SUMMARY
Cardiovascular growth must balance stabilizing signals required to maintain endothelial connections and network integrity with destabilizing signals that enable individual endothelial cells to migrate and proliferate. The cerebral cavernous malformation (CCM) signaling pathway utilizes the adaptor protein CCM2 to strengthen endothelial cell junctions and stabilize vessels. Here we identify a CCM2 paralogue, CCM2L, that is expressed selectively in endothelial cells during periods of active cardiovascular growth. CCM2L competitively blocks CCM2-mediated stabilizing signals biochemically, in cultured endothelial cells, and in developing mice. Loss of CCM2L reduces endocardial growth factor expression and impairs tumor growth and wound healing. Our studies identify CCM2L as a molecular mechanism by which endothelial cells coordinately regulate vessel stability and growth during cardiovascular development as well as postnatal vessel growth.
INTRODUCTION
The heart and blood vessels mediate gas exchange and deliver nutrients, signaling molecules and circulating cells to the tissues of the body. The vertebrate cardiovascular system is lined by specialized endothelial cells that direct its growth and function. During cardiovascular development the heart and vessels are first formed by endothelial cells arise from mesodermal precursors through a process of cardiogenesis and vasculogenesis (Potente et al., 2011; Risau, 1997). After de novo formation of the heart and earliest embryonic vessels, vascular growth occurs through angiogenic sprouting of endothelial cells from pre-existing vessels (Potente et al., 2011).
In the functioning cardiovascular system endothelial cells must be tightly connected to each other through cell-cell junctions to maintain a closed vascular network through which blood can circulate (Dejana et al., 2009). In contrast, during angiogenesis endothelial cells must transiently disconnect from each other and the existing network in order to proliferate and migrate. Endothelial cell junctions and vessel stability must therefore be molecularly regulated during vascular growth in a highly spatially and temporally coordinated manner to allow growth without compromising the integrity of the existing cardiovascular network. Vascular endothelial growth factor (VEGF), a protein that both loosens endothelial junctions and stimulates endothelial proliferation (Murohara et al., 1998; Senger et al., 1983), is one such regulator. However, since tumor vessels are able to overcome the effects of VEGF blockade other molecular mechanisms of regulating vessel stability and vessel growth must exist, and their identification is critical to design more effective therapies.
The cerebral cavernous malformation (CCM) signaling pathway has recently been identified as a critical positive regulator of endothelial junctions and vessel stability. The CCM pathway consists of three adaptor proteins, KRIT1 (aka CCM1), CCM2, and PDCD10 (aka CCM3) that were identified as disease genes in patients with cerebral vascular malformations. The CCM proteins bind each other (Voss et al., 2007) and the HEG receptor (Kleaveland et al., 2009). Human CCMs exhibit defective endothelial junctions (Clatterbuck et al., 2001), and loss of HEG, CCM1, CCM2 or CCM3 function results in abnormal endothelial cell junctions and vascular lumen formation in mice and zebrafish in vivo, and endothelial cells in vitro (Glading et al., 2007; Kleaveland et al., 2009; Stockton et al., 2010; Whitehead et al., 2009; Zheng et al., 2010). Genetic studies in mice and fish have also demonstrated that the CCM signaling pathway plays an essential and conserved role in cardiovascular development. Mice and fish lacking CCM1 or CCM2 fail to develop lumenized branchial arch arteries that connect the heart to the aorta (Whitehead et al., 2009; Whitehead et al., 2004; Zheng et al., 2010), and loss of HEG in both species confers defects in heart growth (Kleaveland et al., 2009; Mably et al., 2006; Mably et al., 2003). We hypothesized that the HEG-CCM signaling pathway must be regulated to permit efficient angiogenesis and cardiogenesis.
To test this hypothesis we searched for novel regulators of angiogenesis that might function through the CCM pathway. We demonstrate that a paralogue of CCM2 (CCM2L) opposes the stabilizing effects of CCM signaling to liberate angiogenic endothelial cells during cardiovascular growth. Biochemical studies and genetic analysis of mice lacking HEG, CCM2 and/or CCM2L reveal that CCM2L functions by competing with CCM2 for binding to the HEG-CCM1 complex and uncoupling these upstream components of the pathway from CCM3, a critical stability effector, while activating expression of factors that support cardiovascular growth. Loss of CCM2L prevents tumor growth in mice and delays wounding healing, findings consistent with a specific role in regulating angiogenesis in vivo. Ccm2LGFP reporter mice reveal that CCM2L expression in vivo is detected only in endothelial cells, especially those that participate in active cardiovascular growth. We propose that CCM2L functions as a molecular mechanism through which CCM signaling converts endothelial cells from a stable to an angiogenic phenotype and by which endothelial responses in vascular disease and growth may be specifically targeted.
RESULTS
Identification of a CCM2 paralogue that binds CCM1 and HEG but not CCM3
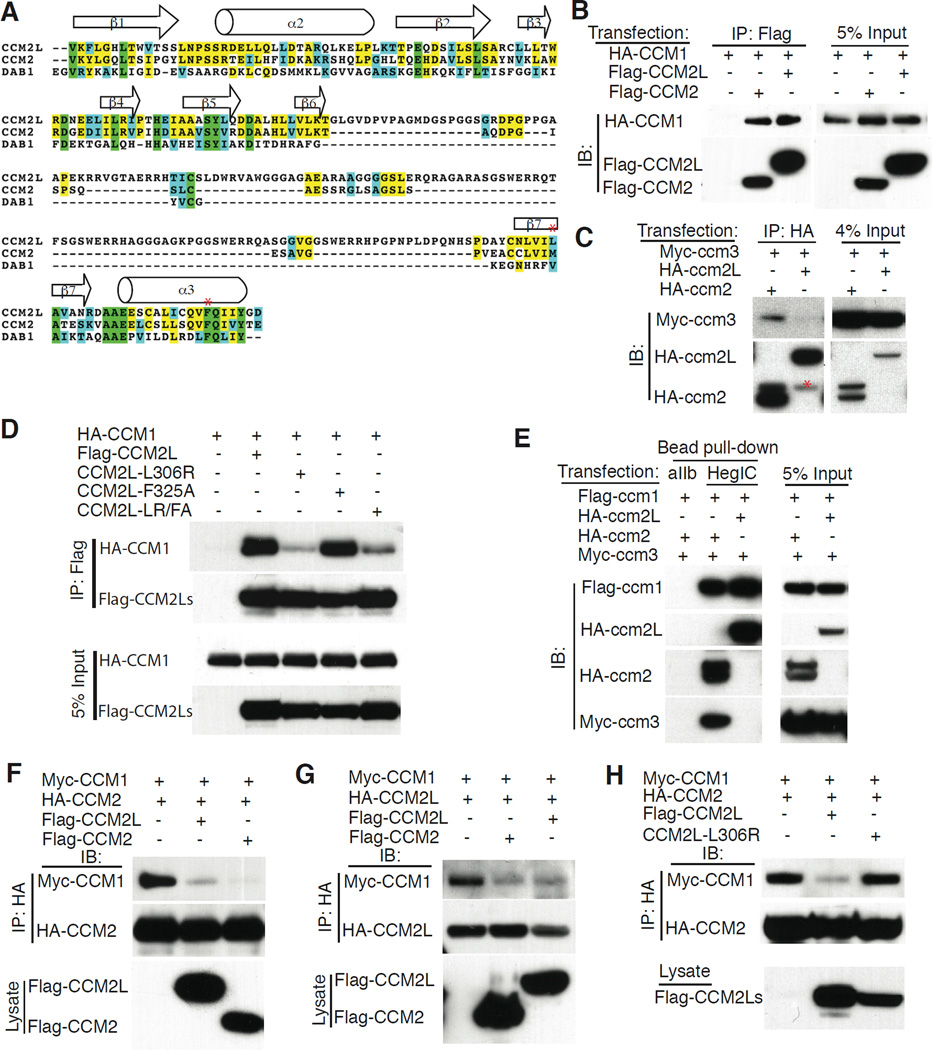
To identify potential novel regulators of the CCM signaling pathway we used BLAST searching of the EST and Ensemble databases to identify genes encoding structurally related proteins. A gene that encodes a protein predicted to be highly homologous to CCM2 that we designated CCM2L (aka BC020535 in the mouse and C20orf160 in the human) was identified in the human, mouse and zebrafish databases (Figure 1A and Supp. Fig. 1). The best-characterized domain of the CCM2 protein is the phospho-tyrosine binding (PTB) domain that mediates interaction with CCM1 (Zawistowski et al., 2005). Ccm2L encodes a PTB domain that contains a long insertion between the β6 and β7 sheets but is highly homologous to that of CCM2 in the β5 strand through which DAB 1-like PTB domains are believed to interact with peptide ligands (Fig. 1A and (Stolt et al., 2003; Uhlik et al., 2005)), suggesting that CCM2L may also bind CCM1.
Figure 1. CCM2L competes with CCM2 for binding to CCM1 but does not bind CCM3.
