Abstract
Size has a profound effect on the structure of the brain. Many brain structures scale allometrically, that is, their relative size changes systematically as a function of brain size. Here we use independent contrasts analysis to examine the scaling of frontal cortex in 43 species of mammals including 25 primates and 15 carnivores. We find evidence for significant differences in scaling between primates and carnivores. Primate frontal cortex hyperscales relative to the rest of neocortex and the rest of the brain. The slope of frontal cortex contrasts on rest of cortex contrasts is 1.18 (95% confidence interval, 1.06-1.30) for primates, which is significantly greater than isometric. It is also significantly greater than the carnivore value of 0.94 (95% confidence interval, 0.82-1.07). This finding supports the idea that there are substantial differences in frontal cortex structure and development between the two groups.
Comparative neuroanatomists have long been interested in the relationship between size and brain structure. Early work focused on how the brain scales with the body, and how gross morphological characteristics such as cortical folding change with size (1, 2). More recently, emphasis has been put on the scaling of various brain structures with each other and with overall brain size (3-6).
The scaling of frontal cortex presents an interesting case. From the beginning, workers have been drawn to this region because of the supposition that volume increases occurred in the line leading to humans. Brodmann's regio frontalis consisted of frontal cortex minus areas 4 and 6 and parts of the cingulate. He described a “progressive” expansion of this region in the primate line going from prosimians to humans, and argued that primates more closely related to humans have a disproportionally larger regio frontalis (7). However, primates more closely related to humans also have larger brains. The disproportionate expansion of the frontal region could be due to allometric scaling only.
Von Bonin (8) explicitly argued that frontal cortex hyperscales with brain size, and man has “precisely the frontal lobe which he deserves by virtue of the overall size of his brain”. A number of subsequent workers used allometric lines as a kind of standard for comparing whether human frontal cortex is bigger or smaller than one would expect for a similarly sized primate (9-11). However, neither Von Bonin nor later workers had adequate data or methods to establish whether frontal cortex hyperscaling is a regular and systematic relationship with size, or simply an artifact of grade differences. As was originally pointed out by Felsenstein (12), the phylogenetic structure of a sample of species can make it appear that there is a systematic relationship between two variables where none exists.
To make the distinction between a series of grade shifts and systematic allometry, one must apply a method such as independent contrasts, which can factor out the effects of phylogeny (12). In addition, one must have data from a phylogenetically wide sample of species. Here we examine the scaling of frontal cortex in a large sample of mammals that includes broad representation in two orders, primates and carnivores. We analyze the resulting data by using the method of independent contrasts.
Materials and Methods
Brains. We examined brains from a total of 55 mammalian species in eight orders. The majority of these are located at the comparative brain collection at the University of Wisconsin (Madison). Brains were embedded in celloidin and stained with thionin. (Our Daubentonia madagascariensis measurement was based on a T2 weighted MRI in conjunction with Nissl-stained frozen sections from the same brain.) We took a systematic random sample from each brain. That is, slices were chosen at a regular sampling interval with the position in the first interval randomized. We used 40 or more slices per brain, digitizing these with a standard office flatbed scanner (Epson Expression 800) at 800 dpi. In cases where a slice we needed was missing, we took an adjacent slice or a slice from a corresponding fiber series. In several cases where no suitable substitute was available, we interpolated between adjacent slices in our series to obtain volume measurements. The digital images were roughly aligned for convenience, and analyzed by using the amira software package. Coefficients of error for our volume measurements were <0.03, using the method of Gundersen et al. (13). Our raw measurements are shown in Table 2, which is published as supporting information on the PNAS web site.
