Abstract
Purpose
To evaluate the effect of aflibercept treatment after developing ranibizumab tachyphylaxis for the treatment of polypoidal choroidal vasculopathy (PCV).
Patients and methods
Ten eyes from ten patients with PCV who developed ranibizumab tachyphylaxis were reviewed. Tachyphylaxis was defined as when repeated intravitreal ranibizumab (IVR) resulted in a complete lack of response after initial treatment response. All treatments were converted to intravitreal aflibercept (IVA) after development of ranibizumab tachyphylaxis. Central retinal thickness (CRT) was compared at baseline, at complete resolution after IVR, at reactivation after IVR, at initial IVA, and at 4 and 12 weeks after initial IVA.
Results
Mean number of IVR treatments before conversion to IVA was 11.3 (range 5–16). All eyes had positive therapeutic responses after conversion to IVA. Mean CRT at 4 and 12 weeks after initial IVA was significantly decreased from baseline initial IVA (P = 0.005).
Conclusion
Switching therapy to aflibercept is effective for patients with PCV who develop tachyphylaxis to ranibizumab.
Keywords: polypoidal choroidal vasculopathy, ranibizumab, aflibercept, tachyphylaxis
Introduction
Polypoidal choroidal vasculopathy (PCV), which was first described by Yannuzzi et al, is characterized by numerous recurrent, bilateral, asymmetric, serosanguinous detachments in the retinal pigment epithelium.1 Several treatment strategies have been reported for PCV.2 Among them, photodynamic therapy3 and intravitreal injection of anti-vascular endothelial growth factor (VEGF) including ranibizumab4 (Lucentis; Genentech, San Francisco, CA, USA) have shown favorable results. However, several problems have been reported with these treatments. Serious complications after photodynamic therapy for PCV were reported, such as massive subretinal hemorrhage and retinal atrophy.5 With anti-VEGF therapy, polypoidal lesions regressed in only 40% of the cases.4 Hence, prolonged treatment might be required for PCV. With prolonged use of ranibizumab, diminution of biological effects adversely affect long-term efficacy. One possible mechanism of diminution is tachyphylaxis, which is defined as phenomena causing reduced drug efficacy by repeated administration.6 The chance of developing tachyphylaxis was 2% after intravitreal ranibizumab (IVR) for age-related macular degeneration (AMD),7 and prevention of tachyphylaxis was important for long-term therapeutic effects against PCV.
One possible countermeasure for tachyphylaxis is cessation of drug treatment;6 however, discontinuation of the treatment might result in irreversible retinal damage by deterioration from PCV. Another possible countermeasure for tachyphylaxis is introduction of a drug with a different mechanism of action. Aflibercept (Eylea; Regeneron, Tarrytown, PA, USA and Bayer HealthCare, Berlin, Germany) is a recombinant soluble decoy receptor fusion protein.8 In contrast to the antibody-based VEGF binding strategy used by ranibizumab, aflibercept incorporates the second binding domain of the VEGF receptor-1 and the third domain of the VEGF receptor-2.9 The binding affinity of intravitreal aflibercept (IVA) to VEGF is higher than that of ranibizumab.9 A rapid improvement after conversion to aflibercept from ranibizumab was reported for eyes with chronic refractory AMD.10 Conversion to aflibercept might be a prospective countermeasure for ranibizumab tachyphylaxis in PCV. In this study, we evaluated the visual and anatomical response of IVA in eyes with PCV after development of ranibizumab tachyphylaxis, and evaluated therapeutic effects 3 months after conversion from ranibizumab to aflibercept.
Material and methods
This retrospective study was performed according to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Boards of Tokyo Medical University and Ibaraki Medical Center. Informed consent for the examination was obtained from all patients. At each visit, all patients received a comprehensive ophthalmologic examination, including best-corrected visual acuity (BCVA), indirect ophthalmoscopy, slit-lamp biomicroscopy with a contact lens, fundus photography, and optical coherence tomography (OCT: 3D OCT-2000; Topcon, Tokyo, Japan). Central retinal thickness (CRT) was measured using an OCT B-scan image.
The clinical diagnosis of PCV was made by identification of polypoidal lesions with indocyanine green angiography. All eyes were initially treated with three consecutive intravitreal injections of 0.5 mg ranibizumab every 4 weeks. An additional IVR was given if any of the following changes were observed: (1) any serous retinal detachment, (2) increasing intraretinal fluid, (3) enlargement of a pigment epithelial detachment, and (4) new macular hemorrhages.
