Abstract
Background
This study examined therapist-patient interactions during clinical management with anti-depressant medication and pill-placebo.
Methods
The sample consisted of 80 patients on active medication and 40 patients in a pill-placebo condition from a randomized controlled trial for moderate to severe depression. Pharmacotherapist-patient interactions were characterized using observer ratings of the therapeutic alliance, pharmacotherapist-offered facilitative conditions, pharmacotherapist adherence to clinical management treatment guidelines, and pharmacotherapist competence. Patients, therapists, and raters were blind to treatment condition and outcome.
Results
Provision of greater nonspecific support (facilitative conditions) in early sessions predicted less subsequent improvement in depressive symptoms for patients receiving pill-placebo but not those receiving active medications, for which none of the process ratings predicted subsequent change. Early symptom change predicted later alliance and adherence in both conditions and therapist competence in the active condition.
Conclusions
Higher levels of support in early sessions predict poorer subsequent response among placebo patients. It remains unclear whether patients who are likely to be refractory elicit greater nonspecific support or whether the provision of such support has a deleterious effect in unmedicated patients. Differences in treatment process variables between conditions late in treatment are likely to be largely a consequence of symptom relief produced by active medications.
Keywords: Depression, Antidepressant Drugs, Placebos, Facilitative Conditions
BACKGROUND
Comparisons of active and control treatments for mental disorders are invaluable to understanding treatment effects. Pill-placebo conditions have emerged as the control condition of choice for the evaluation of the pharmacological properties of psychiatric medications (Klein, 1996). Although well-conducted drug-placebo comparisons can establish specific medication effects (Depression Guideline Panel, 1993), psychosocial factors likely also contribute to the efficacy of treatments (Kirsch, Scoboria, & Moore, 2002; Wampold, 2001). Such factors may be primary determinants of the placebo effect, and their influences are presumably operative in the presence of an active medication. Both factors common to treatments for psychological disorders and techniques specific to pharmacotherapy may be important determinants of symptom change.
In psychotherapy, common factors often have been found to covary with outcome. The most commonly studied of these factors is the working relationship between therapist and patient, often referred to as the therapeutic alliance (Horvath & Luborsky, 1993; Martin, Garske, & Davis, 2000). Another potentially important common factor is therapist-offered facilitative conditions (e.g., therapist warmth, empathy, and involvement), which Rogers (1957) asserted were “necessary and sufficient conditions” for therapeutic change. Although investigators have used pharmacotherapist, patient, and observer ratings and have relied on different measures of process variables, results across studies generally support a positive process-outcome relation for common factors in the treatment of depression (Krupnick et al., 1996; Weiss, Gaston, Propst, Wisebord, & Zicherman, 1997) and substance use disorders (Carroll, Nich, & Rounsaville, 1997; Dundon et al., 2008). Thus, although only a small number of studies have addressed this issue, available evidence suggests that common factors are associated with positive outcomes in the pharmacological treatment of depression.
While investigations of the relation between modality-specific techniques (i.e., techniques specific to a particular treatment orientation) and outcome in psychotherapy have yielded inconsistent findings (Castonguay & Beutler, 2005), similar investigations of pharmacotherapy treatments have not been conducted. Specific techniques in pharmacotherapy may achieve effects in several ways. For example, a clinicians’ adherence to techniques of clinical management (CM), such as providing a biochemical rationale for a patient’s condition or evaluating side effects of medication (Fawcett, Epstein, Fiester, Elkin, & Autry, 1987), may promote compliance and thereby maximize the potential benefits of the medication. These clinician behaviors may also have a salutary effect for non-pharmacological reasons. As such, they may enhance the magnitude of the placebo effect whether or not active medication is provided.
Given previous work, we planned to examine three hypotheses by drawing data from a study of treatments for moderate to severe depression (DeRubeis et al., 2005). First, we expected common factors to be more predictive of response in placebo treatment than in the active medication condition, variation in which we expected to be largely accounted for by individuals’ responses to the pharmacological properties of the medication. Second, we expected factors specific to CM (therapist adherence and competence) to predict subsequent symptom change in both conditions. Finally, we expected that early change in depressive symptoms would predict level of process variables assessed later in treatment in both conditions.
