Abstract
Injury to the premature brain is a major contributor to infant mortality and morbidity, often leading to mental retardation and sensory-motor impairment. The disease process is believed to be caused, sustained, and aggravated by multiple perinatal factors that team up in a multi-hit fashion. Clinical, epidemiological, and experimental studies have revealed that key factors such as inflammation, excitotoxicity, and oxidative stress contribute considerably to white and grey matter injury in premature infants, whose brains are particularly susceptible to damage. Depending on the timing, lesions of the immature brain may influence developmental events in their natural sequence and redirect subsequent development. We review current concepts on molecular mechanisms underlying injury to the premature brain.
Keywords: preterm, premature, infant brain, inflammation, excitotoxicity
Introduction
Consequences of preterm birth are a major health problem worldwide since the incidence of preterm birth has increased and improvements in survival rates have outpaced a concomitant decrease in long-term neurodevelopmental disability rates.1-4 Factors that predispose to injury of the premature brain include hypoxia, ischemia, hyperoxia, and maternal-fetal infection. The premature brain is believed to be particularly susceptible to multiple perinatal impacts that result in processes such as inflammation, excitotoxicity, and oxidative stress. Also, genetic susceptibility plays a role.5, 6 Together, these factors contribute to encephalopathy of prematurity, defined as white and grey matter damage of the premature brain.7, 8 We review current concepts on molecular mechanisms underlying injury to the premature brain.
Periventricular White-Matter Injury
Perinatal brain injury in survivors of premature birth has a unique predilection for the periventricular cerebral white matter. Periventricular white-matter injury is now the most common cause of brain injury in preterm infants and the leading cause of chronic neurological morbidity.7 It affects preterm neonates born between 23 weeks and 32 weeks of gestation and follows successive pathological events from the prenatal to the postnatal period. The spectrum of periventricular white-matter injury includes focal cystic necrotic lesions, so-called periventricular leukomalacia, and a diffuse form. The diffuse form is linked to premyelinating oligodendrocyte vulnerability and leads to a global myelination delay and deep grey matter damage.9 Neuroimaging studies support that the incidence of periventricular leukomalacia is declining, whereas focal or diffuse noncystic injury is emerging as the predominant lesion.10
The neuropathologic hallmarks of periventricular leukomalacia are microglial activation and focal and diffuse periventricular depletion of premyelinating oligodendroglia. Premyelinating oligodendroglia are highly vulnerable to injury caused by glutamate, free radicals, and proinflammatory cytokines. In humans, coagulation necrosis of all cellular elements with loss of cytoarchitecture and tissue vacuolation are the first recorded microscopic neuropathologic findings.11 Moreover, injury to oligodendrocyte progenitors may contribute to the pathogenesis of periventricular white matter injury by disrupting the maturation of myelin-forming oligodendrocytes. Axonal swelling and intense activated microglia reactivity and proliferation have been observed as early as 3 hours after an insult.12, 13 In addition, in the periphery of these focal lesions a marked astrocytic and vascular endothelial hyperplasia characterized the brain tissue reaction at the end of the first week. After 1 week to 2 weeks, macrophage activity with characteristic lipid-laden macrophages was predominant over astrocytic reactivity, with progressive cavitation of the tissue and cyst formation. During subacute and chronic stages of periventricular leukomalacia, swollen axons calcify, accumulate iron, and degenerate, particularly at the periphery of the injured zone.14 Additional minor changes were also found within the grey matter, with some diffuse neuronal loss, especially in lower cortical layers, the hippocampus and the cerebellar Purkinje cell layer.
