Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) infections are associated with significant mortality and health care costs. To improve treatment outcomes for MRSA, a better understanding of the pharmacokinetic/pharmacodynamic parameters of vancomycin is required to develop optimal dosing strategies, particularly in elderly patients (≥75 years of age) with limited renal function. The purpose of this study was to determine whether pharmacokinetic indices for vancomycin are associated with mortality from MRSA hospital-acquired pneumonia in elderly patients.
Methods
We conducted a retrospective observational study with 28-day mortality as the primary outcome for 94 patients with MRSA hospital-acquired pneumonia who had been treated with vancomycin from January 2006 through December 2012. Our most recent sampling of MRSA isolates had a minimum inhibitory concentration (MIC) for vancomycin of 1 μg/mL (86%), indicating that the area under the curve (AUC) was equal to the AUC/MIC in these isolates. The primary data from 28-day survivors and nonsurvivors were compared.
Results
Among 94 elderly patients, the mean age was 82 (75–99) years. Multivariate analyses revealed that, among the factors examined, only the nonoptimal AUC (<250, >450 μg*h/mL) was an independent predictor of 28-day mortality in elderly patients (odds ratio 23.156, 95% confidence interval 6.814–78.687, P < 0.001). We detected a significant difference for increasing nephrotoxicity in nonsurvivors (nine of 32 patients [28%]) compared with survivors (three of 62 patients [4.8%], P = 0.003).
Conclusion
This finding indicates that patients with potentially poor renal function are likely to have increased AUC values and a poor prognosis. Consideration of the pharmacokinetics/pharmacodynamics of vancomycin and targeting an AUC/MIC value of 250–450 μg*h/mL may result in improved treatment outcomes for elderly patients with MRSA hospital-acquired pneumonia.
Keywords: methicillin-resistant Staphylococcus aureus, elderly patients, vancomycin, pharmacokinetics, pharmacodynamics
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) infections are associated with significant mortality and health care costs.1 In nearly all hospitals, MRSA has become one of the Gram-positive bacterial species associated with serious hospital-acquired infections.2–9 The population of patients most vulnerable to acquiring MRSA infections, including hospital-acquired pneumonia, is the elderly, whose immune systems are often affected by aging, underlying diseases, and medical interventions. In Japan, senior citizens aged 75 years and older, termed elderly, constitute over 10.4% of the population and represent the largest and most frequent users of health care facilities, such as hospitals and long-term skilled nursing and residential homes. Improving treatment outcomes for elderly patients with MRSA infections will therefore increase survival and quality of life, and reduce health care expenditure burdens.2
To date, MRSA hospital-acquired pneumonia has primarily been treated with intravenously administered vancomycin. In vitro and animal model studies investigating the pharmacodynamics of vancomycin indicate that the rate of MRSA killing depends primarily upon the duration of exposure to concentrations exceeding the minimum inhibitory concentration (MIC) value of the target strain. Although the area under the curve (AUC)/MIC ratio is the best predictor of efficacy in animal models,10,11 data characterizing the pharmacodynamic properties of vancomycin against MRSA in humans are limited. Although a study that examined clearance of MRSA from sputum suggested that an AUC/MIC ratio >400 may be effective,12 a study involving adult patients with MRSA pneumonia or sepsis failed to show an association between pharmacokinetic/pharmacodynamic parameters of vancomycin and treatment outcomes.13
In elderly individuals, renal clearance is significantly reduced. Creatinine clearance decreases with age but serum creatinine concentration remains relatively stable because elderly persons typically lose muscle mass as they age, often leading to overestimation of renal function in the elderly.14–17 Because vancomycin is eliminated from the body mainly via the kidneys, overestimation of renal function leads to increased vancomycin trough concentrations, which may result in nephrotoxicity. However, it is uncertain whether targeting higher blood concentrations leads to increased efficacy of vancomycin and/or risk of nephrotoxicity in elderly patients. We performed a retrospective observational study to determine whether two pharmacokinetic indices for vancomycin, ie, serum trough concentrations and AUC values, are associated with mortality from MRSA hospital-acquired pneumonia in elderly patients aged 75 years and older.
Materials and methods
Study location and patients
The study was conducted at the National Center for Geriatrics and Gerontology Hospital, Obu, Japan. This 320-bed hospital comprises general (including emergency) services, except for pediatrics, and admits approximately 5,000 patients per year (more than 50% of whom are aged over 75 years). MRSA is endemic in this hospital, and the ratio of MRSA isolates per total S. aureus isolates is approximately 70%.