METHODS
Participants
Participants were drawn from the antidepressant medication (paroxetine) plus clinical management (ADM-CM) and pill-placebo plus clinical management (PL-CM) conditions of a larger trial comparing these conditions to Cognitive Therapy in the treatment of moderate to severe depression (for details see DeRubeis et al., 2005). As part of that trial, 120 patients were randomly assigned to ADM-CM and 60 patients were assigned to PL-CM. Treatment lasted 16 weeks in ADM-CM but lasted only 8 weeks in PL-CM. Only the first 8 weeks of ADM-CM and PL-CM were examined for this report. Patients were excluded from the process ratings if they: (a) discontinued treatment prior to week 8 (n= 21), or (b) did not have the same pharmacotherapist for at least 70% of the sessions during the first 8 weeks of treatment (n= 15). Another 24 patients were excluded on a random basis to yield an equal number of patients across the two study sites, resulting in a total of 80 patients in ADM-CM and 40 patients in PL-CM (67% of the patients initially randomized). The final sample was predominantly female (70%), Caucasian (86%) and middle aged (M = 41.1, SD = 11.6). There were substantial levels of Axis I and Axis II comorbidity (75% and 51%, respectively). The mean age of onset of the first depressive episode was 22.0 (SD = 12.2), and the average number of prior depressive episodes was 2.4 (SD = 2.7). The criterion for chronic depression was met by 60% of the sample, and the average number of prior courses of treatment was 1.7 (SD = 1.9).
Treatments
Clinical Management (CM)
CM was provided in both ADM-CM and PL-CM conditions according to a manualized protocol initially developed for the NIMH Treatment of Depression Collaborative Treatment Program (Fawcett et al., 1987). The five participating pharmacotherapists were all male, board-certified psychiatrists with 9–23 years of experience treating major depression with medications (DeRubeis et al., 2005). Pharmacotherapists at each site met weekly and conferred across sites several times a year. Consultation was provided by Dr. Fawcett on an “as needed” basis and at yearly investigator’s meetings. Techniques and strategies for CM were: (1) medication management: education about medications, adjustment of dosage and dosage schedules, and discussion of side effects; and (2) review of patient’s symptoms of depression and functioning in life spheres. Brief supportive counseling and limited advice giving were allowed, but use of specific psychotherapy techniques was prohibited. Treatment sessions were scheduled weekly for the first four weeks, then biweekly thereafter, and each visit lasted approximately 20 minutes.
Antidepressant Medication (ADM-CM) and Placebo (PL-CM)
Patients in the ADM-CM condition were treated with paroxetine. (Two patients who were unable to tolerate paroxetine were switched to either bupropion or sertraline.) Following week 8, pharmacotherapists could augment ADM-CM patients with lithium or desipramine (and in one case venlafaxine). The PL-CM condition was designed to be identical to ADM-CM with the exception that pharmacotherapists administered a dose-matched pill placebo.
Measures
Process measures -- Common Factors
The therapeutic alliance was assessed using the 12-item short form of the observer-rated Working Alliance Inventory (WAI; Horvath & Greenberg, 1989). The WAI assesses clinician and patient agreement on tasks, goals, and clinician-patient bond. For example, “There is mutual liking between the client and therapist” is a bond-related item that was rated from 1 “never” to 7 “always.” A review of the alliance literature suggests that observer ratings of the alliance, using the WAI, are as likely to detect effects of interest as any other method (Elvins & Green, 2008). The 8-item Facilitative Conditions Scale (FC; Hollon et al., 1988) was used to assess the clinician’s efforts to provide a therapeutic environment (e.g., providing warmth, empathy, and developing rapport). For example, “Did the therapist convey warmth?” was rated from 1 “not at all” to 7 “very much.” Additionally, a single item assessing the percent of the session (rated 0 – 100%) during which the patient was talking was rated at each session.
Process measures -- Specific Factors
Clinician adherence to the clinical management guidelines was measured by the 20-item clinical management scale (Hollon et al., 1988; e.g., “Did the therapist discuss the possibility of side effects and describe those most likely to occur?” rated from 1 “not at all” to 7 “extensively”). This subscale was found to discriminate clinical management almost perfectly from psychotherapy conditions in the Treatment of Depression Collaborative Research Project (Hill, O’Grady, & Elkin, 1992). To assess how well CM was conducted, a 7-item clinician competence scale was used (Hollon, Shelton, Fawcett, & DeRubeis, 2000). Whereas adherence measures reflect the frequency with which targeted behaviors occur, competence measures assess how skillfully those behaviors are performed.
Outcome measure
Outcome was assessed by clinical evaluators using the 17-item version of the Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960; Williams, 1988), which was assessed on weeks 1, 2, 3, 4, 6, and 8. The HRSD was modified to allow for the assessment of atypical symptoms. Evaluators were blind to treatment condition throughout the trial.