A. ClustalW alignment of the predicted PTB domains of mouse CCM2L, CCM2 and DAB1 are shown. Green shading indicates identity in all 3 proteins; yellow shading indicates identity in at least two proteins; blue shading indicates conserved residues in at least two proteins. Red asterisks indicate CCM2L L306 and F325 amino acid residues. The predicted beta strands and alpha helices are ordered according to those of DAB 1 PTB domain. B. CCM2L binds CCM1. Flag-CCM2 or Flag-CCM2L was co-expressed with HA-CCM1 in HEK293T cells and anti-Flag immunoprecipitations (IP) were performed. C. CCM2L does not bind CCM3. HA-ccm2 or HA-ccm2L was co-expressed with Myc-ccm3 in HEK293T cells and anti-HA immunoprecipitations (IP) were performed. Red asterisk notes a weak nonspecific detection of the IgG band. D. The CCM2L L306R but not the CCM2L F325 A mutation disrupts CCM2L-CCM1 interaction. Flag-tagged CCM2L, CCM2L-L306R, CCM2L-F325A, or CCM2L-L306R/F325A was co-expressed with HA-CCM1 and anti-Flag immunoprecipitations (IP) were performed. E. The HEG intracellular tail forms a complex with CCM1 and CCM2L that does not include CCM3. HA-ccm2L or HA- ccm2, FLAG- ccm1 and Myc-ccm3 were expressed in HEK293 cells and pulldowns performed with affinity matrices containing the intracellular tail of either the aIIb integrin subunit (aIIb) or the HEG receptor (HegIC). Total protein expression in pulldown input is shown by immunoblot (IB) analysis (right). F-H. CCM2L and CCM2 compete for CCM1 binding. F. Cell lysates containing the same amount of MYC-CCM1 and HA-CCM2 were mixed with control lysate or lysate containing FLAG-CCM2L (middle lane) or FLAG-CCM2 (right lane) and HA-CCM2 immunoprecipitated with anti-HA antibodies. The amounts of immunoprecipitated HA-CCM2 and co-immunoprecipitated MYC-CCM1 are shown above and the amounts of FLAG-CCM2L and FLAG-CCM2 added are shown below. Note that addition of FLAG-CCM2L or FLAG-CCM2 reduce the level of CCM1 that is co-immunoprecipitated with HA-CCM2 to a similar degree. G. The level of MYC-CCM1 co-immunoprecipitated with HA-CCM2L in the presence of either FLAG-CCM2L or FLAG-CCM2 was measured as in F. H. The level of MYC-CCM1 co-immunoprecipitated with HA-CCM2 is reduced in the presence of FLAG-CCM2L, but not FLAG-CCM2L-L306R.
Biochemical studies have demonstrated that CCM2 binds CCM1 via its PTB domain, and CCM3 through an as yet undefined region of the protein, to nucleate a signaling complex that is recruited to the HEG receptor by CCM1 (Kleaveland et al., 2009; Voss et al., 2009; Zawistowski et al., 2005; Zheng et al., 2010). To determine if CCM2L proteins are also able to complex with CCM1 and CCM3, epitope-tagged CCM2 and CCM2L proteins were co-expressed with CCM1 and/or CCM3 in HEK293T cells and co-immunoprecipitation experiments performed. CCM1 co-immunoprecipitated efficiently with both CCM2 and CCM2L (Fig. 1B). In contrast, CCM3 co-immunoprecipitated with CCM2 and not with CCM2L (Fig. 1C). Studies of CCM2 binding to CCM1 have identified two PTB domain point mutants, L198R and F217A, that disrupt binding to CCM1 (Zawistowski et al., 2005). To further investigate the mechanism by which CCM2L binds CCM1 we next tested whether the equivalent CCM2L PTB domain point mutants, L306R and F325A (see Fig. 1A for comparison to mouse sequence in which L198 is M198), are capable of binding CCM1. The L306R point mutant that is equivalent to a CCM disease-associated mutation in CCM2 (Denier et al., 2004), but not the F325A mutant, conferred severe loss of CCM1 binding by CCM2L (Fig. 1D). Thus CCM2L binds CCM1 via its PTB domain in a manner that is similar but not identical to that of CCM2. Previous studies have demonstrated that the CCM protein complex associates highly efficiently with the HEG receptor intracellular tail (HEG-IC) via CCM1 (Kleaveland et al., 2009; Zheng et al., 2010). Beads coupled to the intracellular (IC) tail of HEG but not integrin αIIb efficiently pulled down a complex of ccm1, ccm2 and ccm3 when those proteins were co-expressed in HEK-293T cells (Fig. 1E). In contrast, when ccm1, ccm2L and ccm3 proteins were co-expressed, HEG-IC beads pulled down a complex containing only ccm1 and ccm2L (Fig. 1E). These findings indicate that both CCM2 and CCM2L interact with CCM1 and HEG via their PTB domains, but that ccm2L does not associate with ccm3, a critical downstream effector of the known CCM signaling pathway.
CCM2L competes with CCM2 for CCM1 binding
The finding that CCM2L binds CCM1 suggested that CCM2 and CCM2L may compete for CCM1 binding. Since endothelial CCM1 and CCM2 levels were too low to detect endogenous protein interaction in cultured endothelial cells and anti-CCM2L antibodies are not yet available, to determine if CCM2L and CCM2 compete for binding to CCM1 we compared the ability of known amounts of FLAG-tagged CCM2 or CCM2L to compete with HA-tagged CCM2 (Fig. 1F) or CCM2L (Fig. 1G) for binding to CCM1. The addition of FLAG-CCM2L or FLAG-CCM2 resulted in a dramatic reduction in the amount of CCM1 associated with HA-CCM2 (Fig. 1F). Conversely, the addition of FLAG-CCM2 or FLAG-CCM2L resulted in a dramatic reduction in the amount of CCM1 associated with HA-CCM2L (Fig. 1G). Finally, addition of CCM2L L306R, a PTB point mutant with severely reduced CCM1 binding (Fig. 1C), failed to competitively block CCM2 binding to CCM1 (Fig. 1H). These studies demonstrate that CCM2 and CCM2L compete for binding to CCM1 and that the expression of CCM2L may thereby prevent HEG-CCM1 complexes from associating with CCM3.
Ccm2L expression is spatially restricted to endothelial cells and temporally linked to cardiovascular growth
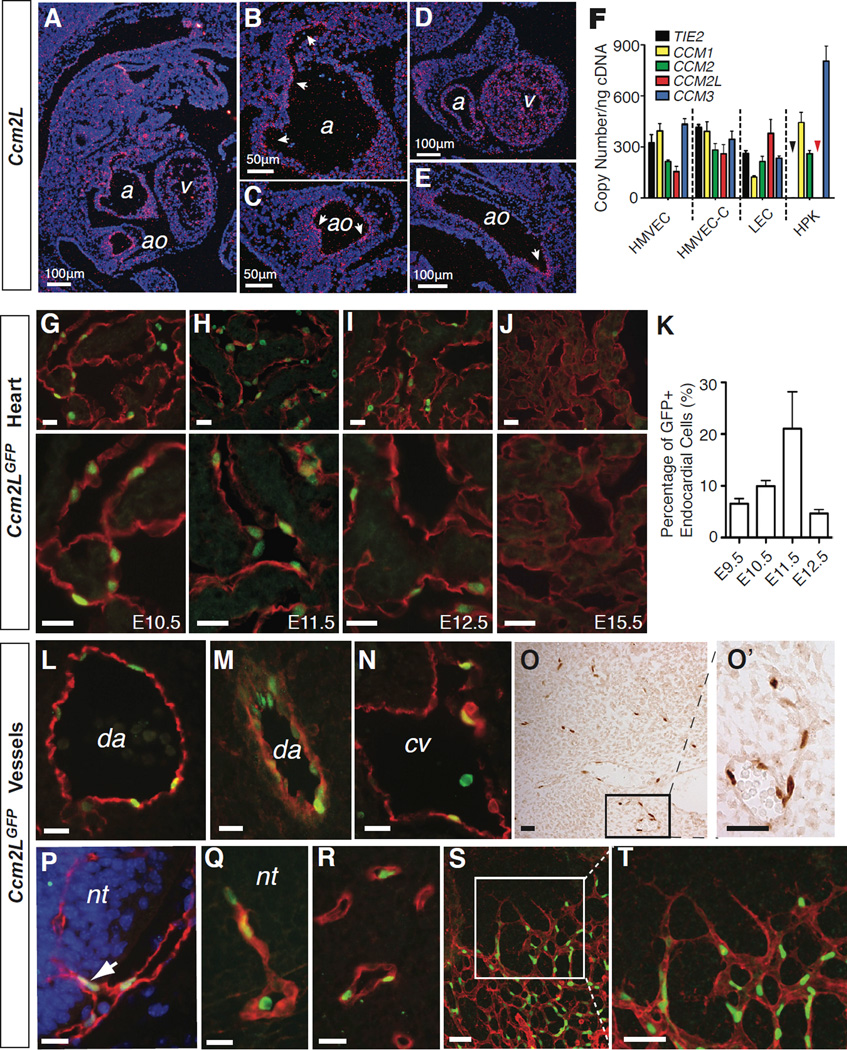
Although an essential function for CCM2 has been demonstrated in endothelial cells using genetic approaches (Boulday et al., 2009; Whitehead et al., 2009), endothelial Ccm2 levels are too low to be detected above background using either LacZ or GFP knockin reporter alleles or radioactive in situ hybridization (Kleaveland et al., 2009; Whitehead et al., 2009). Northern blot analysis of adult mouse tissues revealed Ccm2L mRNA expression in adult heart and lung, organs with large endothelial cell populations (Supp. Fig. 1). To better define the expression pattern of Ccm2L we performed in situ hybridization studies using mouse embryos from E9.5 to E18. Ccm2L mRNA was detected exclusively along the endothelial cell border of the developing heart and vesselsfs (Fig. 2A–E). Ccm2L expression was strongest in the E10.5 heart, but was not detected in the heart or elsewhere after E12.5 using in situ hybridization. Consistent with these findings in the mouse, RT-PCR detected CCM2L expression in primary human microvascular endothelial cells and primary human lymphatic endothelial cells, but not in primary human keratinocytes (Fig. 2F). In contrast to CCM2L and TIE2, a known endothelial specific gene, CCM1, CCM2 and CCM3 were detected in equal abundance in primary keratinocytes and endothelial cells. Measurement of transcript copy numbers revealed that CCM2L is expressed at levels similar to those of CCM1, CCM2 and CCM3 in endothelial cells (Fig. 2F). These studies suggest that CCM2L is expressed in an endothelial-specific pattern, and at a basal level that is similar to those of CCM1, CCM2 and CCM3.