During a 6-year period (from January 2006 through December 2012), all hospitalized patients aged 75 years or older with MRSA pneumonia microbiologically confirmed by sputum or blood cultures and treated with vancomycin therapy were identified using the clinical pharmacokinetics department computer database.
Study design and data collection
We conducted a retrospective 6-year observational study with 28-day mortality as the primary outcome for 94 patients with MRSA pneumonia treated using vancomycin. We also assessed the relationship between the effect of pharmacokinetic indices of vancomycin, including serum trough concentrations and AUC values, and the primary outcome. The clinical characteristics of the study patients were reviewed from hospital medical records. For patients with multiple episodes, only the first episode was counted. This study was approved by the ethics committee at the National Center for Geriatrics and Gerontology Hospital.
Definitions
The definition of hospital-acquired pneumonia was based on American Thoracic Society guidelines for the management of adults with hospital-acquired, ventilator-associated, and health care-associated pneumonia.2 Here, hospital-acquired pneumonia was defined as pneumonia occurring 48 hours or more after hospitalization, with an acute lung infection characterized by cough, fever, purulent sputum, and an abnormal chest X-ray, that was not deemed to be incubating at the time of admission. Among hospital-acquired pneumonia cases, those with MRSA isolated from blood culture or sputum which showed no sign of improvement after treatment with broad-spectrum antibiotics, such as carbapenem, for more than 3 days were defined as MRSA hospital-acquired pneumonia.
The severity rating of pneumonia was defined according to the Japanese Respiratory Society guidelines for management of hospital-acquired pneumonia18 and was used to divide the patients into severe, moderate, and mild groups. The severe group was defined as patients with three or more of the following risk factors or conditions: malignancy or immunocompromised status, impaired consciousness, requiring a fraction of inspired oxygen >35% to maintain oxygen saturation >90%, mean age 70 years or older (woman aged 75 years or older), and oliguria or dehydration. The moderate group was defined as patients with any two of the risk factors described above, and in addition, at least one of the following secondary risk factors: C-reactive protein ≥20.0 mg/L or extent of infiltration on chest X-ray covering at least two thirds of one lung. The mild group was defined as all other patients who were not compatible with severe or moderate criteria. Immunosuppression was defined as need for corticosteroids or immunosuppressive agents.2 Nephrotoxicity resulting from treatment with vancomycin was defined as an increase in serum creatinine of 0.5 mg/dL or a 50% increase from pretreatment levels.10 Patients were divided into 28-day survivors and nonsurvivors from the time of vancomycin administration.
Microbiologic data
Our institutional microbiology laboratory performed antimicrobial susceptibility tests of clinical isolates using a broth microdilution method with the MicroScan Pos Series PC6.1J panel (Siemens, Sacramento, CA, USA) according to Clinical Laboratory and Standards Institute recommendations.19 To determine more accurately the susceptibility of MRSA to vancomycin, the most recent (January 2010 through December 2012) MRSA samples collected from patients (n = 21) were sent to Mitsubishi Chemical Medience Corporation (Tokyo, Japan) for determination of a vancomycin MIC ranging from 0.5 to 2.0 μg/mL.
Pharmacokinetic data
The initial treatment schedule for vancomycin was simulated to achieve a trough concentration of 10–15 μg/mL with TDM software, using patient characteristics, including age, body weight, and serum creatinine (VCM-TDM Microsoft Excel version 3.0, Shionogi and Co, Ltd, Osaka, Japan). Serum concentrations of vancomycin were determined from samples collected on the fifth day from the start of drug administration. Blood samplings were performed twice, ie, once before vancomycin administration (trough), and once one hour after vancomycin administration (peak). The predicted 24-hour AUC values for vancomycin were calculated using TDM software based on peak and trough concentrations.
Statistical analysis
All comparisons were unpaired, and all tests of significance were two-tailed. Continuous variables were compared using the Student’s t-test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Categorical variables were compared using the Chi-square or Fisher’s Exact tests. The primary data from 28-day survivors and nonsurvivors were compared. The multivariable model was used to establish risk factors for mortality after treatment with vancomycin for MRSA pneumonia. Multivariable analysis were used for nephrotoxicity, the Charlson comorbidity index, and nonoptimal AUC.16 The predictive ability of the final model was quantified using Hosmer–Lemeshow statistical tests for goodness of fit. In these tests, a two-sided P-value of less than 0.05 was considered to be statistically significant. Statistical package for the Social Sciences SPSS version 12.0 software (SPSS Inc, Chicago, IL, USA) was used for the statistical analysis.
