our data demonstrate that the HIV virucidal activity of lactic acid is pH dependent. To determine whether the uncharged lactic acid predominantly found at low pH mediates virucidal activity, as distinct from the lactate anion present at neutral pH (Figure 5a), we evaluated the ability of l-lactic acid to inactivate HIVBa-L when neutralized to pH 7.0 compared with l-lactic acid at pH 3.8. In addition, we examined the capacity of l-sodium lactate to inactivate HIV, which provides a source of lactate anion in the absence of acidity. While 1% l-lactic acid at pH 3.8 resulted in a 5.4 × 103-fold decrease in HIVBa-L infectivity, little virucidal activity was observed with either 1% l-lactic acid at pH 7.0 or 1% l-sodium lactate pH 7.0 (Figure 5b). These data demonstrate that it is uncharged protonated lactic acid and not the lactate anion that mediates HIV-1 virucidal activity.
Figure 5.
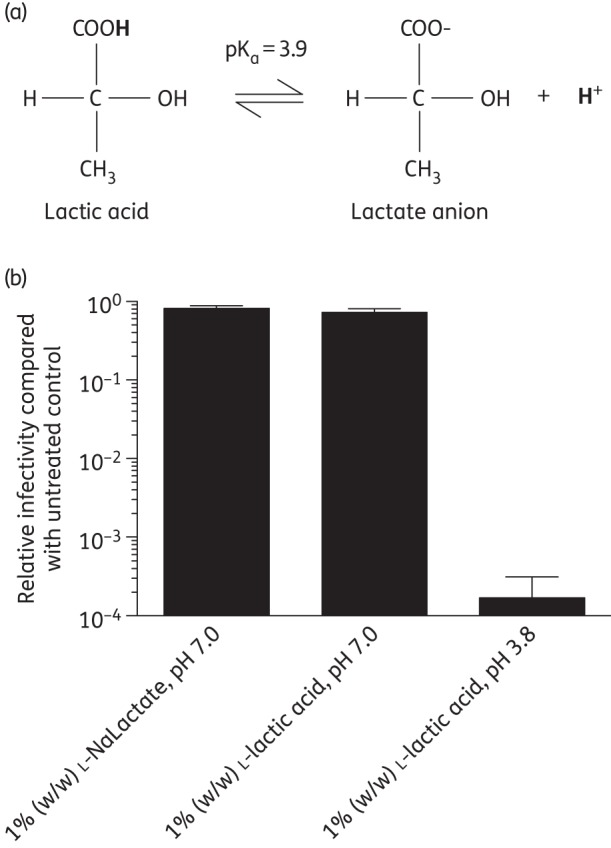
HIV-1 virucidal activity of protonated l-lactic acid compared with l-lactate anion. (a) Protonated lactic acid exists in equilibrium with the lactate anion at pH 3.9. (b) HIVBa-L was incubated at 37°C for 30 min with l-lactic acid adjusted to pH 3.8, l-lactic acid adjusted to pH 7.0 or l-sodium lactate (l-NaLactate) at pH 7.0, with the pH adjusted prior to virus addition. After neutralization, infectivity was determined in TZM-bl cells. The data were obtained from three independent assays and error bars denote the standard error of the mean.
Discussion
Our study shows that l-lactic acid has broad-spectrum HIV virucidal activity that is additional to acidity alone (due to HCl). l-lactic acid's virucidal activity is more potent and rapid compared with acetic acid, a smaller, membrane-permeant carboxylic acid. Complete inactivation of HIV infectivity by lactic acid concentrations ≥0.5%, under conditions where the media were subsequently neutralized prior to titration in TZM-bl cells, strongly suggests that the impact of lactic acid on HIV infectivity is irreversible. We have also shown that it is the protonated form of lactic acid and not the lactate anion that mediates HIV virucidal activity, which is consistent with the pH-dependent anti-HIV activity observed in the absence and presence of SP.