Demarcation of Boundaries. Frontal cortex is neocortex anterior to the motor-somatosensory border. Except for the borders of primate V1, this is the most recognizable and reliable cytoarchitectonic border in the neocortex (14). Several notable features of the cytoarchitecture change here. Motor cortex has large Betz cells in layer 5. In addition, it lacks a granular layer 4, which is present in somatosensory cortex. Several photomicrographs of motor cortex and its border with somatosensory cortex are shown in Figs. 2-5, which are published as supporting information on the PNAS web site. The motor-somatosensory border is also a landmark that has been identified electrophysiologically in a number of species. We referred to this work where available: Hylobates and Pan (15), Allouatta (16), Aotus (17), Perodicticus (18), Otolemur (19), Galago (20), Nycticebus (21), Procyon (22), and Dasypus (23). We also referred to several cytoarchitectonic studies for Lemur and Potos (14) and Choloepus (24). We were able to identify the motor somatosensory border in 43 species of mammals, including 25 primates and 15 carnivores, and we used its position and trajectory to divide cortex in two. On the lateral side in primates, we followed its trajectory until it intersected the sylvian fissure. We then followed the sylvian forward, counting everything anterior of where the sylvian disappears as frontal cortex. In carnivores, on the lateral side we followed the trajectory of the motor somatosensory border until it reached the coronal sulcus. We followed the coronal until it disappeared or until we reached the level of the cruciate sulcus, whichever came first. We counted everything anterior to that as frontal cortex. In the three other mammals, there were not limiting sulci of this type, and we simply followed the trajectory of the motor somatosensory border all of the way to the edge of neocortex. Medially in all mammals, we followed the trajectory of the motor-somatosensory border, through the cingulate down to the level of the corpus callosum. We identified the borders of neocortex in our sample by using well known cytoarchitectonic criteria.
Shrinkage. Celloidin embedding causes shrinkage. We corrected for overall shrinkage by using pictures taken of the brains before embedding. These were from standard views and included a scale bar. By comparing various measurements on these pictures with our scanned images, we were able to make estimates of slice dimensions before celloidin embedding. In Homo sapiens and Propithecus verreauxi, no presectioning picture was available. In these cases, we scaled our measurements so that whole brain volumes would match those measured by Stephan et al. (25).
A second issue relates to differential shrinkage of various structures. Our frontal cortex scaling results have been presented as comparisons between two regions of neocortical gray matter. If gray matter in different regions of cortex shrinks differently, it would present a problem for us. We therefore examined two celloidin shrinkage studies, a beaver and a capybara, from the Wisconsin collection. One hemisphere of these brains was sectioned frozen, whereas the other was sectioned after being embedded in celloidin. We measured shrinkage in the celloidin hemisphere relative to that in the frozen one. We did not feel that we could identify the motor-somatosensory border reliably in these two rodent brains, so we used the caudal end of the corpus callosum as a landmark. We used it to divide the cortex into two parts. In the beaver, we found that gray matter caudal to the corpus callosum shrank to 39.6% of its original volume in the celloidin hemisphere. In our other, rostral division of beaver neocortex, gray matter shrank to 39.1% of its original volume. In the capybara, the values were 33.7% and 32.1% for caudal and rostral divisions, respectively. In both brains, the amount of shrinkage found in the two divisions of neocortex was very similar. We conclude that differential shrinkage of neocortical gray matter within the same brain is not a significant problem for our analysis. We also looked at scaling between neocortical gray matter and the subcortical brain, i.e., whole brain minus neocortical gray and white. In the shrinkage studies, we looked at the shrinkage of the rest of the brain, which is whole brain minus neocortex and cerebellum, which is missing in these studies. Rest of brain shrank to 43.2% and 40.7% of its original volume in the beaver and capybara, respectively. These values differ somewhat from the values for neocortical gray matter, but they do not differ by enough to account for our results, even if they varied systematically with brain size, which they almost certainly do not.
Data Analysis. Data analysis was performed in the r language (26). To calculate independent contrasts (12), we used the ape package for r (27), applied to log-transformed volume data. Phylogenies and dates come from the literature (28-33), and a copy of the tree we used is available in Figs. 6 and 7, which are published as supporting information on the PNAS web site. In cases of soft polytomies, we separated uncertain nodes with branches of length zero and reduced the degrees of freedom correspondingly in our statistical analysis (34). The 95% confidence intervals we report have been calculated with these minimum degrees of freedom, and so represent the maximum range. We used regression forced through the origin to calculate slopes, and followed the recommendations of Garland et al. (35) and Harvey and Pagel (36) to ensure that the requirements for regression were met. Regression coefficients we present were calculated by using least squares. Because our variables were log-transformed volumes of brain structures, regressions were highly significant with high coefficients of determination (>0.93), making it unlikely that the choice of line fitting method affected the results significantly. We also applied a robust line fitting method, iterated reweighted least squares (37), and found that this did not change the results. To determine whether scaling exponents in two groups were significantly different, we regressed their contrasts together and compared the residuals for each group with a t test (38).