Results
Ninety-four elderly patients aged 75 years or older with MRSA pneumonia treated using vancomycin were eligible for the study. The median age of the study patients was 82 (range 75–99) years, with more than half of the patients (71/94 [76%]) residing in a nursing facility before hospital admission. Of the 94 patients, 18 (19%) had MRSA pneumonia and sepsis, while the remaining 76 (81%) were diagnosed with MRSA pneumonia without sepsis. MRSA was coisolated with Pseudomonas aeruginosa (n = 6) and/or Klebsiella pneumoniae (n = 8) and/or Acinetobacter species (n = 7) in 21 of 94 patients (22%). All patients had at least one comorbid disease, such as cardiovascular disease, dementia, or diabetes mellitus (data not shown).
The demographic data of the 28-day survivors (n = 62) and nonsurvivors (n = 32) are summarized in Table 1. The percentage of severe cases among survivors was 45% (28/62), whereas the rate was 78% (25/32) among nonsurvivors (P = 0.004). Mean (± standard deviation [range]) serum creatinine levels in the survivor group was 0.7 ± 0.4 (0.2–2.5) mg/dL while that in the nonsurvivor group was 0.8 ± 0.4 (0.2–2.1) mg/dL (P = 0.145). Survivors tended to have a lower mean Charlson comorbidity index (2.4 ± 1.8 [0–8]) than nonsurvivors (3.1 ± 2.5 [1–10], P = 0.089). Coexistence of MRSA bacteremia did not significantly differ between the two groups (23% versus 19%, respectively, P = 0.375).
Table 1.
Characteristics of study patients with methicillin-resistant Staphylococcus aureus pneumonia
| Characteristic | Survivors (n = 62) | Nonsurvivors (n = 32) | P-value |
|---|---|---|---|
| Age (years) | 83.0 ± 6.2 (75–99) | 81.8 ± 5.2 (75–93) | 0.162a |
| Gender (male/female) | 39/23 | 24/8 | 0.171b |
| Body weight (kg) | 43.1 ± 9.2 (27–70) | 42.5 ± 9.4 (28–72) | 0.388a |
| Serum creatinine (mg/dL) | 0.7 ± 0.4 (0.2–2.5) | 0.8 ± 0.4 (0.2–2.1) | 0.145a |
| Charlson comorbidity index | 2.4 ± 1.8 (0–8) | 3.1 ± 2.5 (1–10) | 0.089b |
| Combination antibiotic therapy | 32 (51.6%) | 20 (63%) | 0.216b |
| Diagnosis Pneumonia/Pneumonia and sepsis | 48/14 | 28/4 | 0.185b |
| Infection severity | |||
| Mild | 9 | 4 | 0.004c,* |
| Moderate | 25 | 3 | |
| Severe | 28 | 25 |
Notes:
P-values were determined by the Student’s t-test
Fisher’s Exact test
Chi-square test
P < 0.01, survivors versus nonsurvivors. Data are presented as the mean ± standard (range) deviation or the number of subjects (percentage).
The pharmacokinetic and pharmacodynamic parameters of vancomycin in survivors and nonsurvivors are summarized in Table 2. Notably, the mean trough and peak concentrations, calculated AUC values, and clearance of vancomycin did not differ significantly between the two groups (Table 2).
Table 2.
Pharmacokinetic and pharmacodynamic parameters for vancomycin between surviving and nonsurviving patients with methicillin-resistant Staphylococcus aureus pneumonia
| PK/PD parameters | Survivors (n = 62) | Nonsurvivors (n = 32) | P-value |
|---|---|---|---|
| Cmax (μg/mL) | 24.5 ± 8.2 | 25.5 ± 8.0 | 0.378 |
| Trough concentration (μg/mL) | 9.2 ± 8.2 | 10.0 ± 5.8 | 0.266 |
| AUC (μg*h/mL) | 344 ± 95.8 | 394.7 ± 209.9 | 0.104 |
| Vd (L) | 62.3 ± 6.6 | 63.6 ± 4.1 | 0.129 |
| T1/2 (hours) | 26.5 ± 13.1 | 31.5 ± 23.2 | 0.064 |
| CLr (mL/min) | 40.8 ± 16.9 | 35.5 ± 18.9 | 0.094 |
| Daily dose (mg/kg/day) | 20.9 ± 10.6 | 18.8 ± 6.6 | 0.119 |
| Dose interval (hours) | 26.5 ± 13.3 | 25.5 ± 7.8 | 0.322 |
Notes:P-values were determined by the Student’s t-test. Data were presented as the mean ± standard deviation.