Figure 2. Ccm2L is expressed in endothelial cells that participate in active cardiovascular growth.
A–E. In situ hybridization reveals endothelial-specific expression of Ccm2L. Shown are saggital sections of an E11.5 mouse embryo. Ccm2L signal is shown in pink (arrows) and DAPI staining of cell nuclei in blue. ao, aorta; cv, cardinal vein; a, atrium; v, ventricle. F. Quantitative RT-PCR measurement of mRNA transcripts encoding CCM1, CCM2, CCM2L, CCM3 and the endothelial-specific control gene TIE2 in cultured human microvascular endothelial cells from the skin (HMVEC) and heart (HMVEC-C), dermal lymphatic endothelial cells (LEC), and human primary keratinocytes (HPK) is shown. The black and red arrowheads indicate that no expression of TIE2 or CCM2L was detected in HPKs. N=4; error bars indicate SEM. G–K Ccm2LGFP expression in the endocardium. Shown are low (above) and high (below) magnification images of cardiac trabeculae from E10.5 -E15.5 hearts following immunostaining for GFP (green) and the endothelial cell marker PECAM (red). K. Quantitation of Ccm2LGFP-expressing endocardial cells during mouse cardiac development. N=5; error bars indicate SEM. L–N. Expression of Ccm2LGFP in the dorsal aorta (da) and cardinal vein (cv) at E10.5. O–T Ccm2LGFP is expressed in the endothelial cells of nascent vessels. Ccm2LGFP was frequently detected in non-lumenized endothelial extensions of existing vessels (O), such as those that invade the neural tube (nt) at E10.5-E11.5 (P, Q), and microvasculature (R), and in newly formed microvasculature of the neonatal retina (S, T). Scale bars indicate 20 µm unless otherwise indicated. See also Supp. Fig. 1 and Supp. Table 1.
To further characterize Ccm2L expression and function in vivo we used homologous recombination in ES cells to place a cDNA encoding nuclear-localized GFP in frame with the start methionine of Ccm2L (Supp. Fig. 1). GFP was detected exclusively in the nuclei of PECAM+ endocardial and endothelial cells of Ccm2LGFP mice (Fig. 2G–T). GFP was not detected in all endothelial cells, but was instead found in a small subset of endothelial cells that varied with embryonic age. Ccm2LGFP was strongly expressed in the endocardium, where nuclear GFP was detected by E9.5, peaked at E11.5 (when 20% of endocardial cell nuclei were GFP+) and declined thereafter (Fig. 2G–K). Ccm2LGFP expression was undetectable in myocardial cells at all timepoints (Fig. 2G–J). Ccm2LGFP expression was strongly detected in the endothelial cells of the dorsal aorta and cardinal vein prior to E12.5 but not at later timepoints (Fig. 2L–N). Throughout gestation Ccm2LGFP expression was detected in a small number of endothelial cells in vessels scattered throughout the embryo (Fig. 2O). These cells were frequently adjacent to one another within a single vessel and often found in non-lumenized extensions from lumenized, blood-containing vessels (Fig. 2O), e.g. endothelial cells invading the neural tube at E10.5 (Fig. 2P, Q), consistent with a role in active vessel and heart growth.
A site of highly active angiogenesis is the retina, where a vascular complex forms rapidly during the first weeks of life (Saint-Geniez and D'Amore, 2004). Consistent with a role in active vessel growth, the endothelial cells of most vessels within the Ccm2LGFP retina were GFP+ at P6 (Figure 2S, T). However, the tip cells that lead retinal vascular growth were mostly GFP- (Fig. 2T, discussed further below). Despite a spatial association between Ccm2LGFP expression and nascent vessels, GFP+ endothelial cells did not exhibit an increase in BrdU uptake (Supp. Fig. 1) indicating that expression of Ccm2L does not correlate directly with endothelial proliferation. These studies reveal that Ccm2L expression is highly specific and restricted to endothelial cells of the growing heart and vessels in a pattern consistent with a role in active angiogenesis and cardiogenesis.
Heg and Ccm2L function in a pathway required for cardiac growth
To test the requirement for CCM2L in vivo we generated Ccm2L−/− mice by intercrossing Ccm2LGFP/+ mice. qPCR and RT-PCR analysis of Ccm2LGFP/GFP embryos and tissues revealed that the Ccm2LGFP allele fails to express any Ccm2L mRNA 3’ of exon 1 and (Supp. Fig. 1) and is therefore a null allele. Ccm2L−/− mice were born in normal numbers from Ccm2L+/− intercrosses and grew to maturity without overt phenotypes on mixed SV129J;C57Bl/6, 100% SV129J and 100% C57Bl/6 genetic backgrounds (Supp. Table 1). Thus, unlike CCM2, CCM2L is not required for mouse cardiovascular development.
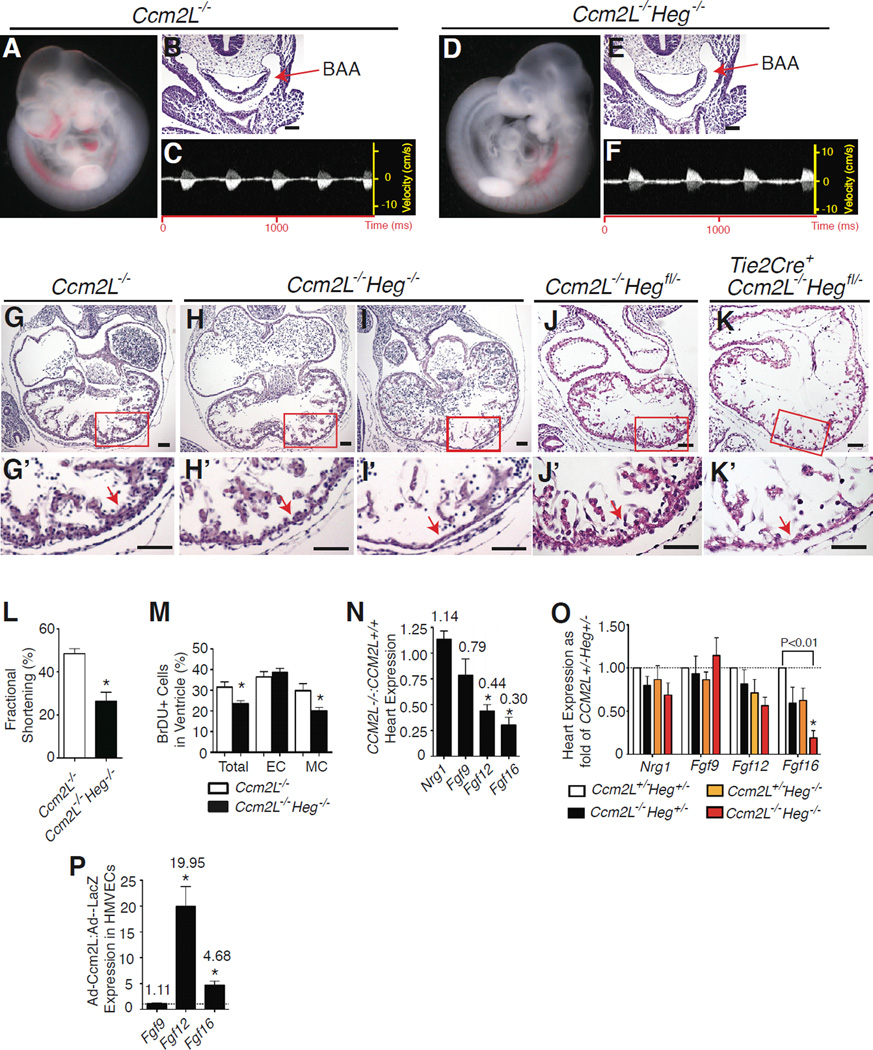
Like Ccm2L, Heg is strongly expressed in endocardial and endothelial cells (Kleaveland et al., 2009). HEG-deficient mice exhibit predominantly postnatal cardiovascular phenotypes, including thinning of the myocardial wall that can result in cardiac rupture and death (Kleaveland et al., 2009). In contrast, CCM1-deficient and CCM2-deficient mouse embryos die by E9.5 due to defective branchial arch artery lumen formation and an inability to circulate blood, a defect reproduced by endothelial-specific loss of CCM2 (Boulday et al., 2009; Whitehead et al., 2009; Whitehead et al., 2004). This phenotype is also observed in Heg−/−;Ccm2+/− embryos (Kleaveland et al., 2009), demonstrating that HEG and CCM2 function in a common pathway and that loss of HEG sensitizes this pathway to partial loss of CCM2. Since CCM2L associates with HEG and CCM1, we next generated animals lacking both Heg and Ccm2L to determine whether and how CCM2L might interact with the known CCM signaling pathway. Analysis of 116 progeny of Heg+/−;Ccm2L+/− intercrosses revealed no liveborn Heg−/−;Ccm2L−/− animals (P<0.01). Heg−/−;Ccm2L+/− and Heg+/−;Ccm2L−/− animals survived to birth, but all Heg−/− ;Ccm2L−/− embryos died prior to E11.5 (Supp. Table 2). In contrast to Ccm2−/− and Heg−/− ;Ccm2+/− embryos, however, Heg−/−;Ccm2L−/− embryos were viable at E9.5, developed patent branchial arch arteries, and had normal blood circulation at this timepoint (Fig. 3A–F and Supp. Table 2). By E10.5 Heg−/−; Ccm2L−/− embryos exhibited severe myocardial thinning, reduced ventricular trabeculation, and dilated atria (Fig. 3G–I). High frequency ultrasound revealed a marked reduction in the systolic fractional shortening of the left ventricle in E10.5 Heg−/−;Ccm2L−/− embryos (Fig. 3L), confirming that Heg−/−;Ccm2L−/−embryos die of heart failure. Analysis of BrdU uptake revealed reduced proliferation of myocardial but not endocardial cells in the hearts of E10.5 Heg−/− ;Ccm2L−/− embryos (Fig. 3M). Thus Heg and Ccm2L operate in a common pathway in vivo that supports cardiac growth, a role distinct from that of HEG-CCM2 signaling and consistent with the expression pattern of Ccm2L.