While it has been reported that l-lactic acid has more potent antibacterial activity than d-lactic acid,50 to our knowledge this is the first report of a carboxylic acid eliciting stereochemical-dependent virucidal activity. The antimicrobial mechanism of action of lactic acid under acidic conditions has been ascribed to its ability to penetrate cell membranes, resulting in cytosol acidification,51 direct action on membranes52 or interference with the enzymatic reactions of the cell.50 HIV does not have a cytosol and the stereochemical-dependent activity of lactic acid suggests that it is targeting protein. In this regard, l-lactic acid has been shown to induce protein unfolding;53 thus, an obvious viral protein target is the HIV gp120 envelope protein exposed on the outside of the virion. However, given the membrane-permeant properties of the uncharged form of lactic acid predominately present at low pH, it is also possible that it could penetrate the viral lipid envelope, resulting in its perturbation and/or adversely affecting the function of proteins embedded in this layer in addition to penetrating the lipid bilayer and targeting viral proteins and enzymes inside the virion. A previous study reported that HIV-1 virions treated with 1% lactic acid at pH 4.0 for 1 h at 37°C retained gp120 in an immunologically recognizable form.6 Further studies are in progress to examine whether similar effects are observed with l-lactic acid at pH 3.8, to correlate this effect to its virucidal activity and elucidate the mechanisms by which it inactivates HIV infectivity.
Our studies clearly show that l-lactic acid inactivates subtype B and non-subtype B HIV-1 strains, even under mildly acidic conditions at pH 4.5, which appears to be at odds with a previous study that reported enhancement of HIV-1 infectivity.40 It is not clear why Connor40 observed this enhancement. In contrast to that study we quantified virus titre by counting blue foci-forming cells rather than luciferase activity, we obtained data from at least two independent assays and measured the final pH after mixing of virus and acid-buffering medium, which can shift from the intended pH. We also reported the final concentration of l-lactic acid in the medium, which along with pH we have found to be critical for HIV virucidal activity. In this regard, 0.2% l-lactic acid at pH 4.2 lacked potent virucidal activity for 9 of the 10 HIV strains tested (Figure 2). We also did not observe dramatic variations in HIV inactivation for the majority of the strains tested in the presence of 0.3% l-lactic acid, which acidified the media to ∼pH 4.0 under our experimental conditions.
It is highly unlikely the concentration of solutes and salts introduced during pH adjustment of lactic acid had an impact on HIV virucidal activity. The HIV inactivation studies were performed in tissue culture medium, which has an osmolality of ∼295 mOsm/kg. If we account for ∼56 mOsm/kg due to dissociated lactate anion present at pH 3.8 in the 1% (w/w) lactic acid solution and the ∼6 mOsm/kg osmolality associated with adjusting the pH of lactic acid-containing medium with HCl to account for chloride ions in the time-course experiments, the total osmolality is ∼357 mOsm/kg. By contrast, 1% sodium lactate (pH 7.0), with a much higher osmolality of ∼473 mOsm/kg (295 mOsm/kg from media and 178 mOsm/kg to account for Na+ and lactate anions from sodium lactate), demonstrated little effect on HIV infectivity compared with the untreated control virus (Figure 5b). Taken together it is unlikely that the small change in osmolality due to HCl addition would have contributed to HIV inactivation. Moreover, the volumes of HCl added to adjust l-lactic acid, acetic acid and media alone to pH 3.8 were similar. Thus, differences in solutes and salt concentrations are unlikely to account for the more potent HIV inactivation profile observed for l-lactic acid compared with the other acids tested.
The superior virucidal activity of l-lactic acid compared with other acids and its capacity to inactivate HIV even in the presence of CVS has significant implications with regard to HIV transmission. Higher concentrations of HIV-1 RNA in male and female genital secretions are associated with a greater risk of heterosexual transmission of HIV-1, even when adjusting for plasma viral load.9 Notably, women harbouring vaginal Lactobacillus spp. have a lower risk of genital HIV-1 shedding whereas the bacteria associated with BV increase this risk.7,8 The reduced presence of HIV-1 RNA in HIV-infected women with a lactobacillus-dominated microbiota may be related to the impact of lactobacilli and/or lactic acid on the innate immune response in the vagina.54–56 Alternatively or additionally, lactic acid may directly inactivate HIV shed into the vagina. However, the impact of lactic acid treatment (in the presence of CVS) on the integrity of viral RNA, which is normally used to measure the presence of HIV in genital secretions, needs to be determined. Regardless, our findings could be exploited by delivering l-lactic acid into the vaginas of HIV-infected women by an intravaginal ring to inactivate HIV and reduce female-to-male transmission.