Abbreviations: AUC, area under the curve; Vd, volume of distribution; CLr, renal clearance; PD, pharmacodynamic; PK, pharmacokinetic; Cmax, concentration max; T1/2, half-life.
Stratification of vancomycin trough concentrations revealed no statistically significant relationships with 28-day mortality at any of the breakpoints evaluated (P = 0.603, Figure 1A). However, the AUC values of <250 or >450 μg*h/mL were significantly associated with 28-day mortality (P< 0.001, Figure 1B).
Figure 1.
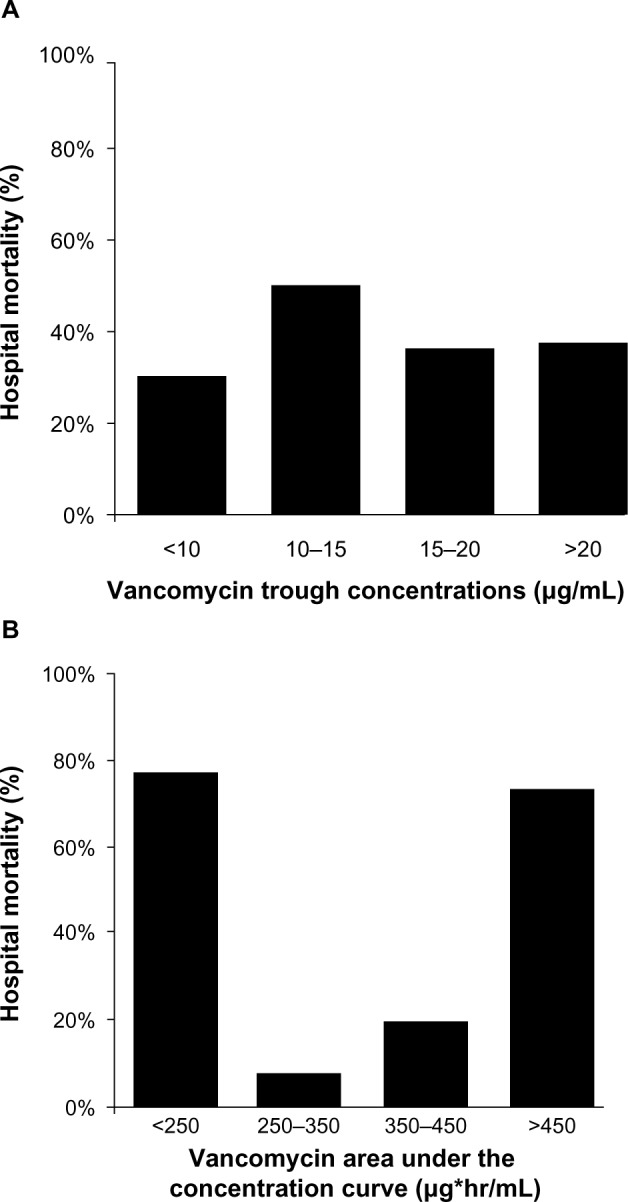
Twenty-eight-day mortality according to stratification of vancomycin trough concentrations and AUC values. Data were presented as percentages. P values were determined by χ2 tests. Stratification of vancomycin trough concentrations revealed no statistically significant relationship with 28-day mortality at any of the breakpoints evaluated (P = 0.603 [A]). However, AUC values of 250–350 μg*h/mL and 350–450 μg*h/mL were associated with significantly lower 28-day mortality than AUC values <250 and >450 μg*h/mL (P < 0.001 [B]).
Abbreviation: AUC, area under the concentration curve.