Figure 3. HEG-CCM2L signaling is required for endocardial growth factor expression during cardiac development.
A-F Ccm2L−/−; Heg−/− embryos exhibit patent branchial arch arteries and normal blood circulation. E9.5 Ccm2L−/− and Ccm2L−/−; Heg−/− embryos are shown. Blood can be visualized in the great vessels (A, D), and patent branchial arch arteries (BAAs) demonstrated by H-E staining of transverse sections (B, E). Doppler ultrasound was used to detect normal systolic flow in the aorta of Ccm2L−/−; Heg−/− embryos (C, F). G–I Ccm2L−/−;Heg−/− embryos exhibit cardiac thinning, reduced trabeculation and heart failure after E10.5. H-E stained transverse sections of E10.5 Ccm2L−/− and Ccm2L−/−; Heg−/− embryos are shown, with magnification of the boxed regions below. In addition to marked thinning of the heart wall (arrows, H’ & I’) and reduced ventricular trabeculation, Ccm2L−/−; Heg−/− embryos develop dilated atria, a sign of cardiac failure. J–K. HEG-CCM2 signaling is required in the endocardium for myocardial growth. H–E stained transverse sections of E10.5 Ccm2L−/−;Hegfl/− and Ccm2L−/−;Tie2-Cre;Hegfl/− embryos are shown, with magnification of the boxed regions below (j’ and k’). L Ccm2L−/−;Heg−/− embryo hearts exhibit reduced systolic function. Fractional shortening of littermate embryo hearts was measured in utero at E10.5 with high frequency ultrasound. N= 4. M Ccm2L−/−; Heg−/− embryo hearts have reduced BrdU uptake in myocardial but not endocardial cells at E10.5. N= 3. Scale bars indicate 50nm. N Ccm2L−/− embryo hearts exhibit reduced Fgf12 and Fgf16 mRNA expression at E9.5. mRNA levels of the indicated growth factors were measured using qPCR and represented as the ratio of Ccm2L−/− Ccm2L+/+ expression. N=5 for each genotype. O Ccm2L−/−; Heg−/− embryo hearts exhibit severely reduced levels of Fgf16 mRNA. mRNA levels of the indicated growth factors were measured using qPCR and represented as the ratio of the indicated genotypes to Ccm2L+/−; Heg+/− expression. N=4 for each genotype. P. CCM2L drives expression of FGF12 and FGF16 in cultured endothelial cells. Human microvascular endothelial cells were exposed to adenoviral vectors encoding Ccm2L or LacZ and the indicated mRNAs measured using qPCR after 48 hours. N=3 for FGF9; N=10 for both FGF12 and FGF16. * indicates P<0.01. Error bars in L-P indicate SEM. See also Supp. Fig. 2 and Supp. Table 2.
HEG-CCM2L signaling regulates endocardial growth factor expression
Since HEG and CCM2L physically associate and are expressed strongly in endocardial cells, the reduced myocardial proliferation observed in Heg−/−; Ccm2L−/− embryos suggested that HEG-CCM2L signaling might regulate the endocardial production of growth factors required to support myocardial growth at this timepoint (e.g. neuregulin and FGF family members, (Gassmann et al., 1995; Iwamoto et al., 2003; Lavine et al., 2005; Lu et al., 2010)). To test the requirement for HEG-CCM2L signaling specifically in endothelial cells, we generated mice carrying a conditional Heg allele using homologous recombination in ES cells (Supp. Fig. 2). Cre-mediated recombination of the Hegfl allele results in deletion of exon 1 and creation of a Heg allele that we have previously shown to be null (Supp. Fig. 2 and (Kleaveland et al., 2009)). Analysis of 44 offspring of Tie2-Cre;Ccm2L−/−Heg+/− × Ccm2L−/−Hegfl/fl matings revealed no liveborn Tie2-Cre;Hegfl/−;Ccm2L−/− animals (P<0.001). Tie2-Cre;Hegfl/−;Ccm2L−/− animals exhibited embryonic lethality at E11 associated with myocardial thinning, a phenotype identical to that observed in Heg−/−;Ccm2L−/− animals (Fig. 3J, K). These findings and the endothelial-specific expression pattern of Ccm2L indicate that HEG and CCM2L function in a common endothelial pathway during cardiovascular development.
To determine if HEG-CCM2L signaling regulates endocardial growth factor expression qPCR was performed to measure the levels of Neuregulin, Fgf9, Fgf12 and Fgf16 in the hearts of mice lacking CCM2L, HEG or both HEG and CCM2L. Although CCM2L-deficient embryos exhibited normal cardiac growth, significantly reduced levels of Fgf12 and Fgf16 but not Fgf9 or Neuregulin mRNA were detected in E10.5 Ccm2L−/− hearts at this timepoint (Fig. 3N). At E9.5, a timepoint prior to any detectable cardiac phenotype, when compared to Heg+/−;Ccm2L+/− littermates Heg−/−;Ccm2L+/−, Heg+/− ;Ccm2L−/− and Heg−/−;Ccm2L−/− hearts exhibited a graded loss of expression of Fgf16, a gene required to stimulate myocardial growth at this timepoint (Lavine et al., 2005; Lu et al., 2008) (Fig. 3O). Consistent with these loss-of-function findings in vivo, adenoviral over-expression of CCM2L in endothelial cells in vitro conferred an increase in FGF12 and FGF16 expression (Fig. 3P). These studies demonstrate that HEG-CCM2L signaling regulates the expression of myocardial growth factors by endothelial cells in the E9.5–10.5 heart and explain why combined deficiency of HEG and CCM2L is lethal at this timepoint.
Ccm2L and Ccm2 play opposing roles during cardiovascular development
The studies described above and published studies (Boulday et al., 2009; Whitehead et al., 2009) establish that HEG, CCM2 and CCM2L all function in endothelial cells during cardiovascular development. The observations that (i) CCM2L competes with CCM2 for CCM1 binding but does not bind CCM3, (ii) Ccm2L is expressed in a dynamic manner in endothelial cells, and (iii) Heg−/−;Ccm2L−/− embryos exhibit a cardiovascular phenotype distinct from that of Ccm2−/− and Heg−/−;Ccm2+/− embryos suggested either that CCM2L and CCM2 operate in discrete endothelial signaling pathways downstream of HEG, or that these two pathways may compete with each other in a dynamic manner determined by the relative levels of CCM2L and CCM2. To determine if CCM2 and CCM2L function in discrete or intersecting pathways we next performed genetic experiments to test the relationship between Ccm2 and Ccm2L during cardiovascular development.
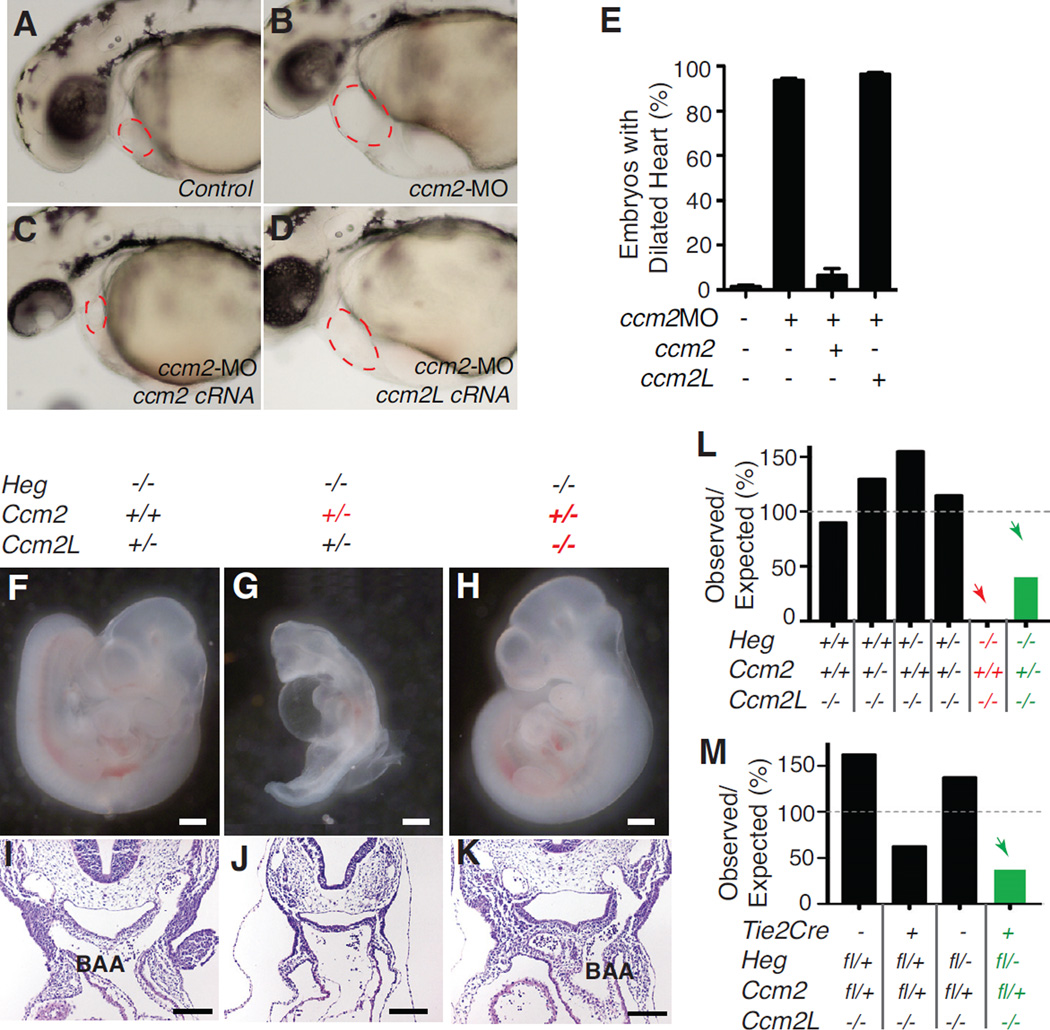
To test for functional redundancy between CCM2 and CCM2L we first generated Ccm2L−/−;Ccm2+/− compound mutant animals. Ccm2L−/−;Ccm2+/− mice were born in normal numbers and appeared healthy and fertile (data not shown), suggesting that CCM2 and CCM2L are not redundant in function. To further address whether CCM2 and CCM2L are functionally redundant in vivo we performed complementation experiments in zebrafish embryos. The cardiac phenotype conferred by loss of ccm2 in zebrafish embryos can be efficiently rescued by the injection of wild-type ccm2 cRNA (>90% rescue), but not by cRNA encoding a ccm2 PTB domain mutant (Kleaveland et al., 2009). To determine if ccm2L can compensate for loss of ccm2 in vivo we co-injected ccm2 or ccm2L cRNAs with ccm2 morpholinos into zebrafish embryos. cRNA encoding ccm2 but not ccm2L efficiently rescued the cardiovascular phenotype of ccm2-morphant embryos (Fig. 4A–E), despite successful expression of ccm2L protein (data not shown). These studies, and the finding that CCM2 but not CCM2L can bind CCM3, indicate that CCM2 and CCM2L do not play functionally redundant roles downstream of HEG.