The main rationale for performing the HIV inactivation studies in CVS was to determine whether exogenously added lactic acid would be active in an environment comprising viscous mucus and proteins found in the vagina. The studies were not designed to determine the impact on HIV of endogenous lactic acid that might be present in these samples. The pH of the pooled CVS used in our experiments was around 5.4. Vaginal pH >5.0 has been reported in asymptomatic women and is associated with bacterial community group IV that is not dominated by Lactobacillus spp.11 In the context of media, 0.1% lactic acid results in a pH of 4.9 (Figure 2a). This suggests that the CVS sample had very little protonated lactic acid. This lack of lactic acid in the sample is consistent with our observation of similar HIV inactivation profiles in the absence and presence of 50% CVS (Figure 4a).
Our experience with handling CVS indicates that freezing of the sample is critical to minimize exposure of the sample to oxygen in air, which can result in a decrease in lactic acid levels. This is manifested owing to the altered metabolism of lactobacilli in the presence of oxygen, which results in the production of acetic acid instead of lactic acid and/or loss of CO2 (which partially acidifies CVS) from the sample. We have found that the pH of fresh CVS (collected using the SoftCup)57 compared with the pH of the same sample measured following freezing at −80°C and thawing increases on average by 0.1 pH unit (T. Moench and G. Tachedjian, unpublished data). This small increase could be due to loss of CO2 and/or lactic acid levels due to exposure to air. Thus, it is unlikely that limited freeze–thawing per se would substantially affect lactic acid levels, provided that exposure of samples to oxygen is minimized. In addition, the samples we used remained viscous following freeze–thaw, indicating lack of breakdown of mucus.
In contrast to CVS we found that SP attenuates the anti-HIV activity of l-lactic acid, most likely due to its buffering capacity, which increases the pH thereby decreasing levels of the active protonated form of lactic acid. Accordingly, in the absence of buffering to maintain low pH, supra-physiological concentrations of lactic acid would likely be needed in the vagina to completely block male-to-female transmission of HIV. Whether endogenously produced lactic acid has a role in attenuating HIV transmission from males to females would depend on several factors. These include the rate of re-acidification following deposition of semen in the vaginal tract and how quickly HIV is able to penetrate physical barriers in the vagina to encounter target cells in the mucosa, which are critical for establishing infection.58 Lactobacilli found in the vagina acidify at a rate of ∼0.5 pH units/h, which is consistent with lactobacilli being responsible for re-acidifying the vagina within several hours of coitus, following neutralization by semen.28 While the time required for HIV to infect target cells after introduction in the vagina is unknown, a study where rhesus macaques were vaginally infected with a high inoculum of simian immunodeficiency virus (SIV) reveals that cell-free virus penetrates the cervicovaginal epithelium and infects epithelial dendritic cells within 60 min of exposure.59 However, macaques are sparsely colonized with lactobacilli and have low levels of lactic acid, and consequently rarely have acidic vaginas.31 In addition to the direct inactivation of HIV, CVS from women with a lactobacillus-dominated vaginal flora that is acidified to pH ∼4 traps HIV, while neutralizing the same CVS sample in parallel abolishes this effect.6 An uncharacterized factor in mucus that immobilizes HIV in a pH-dependent manner has also been described.10 Thus, provided that acidification following neutralization with semen occurs before HIV can encounter target cells, endogenously produced lactic acid could potentially attenuate HIV infection by both direct inactivation and by preventing its ability to diffuse through cervicovaginal mucus.