We wanted to ensure that our observed hyperscaling relationships are not caused by the confounding effect of several categorical variables: diet, activity pattern, and social structure. To do this, we used a simple method that involves performing independent contrasts separately on each category. For example, if we wish to know whether the apparent hyperscaling between frontal gray volume and rest of cortex volume is actually caused by the confounding effect of activity level, we can do the following. We perform independent contrasts on frontal cortex and rest of cortex for nocturnal and diurnal primates separately. We can regress the results for nocturnal and diurnal separately as well, and if the hyperscaling persists, be confident that activity pattern is not affecting the scaling relationship. However, this has the disadvantage of dividing the data into parts and reducing the sample size used in any individual regression. Instead, we can take the contrasts that were calculated separately for nocturnal and diurnal primates and regress them through the origin together. As long as the phylogenies for nocturnal and diurnal primates are drawn from the same underlying phylogeny and have been treated in the same way (e.g., put through the same transformations on branch lengths), this will give a valid result. Again, if the hyperscaling persists, we can conclude that it is not caused by the confounding influence of activity level.
To test whether group size is related to relative frontal cortex size, we calculated the ratio of frontal cortex to rest of cortex. We performed Pearson's correlation between this ratio and log group size. We also calculated the residuals of frontal cortex contrasts on rest of cortex contrasts and regressed these against group size contrasts.
Our group size numbers were population group size from Wrangham et al. (39). Our categories for diet, activity, and social structure in primates were based on Rowe (40). For diet, we divided the primates into three groups: insect eaters, leaf eaters, and those who eat primarily high quality food of limited availability (fruit, gums, and seeds). For social structure, we used six groups: monogamous, fission fusion, troop, solitary, harem, and human.
Results
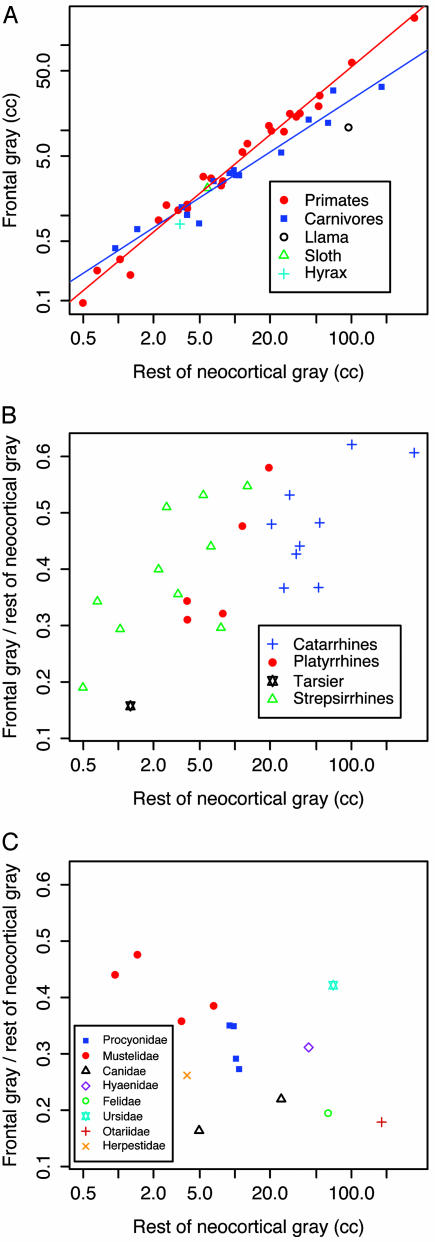
We find significant differences in frontal cortex scaling between primates and carnivores (Fig. 1A). In primates, the slope of frontal gray matter contrasts on rest of cortex contrasts is 1.18 and the 95% confidence interval is 1.06-1.30 (Table 1 gives a summary of independent contrasts results). The lower bound of the primate 95% confidence interval is >1 and the scaling is therefore significantly greater than isometric. Fig. 1B illustrates primate hyperscaling in a different way.
Fig. 1.
Frontal cortex scaling. (A) Log-log plot of frontal gray matter volume vs. rest of cortex gray matter volume for primates, carnivores, and other mammals. Included are least squares regression lines for primates (red) and carnivores (blue). Plots B and C take the form of a ratio on the y axis, plotted against its own denominator on a logarithmic x axis. (B) Ratio of primate frontal gray matter volume to rest of neocortical gray matter volume plotted against rest of neocortical gray matter volume. (C) Same as B, but showing carnivores.