Since these early studies, many conventional neuropathology studies have noted a widespread diffuse central cerebral white matter astrocytosis, often with abnormal glial cells,15-17 referred to as “perinatal telencephalic leukoencephalopathy.”15-17 On the basis of these studies, Leviton and Gilles differentiated between focal and diffuse white matter damage.18 More recent neuropathological studies revealed that diffuse white matter damage is macroscopically characterized by a lack of white matter, thinning of the corpus callosum, and, in later stages, ventriculomegaly as well as delayed myelination.18, 19 Through the use of immunocytochemical techniques, assessment of autopsy tissue has further localized white matter damage and defined the cells involved. Deep periventricular white matter was prone to focal necrosis, regionally consistent with vascular end-zones/border zones, whereas diffuse injury to peripheral white matter could be characterized by preferential death of late oligodendrocyte progenitors.20
Vulnerability of Oligodendroglial Precursors
Several lines of evidence implicate damage to immature oligodendrocytes during a specific window of vulnerability as a significant underlying factor in the pathogenesis of periventricular leukomalacia. Premyelinating oligodendrocytes are the main component of white matter between 23 weeks and 32 weeks postconception. Findings from animal models show a maturation-dependent vulnerability of oligodendrocyte lineage to the detriment of premyelinating oligodendrocytes, acting through several cytotoxic pathways. Oligodendrocyte progenitor cells proliferate and die by programmed cell death regulated by trophic factors such as platelet-derived growth factor (PDGF) and insulin-like growth factor (IGF).21 Activation of cytokine receptors on the surface of oligodendrocytes can lead to the death of these cells.
Studies have shown that the inflammatory cytokines tumor necrosis factor (TNF)-α and interferon (IFN)-γ are toxic to cultured oligodendrocyte progenitor cells in vitro.22 Selective injury to oligodendrocytes is mediated by induction of “death” receptors such as Fas on the surface of oligodendrocytes. Direct axonal contact appears to be another important factor for the survival of oligodendrocytes.23 These cells are further susceptible to oxidative damage mediated by free radicals such as reactive oxygen and nitrogen species and as a consequence of the depletion of the main antioxidant glutathione.24 Injury-induced swelling and disruption to axons within the white matter leads to locally elevated glutamate, which also induces oligodendrocyte cell death. Glutamate toxicity depends on the maturational stage of the oligondendrocyte and is mediated via the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor.25, 26
Microglial Activity
Specific immunocytochemical markers (eg, CD68) have identified marked increase of activated microglia in diffuse white matter injury.27 Microglia are already widely dispersed throughout the immature white matter by 22 weeks of gestation. These cells are fully capable of producing potentially toxic inflammatory mediators, free radicals, and reactive oxygen intermediates.28 The phagocytic activity of microglia and their capacity for oxidative stress-mediated injury are potently enhanced by inflammatory mediators (IFN-γ, TNF-α, interleukin (IL)-β, and bacterial lipopolysacharide). Recent studies of preoligodendrocytes of the same maturational stage as those populated in the immature white matter of the human premature infant show that cells are exquisitely vulnerable to attack by reactive oxygen species (and reactive nitrogen species produced by activated microglia.30 Presence of activated microglia inducing cell death in immature white matter, both in preoligodendrocytes as well as in astrocytes, has been widely confirmed.31, 32 So far, it also seems that microglia and resident mononuclear phagocytes are the primary sources for the pro-inflammatory cytokines in brains with periventricular leukomalacia.33
Axonal Damage
Axonal damage in periventricular leukomalacia was assessed indirectly by immunostaining for beta-amyloid precursor protein, a neuronoaxonal protein. Damaged axons were detected predominantly in the acute phase of periventricular leukomalacia by immunostaining, but were no longer apparent in the chronic stage.12 Swollen axons calcify (probably due to glutamatergic overactivation), accumulate iron, and degenerate; this has been shown to occur without overt coagulation necrosis of all tissue components.34 Axons from corticospinal tract, thalamocortical fibers, optic radiation, superior occipito-frontal fasciculus, and the superior longitudinal fasciculus may be affected and result in motor, sensory, visual, and higher cortical functional deficits. Thalamocortical projections that course through the white matter develop prior to the functional development of cortical neurons. Therefore, the ensuing disruption to these circuits and to the subcortical plate not only may affect the function, but also the density, survival, and organization of cortical neurons and the cortex itself.35