Lactic acid production by lactobacilli may also have an indirect impact on HIV transmission by potentially preventing BV, which is associated with an increased risk of HIV acquisition in both males and females.18–20 Virulence factors released or induced by BV organisms may be responsible for some of the risks for STIs. In this regard, metabolic products such as acetic acid, propionic acid and butyric acid released by BV-associated bacteria increase inflammation and potentially activate target cells in the vagina known to promote HIV infection.60 Alternatively, lactobacilli and lactic acid production in the context of a lactobacillus-dominated vaginal microbiota might reduce the risk of BV, and hence indirectly HIV, by selectively suppressing growth of BV-associated bacteria.23 Several clinical trials have evaluated the use of lactobacillus-based probiotics for the treatment or prevention of BV recurrence. However, while the use of probiotics appears to be promising the data are inconclusive, with some studies demonstrating BV cure and/or reduced BV recurrence compared with placebo while other studies showed no significant difference.60–63 The use of distinct Lactobacillus spp. that may differ in their metabolic output of lactic acid26 or other antimicrobial factors3 and challenges associated with establishing and maintaining vaginal colonization of strains may explain the apparently discordant data.64
Another alternative strategy is to use lactic acid instead of probiotics. In this regard, a multicentre, open-label, controlled, randomized study in 90 women demonstrated that lactic acid gel (Lactacyd, comprising 4.5% lactic acid and 5 g of glycogen) is safe and as effective as metronidazole in the treatment of BV.65 Furthermore, the combination of metronidazole and lactic acid gel was superior to metronidazole alone in promoting lactobacilli colonization and decreasing BV recurrence.65 Other unblinded studies have reported that lactate gel inserted into the vagina for 7 days is as effective as oral metronidazole in the treatment of BV66 and that topical use of lactic acid and lactoserum intimate liquid soap following standard oral metronidazole treatment reduced BV recurrence.67 Further blinded controlled studies are warranted to examine the potential for l-lactic acid, particularly applied continuously via an intravaginal ring, for BV treatment and prevention.
This study focused on the impact of lactic acid on cell-free virus. However, HIV-infected cells have also been reported in the cervical mucus and semen of infected individuals, which could initiate infection.68 In this regard, studies in mouse and macaque models demonstrate that cell-associated HIV69 and SIV,70 respectively, can establish infection via the vagina. While a clinical study suggests that cell-associated HIV transmission in semen is possible,71 a second study showed that HIV transmission was attributable to cell-free virus in SP rather than seminal cells, as determined by phylogenetic analysis.72 Our previous studies demonstrate that low pH (4.5) immobilizes and kills human leucocytes and prevents transmission of cell-associated HIV in a mouse model.73 These experiments were performed with Buffer Gel, which is acidified by HCl and found to be less potent than lactic acid in the inactivation of cell-free HIV in the current study. Thus, studies to examine the impact of lactic acid on transmission of cell-associated HIV would be of interest.
Under conditions where the pH was carefully monitored throughout the experiment we found that acidity alone, even at pH 3.8 found in a lactobacillus-dominated vagina, resulted in not more than a 29-fold reduction in titre and was 600-fold to 3500-fold less effective at inactivating a laboratory strain and two transmitter/founder strains of HIV-1 than l-lactic acid at the same pH. Our data appear to differ from a previous report showing that a 10 min incubation of HIV-1 in medium acidified with HCl results in almost a 104-fold reduction in titre.38 However, that study used an X4 laboratory strain of HIV, which may be intrinsically more susceptible to acid than the more biologically relevant R5 strains tested in this study. Our findings may inform microbicide formulations, which are normally acidified to a pH compatible with the natural acidity of the vagina. In this regard, the acid-buffering microbicide BufferGel®, while safe, was not effective in protecting women from the sexual transmission of HIV from their male partners.74 Notably, BufferGel® comprises a mucoadhesive and acid-buffering Carbopol® polymer gel that contains no lactic acid and provides only a low pH, which we have shown to be dramatically less virucidal and less effective against BV-associated bacteria than lactic acid.23 Thus, l-lactic acid could potentially be used as an acidifying agent in topical microbicides instead of acidity alone, and may favour a lactobacillus-dominated vaginal microbiota in addition to having a role in directly inactivating HIV in the vagina.
Thus, we have shown that l-lactic acid, a naturally occurring microbicidal agent found in the vagina, also has potent, broad-spectrum HIV virucidal activity that could help prevent both female-to-male and male-to-female spread of the virus by both direct inactivation and indirectly through prevention of BV, which contributes to HIV transmission. The potential use of lactic acid as a microbicide or in microbicide formulations, especially in combination with other potential microbicides such as antiretroviral drugs, warrants further evaluation.