Table 1. Independent contrasts results.
| Structures compared | Slope | 95% CI | No. of contrasts | |
|---|---|---|---|---|
| Primates | Frontal neog vs. rest of neog | 1.18 | 1.06-1.30 | 24 |
| Frontal neog vs. subcort brain | 1.38 | 1.22-1.53 | 24 | |
| Rest of neog vs. subcort brain | 1.13 | 0.98-1.28 | 24 | |
| Total neog vs. subcort brain | 1.10 | 1.01-1.18 | 37 | |
| Carnivores | Frontal neog vs. rest of neog | 0.94 | 0.82-1.07 | 14 |
| Frontal neog vs. subcort brain | 1.13 | 0.98-1.27 | 14 | |
| Rest of neog vs. subcort brain | 1.17 | 1.03-1.31 | 14 | |
| Total neog vs. subcort brain | 1.14 | 1.02-1.26 | 14 | |
| All mammals | Total neog vs. subcort brain | 1.17 | 1.11-1.24 | 93 |
Slopes and 95% confidence intervals (CI) for regression through the origin of independent contrasts of frontal cortex gray matter (neog), rest of cortex gray matter, and subcortical (subcort) brain. Data for all mammals and for primate total neocortex consists of our own data combined with that of Frahm et al. (1).
The scaling exponent for carnivore frontal cortex contrasts vs. rest of cortex contrasts is significantly less than for primates (P = 0.03, t = 2.22, df = 35). The carnivores show no tendency toward frontal cortex hyperscaling (Fig. 1C). Their exponent is 0.94 (95% confidence interval, 0.82-1.07), which is not significantly different from isometric scaling.
We see that primates and carnivores differ in how these two regions of cortex scale with each other. Does this result primarily from differences in frontal cortex, rest of cortex, or both? We can examine this by looking at the scaling of the cortical regions with the subcortical brain. Table 1 makes it clear that the scaling exponent for rest of cortex vs. the subcortical brain does not differ significantly in primates and carnivores. However, the exponent for frontal cortex vs. subcortical brain does differ in the two groups. Table 1 also shows that, consistent with previous claims, neocortex as a whole scales up relative to the rest of the brain in mammals (1, 41). Taken separately, primates and carnivores show neocortex vs. subcortical brain scaling trends similar to the rest of mammals and to each other. Thus, primates and carnivores show similar scaling relationships for neocortex as a whole and for the nonfrontal parts of the cortex. But frontal cortex scaling in the two groups differs significantly.
Fig. 1B hints at the possibility of grade differences within primates. For a given volume of rest of cortex, strepsirrhines seem to have a larger frontal cortex than haplorhines. To demonstrate that this is a grade difference, we would want to show that strepsirrhines and haplorhines have the same scaling exponent. In our sample, their exponents are not significantly different (P = 0.38, t = 0.91, df = 22), although this may reflect our small sample size for the individual primate groups. In any event, Fig. 1B shows that, where their cortex sizes overlap, strepsirrhines tend to have a larger frontal cortex than haplorhines.
We also examine whether the allometric scaling of frontal cortex in primates can be accounted for by confounding relationships with the ecological variables diet, activity pattern, and social structure. When we calculate the scaling exponents for frontal vs. rest of cortex contrasts separately for each category within a variable (e.g., nocturnal and diurnal within activity pattern) and regress them together, we find that hyperscaling persists in all three variables (activity pattern: 1.18, 95% confidence interval, 1.05-1.30; diet: 1.11, 95% confidence interval, 0.99-1.24; social structure: 1.20 95% confidence interval, 1.01-1.40). For all three, the slope remains high, and for two, social structure and activity pattern, the 95% confidence interval still excludes 1. In fact, in many cases there is strong hyperscaling even within single categories (e.g., nocturnal primates 1.33, 95% confidence interval 1.11-1.56).
We compared the ratio of frontal gray over rest of cortex to log group size for nine primates and eight carnivores. Pearson's correlation between these two quantities was not significant (P > 0.3). We also calculated the residuals for a regression of frontal cortex contrasts on rest of cortex contrasts. We regressed these against groups size contrasts, again finding no relationship. This suggests that group size and relative frontal cortex size are not related.