Figure 4. Ccm2 and Ccm2L play opposing rather than redundant roles during cardiovascular development.
A–E. ccm2L does not rescue loss of ccm2 in zebrafish embryos in vivo. Zebrafish embryos were injected with control morpholino alone (A), morpholino directed against ccm2 alone (B), with ccm2 cRNA (C) or with ccm2L cRNA (D) and the presence of a dilated heart (red circle) phenotype scored 48 hpf. ccm2 cRNA conferred highly efficient (>90%) rescue of the ccm2 morphant phenotype, but ccm2L cRNA did not (E). N=6; error bars indicate SEM. F-K. Loss of Ccm2L rescues loss of Ccm2 during cardiovascular development. E9.5 embryos lacking different numbers of Ccm2 and Ccm2L alleles were generated on a Heg−/− background to test for genetic interaction. Loss of Ccm2L rescued the defect in branchial arch artery (BAA) lumenization and embryonic lethality observed with loss of Ccm2 (I-K). White scale bars indicate 500µm; black scale bars indicate 100µm. L. The ratio of observed/expected offspring of Ccm2L−/−Heg+/− × Ccm2L−/−Heg+/−Ccm2+/− matings at P14 is shown. M. The ratio of observed/expected offspring of Tie2-Cre;Ccm2L−/−Heg+/− × Ccm2L−/−Hegfl/flCcm2fl/fl matings at P14 is shown. Red lettering and arrows indicate combinatorial lethality; green lettering and arrows indicate combinatorial rescue of lethality. See also Supp. Fig. 3 and Supp. Table 3.
Biochemical studies revealed that CCM2L and CCM2 compete for binding to CCM1 (Fig. 1F, G), suggesting that expression of CCM2L could reduce CCM2 signaling in endothelial cells. To determine if CCM2L might modulate signaling by CCM2 we next tested the effect of changes in the balance of Ccm2 and Ccm2L gene dosage on cardiovascular development by examining the effect of loss of a Ccm2 allele on Heg-Ccm2L compound mutants, and vice versa. Although all Heg−/−;Ccm2L−/− embryos and all Heg−/−;Ccm2+/− embryos died prior to E12 due to defects in heart and branchial arch artery development respectively, approximately half of Heg−/−;Ccm2L−/−;Ccm2+/− animals generated by Heg+/−;Ccm2L−/− × Heg+/−;Ccm2L−/−;Ccm2+/− matings survived (Fig. 4F–L, P<0.05). Matings between Heg+/−;Ccm2L−/−;Ccm2+/− animals and surviving Heg−/− ;Ccm2L−/−;Ccm2+/− animals confirmed that all Heg−/−;Ccm2L−/−;Ccm2+/+ offspring died in utero while half of Heg−/−;Ccm2L−/−;Ccm2+/− offspring exhibited normal cardiovascular development (Fig. 4F–K and Supp. Table 3, P<0.01). Finally, intercrosses of surviving Heg−/−;Ccm2L−/−;Ccm2+/− animals revealed that all live-born offspring were Heg−/−;Ccm2L−/− ;Ccm2+/−, while all Heg−/−;Ccm2L−/−;Ccm2+/+ and all Heg−/−;Ccm2L−/−;Ccm2−/− embryos died in utero (data not shown, P<0.01). Thus the survival of Heg−/−;Ccm2L−/−;Ccm2+/− animals is an effect of Ccm2 vs. Ccm2L gene dosage and not selection for a favorable background strain. Finally, to further test whether HEG, CCM2 and CCM2L interact specifically in endothelial cells we crossed Tie2-Cre;Heg+/−;Ccm2L−/− and Hegfl/fl;Ccm2L−/−;Ccm2fl/flanimals (Supp. Fig. 3). Although all Tie2-Cre;Hegfl/−;Ccm2L−/− animals die before E11 due to cardiac failure (Fig. 3K–L), liveborn Tie2-Cre;Hegfl/−;Ccm2L−/−;Ccm2fl/+ animals were observed, demonstrating rescue with endothelial-specific loss of CCM2 (Fig. 4M). These genetic studies identify an endothelial cell autonomous signaling pathway and reveal that loss of CCM2L can compensate for loss of CCM2 during development and vice versa. CCM2L and CCM2 therefore play roles in opposing pathways downstream of HEG in endothelial cells in vivo, and an appropriate balance of CCM2 and CCM2L expression is critical at multiple timepoints during cardiovascular development.
CCM2L inhibits endothelial lumen formation and loosens endothelial cell junctions in vitro
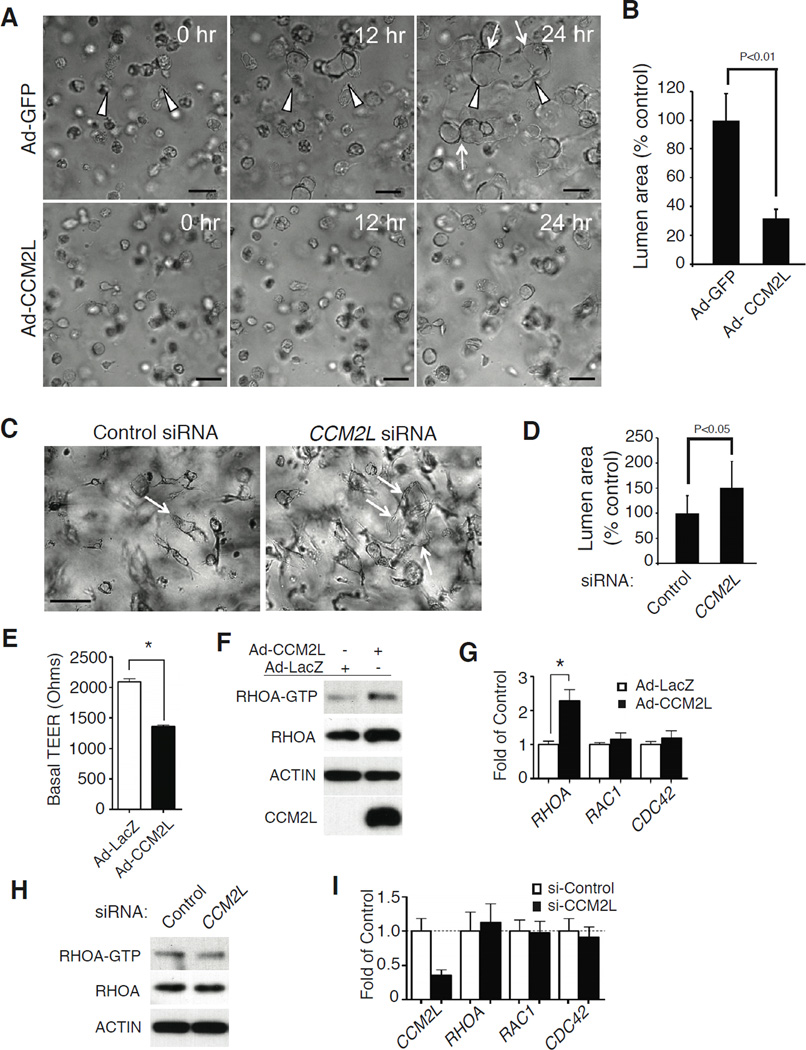
Endothelial loss of CCM2 prevents lumen formation in collagen gels in vitro and results in a non-lumenized branchial arch artery in vivo (Kleaveland et al., 2009; Whitehead et al., 2009). The observation that loss of CCM2L can rescue branchial arch artery lumenization in Heg−/−;Ccm2+/− embryos suggested that CCM2L may also oppose the known role of CCM2 in lumen formation. Ccm2LGFP expression is only detected in the endothelial cells of growing cardiovascular organs in vivo, and Ccm2L expression in cultured endothelial cells was also extremely low when measured by RT-PCR (Supp. Fig. 4). We therefore used adenoviral vectors to express CCM2L or the control proteins β-galactosidase (LacZ) or GFP in cultured endothelial cells. Expression of CCM2L, but not LacZ or GFP, significantly inhibited lumen formation by cultured HUVECs (Fig. 5A, B and Supp. Movies 1– 3). Conversely, siRNA directed against CCM2L (Supp. Fig. 4) conferred an increase in endothelial lumen formation (Fig. 5C, D).