Blinds among Pharmacotherapists, Symptom Evaluators, and Process Raters
Pharmacotherapists and patients were blind to treatment condition for the first 8 weeks. The blind was broken at the conclusion of the week 8 visit for the pharmacotherapists and patients, but maintained through the end of active treatment and beyond for the independent evaluators who assessed depressive symptoms. Observers who provided ratings of process variables were blind to both treatment condition and information about subsequent outcome.
Procedure
Two videotaped pharmacotherapy sessions were selected and rated for each patient included in this study: session 2 and the session from week 8. Due to technical problems (i.e., poor audio quality), some tapes could not be used; therefore, only 223 of the intended 240 sessions (93%) were rated. Because not all of the targeted sessions were available for all patients, there are slight fluctuations in sample sizes used in different analyses. Seven undergraduate students in psychology made the process ratings. Prior to conducting study ratings, these raters each completed 70 hours of rater training led by two of the authors (DRS and MOS). Retraining occurred periodically to prevent rater drift. Raters were paired according to a balanced incomplete block design (Fleiss, 1981) so that every session was to be rated by two raters and each rater was to be paired with every other rater an equal number of times. However, due to some raters providing only partial data, only 77% of sessions were rated by two raters.
Reliability of Ratings
Intraclass correlation coefficients (ICCs; Shrout & Fleiss, 1979) were computed to assess the reliability of pooled raters’ judgments for all double-rated sessions. ICCs were .75 for clinician adherence, .53 for clinician competence, .52 for the therapeutic alliance, .43 for FC, and .53 for patient talking. Although the ICCs were modest, they are on par with the ICCs reports in other studies of clinical management (Hill et al., 1992; Krupnick et al., 1996). The ICC reported for the HRSD in the original trial from which these tapes were derived was a quite respectable .96 (DeRubeis et al., 2005).
Statistical Analysis
To examine process measures as predictors of subsequent change, each process measure was examined as a predictor of change in a separate regression model predicting HRSD difference scores from week 2 to week 8 while controlling for HRSD scores at week 2. Regression analyses also examined prior symptom change as a predictor of week 8 process measures. In these models, a change score reflecting HRSD scores from intake to week 8 was examined as a predictor of each process measure assessed at week 8 while controlling for individual differences in HRSD scores at intake.
RESULTS
The four process scales as assessed at session 2 were all positively correlated with one another (rs ranged from .33 to .63; all ps < .001). Alliance and clinician adherence, measures of the constructs that were most distinct conceptually, exhibited the lowest of the correlations obtained (r = .33). Conceptually similar constructs, alliance and FC on the one hand (r = .62) and clinician adherence and competence on the other (r = .63), exhibited higher correlations. This pattern of convergent and discriminant validity speaks to the construct validity of the process measures (Campbell & Fiske, 1959).
Means and standard deviations for process measures and HRSD by condition are presented in Table 1. To place these means in context, we compared them to a study examining FC and adherence in the Treatment of Depression Collaborative Research Program. They were quite comparable (Hill et al., 1992). As the table shows, there were no significant differences between conditions on the process measures at session 2. However, in the session occurring during week 8 of the trial, differences between conditions emerged on three of the four process measures and on the HRSD. With regard to process differences, the ADM-CM condition was characterized by stronger therapeutic alliance, greater clinician adherence, and greater clinician competence than PL-CM.
Table 1.
Descriptive Statistics for Process Measures and Depression Scores by Condition at Intake, Week 2, and Week 8
| Intake | Week 2 | Week 8 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | n | M | SD | n | M | SD | n | |
| Hamilton Rating Scale for Depression | |||||||||
| ADM-CM | 22.6 | 3.2 | 80 | 17.7 | 5.4 | 77 | 11.4a | 6.5 | 80 |
| PL-CM | 23.1 | 3.2 | 40 | 19.2 | 5.3 | 39 | 15.4 | 7.0 | 40 |
| Therapeutic Alliance | |||||||||
| ADM-CM | 4.88 | .57 | 75 | 5.22b | .58 | 74 | |||
| PL-CM | 5.00 | .50 | 36 | 4.98 | .60 | 37 | |||
| Facilitative Conditions | |||||||||
| ADM-CM | 26.2 | 4.7 | 75 | 27.9 | 4.4 | 74 | |||
| PL-CM | 26.6 | 4.3 | 36 | 27.1 | 3.8 | 37 | |||
| Clinician Adherence | |||||||||
| ADM-CM | 41.8 | 6.9 | 75 | 41.7a | 8.1 | 74 | |||
| PL-CM | 41.5 | 6.7 | 36 | 36.8 | 8.9 | 37 | |||
| Clinician Competence | |||||||||
| ADM-CM | 19.6 | 4.6 | 75 | 21.3a | 4.3 | 74 | |||
| PL-CM | 19.4 | 4.6 | 36 | 17.7 | 4.9 | 37 | |||
Note. Scores have a possible range of 1–7 (Therapeutic Alliance), 8–56 (Facilitative Conditions), 20–140 (Clinician Adherence), and 0–42 (Clinician Competence).