Discussion
We have provided evidence for significant differences between primates and carnivores in frontal cortex scaling. In primates, frontal cortex hyperscales relative to the rest of cortex; in carnivores, it does not. This suggests important differences in the development and composition of frontal cortex in the two groups, and supports the claim that primate frontal cortex differs from that of other mammals (42).
In addition, our use of the method of independent contrasts demonstrates that the hyperscaling of primate frontal cortex is a regular and systematic relationship with size. It is not due to a series of grade shifts in the line leading to humans.
Our results also provide some new context for old arguments. Much interest has focused on the question of whether humans have an unusually large frontal cortex compared to other primates. Semendeferi et al. (10) showed that frontal cortex occupies about the same proportion of total cortex in humans as it does in the great apes. Fig. 1B shows the ratio of frontal to rest of cortex for the wider range of primates in our data set. Ratios of frontal to rest of cortex for individual species can be found in Table 2. Note that catarrhines are not the only group where species with a high frontal cortex proportion have evolved. Such species have also evolved independently in platyrrhines (e.g., the spider monkey) and strepsirrhines (e.g., the aye aye). This is broadly consistent with the proposition that humans are not special with regard to the portion of their cortex devoted to frontal cortex. Indeed, the presence of a hypermetric scaling relationship in primate frontal cortex only serves to reinforce the point.
In addition, our data suggest that there may be a grade difference between strepsirrhines and haplorhines, with haplorhines actually having a smaller frontal cortex for a given rest of cortex size. This appears to turn on its head Brodmann's notion of a progressive expansion of frontal cortex in the line leading to humans.
Our results also shed some light on an Aegyptopithecus zeuxis endocast described by Simons (43). Aegyptopithecus is an early catarrhine from the Oligocene of Egypt. On this endocast (DUPC 5401), we examined the position of the central sulcus. The sulcus is ≈40% of the way back as you move along the top of the brain from the frontal to the occipital pole. In comparison, in a 3D reconstruction we made of the brain of the living mandrill, the sulcus is ≈50% of the way back. The ratio of frontal cortex to rest of cortex in the mandrill is at the bottom of the range among catarrhines in our sample. This shows that the central sulcus in Aegyptopithecus was placed relatively far forward, and implies that the animal had a small frontal cortex compared with living catarrhines. Aegyptopithecus had a brain volume of ≈27 cc (43), which is also below the range of living catarrhines. The small brain volume likely explains the relatively small frontal cortex. With its small brain, Aegyptopithecus represents an element of catarrhine variation that no longer exists today.
The difference in scaling between primates and carnivores is striking. Perhaps the most general conclusion to be drawn is that there are important differences between the two orders in the molecular regulation of cortical development. We discuss possible explanations for the scaling difference below, several of which do not have an immediate connection with development. However, even in these cases, the ultimate mechanisms behind the difference must be the mechanisms of development.
Now let us consider several possible explanations for the scaling difference. In other parts of the brain, allometry has been argued to be the result of a fixed order of neurogenesis, which causes later developing structures to become disproportionately large (3). This theory was intended to explain scaling differences between structures (e.g., why does neocortex scale up and hippocampus scale down). It was based on the suggestion that mammals share a rigid developmental program in which the order of development for different structures does not change. Clearly, the theory as originally specified does not explain our data, where we find that a single structure scales differently within two mammalian orders. One might propose that primates and carnivores have a different rigid developmental program in cortex. To explain our scaling data, primates would need to have a gradient of neurogenesis that moved from posterior to anterior. However, this is inconsistent with the known facts. In mammals that have been examined so far, including primates, anterior areas of neocortex complete neurogenesis before posterior areas do (44-46).
Another possible explanation is that the apparent relationship with size in primates is actually driven by some other variable that is itself correlated with size. In primates, ecological variables such as diet, activity pattern, and social structure are related to body size. Primate frontal cortex might contain structures, absent in carnivores, whose relative size correlates with such variables. This would make it appear that relative frontal cortex size is correlated with absolute size in primates. We calculated scaling exponents so as to remove the effect of the categorical variables diet, activity pattern, and social structure. Our results suggest that the scaling of frontal cortex is not due to confounding with these variables.