Figure 5. CCM2L opposes CCM2 in the regulation of lumen formation and endothelial junctions.
A, B. Expression of CCM2L inhibits endothelial lumen formation in vitro. Adenoviral vectors were used to express CCM2L or the control proteins GFP or LacZ in human umbilical vein endothelial cells (HUVECs) and lumen formation measured in a 3D collagen gel. Shown are images of endothelial cells in representative collagen gels at the indicated time points (A), and the % lumen area relative to control vector-treated cells (B). N=20 collagen gels for the adeno-GFP controlled experiment and N=10 for the adeno-CCM2L experiment. Scale bar is 50 µM. Arrowheads indicate developing multi-cellular lumens and arrows indicate the final border of the lumen structure. C, D. Loss of CCM2L accelerates endothelial lumen formation in vitro. HUVEC lumen formation was measured following treatment with siRNA directed against CCM2L or control siRNA. Shown are images of endothelial cells in representative collagen gels at 24 hours (C) and % lumen area relative to control vector-treated cells (D). N=40 collagen gels analyzed for each group. Arrows indicate lumenized structures. E. CCM2L expression reduces microvascular endothelial cell trans-endothelial electrical resistance (TEER). Microvascular endothelial cells were treated with adenovirus to express the control protein b-galactosidase (LacZ) or FLAG-CCM2L and TEER measured on confluent cell monolayrers. N= 4. Asterisk indicates P<0.01. F. CCM2L expression increases the levels of RHO-A activation and total protein. Shown are representative immunoblots to detect RHOA-GTP, RHOA, FLAG-CCM2L and ACTIN in the microvascular endothelial cell lysate after exposure to adeno-β-galactosidase and adeno-CCM2L. N=5 for total RHOA, N=2 for RHOA-GTP. G. CCM2L expression raises the levels of RHOA but not RAC1 or CDC42 mRNA as detected by qPCR. N= 5. Asterisk indicates P<0.01. H. Loss of CCM2L does not significantly alter the level of RHOA activation or protein expression in microvascular endothelial cells. The blot shown is representative of 2 independent experiments. I. Loss of CCM2L does not significantly alter expression of RHOA, RAC1 or CDC42 in microvascular endothelial cells. mRNA levels were detected using RT-qPCR. N= 4; error bars in B, D, E, G and I indicate SEM. See also Supp. Fig. 4.
In vivo and in vitro studies have established that HEG-CCM2 signaling also positively regulates endothelial cell junctions, in part by reducing RHO activity and expression level (Borikova et al., 2010; Glading et al., 2007; Kleaveland et al., 2009; Whitehead et al., 2009; Zheng et al., 2010). Expression of CCM2L significantly reduced transendothelial resistance (TEER, a measure of endothelial junction tightness) (Fig. 5E), an effect similar to that previously observed with loss of CCM2 or CCM3 (Whitehead et al., 2009; Zheng et al., 2010). Forced expression of CCM2L also raised the levels of both activated RHOA (RHOA-GTP), total RHOA protein and RHOA, but not RAC1 or CDC42, transcripts in microvascular endothelial cells (Fig. 5F, G). Reduction of the low basal level of CCM2L levels in these cells did not significantly alter the level of activated or total RHOA protein or RHOA mRNA (Fig. 5H, I). These findings demonstrate that CCM2L and CCM2 have opposing effects in endothelial cells in vitro as well as in vivo.
Loss of CCM2L retards tumor growth and wound healing
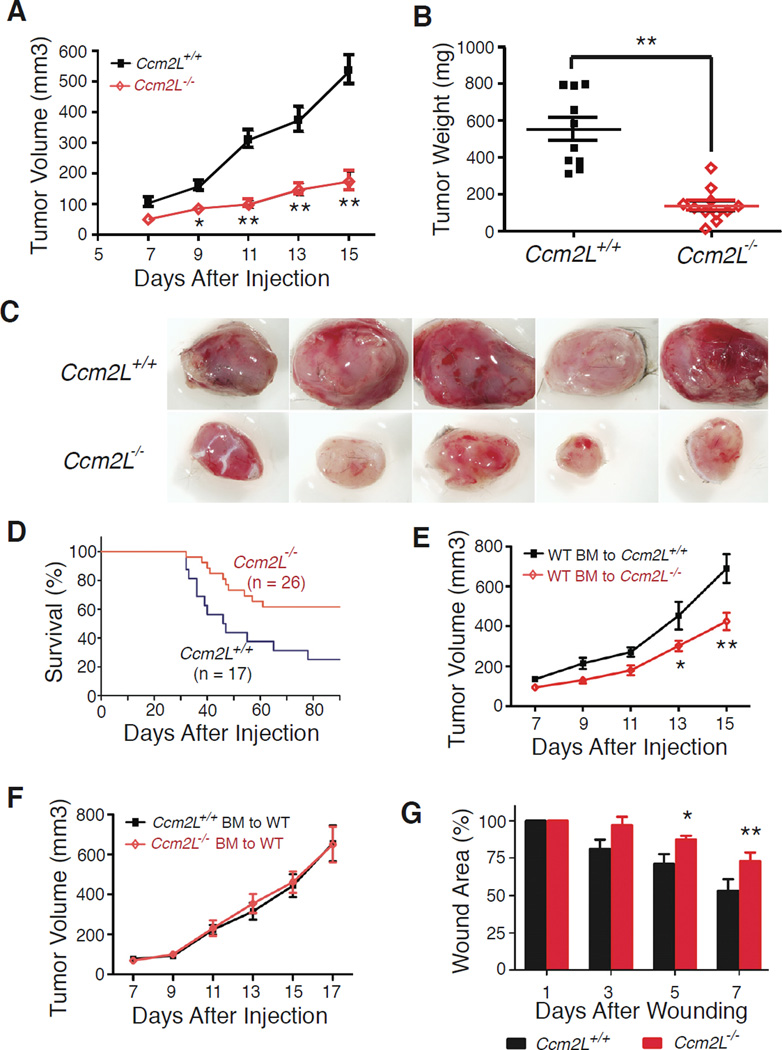
The studies described above suggested that CCM2L may play an important role in promoting vascular growth despite the fact that it is not required during cardiovascular development. To further test a potential angiogenic role for CCM2L we next examined tumor growth and wound healing, processes known to require robust vessel growth (Conway et al., 2001; Lyden et al., 2001). To test tumor growth 4 × 105 Lewis Lung Carcinoma (LLC) cells were injected into the flank of Ccm2L−/− mice and littermate controls. LLC tumor growth was markedly impaired in Ccm2L−/− mice relative to wild-type littermates, and tumors grown in Ccm2L−/− animals appeared pale in comparison with those in control animals (Fig. 6A–C). Ccm2L−/− mice were also protected from lethality due to pulmonary metastases following tail vein injection of 5 × 105 LLC cells (Fig. 6D). These findings suggested that endothelial CCM2L is required for optimal tumor angiogenesis, but Ccm2LGFP expression could not be detected in the tumors of Ccm2L+/− animals. To further address the source of CCM2L LLC cells were injected into lethally irradiated Ccm2L−/− animals that were reconstituted with wild-type bone marrow (Fig. 6E) and wild-type mice reconstituted with Ccm2L−/− bone marrow (Fig. 6F). These studies demonstrated a requirement for CCM2L in non-hematopoietic cells, consistent with low level expression of CCM2L in the endothelium of tumor vessels. To test the role of CCM2L in a more physiologic angiogenic process we compared wound healing following full thickness skin punch biopsy in Ccm2L−/− mice and wild-type littermates. Wound healing was significantly delayed in Ccm2L−/− mice relative to controls (Fig. 6E). These findings suggest that although CCM2L facilitates rapid angiogenic responses in postnatal animals.
Figure 6. CCM2L is required for rapid tumor growth and wound healing in vivo.
A–C. Growth of Lewis Lung Carcinoma (LLC) cells is severely retarded in CCM2L-deficient mice. 4 × 105 LLC cells were injected into the flank of mature mice and tumor volume measured at the indicated times after injection. Tumor weight was measured after harvest at 15 days. N=10. Representative tumors are shown. Note the small size and lack of vascularity in tumors harvested from Ccm2L−/− animals. D Ccm2L−/− mice are protected from death after tail vein injection of LLC cells. Survival curves following tail vein injection of 5 × 105 LLC cells are shown. E, F. Growth of Lewis Lung Carcinoma (LLC) cells is inhibited in irradiated Ccm2L−/− mice reconstituted with wild-type bone morrow (E, N=10), but not irradiated wild-type mice reconstituted with Ccm2L−/− bone morrow (F, N=12). G. Wound healing is retarded in Ccm2L−/− mice. 8 mm full thickness wounds were made in wild-type and Ccm2L−/− animals and the percentage of the original wounded area calculated at the indicated timeponits after wounding. N=8; error bars in A, B, E, F and G indicate SEM.