p < .01;
p < .05 for between condition comparisons at each time point (t-test)
Process Measures as Predictors of Subsequent Symptom Change in ADM-CM and PL-CM
Before examining the relationship between session 2 process measures and subsequent symptom change, it is worth noting that, with the exception of alliance in ADM-CM (β = .28, p = .03), prior change in depression did not predict process measures at session 2 (all ps >.10).
In the analyses of session 2 predictors of subsequent change, only FC predicted subsequent symptom change, and then only in the PL-CM condition (see Table 2). Surprisingly, greater provision of FC was predictive of less subsequent symptom improvement. This relationship was the only significant finding of the eight tests reported in Table 1. However, with a p-value of .001, the test survives a Bonferroni correction requiring a significance level of p < .006. The presence of a significant treatment by FC interaction in predicting subsequent symptom change (F = 7.37, p = .008) further shows that the identified relationship involving FC in PL-CM was significantly greater than that found in ADM-CM. A non-significant trend for the alliance to predict subsequent symptom change in the PL-CM condition was in the same direction (meaning that the more positive the alliance, the less the subsequent symptom change). There was a parallel non-significant trend for the interaction of alliance and treatment in predicting subsequent symptom change (F = 3.61, p = .06).
Table 2.
Prediction of Subsequent Symptom Change as a Function of Condition
| Pharmacotherapy | Placebo | |
|---|---|---|
| n =76 | n =37 | |
| Therapeutic Alliance | .11 | −.25# |
| Facilitative Conditions | .01 | −.47* |
| Clinician Adherence | .08 | −.13 |
| Clinician Competence | −.07 | −.22 |
Note. Signs have been adjusted so that positive standardized beta weights indicate that process variables were associated with greater subsequent symptom change.
p < .10
p < .01
In an exploratory analysis, we examined patient involvement / talkativeness at session 2. Patient talkativeness was correlated with FC (r = .23, p = .01). In the PL-CM condition, patient talkativeness predicted less improvement on the HRSD by week 8, after controlling for symptoms at week 2 (β = −.36, p = .02). This was not the case in ADM-CM (β = .06, ns). The interaction of patient talkativeness and treatment in predicting subsequent symptom change was at the level of a non-significant trend (F = 2.87, p = .09). It is of particular interest that this finding parallels the finding for FC, as therapist provision of FC would be expected to foster patient talkativeness.
Potential Determinants of Facilitative Conditions: Pharmacotherapists and Patient Characteristics
Given our unexpected finding involving FC, we next examined the potential role of patient characteristics and pharmacotherapist differences in determining the level of FC at session 2. We examined six patient characteristics which might have played a role in determining the level of FC pharmacotherapists provided. These were: presence of an Axis I condition other than depression; presence of an Axis II personality disorder; age at onset of first depressive episode; number of prior depressive episodes; whether the patients met criteria for chronic depression (i.e., 2 or more years); and whether patients had been in treatment previously. When these variables were examined individually, only Axis I and Axis II comorbidity were significantly related to FC; greater comorbidity was associated with lower levels of FC. These two predictors together accounted for 8% of the variance in FC (F = 3.00, p = .03). In contrast, pharmacotherapist accounted for 22% of the variance in FC (F = 7.47, p < .0001)1. When these predictors (i.e., Axis I comorbidity, Axis II comorbidity, and pharmacotherapist) were entered in the same model, pharmacotherapist remained a significant predictor (F = 6.16, p = .0002), whereas Axis I and Axis II comorbidity did not (test for both predictors: F = 2.38, p = .09).
Patient Characteristics and the Facilitative Conditions – Outcome Association
We explored whether the association between FC and subsequent symptom change was independent of patient characteristics. A regression analysis was conducted to examine FC as a predictor of subsequent symptom change in the PL-CM condition with the following variables entered as covariates: presence of an Axis I condition other than depression, presence of an Axis II personality disorder, age at onset of first depressive episode, number of prior depressive episodes, number of times patients had been treated previously, whether patients met criteria for recurrent depression (i.e., 3 or more episodes), whether the patients met criteria for chronic depression (i.e., 2 or more years), whether patients’ blind to treatment condition was broken during the rated session at week 8, and the dose of medication prescribed at session 2. Even after all of these covariates were entered, the assessment of FC at session 2 was still a significant negative predictor of subsequent symptom change (β = .53, p = .009) in the PL-CM condition. Thus, we failed to identify patient characteristics that accounted for the relationship between FC and outcome in the PL-CM condition.