A third alternative follows from a more functional explanation for scaling. In other contexts, biologists think of scaling in functional terms, for example the relationship between bone thickness and body weight (47). In the brain, too, certain scaling phenomena have been explained functionally. White matter hyperscales relative to gray matter in neocortex, and this has been seen as a consequence of the need to maintain connectivity as brain size increases or as a reflection of systematic changes in axon diameter (1, 48).
Perhaps a more relevant example can be found in the hyperscaling of V1 relative to lateral geniculate nucleus (LGN), which provides all of V1's input. Stevens (6) pointed out that this might be a reflection of the information the two structures represent. In V1, a number of features are represented explicitly that are implicit in LGN. Edge orientation is an example. As retinal and LGN resolution increase, the resolution of edge orientation should also increase. The result is that the total number of cells involved in representing edge orientation will increase disproportionately with size (6).
Sensory information is repeatedly transformed in the brain, eventually finding its way back into the world as behavior. Perhaps the situation described for V1 and lateral geniculate nucleus is not uncommon. The case of primate frontal cortex could be seen in this light. Perhaps primates have evolved machinery in their frontal cortex that is absent in carnivores. This machinery, because of the nature of the circuits it uses and the information it represents, increases disproportionately with size.
Supplementary Material
Acknowledgments
We thank Wally Welker and Inge Siggelkow for access to the Wisconsin brain collection. We also thank Eric Ahrens for kindly providing MR data and Elwyn Simons for providing an Aegyptopithecus zeuxis endocast. Rizvan Mamet made suggestions on the analysis, and Wulf Haubensak provided German translation help. This work was supported by National Institutes of Health Grant EY11759 and the W. M. Keck Foundation Fund for Discovery in Basic Medical Research at the California Institute of Technology.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Baillarger, J. (1845) Gazette des Hopitaux 18, 179. [Google Scholar]
- 2.Dubois, E. (1913) Verh. Kon. Akad. Wetenschappen Amsterdam 16, 647. [Google Scholar]
- 3.Frahm, H. D., Stephan, H. & Stephan, M. (1982) J. Hirnforsch. 23, 375-389. [PubMed] [Google Scholar]
- 4.Haug, H. (1987) Am. J. Anat. 180, 126-142. [DOI] [PubMed] [Google Scholar]
- 5.Finlay, B. L. & Darlington, R. B. (1995) Science 268, 1578-1584. [DOI] [PubMed] [Google Scholar]
- 6.Stevens, C. (2001) Nature 411, 193-195. [DOI] [PubMed] [Google Scholar]
- 7.Brodmann, K. (1912) Suppl. Anat. Anz. 41, 157-216. [Google Scholar]
- 8.Von Bonin, G. (1947) Assoc. Res. Nervous Mental Dis. 27, 67-83. [PubMed] [Google Scholar]
- 9.Uylings, H. & VanEden, C. (1990) Progr. Brain Res. 85, 31-62. [DOI] [PubMed] [Google Scholar]
- 10.Semendeferi, K., Lu, A., Schenker, N. & Damasio, H. (2002) Nat. Neurosci. 5, 272-276. [DOI] [PubMed] [Google Scholar]
- 11.Passingham, R. (2002) Nat. Neurosci. 5, 190-192. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein, J. (1985) Am. Nat. 125, 1-15. [Google Scholar]
- 13.Gundersen, H., Jensen, E., Kieu, K. & Nielsen, J. (1999) J. Microsc. (Oxford) 193, 199-211. [DOI] [PubMed] [Google Scholar]
- 14.Brodmann, K. (1994) Brodman's Localization in the Cerebral Cortex, trans. Garey, L. G. (Smith-Gordon, London).
- 15.Welt, C. (1962) Ph.D. thesis (University of Chicago, Chicago).
- 16.Vogt, C. & Vogt, O. (1907) Elektrisch Erregbaren Hirnrinden-Gebiete bei den Saugetieren. (Verlag Von Johann Ambrosius Barth, Leipzig).