DISCUSSION
CCM2L promotes vascular remodeling by blocking canonical CCM signals
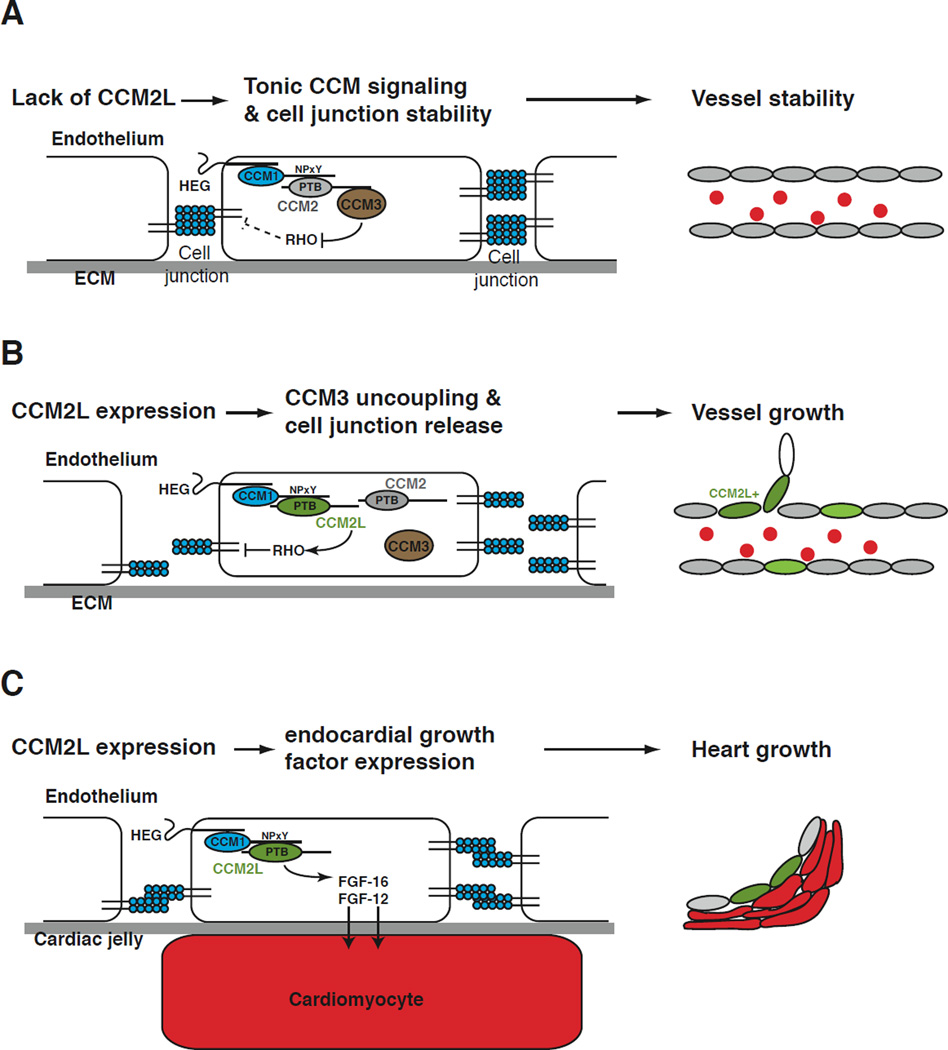
Diseases such as ischemia and cancer are characterized by inadequate or excessive vascular growth. In the former vessels remain excessively stable and fail to sprout new vessels to feed blood-starved tissues. In the latter there is excess vascular permeability and new vessel formation that fuels tumor growth and inflammation. Understanding the molecular signals that balance vascular stability and growth is essential to devise effective angiogenic therapies. The CCM pathway has recently been identified as a positive regulator of endothelial cell junctions and vessel stability in both developing and mature animals (Kleaveland et al., 2009; Stockton et al., 2010; Whitehead et al., 2009). Ccm2 is expressed at low levels in endothelial cells, and increased permeability in Ccm2+/− mice (Stockton et al., 2010) is consistent with a role for CCM2 in generating tonic stabilizing signals in the cardiovascular system. In the present study we identify CCM2L as an endothelial-specific modulator of CCM signaling that is linked to cardiovascular growth. Our studies suggest that CCM2L both opposes CCM2-mediated endothelial and vascular stability and activates endothelial growth factor expression in the heart. These findings are consistent with a model in which dynamic expression of CCM2L functions as a molecular means of converting a tightly connected, quiescent endothelial cell to a one that is disconnected and actively participating in vessel or cardiac growth (Fig. 7). CCM2L upregulation therefore provides a simple and elegant molecular mechanism by which stabilizing signals are turned off and growth signals turned on in a coordinated manner via changes in a single endothelial cell pathway.
Figure 7. Model of CCM2L function during cardiovascular growth.
A. In the absence of CCM2L, constitutive expression of CCM2 confers tonic positive regulation of endothelial junctions and vessel stability. B. In response to as yet unidentified angiogenic signals, CCM2L is expressed in a restricted number of endothelial cells in actively growing cardiovascular organs. CCM2L competes with CCM2 for CCM1 and uncouples CCM1 from CCM3 to break the tonic CCM signal. In the absence of CCM2-CCM3 signaling, CCM2L-expressing endothelial cells are able to uncouple from neighboring endothelial cells in preparation for cellular proliferation and migration. C. HEG-CCM2L signaling in the developing heart drives endocardial expression of growth factors and myocardial proliferation.
Tight regulation of CCM2L expression reflects its role in balancing vessel stability versus growth through the CCM pathway
Comparison of CCM2L expression with that of CCM2 reveals important differences and similarities. Studies of Ccm2LacZ animals reveal broad Ccm2 expression in vivo with no detectable vascular pattern or dynamic changes in gene expression level (Kleaveland et al., 2009). In contrast to Ccm2, studies of Ccm2LGFP animals reveal tight precise spatial and temporal control of Ccm2L expression in vivo. Spatially Ccm2LGFP is entirely restricted to endothelial cells, consistent with a highly specific role in regulating cardiovascular CCM signaling. Temporally, Ccm2LGFP expression is very dynamic and highest during peak cardiac and vessel growth (e.g. in the E11 heart and neonatal retina). Like both Ccm1 and Ccm2, however, Ccm2L expression is undetectable in a majority of endothelial cells in vivo, even when deficient animals exhibit significant cardiovascular phenotypes such as defects in tumor angiogenesis and wound healing. Our biochemical, cellular and genetic studies suggest that a primary role of CCM2L is to oppose CCM2 by competing for binding to CCM1 and thereby dynamically regulate CCM signaling. Dynamic modulation of CCM signaling using this mechanism is only possible if the relative levels of these three proteins is in a similar range so that endothelial cells can transition rapidly between stable, non-angiogenic states and unstable, angiogenic states by altering CCM2L expression. Consistent with such a mechanism, we detected similar levels of mRNA transcripts encoding CCM2L and other CCM proteins in cultured endothelial cells (Fig. 2F). While CCM2L may also affect CCM signaling through an as yet unidentified indirect mechanism, these studies support a model in which endothelial CCM signaling is mediated by low levels of CCM1, CCM2, and CCM2L that are tightly balanced by expression of CCM2L to regulate endothelial cell stability versus growth in the functioning vascular network.
The role of CCM2L in angiogenesis may be to enable but not stimulate endothelial proliferation
The in vivo phenotypes of CCM2L-deficient mice support a role for CCM2L in angiogenesis. Most of the angiogenic regulators identified to date that act on or within endothelial cells have been shown to regulate endothelial proliferation. Despite a correlation with active angiogenic states, however, GFP+ endothelial cells in Ccm2LGFP animals are not more BrdU+ than GFP- endothelial cells, we do not detect high Ccm2LGFP expression in retinal tip cells, and Ccm2L levels in cultured endothelial cells are not increased by VEGF (Supp. Fig. 4B). Instead, our biochemical, cellular and genetic studies support a specific role for CCM2L in opposing the stabilizing signals mediated by CCM2 and CCM3. These findings are consistent with a model in which CCM2L enables endothelial cells to respond effectively to growth factors such as VEGF by releasing them from neighboring cells, but is not a proliferative signal itself. For example, in the retina tip cells arise by active competition among stalk cells for VEGF signaling(Jakobsson et al., 2010). By the time an endothelial cell assumes a tip cell position it has already responded to VEGF signals and been released from the constraint of other endothelial cells and perhaps down-regulated Ccm2L expression.
CCM2L couples CCM signaling to cardiac growth
Our studies identify two lethal loss of function phenotypes, defective branchial arch artery formation in E9 Heg−/−;Ccm2+/− embryos and inadequate cardiac growth in E10.5 Heg−/−;Ccm2L−/− embryos, that can be reversed by further loss of either CCM2L or CCM2 to restore the balance of these two proteins. Defective branchial arch artery formation in Ccm2−/− and Heg−/−;Ccm2+/− embryos occurs when endothelial cells align correctly along the branchial arch but fail to associate into a lumenized vessel that connects the heart to the dorsal aorta (Boulday et al., 2009; Kleaveland et al., 2009; Whitehead et al., 2009). This in vivo phenotype strongly resembles the failure of CCM2-deficient endothelial cells to lumenize in collagen gels in vitro(Whitehead et al., 2009), and is consistent with the known role for CCM signaling in regulating endothelial cell-cell association and lumen formation. In contrast, HEG-CCM2L signaling is required for cardiac trabeculation and myocardial growth at E10–11 (Fig. 3), and expression studies of embryonic hearts lacking one or both of these proteins as well as endothelial cells that over-express CCM2L support reduced endothelial expression of essential myocardial growth factors such as FGF16 (Lu et al., 2008) as the mechanism for this phenotype. One possible explanation for the drop in endocardial growth factor expression in these animals is a failure of endocardial cells to disengage and expand due to inadequate suppression of CCM2 signaling, but endocardial proliferation is not reduced in these hearts (Fig. 3M). These findings therefore suggest that HEG-CCM2L signaling supports cardiac growth through regulation of endothelial growth factor expression. The observation that cardiac lethality in Heg−/−;Ccm2+/− embryos can be reversed by loss of one Ccm2 allele is consistent with bidirectional competition and a mechanism by which myocardial growth is coupled to endocardial growth in the developing embryo. Further studies to test the role of HEG-CCM2L signaling and identify the downstream effectors by which it controls the expression of endocardial growth factors is an important next step in these studies.