After centering predictors to a mean of zero, gender was examined as a potential moderator of the relationship between FC and subsequent symptom change, controlling for current level of depressive symptoms. Among patients in the PL-CM condition, the interaction of gender and FC was significant (β = .33, p = .009). For men, there was no significant relationship (β = .18, ns). For women, the relationship was strongly positive (β = .67, p =.0004). Thus, the relationship between FC (at session 2) and subsequent symptom change in the PL-CM condition appeared to be driven by female patients. In the placebo condition, those female patients who received the highest levels of FC in early sessions were the ones whose symptoms improved the least in the period between the early session and the session at week 8. There was a non-significant trend level difference favoring women in terms of greater provision of FC at session 2 in PL-CM (t(34) = −2.43, p = .09).
Pharmacotherapists and the Facilitative Conditions – Outcome Association
To examine the potential role of pharmacotherapists in the FC-outcome association identified in PL-CM, we first examined whether pharmacotherapists differed on outcome either across or within treatment conditions (HRSD scores at week 8 covarying HRSD scores an intake). There was no evidence of such pharmacotherapist effects (ps > .3). In addition, there was no evidence of any pharmacotherapist-by-process variable interactions in predicting subsequent symptom change (for all interactions, ps > .10). We also examined FC as a predictor in PL-CM while covarying pharmacotherapist. FC remained significant in this model (β = .45, p = .02).
Association of Process Measures with Prior Symptom Change in ADM-CM and PL-CM
Table 3 presents the results of the regression analyses examining prior change in depressive symptoms and subsequent levels of each process measure as assessed at week 8. As the table shows, greater symptom relief predicted higher alliance ratings at week 8 in both conditions. Thus, patients who experienced greater symptom relief were rated as having stronger alliances at week 8. FC at week 8 was unrelated to prior symptom change in either condition.
Table 3.
Prior Symptom Change and the Prediction of Subsequent Process as a Function of Condition
| Pharmacotherapy | Placebo | |
|---|---|---|
| n = 74 | n = 37 | |
| Alliance | .41** | .33* |
| Facilitative Conditions | .01 | −.17 |
| Clinician Adherence | −.36** | −.37* |
| Clinician Competence | −.37** | −.11 |
Note. Standardized beta weights shown are for change on the Hamilton Rating Scale for Depression from intake to week 8 (as indexed by a week 8 minus intake difference score) as a predictor of each process measure. Intake scores on the Hamilton Rating Scale for Depression were included as a covariate in each model. Positive beta weights indicate that greater prior symptom reductions predicted greater values of process variables at week 8.
p < .05
p < .01
Clinician adherence also was significantly related to prior symptom change in both conditions, although, in this instance, therapists were rated as being less adherent late in treatment when working with patients who had experienced greater symptom relief. A similar relationship was observed for clinician competence in the ADM-CM condition, but not the PL-CM condition. Thus, greater response to treatment predicted lower ratings of clinician adherence and lower competence ratings at week 8.
We also conducted analyses to examine whether any treatment differences in process variables at week 8 persisted after controlling for residualized symptom change from intake to week 8. Significant treatment differences in adherence and competence emerged in these analyses (adherence: β = .36, p < .0001; competence: β = .43, p < .0001), but there were no differences between conditions in alliance or FC in these models (ps > .1).
CONCLUSIONS
This study examined the role of process variables in ADM-CM and PL-CM treatments for depression. The most striking finding was that greater provision of FC early in treatment predicted less symptom change among placebo patients. Our confidence in this finding is bolstered by parallel findings for the therapeutic alliance and patient talkativeness (albeit at the level of non-significant trends). There are two classes of explanation for the relation that we found: (1) patient characteristics elicit higher levels support from pharmacotherapists in early sessions; or (2) high levels of support have a deleterious effect in the absence of active medications. Patients with comorbid conditions were less likely to elicit high levels of FC. However, we failed to find any patient characteristic that accounted for the relationship between FC and outcome. While our results were particularly pronounced for females, they could not be accounted for by any patient characteristic examined. Thus, these results raise the possibility that high levels of support have a deleterious effect in the absence of active medications.