Funding
This work was supported by the National Health and Medical Research Council of Australia (NHMRC) (Project Grant 1028294), the Australian Centre for HIV and Hepatitis Virology Research (ACH2) and in part by the National Institute of Allergy and Infectious Diseases, National Institute of Health Grant (Grant number U19 AI060598). G. T. was supported by the NHMRC Senior Research Fellowship (Grant 543105) and M. A. by an Australian Postgraduate Award through Monash University. The authors gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program received by the Burnet Institute.
Transparency declarations
T. M. and R. C. own equity in ReProtect Inc., which has pending patent applications relating to sustained release of lactic acid. The remaining authors have none to declare.
Supplementary data
Figures S1–3 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
This study was presented in part at the International Microbicides Conference: Building bridges in HIV Prevention, Pittsburgh, PA, USA 22–25 May 2010 (Abstract 354), the 13th International Union against Sexually Transmitted Infections (IUSTI) World Congress, Melbourne, Australia 14–16 October 2012 (Abstract 416) and the International Workshop on the Biology of Mucosally Transmitted Sexual Infection: Translating the Basic Science into Novel HIV Intervention, Sydney, Australia 15 April 2012. We thank the NIH AIDS Research and Reference Reagent Program for providing the HIV isolates: HIVBa-L, 92RW016, 92BR025, CMU02, 93BR020, CDC310319 and the infectious molecular clones pRHPA.c/2635 and pCH058.c/2960 used in this study. TZM-bl cells were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH that were contributed by John C. Kappes, Xiaoyun Wu and Transzyme Inc. We also thank Dana Gabuzda for providing MACS3-LN, MACS1-spln and CB1-br.
References
- 1.Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6:20–8. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 2.Saidi H, Jenabian MA, Belec L. Understanding factors that modulate HIV infection at the female genital tract mucosae for the rationale design of microbicides. AIDS Res Hum Retroviruses. 2012;28:1485–97. doi: 10.1089/AID.2012.0049. [DOI] [PubMed] [Google Scholar]
- 3.Boris S, Barbes C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2:543–6. doi: 10.1016/s1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 4.Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Lai SK, Wang YY, Hida K, et al. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci USA. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai SK, Hida K, Shukair S, et al. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell C, Balkus JE, Fredricks D, et al. Interaction between lactobacilli, bacterial vaginosis-associated bacteria, and HIV Type 1 RNA and DNA genital shedding in U.S. and Kenyan women. AIDS Res Hum Retroviruses. 2013;29:13–9. doi: 10.1089/aid.2012.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sha BE, Zariffard MR, Wang QJ, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukair SA, Allen SA, Cianci GC, et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013;6:427–34. doi: 10.1038/mi.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allsworth JE, Lewis VA, Peipert JF. Viral sexually transmitted infections and bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Sex Transm Dis. 2008;35:791–6. doi: 10.1097/OLQ.0b013e3181788301. [DOI] [PubMed] [Google Scholar]
- 14.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–50. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 15.Thoma ME, Gray RH, Kiwanuka N, et al. The natural history of bacterial vaginosis diagnosed by gram stain among women in Rakai, Uganda. Sex Transm Dis. 2011;38:1040–5. doi: 10.1097/OLQ.0b013e3182275499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaul R, Nagelkerke NJ, Kimani J, et al. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Infect Dis. 2007;196:1692–7. doi: 10.1086/522006. [DOI] [PubMed] [Google Scholar]
- 18.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Atashili J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamey TA, Timothy MM. Studies of introital colonization in women with recurrent urinary infections. I. The role of vaginal pH. J Urol. 1975;114:261–3. doi: 10.1016/s0022-5347(17)67003-4. [DOI] [PubMed] [Google Scholar]
- 22.Hanna NF, Taylor-Robinson D, Kalodiki-Karamanoli M, et al. The relation between vaginal pH and the microbiological status in vaginitis. Br J Obstet Gynaecol. 1985;92:1267–71. doi: 10.1111/j.1471-0528.1985.tb04874.x. [DOI] [PubMed] [Google Scholar]