- 17.Stepniewska, I., Preuss, T. & Kaas, J. (1993) J. Comp. Neurol. 330, 238-271. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick, K., Carlson, M. & Charlton, J. (1982) J. Comp. Neurol. 204, 296-310. [DOI] [PubMed] [Google Scholar]
- 19.Fogassi, L., Gallese, V., Gentilucci, M., Luppino, G., Matelli, M. & Rizzolatti, G. (1994) Behav. Brain. Res. 60, 91-113. [DOI] [PubMed] [Google Scholar]
- 20.Sur, M., Nelson, R. & Kaas, J. (1980) J. Comp. Neurol. 189, 381-402. [DOI] [PubMed] [Google Scholar]
- 21.Sanides, F. & Krishnamurti, A. (1967) J. Hirnforsch. 9, 225-252. [PubMed] [Google Scholar]
- 22.Welker, W. & Seidenstein, S. (1959) J. Comp. Neurol. 111, 469-501. [DOI] [PubMed] [Google Scholar]
- 23.Royce, G. J., Martin, G. F. & Dom, R. M. (1975) J. Comp. Neurol. 164, 495-521. [DOI] [PubMed] [Google Scholar]
- 24.Gerebtzoff, M. A. & Goffart, M. (1966) J. Comp. Neurol. 126, 523-533. [PubMed] [Google Scholar]
- 25.Stephan, H., Frahm, H. & Baron, G. (1981) Folia Primatol (Basel) 35, 1-29. [DOI] [PubMed] [Google Scholar]
- 26.Ihaka, R. & Gentleman, R. (1996) J. Comput. Graph. Stat. 5, 299-314. [Google Scholar]
- 27.Paradis, E., Claude, J. & Strimmer, K. (2004) Bioinformatics 20, 289-290. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, W., Eizirik, E., O'Brien, S., Madsen, O., Scally, M., Douady, C., Teeling, E., Ryder, O., Stanhope, M., de Jong, W. & Springer, M. (2001) Science 294, 2348-2351. [DOI] [PubMed] [Google Scholar]
- 29.Springer, M., Murphy, W., Eizirik, E. & O'Brien, S. (2003) Proc. Natl. Acad. Sci. USA 100, 1056-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douady, C., Catzeflis, F., Kao, D., Springer, M. & Stanhope, M. (2002) Mol. Phylogenet. Evol. 22, 357-363. [DOI] [PubMed] [Google Scholar]
- 31.Bininda-Emonds, O., Gittleman, J. & Purvis, A. (1999) Biol. Rev. Cambridge Philos. Soc. 74, 143-175. [DOI] [PubMed] [Google Scholar]
- 32.Purvis, A. (1995) Philos. Trans. R. Soc. London Ser. B 348, 405-421. [DOI] [PubMed] [Google Scholar]
- 33.Kumar, S. & Hedges, S. (1998) Nature 392, 917-920. [DOI] [PubMed] [Google Scholar]
- 34.Purvis, A. & Garland, T. (1993) Syst. Biol. 42, 569-575. [Google Scholar]
- 35.Garland, T., Harvey, P. & Ives, A. (1992) Syst. Biol. 41, 18-32. [Google Scholar]
- 36.Harvey, P. & Pagel, M. (1991) The Comparative Method in Evolutionary Biology (Oxford Univ. Press, Oxford).
- 37.Huber, P. (1981) Robust Statistics (Wiley, New York).
- 38.Barton, R. & Harvey, P. (2000) Nature 405, 1055-1058. [DOI] [PubMed] [Google Scholar]
- 39.Wrangham, R., Gittleman, J. & Chapman, C. (1993) Behav. Ecol. Sociobiol. 32, 199-209. [Google Scholar]
- 40.Rowe, N. (1996) The Pictorial Guide to the Living Primates (Pogonias, East Hampton, NY).
- 41.Hofman, M. (1989) Prog. Neurobiol. 32, 137-158. [DOI] [PubMed] [Google Scholar]
- 42.Preuss, T. (1995) J. Cognit. Neurosci. 7, 1-24. [DOI] [PubMed] [Google Scholar]
- 43.Simons, E. (1993) Am. J. Sci. 293A, 383-390. [Google Scholar]
- 44.Rakic, P. (1988) Science 241, 170-176. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson, K. J. & Weller, W. L. (1990) Brain Res. Dev. Brain Res. 55, 269-274. [DOI] [PubMed] [Google Scholar]
- 46.Bayer, S. & Altman, J. (1991) Neocortical Development (Raven, New York).
- 47.Schmidt-Nielsen, K. (1984) Scaling: Why Is Animal Size So Important? (Cambridge Univ. Press, Cambridge, U.K.).
- 48.Bush, E. & Allman, J. (2003) Brain Behav. Evol. 61, 1-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.