Subplate Damage
Damage to the early developing subplate neurons with their critical role for the organization of the cortical plate have long been postulated as a possible mechanism by which injury to the immature brain results in long-lasting motor and cognitive deficits.36 Recently, McQuillen and colleagues were able to show specific cell death in subplate neurons after hypoxia-ischemia in very immature animals.37, 38 These subplate neurons play an important role in axonal guidance and cortical organization.37, 39
Disturbance of White-Matter Fiber Tracts
Preterm infants with white- and grey-matter damage also tend to have central white-matter fiber tracts that differ in orientation and organization from those of other preterm infants.40-42
Grey-Matter Injury
For decades, the emphasis has been on white-matter damage in the preterm newborn, and only recently has the spotlight turned to the neuronal loss that accompanies white-matter damage. Recent advances in corticogenesis suggest that neurons migrate from the germinative zones through the white matter to the cortex at a time when the white matter is most vulnerable and perhaps is being injured. Advances in developmental neuroscience also suggest that the excitotoxic and inflammatory processes that contribute to white-matter damage are able to damage developing neurons. In view of these data, we recently hypothesized that white-matter damage in the preterm newborn is accompanied by the death of neurons as they migrate through the dangerous minefield of white matter undergoing injury.43
Magnetic resonance imaging-based neuroimaging techniques provide greater diagnostic sensitivity for periventricular leukomalacia than does head ultrasonography and often document the involvement of telencephalic grey matter and long tracts in addition to periventricular white matter.10 In addition, decreased concentrations of the calcium-binding protein parvalbumin in the cerebral cortex of brains with diffuse white-matter damage are viewed as evidence of a loss of thalamocortical neurons.35 Many of the apoptotic cells seen in the white matter of brains with white-matter injury have characteristics of neurons.39 Expression of nestin, a cytoskeletal protein involved in normal development, is often reduced in the cortical grey matter during the subacute stage of white-matter damage but is increased in later stages, suggesting acute impairment with subsequent compensation leading to repair and plasticity.44 Many neurons in the neocortex, hippocampus, basal ganglia, and thalamus in brains with perinatal white-matter damage have strong inflammatory cytokine immunoreactivity.45 These findings show that neuronal loss and impaired neuronal guidance accompany neonatal white-matter damage and support the view that some dysfunctions seen in preterm infants reflect reduced “connectivity” between areas of the brain needed for integrating information.46-48
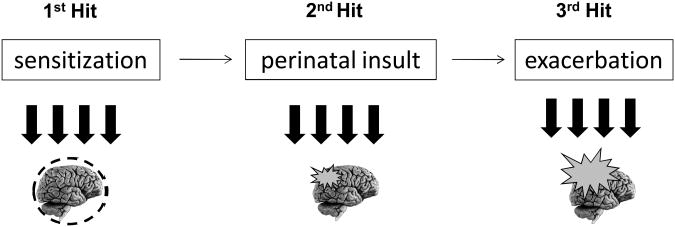
Until a few years ago, periventricular white matter disease was thought to result only from a hypoxic-ischemic mechanism, caused by a decrease in cerebral blood flow and poor development of the vasculature.9 However, it is now recognized that several risk factors are implicated and are frequently associated with periventricular white matter disease pathogenesis in animal models. These comprise prenatal risk factors such as 1) inflammation/cytokine release and maternal stress, 2) perinatal factors such as hypoxic-ischemic stimuli, and 3) postnatal factors such as growth factor deprivation, inflammation/cytokine release, drug side effects, and pain. Furthermore, a combination of such factors in experimental models has resulted in the emergence of a multiple-hit hypothesis, which consists of a sensitization state created by a mild first event, leading to increased susceptibility to a second injury (Figure 1).
Figure 1.
Multiple-hit hypothesis for the development of encephalopathy of prematurity. Schematic representation illustrates the multiple-hit hypothesis, including pre-, peri-, and postnatal factors.