We next investigated the risk factors for mortality after treatment of MRSA pneumonia with vancomycin (Table 3). Univariate analysis was used to compare survivors and nonsurvivors. The following risk factors were found to be associated with 28-day mortality: nephrotoxicity and Charlson comorbidity index, nonoptimal AUC (<250, >450 μg*h/mL, Table 3). These risk factors were analyzed by multivariate analysis with multiple logical regression, finding the following as 28-day mortality: nephrotoxicity (P = 0.309, odds ratio [OR] 2.544; 95% confidence interval [CI] 0.421–15.371), Charlson comorbidity index (P = 0.128, OR 1.236; 95% CI 0.941–1.625), nonoptimal AUC (P < 0.001, OR 23.156; 95% CI 6.814–78.687, Table 3). The multiple logistic regression model was well calibrated among deciles of observed and expected risk in survivors and nonsurvivors (Hosmer–Lemeshow test, P = 0.194, Table 3).
Table 3.
Risk factors for mortality after treatment of methicillin-resistant Staphylococcus aureus pneumonia with vancomycin
| Variable | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Age | 0.983 | 0.881–1.097 | 0.763 | |||
| Nephrotoxicity | 2.570 | 0.420–15.734 | 0.307 | 2.544 | 0.421–15.371 | 0.309 |
| Infection severity | 1.021 | 0.268–3.889 | 0.976 | |||
| Charlson comorbidity index | 1.236 | 0.924–1.654 | 0.154 | 1.236 | 0.941–1.625 | 0.128 |
| Combination antibiotic therapy | 0.745 | 0.195–2.852 | 0.667 | |||
| Nonoptimal AUC (<250, >450 μg*h/mL) | 25.377 | 5.791–111.203 | < 0.001 | 23.156 | 6.814–78.687 | < 0.001 |
Note: Hosmer–Lemeshow test, P = 0.134.
Abbreviations: AUC, area under the concentration curve; CI, confidence interval.
Our institutional microbiology laboratory determined that all MRSA isolates from study patients (n = 94) had a vancomycin MIC of less than 2.0 μg/mL. To determine more accurately the vancomycin MIC, recent consecutive MRSA isolates (n = 21) were sent out and subjected to susceptibility testing by the microdilution method at a commercial microbiology laboratory. The majority of isolates were determined to have a vancomycin MIC of 1 μg/mL (n = 18, 86%), with the remaining isolates having a vancomycin MIC of 0.5 μg/mL (n = 3, 14%).
We also examined adverse effects and found that 12 of 94 (13%) developed nephrotoxicity on vancomycin (Table 4). We detected a significant difference for increasing nephrotoxicity in nonsurvivors (nine of 32 patients [28%]) compared with survivors (three of 62 patients [4.8%], P = 0.003).
Table 4.
Adverse effects of vancomycin among survivors and nonsurvivors
| Adverse effect | Survivors (n = 62) | Nonsurvivors (n = 32) | P-value |
|---|---|---|---|
| Nephrotoxicity | 3 (4.8%) | 9 (28%) | 0.003* |
| Liver dysfunction | 3 (4.8%) | 0 (0%) | 0.282 |
| Skin rash | 2 (3.2%) | 1 (3.2%) | 0.718 |
Notes:P-values were determined by Fisher’s Exact test
P < 0.01, survivors versus nonsurvivors. Data are presented as the number of subjects (percentage).
Discussion
This retrospective observational study shows that a vancomycin AUC value of 250–450 μg*h/mL predicted 28-day mortality in elderly patients with MRSA pneumonia. Our findings suggest that a better understanding of the pharmacodynamics of vancomycin in the elderly may allow improved dosing strategies and treatment outcomes for MRSA infections in this vulnerable population.
There are studies20–22 showing that bacteremia during MRSA hospital-acquired pneumonia impacts on the prognosis, and comorbid conditions such as malignancy lead to a poor outcome.20 Among the elderly patients with MRSA pneumonia examined in our study, many had severe comorbid conditions, such as malignancy, cardiovascular disease, and severe diabetes mellitus (Table 1). Further, the Charlson comorbidity index tended to be higher in nonsurvivors compared with survivors. In addition, in univariate analyses, the percentage of patients with sepsis was not significantly different between survivors and nonsurvivors, and the percentage of severe cases among nonsurvivors was higher than that among survivors (P = 0.004, Table 1). However, no significant differences in these factors were identified between the two groups in multivariate analysis. Although these factors were shown to impact on the prognosis in previous studies, our results suggest that sepsis and severity of infection were lower risk factors for mortality compared with nonoptimal AUC. There was a difference in the age groups between our study and other study,20–22 we considered that aging had an impact on the prognosis.