Experimental Procedures
Mice
The Ccm2L gene was disrupted in SV129 ES cells by replacing the coding portion of exon 1, intron 1 and exon 2 with a cassette expressing nuclear-GFP using recombineering-based gene targeting techniques. HEG-deficient and CCM2-deficient mice have been described previously (Kleaveland et al., 2009). The conditional Heg allele was generated using gene targeting as described in Supp. Fig. 2. The conditional Ccm2 allele was generated using gene targeting as described in Supp. Fig. 3. Tie2-Cre transgenic mice were obtained from Jackson Research Laboratories (Bar Harbor, ME). The University of Pennsylvania Institutional Animal Care and Use Committee approved all animal protocols.
Histology
Embryos at desired developmental stages were dissected and analyzed as previously described (Kleaveland et al., 2009). The primers used to generate a Ccm2L in situ hybridization probe are listed in supplemental methods. The following antibodies were used for immunostaings: goat anti-GFP (1:100, Abcam), rat anti-Flk1 (1:50, BD PharMingen), rat anti-Pecam (1:500, BD PharMingen), and mouse anti-BrDU (1:10, Hybridoma Bank).
In vivo BrDU incorporation assay
Pregnant mice were injected intraperitoneally with 100 µg/g body weight BrDU. The embryos were dissected 1.5 hour after the injection, fixed and embedded in paraffin. Paraffin sections were immunostained with anti-BrDU and anti-PECAM antibodies and nuclei visualized with DAPI.
Tumor xenograft, bone marrow transplant and wound healing studies
Three month-old Ccm2L−/− and littermate control mice were injected with 4×105 Lewis Lung Carcinoma (LLC) cells subcutaneously on the flank. Starting one week after injection tumor size was measured every two days. Tumors were excised and weighed 15 days after injection. For radiation chimera experiments bone marrow was isolated from Ccm2L−/− or control littermate animals and transplanted by retro-orbital injection into recipient Ccm2L−/− or control littermate animals conditioned with a split lethal dose of 10 Gy irradation. LLC cells were injected subcutaneously 8 weeks after transplantation. To test the survival following lung metastasis, three month-old Ccm2L−/− and littermate control mice were injected with 5×105 LLC cells via tail vein and survival monitored daily. Full thickness wounds were made on the dorsum of three month-old Ccm2L−/− and littermate control mice using an 8-mm skin biopsy punch. Wound dimensions were measured every other day and the wound area calculated.
Fetal ultrasound
Pregnant mice were lightly anesthetized with 1–2% isoflurane and trans-uterine embryonic ultrasound performed as previously described (Lee et al., 2006). Left ventricular systolic function was estimated by the percent change in fractional area (FAC %), which was derived using the formula: (Vd-Vs) / Vd × 100. Doppler ultrasound was used to detect blood flow in the dorsal aorta.
Zebrafish studies
Tuebingen long-fin wild-type zebrafish were maintained and with approval of the Institutional Animal Care and Use Committee of the University of Pennsylvania. Antisense morpholino oligonucleotides (Gene Tools) that interfere with the splicing of ccm2 (Mably et al., 2006; Mably et al., 2003) were injected into the yolk of one-cell stage embryos at a dose of 5 ng. To rescue the phenotype conferred by ccm2 morpholinos, 100 pg of cRNA encoding ccm2 or ccm2L was co-injected with the ccm2 morpholino oligonucleotides.
Measurement of trans-endothelial electrical resistance (TEER)
Human dermal micro-vascular endothelial cell (HMEC) barrier function was assayed by measuring the resistance of a cell-covered electrode using an ECIS instrument (Applied BioPhysic) as previously described (Zheng et al., 2010). Cells were infected with adenovirus expressing p-galactosidase or CCM2L at 5,000gc/cell. TEER was measured 72 hours after infection.
Endothelial lumen formation
Endothelial cell lumen formation assays were performed in 3D collagen matrices, realtime movies acquired, and cultures fixed and quantitated for lumen formation as described as described (Koh et al., 2008).
Immunoprecipitation, immunoblotting and RHO activation assays
Biochemical studies of epitope-tagged proteins heterologously expressed in HEK293T cells were performed as previously described (Kleaveland et al., 2009; Zheng et al., 2010). RHO activation was measured using a RHO Activation Assay Biochem Kit (Cytoskeleton). Anti-ACTIN staining (1:5,000, Cell Signaling) was used as a loading control.
Statistics
P values were calculated using an unpaired 2-tailed Student’s t-test, ANOVA, or Chi Square analysis as indicated.
Supplementary Material
Highlights.
CCM2L opposes CCM2 biochemically, in endothelial cells, and during mouse development
CCM2L is expressed selectively in endothelial cells during cardiovascular growth
CCM2L signaling drives endocardial growth factor expression during heart development
CCM2L inhibits cardiovascular stability and promotes cardiovascular growth
Acknowledgements
We thank the members of the Kahn lab for their thoughtful comments during the course of this work, and Katherine Speichinger and Matthew Davis for their technical assistance. We would also like to thank M. Ginsberg and J.J. Liu for generously providing us with HEG-IC and aIIb-IC beads. These studies were supported by National Institute of Health grants R01HL094326 (MLK), R01HL102138 (MLK), R01HL059373 (GED), T32HL07971 (XZ) and American Heart Association grant 11SDG7430025 (XZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borikova AL, Dibble CF, Sciaky N, Welch CM, Abell AN, Bencharit S, Johnson GL. Rho kinase inhibition rescues the endothelial cell cerebral cavernous malformation phenotype. J Biol Chem. 2010;285:11760–11764. doi: 10.1074/jbc.C109.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulday G, Blecon A, Petit N, Chareyre F, Garcia LA, Niwa-Kawakita M, Giovannini M, Tournier-Lasserve E. Tissue-specific conditional CCM2 knockout mice establish the essential role of endothelial CCM2 in angiogenesis: implications for human cerebral cavernous malformations. Dis Model Mech. 2009;2:168–177. doi: 10.1242/dmm.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001;71:188–192. doi: 10.1136/jnnp.71.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Molendini C, Baluk P, McDonald DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res. 2009;335:17–25. doi: 10.1007/s00441-008-0694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denier C, Goutagny S, Labauge P, Krivosic V, Arnoult M, Cousin A, Benabid AL, Comoy J, Frerebeau P, Gilbert B, et al. Mutations within the MGC4607 gene cause cerebral cavernous malformations. Am J Hum Genet. 2004;74:326–337. doi: 10.1086/381718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K, Raab G, et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci U S A. 2003;100:3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- Kleaveland B, Zheng X, Liu JJ, Blum Y, Tung JJ, Zou Z, Sweeney SM, Chen M, Guo L, Lu MM, et al. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nat Med. 2009;15:169–176. doi: 10.1038/nm.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 2008;443:83–101. doi: 10.1016/S0076-6879(08)02005-3. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, et al. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Lu SY, Jin Y, Li X, Sheppard P, Bock ME, Sheikh F, Duckworth ML, Cattini PA. Embryonic survival and severity of cardiac and craniofacial defects are affected by genetic background in fibroblast growth factor-16 null mice. DNA Cell Biol. 2010;29:407–415. doi: 10.1089/dna.2010.1024. [DOI] [PubMed] [Google Scholar]
- Lu SY, Sheikh F, Sheppard PC, Fresnoza A, Duckworth ML, Detillieux KA, Cattini PA. FGF-16 is required for embryonic heart development. Biochem Biophys Res Commun. 2008;373:270–274. doi: 10.1016/j.bbrc.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Mably JD, Chuang LP, Serluca FC, Mohideen MA, Chen JN, Fishman MC. santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development. 2006;133:3139–3146. doi: 10.1242/dev.02469. [DOI] [PubMed] [Google Scholar]
- Mably JD, Mohideen MA, Burns CG, Chen JN, Fishman MC. heart of glass regulates the concentric growth of the heart in zebrafish. Curr Biol. 2003;13:2138–2147. doi: 10.1016/j.cub.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010 doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt PC, Jeon H, Song HK, Herz J, Eck MJ, Blacklow SC. Origins of peptide selectivity and phosphoinositide binding revealed by structures of disabled-1 PTB domain complexes. Structure. 2003;11:569–579. doi: 10.1016/s0969-2126(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol. 2005;345:1–20. doi: 10.1016/j.jmb.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Voss K, Stahl S, Hogan BM, Reinders J, Schleider E, Schulte-Merker S, Felbor U. Functional analyses of human and zebrafish 18-amino acid in-frame deletion pave the way for domain mapping of the cerebral cavernous malformation 3 protein. Hum Mutat. 2009;30:1003–1011. doi: 10.1002/humu.20996. [DOI] [PubMed] [Google Scholar]
- Voss K, Stahl S, Schleider E, Ullrich S, Nickel J, Mueller TD, Felbor U. CCM3 interacts with CCM2 indicating common pathogenesis for cerebral cavernous malformations. Neurogenetics. 2007 doi: 10.1007/s10048-007-0098-9. [DOI] [PubMed] [Google Scholar]
- Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Jones CA, Zhu W, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009;15:177–184. doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccm1 is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development. 2004;131:1437–1448. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- Zawistowski JS, Stalheim L, Uhlik MT, Abell AN, Ancrile BB, Johnson GL, Marchuk DA. CCM1 and CCM2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Hum Mol Genet. 2005;14:2521–2531. doi: 10.1093/hmg/ddi256. [DOI] [PubMed] [Google Scholar]
- Zheng X, Xu C, Di Lorenzo A, Kleaveland B, Zou Z, Seiler C, Chen M, Cheng L, Xiao J, He J, et al. CCM3 signaling through sterile 20-like kinases plays an essential role during zebrafish cardiovascular development and cerebral cavernous malformations. J Clin Invest. 2010;120:2795–2804. doi: 10.1172/JCI39679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.