All eyes were converted to intravitreal injection of 2 mg aflibercept after diagnosis of ranibizumab tachyphylaxis. Tachyphylaxis was defined as the phenomenon in which the repeated intravitreal ranibizumab resulted in a complete lack of response after initial treatment response.7 Initial treatment response was defined as complete resolution of intra/subretinal fluid and pigment epithelial detachment after the initial treatment regimen. Lack of response was considered as an increase in intra/subretinal fluid or pigment epithelial detachment despite two or more intravitreal injections of ranibizumab. After an initial single injection of aflibercept, all eyes were examined every 4 weeks. Retreatment was applied according to the same protocol for intravitreal ranibizumab, at every 4 weeks, and retreatment was applied at least every 8 weeks, regardless of the findings.
For statistical analysis, BCVA, as measured with a Landolt chart, was converted to a logarithm of the minimal angle of resolution. CRT was manually measured using an OCT B-scan image. The BCVA and CRT were compared at baseline, at complete resolution after IVR, at reactivation after IVR, at initial IVA, and at 4 and 12 weeks after initial IVA. Statistical significance was calculated using a Wilcoxon signed-rank test and defined as P < 0.05. Statistical software (Statview 5.0; SAS Institute, Cary, NC, USA) was used for statistical analyses.
Results
This study included ten eyes of ten Japanese patients (seven males, three females) with PCV, ranging from 61 years to 85 years of age (mean: 73.3 years). All eye treatments were converted from ranibizumab to aflibercept because of ranibizumab tachyphylaxis. The mean greatest linear dimension at baseline was 3925 μm (range 850–7600 μm) based on fluorescein angiography, and 2830 μm (range 800–4600 μm) based on indocyanine green angiography. The mean BCVA at baseline was significantly improved at the initial treatment response by IVR (P = 0.025, Table 1). The mean CRT at baseline was significantly reduced at the initial treatment response period (P = 0.008, Table 1). The mean number of IVR for initial inactivation was 3.4 (range 3–5).
Table 1.
Treatment response to ranibizumab and aflibercept
| Mean visual acuity (logMAR) | Mean central retinal thickness (μm) | |
|---|---|---|
| Baseline (SD) | 0.53 (0.39) | 469 (148) |
| Stage of inactive disease (SD) | 0.32 (0.35) | 258 (76) |
| Stage of reactivation (SD) | 0.35 (0.20) | 341 (130) |
| Day of conversion to intravitreal aflibercept (SD) | 0.35 (0.32) | 385 (119) |
| 4 weeks after conversion (SD) | 0.31 (0.32) | 183 (48) |
| 12 weeks after conversion (SD) | 0.36 (0.31) | 223 (95) |
Abbreviations: logMAR, logarithm of the minimal angle of resolution; SD, standard deviation.
The mean number of IVR at reactivation was 7.9 (range 4–14), and the mean duration from initial IVR was 308 days (range 105–518 days). The mean CRT at reactivation was increased from the initial treatment response (P = 0.09, Table 1). After reactivation, the mean CRT was increased (P = 0.11, Table 1) despite monthly IVR (mean 4.4 injections, range 2–8 injections). All eyes were judged with ranibizumab tachyphylaxis based on complete lack of response, and were converted to IVA. Mean number of IVR before conversion to IVA was 11.3 (range 5–16). Indocyanine green angiography images at conversion to IVA showed polypoidal lesions in nine of ten eyes (Figure 1), and polypoidal lesion could not be detected in one eye. At 4 weeks after a single injection by IVA, OCT findings were improved in all eyes (Figure 1), and mean CRT was significantly reduced from time of first IVA (P = 0.005, Table 1). In the 12 weeks after conversion to IVA, three injections by IVA were applied to one eye, and two injections by IVA were applied to nine eyes. The mean CRT at 12 weeks after conversion was significantly reduced from the time of first IVA (P = 0.005, Table 1). In one eye with three injections, intra/subretinal fluid was gradually decreased throughout the observation period, and intra/subretinal fluid disappeared at 12 weeks after the first IVA. Among the eyes with two injections, five eyes showed increased intra/subretinal fluid at 8 weeks after IVA, and intra/subretinal fluid disappeared again at 4 weeks after retreatment. In the other four eyes with two injections, retreatment was applied at 8 week intervals without any recurrences. No significant changes in the mean BCVA were observed throughout the follow-up after initial treatment response.