While process variables may cause outcome, it is also possible that symptom improvement causes changes in process variables. At week 2, only the alliance was predicted by prior symptom change. However, at week 8 (after more symptom change had occurred), there was evidence that prior symptom change was related to both alliance and adherence (and competence in ADM-CM only). As patients improved, alliances improved and pharmacotherapists were less adherent (and exhibited lower competence in ADM-CM). This pattern is consistent with the possibility that changes in outcome caused changes in process measures—particularly later in treatment. Nonetheless, FC was not predicted by prior symptom change at any point in treatment. Thus, for the one measure found to predict subsequent symptom change in either condition (i.e., FC in PL-CM), we failed to find any evidence to suggest that this measure might be a consequence of symptom improvement.
Interestingly, there were marked differences in mean levels of FC (and the other process variables) across pharmacotherapists. These differences persisted even after accounting for patient characteristics such as comorbidity. These findings tempt us to conclude that the relation between FC and outcome is driven by pharmacotherapists; however, the lack of pharmacotherapist differences in outcomes leaves us unable to make a strong case for this conclusion. Thus, we suspect that the relationship between FC and outcome may have been driven by multiple factors: pharmacotherapists and perhaps either unobserved patient characteristics or complex interactions between pharmacotherapists and patient characteristics.
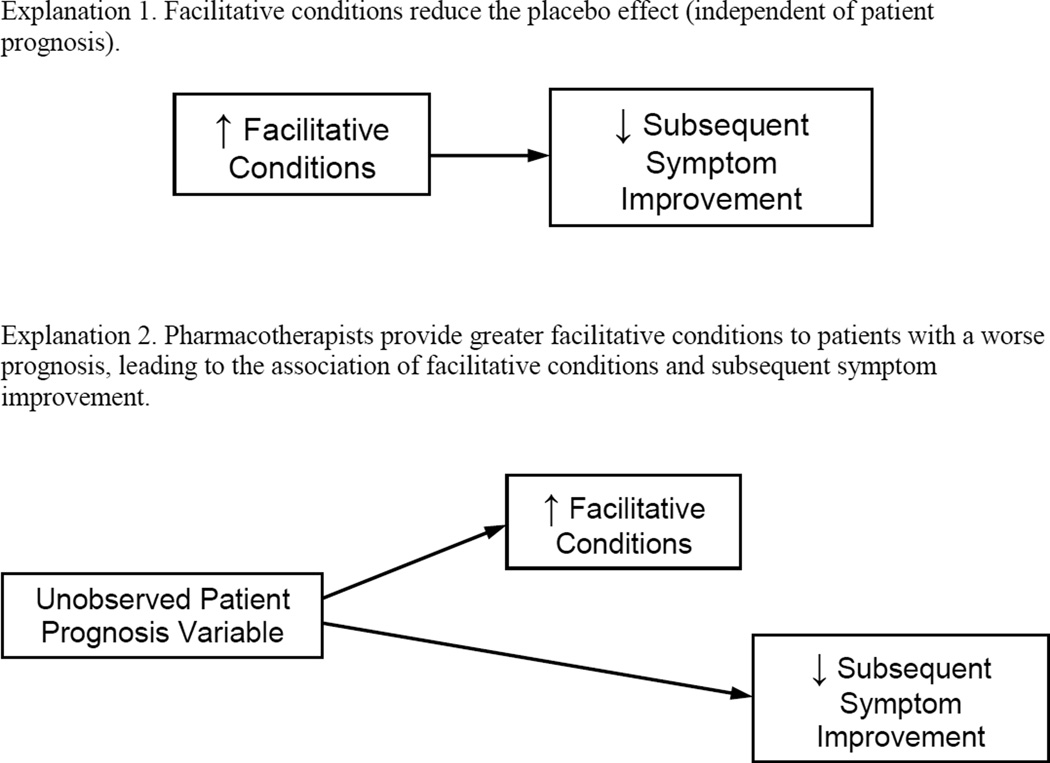
There are two kinds of accounts that could explain the pattern of data we obtained wherein facilitative conditions predicted poor response to PL-CM, as depicted in Figure 1. Higher levels of facilitative conditions may reduce the magnitude of the placebo effect or, alternatively, it could be that pharmacotherapists provide higher levels of facilitative conditions to those patients who are least likely to respond to PL-CM. We explored our data for evidence of the latter, and did not find that any patient characteristic could explain the association between facilitative conditions and subsequent symptom change. Nonetheless, it is possible that a variable that we did not measure, or that was measured poorly, could explain the association.
Figure 1.
Two Possible Explanations for the Finding that Facilitative Conditions Predicts a Poorer Response to Placebo with Clinical Management.