Several studies have examined the relationship between vancomycin treatment outcomes and the in vitro susceptibility of MRSA strains.23–27 The results of these studies prompted an expert recommendation for administration of higher vancomycin doses with targeted trough levels of 15–20 μg/mL when treating MRSA pneumonia.13,24 However, the prevalence of MRSA clinical strains with a high vancomycin MIC and whether higher trough concentrations increase the efficacy of vancomycin and/or risk of nephrotoxicity remain uncertain, particularly in the elderly. In the present study, nephrotoxicity attributable to vancomycin was observed in 13 of 94 patients. In nonsurvivors (n = 32), the rate of nephrotoxicity was 28%, whereas that among survivors (n = 62) was only 4.8% (P = 0.003, Table 4). We identified a significant difference in the increased nephrotoxicity associated with vancomycin between surviving and nonsurviving patients. In our study, the mortality rate in patients with an AUC >450 μg*h/mL was 73% (11 of 15 patients), and these 11 patients showed inferior renal clearance of vancomycin compared with the 83 patients with an AUC < 450 μg*h/mL (P = 0.005, data not shown). This finding indicates that patients with potentially poor renal function are likely to have increased AUC values and a poor prognosis.
A few limitations of our study warrant mention. First, the number of patients with MRSA pneumonia analyzed in this study was relatively small. Second, the AUC values were not adjusted with the MIC of the MRSA isolates. The relationship between vancomycin treatment outcomes and the in vitro susceptibility of MRSA strains has been examined in several studies, which have demonstrated that clinical failure rates are significantly higher in patients infected by MRSA isolates with a vancomycin MIC of ≥2 μg/mL.23–25 Further, for the treatment of infections due to MRSA isolates with a vancomycin MIC >1 μg/mL, optimal pharmacodynamic targets may not be achievable using standard vancomycin doses, even though these isolates are reported as susceptible by Clinical Laboratory and Standards Institute standards.26,27 Therefore, the resulting AUC/MIC ratio would be significantly affected by only small variations in the MIC. During the 6-year period of the present study, all MRSA isolates had a vancomycin MIC ≤ 2 μg/mL, as determined at our institute. Further, in our most recent sampling survey of MRSA (n = 21), the 18 MRSA isolates had a vancomycin MIC of 1 μg/mL (86%), indicating that these isolates had an AUC equal to AUC/MIC (the remaining three isolates showed a vancomycin MIC of 0.5 μg/mL [14%]). Although therapeutic guidelines for vancomycin recommend a target AUC/MIC for vancomycin of >400 μg*h/mL,10 our present findings suggest that an AUC of 250–450 μg*h/mL is suitable for treatment of MRSA pneumonia with vancomycin in the elderly. As for the reason of this discrepancy, all isolates from study patients were considered to have a vancomycin MIC #1 μg/mL ≤1 μg/mL, and those elderly patients aged 75 years older with potentially poor renal function had increased AUC values and displayed poor prognosis. Notably, all patients who had an AUC < 250 μg*h/mL died, meaning that we have to consider alternative agents rather than vancomycin for elderly patients aged 75 years and older with poor renal function who have difficulty controlling the correct dosage of vancomycin.
Conclusion
To the authors’ knowledge, this is the first study to examine the pharmacokinetics of vancomycin in elderly patients aged 75 years and older with MRSA pneumonia. Our results suggest that an AUC of 250–450 μg*h/mL is a suitable target for initial empiric treatment of MRSA pneumonia in the elderly until the vancomycin MIC is determined. In elderly patients with potentially poor renal function, administration of vancomycin may not be suitable and it may be necessary to consider alternative agents in such cases. We recommend that once the MIC is determined, the dose should be adjusted according to the MIC. We also consider that regular surveillance of the institutional antibiogram (such as vancomycin MIC values) will contribute to optimal use of anti-MRSA agents, including vancomycin, resulting in more effective and safer treatment for elderly patients infected with MRSA.