- 23.O'Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–5. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 25.Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J Reprod Fertil. 1973;33:69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- 26.Bai G, Gajer P, Nandy M, et al. Comparison of storage conditions for human vaginal microbiome studies. PloS One. 2012;7:e36934. doi: 10.1371/journal.pone.0036934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boskey ER, Cone RA, Whaley KJ, et al. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16:1809–13. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 28.Boskey ER, Telsch KM, Whaley KJ, et al. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67:5170–5. doi: 10.1128/iai.67.10.5170-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregoire AT, Kandil O, Ledger WJ. The glycogen content of human vaginal epithelial tissue. Fertil Steril. 1971;22:64–8. doi: 10.1016/s0015-0282(16)37989-4. [DOI] [PubMed] [Google Scholar]
- 30.Paavonen J. Physiology and ecology of the vagina. Scand J Infect Dis Suppl. 1983;40:31–5. [PubMed] [Google Scholar]
- 31.Mirmonsef P, Gilbert D, Veazey RS, et al. A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: implications for HIV and SIV susceptibility. AIDS Res Hum Retroviruses. 2012;28:76–81. doi: 10.1089/aid.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meysick KC, Garber GE. Interactions between Trichomonas vaginalis and vaginal flora in a mouse model. J Parasitol. 1992;78:157–60. [PubMed] [Google Scholar]
- 33.Klebanoff SJ, Coombs RW. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991;174:289–92. doi: 10.1084/jem.174.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Hanlon DE, Lanier BR, Moench TR, et al. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis. 2010;10:120. doi: 10.1186/1471-2334-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graver MA, Wade JJ. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob. 2011;10:8. doi: 10.1186/1476-0711-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ongradi J, Ceccherini-Nelli L, Pistello M, et al. Acid sensitivity of cell-free and cell-associated HIV-1: clinical implications. AIDS Res Hum Retroviruses. 1990;6:1433–6. doi: 10.1089/aid.1990.6.1433. [DOI] [PubMed] [Google Scholar]
- 37.Kempf C, Jentsch P, Barre-Sinoussi FB, et al. Inactivation of human immunodeficiency virus (HIV) by low pH and pepsin. J Acquired Immune Defic Syndr. 1991;4:828–30. [PubMed] [Google Scholar]
- 38.Martin LS, McDougal JS, Loskoski SL. Disinfection and inactivation of the human T lymphotropic virus type III/Lymphadenopathy-associated virus. J Infect Dis. 1985;152:400–3. doi: 10.1093/infdis/152.2.400. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor TJ, Kinchington D, Kangro HO, et al. The activity of candidate virucidal agents, low pH and genital secretions against HIV-1 in vitro. Int J STD AIDS. 1995;6:267–72. doi: 10.1177/095646249500600409. [DOI] [PubMed] [Google Scholar]
- 40.Connor RI. Sensitivity of non-clade B primary HIV-1 isolates to mildly acidic pH. J Acquir Immune Defic Syndr. 2006;43:499–501. doi: 10.1097/01.qai.0000243048.50451.18. [DOI] [PubMed] [Google Scholar]
- 41.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–89. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyssen D, Henderson SA, Johnson A, et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PloS One. 2010;5:e12309. doi: 10.1371/journal.pone.0012309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorry PR, Bristol G, Zack JA, et al. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol. 2001;75:10073–89. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wapling J, Moore KL, Sonza S, et al. Mutations that abrogate human immunodeficiency virus type 1 reverse transcriptase dimerization affect maturation of the reverse transcriptase heterodimer. J Virol. 2005;79:10247–57. doi: 10.1128/JVI.79.16.10247-10257.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yap SH, Sheen CW, Fahey J, et al. N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Med. 2007;4:e335. doi: 10.1371/journal.pmed.0040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price CF, Tyssen D, Sonza S, et al. SPL7013 Gel (VivaGel(R)) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PloS One. 2011;6:e24095. doi: 10.1371/journal.pone.0024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26:459–69. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 50.McWilliam Leitch EC, Stewart CS. Escherichia coli O157 and non-O157 isolates are more susceptible to L-lactate than to D-lactate. Appl Environ Microbiol. 2002;68:4676–8. doi: 10.1128/AEM.68.9.4676-4678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell JB, Diez-Gonzalez F. The effects of fermentation acids on bacterial growth. Adv Microb Physiol. 1998;39:205–34. doi: 10.1016/s0065-2911(08)60017-x. [DOI] [PubMed] [Google Scholar]