Infection and Inflammation
Systemic administration of lipopolysaccharide to immature cats, dogs, rabbits, or rats induces white matter lesions.49-52 Systemic administration of lipopolysaccharide can induce a marked systemic inflammation and immune changes in the central nervous system such as an increased expression of CD14. High doses of lipopolysaccharide can also induce other factors potentially predisposing to brain damage: hypotension, hypoglycemia, hyperthermia, and lactic acidosis. Moreover, even low doses of lipopolysaccharide, which do not induce significant hypotension, were shown to induce white matter damage in fetal sheep.53, 54 In fetal sheep, the comparison of white damage induced by cord occlusion and by lipopolysaccharide injection revealed distinct patterns of microglia-macrophage activation, suggesting separate or partly separate underlying mechanisms.54 Systemic administration of lipopolysaccharide to pregnant rats induced hypomyelinization in the internal capsule, cell death in the deep grey matter, and an increase in proinflammatory cytokines in their fetuses.52
Live infectious agents have also been used by a few research groups to produce models of white matter lesions. Ureaplasma, the microorganism most frequently associated with chorioamnionitis, preterm birth, and pulmonary morbidity, has been recently linked to intrauterine inflammation and perinatal brain damage in a mouse model.55 Moreover, ascending intrauterine infection with Escherichia coli (E. coli) causes focal white matter damage in 6% of live rabbit fetuses,56 while direct inoculation of E. coli in the uterine cavity combined with early antibiotics induced focal white matter cysts in about 20% of live fetuses and diffuse white matter cell death in almost all live fetuses.57, 58 Cystic lesions are accompanied by microglia-macrophage activation and reactive astrogliosis, while diffuse white matter cell death does not induce such glial responses. These results suggest these 2 types of brain damage have distinct pathophysiological mechanisms.
Lastly, intrauterine inoculation of Border disease virus to pregnant sheep induces decreased expression of white matter molecules, including myelin basic protein, in the fetus.59 However, the virus also infects the thyroid and the pituitary gland, raising the question of the precise etiology of the white matter damage (low thyroid hormones vs. infectious-inflammatory insult).
Excitotoxicity and Oxidative Stress
Glutamate can act on several types of receptors, including N-methyl-D-aspartate (NMDA), AMPA, kainite, and metabotropic glutamate receptors. Excess release of glutamate may represent a molecular mechanism common to some of the risk factors for brain lesions associated with cerebral palsy. In keeping with this possibility, injection of glutamate agonists into the striatum, neocortex, or periventricular white matter of newborn rodents, rabbits, or kittens produces, according to the stage of brain maturation, histological lesions that mimic those seen in humans with cerebral palsy, such as neuronal migration disorders, polymicrogyria, cystic periventricular leukomalacia, and hypoxic-ischemic or ischemic-like cortical and striatal lesions.60-67
Studies exploring the pathophysiology of these excitotoxic white matter lesions in newborn rodents and rabbits have permitted the following contributions:31, 32, 62, 66, 68 1) Both NMDA and AMPA-kainate agonists can induce periventricular cystic white matter lesions; 2) NMDA receptor-mediated white matter lesions involve an early microglia-macrophage activation and astrocyte cell death, while AMPA-kainate receptor-mediated lesions involve preoligodendrocyte cell death; 3) the periventricular white matter of newborn rodents and rabbits exhibit a window of susceptibility to excitotoxic insults; 4) transient higher expression of NMDA receptors on white matter microglia-macrophages and transient higher expression of high levels of AMPA-kainate receptors on preoligodendrocytes are likely important factors to explain the window of sensitivity of the white matter to neonatal excitotoxic insults; 5) the study of NMDA receptor-mediated white matter lesions in newborn rabbits revealed that excitotoxic white matter lesion extended into the subplate but not in the overlying neocortical layers; based on the use of antioxidant molecules, excitotoxic white matter lesions involve excess production of reactive oxygen species, which play an important role in the pathophysiology of the lesions. Extensive in vitro studies have confirmed the exquisite susceptibility of preoligodendrocytes to AMPA-kainate agonists and to oxidative stress.9