Acknowledgments
This work was supported by Research Funding for Longevity Sciences (21A-20) from the National Center for Geriatrics and Gerontology, Japan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.NNIS System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued Aug 2003. Am J Infect Control. 2003;31:481–498. doi: 10.1016/j.ajic.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 4.Diekema DJ, Pfaller MA, Schmitz FJ, et al. SENTRY Participants Group Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;15:S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 6.Osmon S, Ward S, Fraser VJ, Kollef MH. Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest. 2004;125:607–616. doi: 10.1378/chest.125.2.607. [DOI] [PubMed] [Google Scholar]
- 7.Chung DR, Song JH, Kim SH, et al. Asian Network for Surveillance of Resistant Pathogens Study Group High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 8.Ucgun I, Dagli CE, Kiremitci A, Yildirim H, Ak G, Aslan S. Effects of isolation rooms on the prevalence of hospital acquired pneumonia in a respiratory ICU. Eur Rev Med Pharmacol Sci. 2013;1:2–8. [PubMed] [Google Scholar]
- 9.Tacconelli E, De Angelis G. Pneumonia due to methicillin-resistant Staphylococcus aureus: clinical features, diagnosis and management. Curr Opin Pulm Med. 2009;15:218–222. doi: 10.1097/MCP.0b013e3283292666. [DOI] [PubMed] [Google Scholar]
- 10.Martin JH, Norris R, Barras M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society Of Infectious Diseases Pharmacists. Clin Biochem Rev. 2010;31:21–24. [PMC free article] [PubMed] [Google Scholar]
- 11.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 12.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 13.Jeffres MN, Isakow W, Doherty JA, et al. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest. 2006;130:947–955. doi: 10.1378/chest.130.4.947. [DOI] [PubMed] [Google Scholar]
- 14.Feely J, Coakley D. Altered pharmacodynamics in the elderly. Clin Geriatr Med. 1990;6:269–283. [PubMed] [Google Scholar]
- 15.Liu GG, Christensen DB. The continuing challenge of inappropriate prescribing in the elderly: an update of the evidence. J Am Pharm Assoc. 2002;42:847–857. doi: 10.1331/108658002762063682. [DOI] [PubMed] [Google Scholar]
- 16.Hanlon JT, Schmader KE, Koronkowski MJ, et al. Adverse drug events in high risk older outpatients. J Am Geriatr Soc. 1997;45:945–948. doi: 10.1111/j.1532-5415.1997.tb02964.x. [DOI] [PubMed] [Google Scholar]
- 17.Corsonello A, Pedone C, Incalzi RA. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem. 2010;17:571–584. doi: 10.2174/092986710790416326. [DOI] [PubMed] [Google Scholar]
- 18.Seki M, Watanabe A, Mikasa K, Kadota J, Kohno S. Revision of the severity rating and classification of hospital-acquired pneumonia in the Japanese Respiratory Society guidelines. Respirology. 2008;13:880–885. doi: 10.1111/j.1440-1843.2008.01348.x. [DOI] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. M100-S18. Performance standards for antimicrobial susceptibility testing, 18th informational supplement. Clinical and Laboratory Standards Institute: Wayne, PA; 2008. [Google Scholar]
- 20.Kızılarslanoğlu MC, Sancak B, Yağcı S, Hasçelik G, Unal S. Evaluation of methicillin-resistant Staphylococcus aureus bacteremia and comparison of prognosis according to vancomycin MIC values: experience of the last ten years. Mikrobiyol Bul. 2013;47:199–210. doi: 10.5578/mb.4530. Turkish. [DOI] [PubMed] [Google Scholar]
- 21.Castillo JS, Leal AL, Cortes JA, et al. Mortality among critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia: a multicenter cohort study in Colombia. Rev Panam Salud Publica. 2012;32:343–350. doi: 10.1590/s1020-49892012001100004. [DOI] [PubMed] [Google Scholar]
- 22.Chen LY, Chen LK, Chang CW, et al. Treatment of community-onset methicillin-resistant Staphylococcus aureus (MRSA) bacteremia: a hospital-based study. Arch Gerontol Geriatr. 2012;55:152–156. doi: 10.1016/j.archger.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 25.Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52:3315–3320. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuti JL, Kiffer CR, Mendes CM, Nicolau DP. Pharmacodynamic comparison of linezolid, teicoplanin and vancomycin against clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci collected from hospitals in Brazil. Clin Microbiol Infect. 2008;14:116–123. doi: 10.1111/j.1469-0691.2007.01885.x. [DOI] [PubMed] [Google Scholar]
- 27.Mohr JF, Murray BE. Point: vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2007;44:1536–1542. doi: 10.1086/518451. [DOI] [PubMed] [Google Scholar]