- 52.Alakomi HL, Skytta E, Saarela M, et al. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000;66:2001–5. doi: 10.1128/aem.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang HM, Ou WB, Zhou HM. Effects of lactic acid and NaCl on creatine kinase from rabbit muscle. Biochem Cell Biology. 2003;81:1–7. doi: 10.1139/o02-168. [DOI] [PubMed] [Google Scholar]
- 54.Fichorova RN, Yamamoto HS, Delaney ML, et al. Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action. mBio. 2011;2:e00168–11. doi: 10.1128/mBio.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mossop H, Linhares IM, Bongiovanni AM, et al. Influence of lactic acid on endogenous and viral RNA-induced immune mediator production by vaginal epithelial cells. Obstet Gynecol. 2011;118:840–6. doi: 10.1097/AOG.0b013e31822da9e9. [DOI] [PubMed] [Google Scholar]
- 56.Witkin SS, Alvi S, Bongiovanni AM, et al. Lactic acid stimulates interleukin-23 production by peripheral blood mononuclear cells exposed to bacterial lipopolysaccharide. FEMS Immunol Medical Microbiol. 2011;61:153–8. doi: 10.1111/j.1574-695X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- 57.Boskey ER, Moench TR, Hees PS, et al. A self-sampling method to obtain large volumes of undiluted cervicovaginal secretions. Sex Transm Dis. 2003;30:107–9. doi: 10.1097/00007435-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–39. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 59.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–95. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mirmonsef P, Zariffard MR, Gilbert D, et al. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am J Reprod Immunol. 2012;67:391–400. doi: 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;Issue 4:CD006289. doi: 10.1002/14651858.CD006289.pub2. [DOI] [PubMed] [Google Scholar]
- 62.Falagas ME, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect. 2007;13:657–64. doi: 10.1111/j.1469-0691.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 63.Bradshaw CS, Pirotta M, De Guingand D, et al. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial. PloS One. 2012;7:e34540. doi: 10.1371/journal.pone.0034540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antonio MA, Meyn LA, Murray PJ, et al. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous lactobacilli. J Infect Dis. 2009;199:1506–13. doi: 10.1086/598686. [DOI] [PubMed] [Google Scholar]
- 65.Decena DC, Co JT, Manalastas RM, Jr, et al. Metronidazole with Lactacyd vaginal gel in bacterial vaginosis. J Obstet Gynaecol Res. 2006;32:243–51. doi: 10.1111/j.1447-0756.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 66.Andersch B, Forssman L, Lincoln K, et al. Treatment of bacterial vaginosis with an acid cream: a comparison between the effect of lactate-gel and metronidazole. Gynecol Obstet Invest. 1986;21:19–25. doi: 10.1159/000298923. [DOI] [PubMed] [Google Scholar]
- 67.Bahamondes MV, Portugal PM, Brolazo EM, et al. Use of a lactic acid plus lactoserum intimate liquid soap for external hygiene in the prevention of bacterial vaginosis recurrence after metronidazole oral treatment. Rev Assoc Med Bras. 2011;57:415–20. doi: 10.1590/s0104-42302011000400015. [DOI] [PubMed] [Google Scholar]
- 68.Anderson DJ, Politch JA, Nadolski AM, et al. Targeting Trojan Horse leukocytes for HIV prevention. AIDS. 2010;24:163–87. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khanna KV, Whaley KJ, Zeitlin L, et al. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J Clin Invest. 2002;109:205–11. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salle B, Brochard P, Bourry O, et al. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis. 2010;202:337–44. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 71.Zhu T, Wang N, Carr A, et al. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Butler DM, Delport W, Kosakovsky Pond SL, et al. The origins of sexually transmitted HIV among men who have sex with men. Sci Transl Med. 2010;2:18re1. doi: 10.1126/scitranslmed.3000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olmsted SS, Khanna KV, Ng EM, et al. Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect Dis. 2005;5:79. doi: 10.1186/1471-2334-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdool Karim SS, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25:957–66. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.