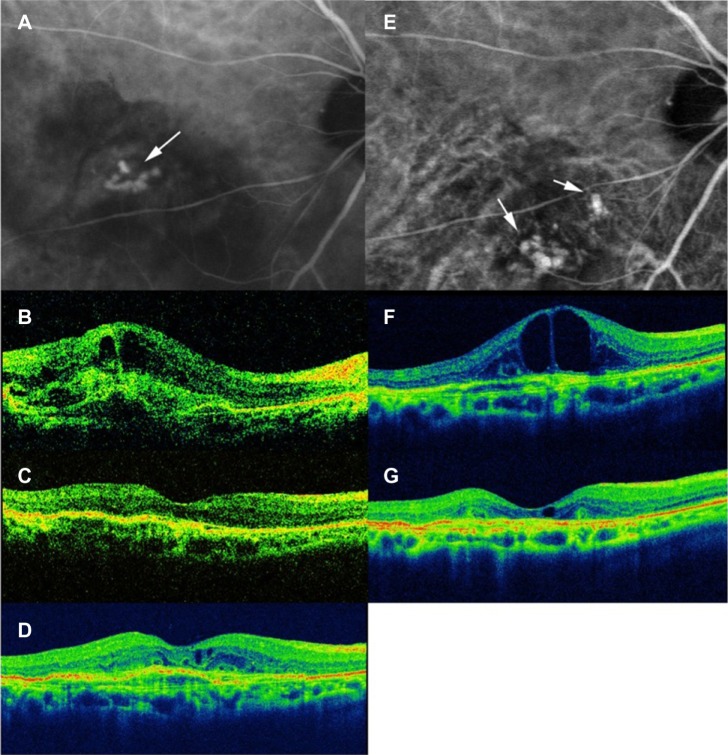
Figure 1.
Right eye of an 80-year-old female with polypoidal choroidal vasculopathy.
Notes: Indocyanine angiography image (A) at baseline shows polypoidal lesions (white arrow). Optical coherence tomography at baseline (B) shows intraretinal and subretinal fluid. Baseline visual acuity was 0.09. After three consecutive injections of IVR, intraretinal or subretinal fluid disappeared (C), and visual acuity was improved to 0.4. After eight as-needed injections of IVR, intraretinal fluid increased (D), and visual acuity was 0.4. Intraretinal fluid increased despite three consecutive IVR injections (F), and visual acuity was decreased to 0.3. Indocyanine angiography image (E) shows polypoidal lesions (white arrows) at different locations from baseline. Four weeks after three consecutive intravitreal aflibercept injections, intraretinal fluid decreased (G), and visual acuity improved to 0.4.
Abbreviation: IVR, intravitreal ranibizumab.
Discussion
Ranibizumab was approved by the United States Food and Drug Administration in December 2005, and ranibizumab became a mainstay for the treatment of AMD.11 In contrast, aflibercept was approved in November 2011, and accordingly, clinicians have only limited clinical experience with aflibercept compared to ranibizumab. The predicted biological activity of aflibercept is higher than ranibizumab,9,12 hence, aflibercept is used as a rescue drug for refractory AMD with ranibizumab. In this study, switching to aflibercept resulted in significant improvement in anatomical outcome in eyes with PCV with ranibizumab tachyphylaxis.
After approval of ranibizumab, monotherapy with ranibizumab became a leading therapeutic modality for AMD.11 Eyes with AMD might receive repeated IVR for a long time, and thereby the risk of tachyphylaxis or tolerance may potentially increase. In our study, all cases showed increases in intra/subretinal fluid or pigment epithelial detachment despite repeated intravitreal ranibizumab, which could be considered as tachyphylaxis rather than tolerance.7 One possible mechanism of ranibizumab tachyphylaxis is the development of emerging anti-ranibizumab antibodies.13 To prevent antibody development, use of drugs with different molecular structure could be an effective alternative. Switching the treatment to bevacizumab achieved 81% success rate after development of ranibizumab tachyphylaxis for AMD.14 In our study, conversion to aflibercept showed better success rates than bevacizumab. Bevacizumab and ranibizumab have similar protein structures.15 In contrast, aflibercept has a different molecular structure than ranibizumab,15 and this dissimilarity might produce a better success rate than bevacizumab.