If high levels of FC, and possibly alliance, are indeed detrimental in brief sessions of PL-CM, how might that be the case? Perhaps more extensive use of FC encourages patients to discuss matters outside of the realm of medication management and leads patients to treat CM more like a formal psychotherapy. It is possible that such interventions are not helpful when sessions are brief and infrequent (as compared to standard psychotherapy treatments) or when a pharmacologically active medication is not provided: Emotional issues may be raised without sufficient therapy time left for developing a resolution. This does not imply that harsh or insensitive treatment will promote better outcomes in PL-CM. All of the pharmacotherapists in our trial sought to establish good working relationships with their patients and the overall level of support was high. It was within this context that PL-CM patients who were offered the highest levels of FC were those who improved the least. Thus, a “kind but efficient” approach on the part of the pharmacotherapist may yield the best outcomes.
Our finding for PL-CM departs markedly from the literature showing that common factors such as the alliance predict subsequent symptom change in psychotherapy (e.g., Barber, Connolly, Crits-Christoph, Gladis, & Siqueland, 2000). The only known published work that could have addressed this issue for PL-CM was that reported by Krupnick and colleagues (1996). However, Krupnick et al. relied on a potentially underpowered interaction to detect the effect of interest. While they failed to find that the alliance-outcome relation differed among their four treatments, they did not report the alliance-outcome relation within PL-CM alone. It would be interesting to examine that relation specifically to compare it to the findings of the current study.
None of the hypothesized specific factors of CM (i.e., adherence and competence) predicted subsequent outcome in either treatment. However, it is possible that the high levels of adherence and competence or the modest reliability of the process measures made it difficult to detect these effects. Furthermore, neither alliance nor therapist-offered FC predicted outcome in the ADM-CM condition. It may be that the positive effects of the pharmacologically active medication override any effects of treatment process; that is, active medication could have produced a positive response in a way that was independent of therapeutic process.
This study has several limitations that merit comment. First, whereas differences between treatments in their impact on process and outcome were observed in the context of a randomized experiment, and as such they provided a suitable basis for drawing causal inferences, differences in patterns of covariation between process and outcome were correlational in nature and are thus open to multiple interpretations. Unidentified third variables may explain these relationships. For example, it may be that some unmeasured patient characteristic both led therapists to provide greater FC in early sessions and produced poorer response to treatment and that there was no direct causal link between the two.
Second, patients who did not complete treatment were excluded from the process ratings. This was done to ensure that measures of patient outcomes would be available for patients at week 8, but we could have accomplished the same purpose by using analyses to estimate the likely scores for dropouts. Similarly, in retrospect, there was no good reason to eliminate a random subset of the tapes just to ensure comparable numbers of cases across the sites. Exclusion of patients who were seen by multiple therapists, however, still strikes us as necessary.
Third, the process measures had only modest reliability. This was a problem and likely reduced our capacity to detect process-outcome relations that truly do exist (Type II errors). However, reliability was comparable to previous research examining clinical management (Hill et al., 1992; Krupnick et al., 1996). There is no reason to believe that random error would have introduced bias or generated false relations among study variables. Relatedly, there are limitations associated with using non-expert process raters. We suspect this may be particularly true for judgments of pharmacotherapist competence.
The findings of this study challenge the implicit notion that relational aspects of common factors (at least as they are often conceptualized) are important determinants of positive response in placebo treatments for moderate to severe depression. One possible interpretation of our results is that in a placebo condition, absent active medication or formal psychotherapy, facilitative conditions may have deleterious effects on therapeutic outcome. We believe that understanding the mechanisms by which the placebo effects occur is of importance in its own right. Though the practical utility of this understanding is likely limited because clinicians may not intentionally prescribe an inert pill-placebo in clinical practice, the findings obtained here in the PL-CM condition may also apply to contexts in which the specific medication chosen has no true pharmacological effect.
Acknowledgment
This work was supported in part by NICHD Grant P30HD15052 and in part from an NIMH training grant (T32-MH18921) (Dr. Stewart). The larger project was supported by grants MH50129 (R10) (Dr. DeRubeis) and MH55875 (R10) (Dr. Hollon) from the National Institute of Mental Health, Bethesda, MD. GlaxoSmithKline, Brentford, Middlesex, United Kingdom, provided medications and pill placebos for the trial. We thank the following people who served as raters for this project: Erica Clark, Nicole Higa, Jacqueline Lloyd, Arica Pittman, Brian Pollock, Victoria Radin, and Naomi Richardson.
Footnotes
Pharmacotherapists also differed significantly on each of the other three process measures assessed at session 2: alliance (F = 2.82, p = .03, R2 = .09); adherence (F = 6.94, p = .0001, R2 = .20); and competence (F = 11.98, p = .0001, R2 = .31).
REFERENCES
- Barber JP, Connolly MB, Crits-Christoph P, Gladis L, Siqueland L. Alliance predicts patient’s outcome beyond in-treatment change in symptoms. Journal of Consulting & Clinical Psychology. 2000;68:1027–1032. doi: 10.1037//0022-006x.68.6.1027. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Fiske DW. Convergent and discriminant validation by the multi-trait multi-method matrix. Psychological Bulletin. 1959;56:81–105. [PubMed] [Google Scholar]
- Carroll KM, Nich C, Rounsaville BJ. Contribution of the therapeutic alliance to outcome in active versus control psychotherapies. Journal of Consulting & Clinical Psychology. 1997;6:510–514. doi: 10.1037//0022-006x.65.3.510. [DOI] [PubMed] [Google Scholar]
- Castonguay LG, Beutler LE, editors. Principles of therapeutic change that work. New York: Oxford University; 2005. [Google Scholar]
- Depression Guideline Panel. Depression in primary care: Vol. 2. Treatment of major depression. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1993. (Clinical Practice Guideline No. 5, AHCPR Publication No. 93-0551). [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Dundon WD, Pettinati HM, Lynch KG, Xie H, Varillo KM, Makadon C, et al. The therapeutic alliance in medical-based interventions impacts outcome in treating alcohol dependence. Drug and Alcohol Dependence. 2008;95:230–236. doi: 10.1016/j.drugalcdep.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvins R, Green J. The conceptualization and measurement of therapeutic alliance: An empirical review. Clinical Psychology Review. 2008;28:1167–1187. doi: 10.1016/j.cpr.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management-imipramine / placebo administration manual: NIMH Treatment of Depression Collaborative Research Program. Psychopharmacology Bulletin. 1987;23:309–324. [PubMed] [Google Scholar]
- Fleiss JL. Balanced incomplete block designs for inter-rater reliability studies. Applied Psychological Measurement. 1981;5:105–112. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, O’Grady KE, Elkin I. Applying the Collaborative Study Psychotherapy Rating Scale to rate therapist adherence in cognitive-behavior therapy, interpersonal therapy, and clinical management. Journal of Consulting and Clinical Psychology. 1992;60:73–79. doi: 10.1037//0022-006x.60.1.73. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Evans MD, Auerbach A, DeRubeis RJ, Elkin I, Lowery A, et al. Development of a system for rating therapies for depression: Differentiating cognitive therapy, interpersonal psychotherapy and clinical management pharmacotherapy. Nashville, TN: Vanderbilt University; 1988. Unpublished manuscript. [Google Scholar]
- Hollon SD, Shelton RM, Fawcett J, DeRubeis RJ. Pharmacotherapy Management Scale. Nashville, TN: Vanderbilt University; 2000. Unpublished manuscript. [Google Scholar]
- Horvath AO, Greenberg LS. Development and validation of the working alliance inventory. Journal of Counseling Psychology. 1989;2:223–233. [Google Scholar]
- Horvath AO, Luborsky L. The role of the therapeutic alliance in psychotherapy. Journal of Consulting & Clinical Psychology. 1993;61:561–573. doi: 10.1037//0022-006x.61.4.561. [DOI] [PubMed] [Google Scholar]
- Klein DF. Preventing hung juries about therapy studies. Journal of Consulting & Clinical Psychology. 1996;64:81–87. doi: 10.1037//0022-006x.64.1.81. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Scoboria A, Moore TJ. Antidepressants and placebos: Secrets, revelations, and unanswered questions. Prevention and Treatment. 2001;5 Article 33. [Google Scholar]
- Krupnick JL, Sotsky SM, Simmens S, Moyer J, Elkin I, Watkins J, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting & Clinical Psychology. 1996;64:532–539. doi: 10.1037//0022-006x.64.3.532. [DOI] [PubMed] [Google Scholar]
- Martin DJ, Garske JP, Davis K. Relation of the therapeutic alliance with outcome and other variables: A meta-analytic review. Journal of Consulting & Clinical Psychology. 2000;68:438–450. [PubMed] [Google Scholar]
- Rogers CR. The necessary and sufficient conditions of therapeutic personality change. Journal of Consulting & Clinical Psychology. 1957;21:95–103. doi: 10.1037/h0045357. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Wampold BE. The great psychotherapy debate: Models, methods, and findings. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. [Google Scholar]
- Weiss M, Gaston L, Propst A, Wisebord S, Zicherman V. The role of the alliance in the pharmacologic treatment of depression. Journal of Clinical Psychiatry. 1997;58:196–204. doi: 10.4088/jcp.v58n0504. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]