Another possible mechanism of ranibizumab tachyphylaxis is the presence of another pathway other than the VEGF-A family. VEGF-A is the target of ranibizumab,16 and is considered important in the development of choroidal neovascularization through its potent angiogenic and vasopermeable abilities.17,18 However, for PCV vascular lesions, ranibizumab could regress the polypoidal lesions in PCV only in 40% of the cases, and ranibizumab had no effect on the regression of branching vascular networks.4,19 In addition to VEGF-A, other members of the VEGF family, including placental growth factor20 and VEGF-B21 have critical roles for angiogenesis and hyperpermeability, and these substances might be related to the activities of PCV vascular lesions. Afibercept could suppress these VEGF effects, and might have greater effectiveness for suppression of PCV vascular lesions.9 Another potential mechanism is upregulation of the receptors on the vascular lesions, thus the amount of given ranibizumab might be insufficient to suppress the PCV vascular lesions. The binding affinity of aflibercept to VEGF is higher than that of ranibizumab;9 hence aflibercept could have greater ability to treat this upregulation.
In the VIEW (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD) study, treatment every 2 months after three initial monthly shots with aflibercept was effective for treatment-naïve AMD.8 For eyes treated after development of ranibizumab tachyphylaxis, there is no established protocol for aflibercept treatment. In this study, five eyes showed increased intra/subretinal fluid at 8 weeks after IVA. This high recurrence rate suggests the necessity of more frequent injections, including three initial monthly injections. The limitations of our study include the small number of cases, and short follow-up periods after conversion to aflibercept. A long-term follow-up after conversion to aflibercept will be necessary to determine the appropriate treatment regimen. In this case series, patients did not have periodic indocyanine green angiography after baseline. This limits the study regarding the development of ranibizumab tachyphylaxis and efficacy of aflibercept for PCV. Photodynamic therapy might be an additional procedure to evaluate in treatment regimens. Further studies are required to establish the strategy of aflibercept treatment for PCV.
Conclusion
In conclusion, conversion to aflibercept is effective for PCV after development of ranibizumab tachyphylaxis.
Acknowledgments
This project was supported in part by KAKENHI (24592682).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina (Philadelphia, Pa) 1990;10(1):1–8. [PubMed] [Google Scholar]
- 2.Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29(1):19–29. doi: 10.1016/j.preteyeres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Gomi F, Ohji M, Sayanagi K, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115(1):141–146. doi: 10.1016/j.ophtha.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Hikichi T, Higuchi M, Matsushita T, et al. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol. 2012;154(1):117–124. e1. doi: 10.1016/j.ajo.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Akaza E, Yuzawa M, Mori R. Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2011;55(1):39–44. doi: 10.1007/s10384-010-0886-x. [DOI] [PubMed] [Google Scholar]
- 6.Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012;96(1):1–2. doi: 10.1136/bjophthalmol-2011-301236. [DOI] [PubMed] [Google Scholar]
- 7.Eghøj MS, Sørensen TL. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2012;96(1):21–23. doi: 10.1136/bjo.2011.203893. [DOI] [PubMed] [Google Scholar]
- 8.Heier JS, Brown DM, Chong V, et al. VIEW 1 and VIEW 2 Study Groups Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel KH, Chow CC, Rathod R, et al. Rapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumab. Eye (Lond) 2013;27(5):663–667. doi: 10.1038/eye.2013.31. quiz 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell P, Korobelnik JF, Lanzetta P, et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol. 2010;94(1):2–13. doi: 10.1136/bjo.2009.159160. [DOI] [PubMed] [Google Scholar]
- 12.Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92(5):667–668. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, ANCHOR Study Group Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65. e5. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Gasperini JL, Fawzi AA, Khondkaryan A, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012;96(1):14–20. doi: 10.1136/bjo.2011.204685. [DOI] [PubMed] [Google Scholar]
- 15.Chong V. Biological, preclinical and clinical characteristics of inhibitors of vascular endothelial growth factors. Ophthalmologica. 2012;227(Suppl 1):2–10. doi: 10.1159/000337152. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 17.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 18.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hikichi T, Higuchi M, Matsushita T, et al. Results of 2 years of treatment with as-needed ranibizumab reinjection for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2013;97(5):617–621. doi: 10.1136/bjophthalmol-2012-302652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(7):3186–3193. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F, Tang Z, Hou X, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009;106(15):6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]