Combined Insults
To further support the hypothesis of a multifactorial hypothesis of perinatal brain damage, various groups have combined insults in newborn rodents. For example, pre-treatment of newborn mice with systemic pro-inflammatory cytokines (eg, IL-1β, IL-6, or TNF--α) prior to an excitotoxic insult significantly exacerbated excitotoxic white matter lesions, demonstrating a causative link between circulating pro-inflammatory cytokines and white matter damage.69 Preliminary results suggest this effect of pro-inflammatory cytokines is more pronounced with NMDA receptor agonists compared with AMPA-kainate receptor agonists. The precise mechanism by which these cytokines systemically act on white matter excitotoxicity remains to be determined but could potentially involve activation of brain cyclooxygenase or activation of microglia with increased white matter production of reactive oxygen species and cytokines. Similarly, systemic pre-treatment with IL-9, a Th2 cytokine, was shown to exacerbate NMDA receptor-mediated white matter lesions.69, 70 The mechanism of IL-9 toxicity involves brain mast cell degranulation and excess release of histamine. Interestingly, increased circulating levels of IL-9 around birth had been noted in a subgroup of human infants who later developed cerebral palsy.71 Recently, chronic mild stress of pregnant mice was shown to induce a significant exacerbation of excitotoxic white matter lesions in pups. Lipopolysaccharide was also used to sensitize the newborn brain to hypoxia-ischemia. A low dose of this endotoxin, given 4 hours prior to a mild hypoxic-ischemic insult in P7 rat, induced extensive brain damage. In contrast, each insult, given separately, did not induce any detectable brain lesion.72 Antenatal bacterial endotoxin also sensitizes the immature rat brain to postnatal excitotoxic injury through ibutenate.73
Conclusion
As our understanding of the pathogenesis of encephalopathy of prematurity improves, new strategies for prevention of brain injury in premature infants are anticipated. Recent improvements in neurological outcome of neonates have largely been due to changes in neonatal care, such as commitment to temperature homeostasis, the use of prenatal betamethasone, the withdrawal of postnatal dexamethasone, the prevention of perinatal infection/sepsis, and sparing use of medication that may cause further brain damage. Moreover, adequate analgesia and reduced use of oxygen therapy have done their share. Recent studies have focused on the protective effects of caffeine, recombinant erythropoietin, ibuprofen, magnesium sulfate, inhaled nitric oxide, xenon, and hypothermia in neonatal medicine.
Table 1. Risk Factors for the Development of Encephalopathy of Prematurity.
| Antenatal Factors | Perinatal Factors | Postnatal Factors |
|---|---|---|
| Inflammation | Hypoxia-ischemia | Oxidative stress |
| Hypoxia-ischemia | Excitotoxicity | Inflammation |
| Toxins | Oxidative stress | Pain |
| Malnutrition | Loss of maternal GF | Excitotoxicity |
| Maternal stress | Drugs | Drugs |
| Genetic factors | Genetic factors | Loss of maternal GF |
| Genetic factors |
GF, growth factor.
Acknowledgments
This work was supported by Inserm, Université Paris 7, PremUP, Sixth Framework Program of the European Commission (contract no LSHM-CT-2006-036534/neobrain), the Fondation des Gueules Cassées, the Fondation Motrice, the ELA Foundation, and the Fondation Grace de Monaco. Presented at the Neurobiology of Disease in Children Conference: Symposium on Injury to the Preterm Brain and Cerebral Palsy, in conjunction with the 37th Annual Meeting of the Child Neurology Society, Santa Clara, California, November 5, 2008. Supported by grants from the National Institutes of Health (5R13NS040925-09), the Cerebral Palsy International Research Foundation, the Kennedy Krieger Institute, and the Child Neurology Society.
References
- 1.Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21:123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- 2.Robertson CM, Watt MJ, Yasui Y. Changes in the prevalence of cerebral palsy for children born very prematurely within a population-based program over 30 years. JAMA. 2007;297:2733–2740. doi: 10.1001/jama.297.24.2733. [DOI] [PubMed] [Google Scholar]
- 3.Vincer MJ, Allen AC, Joseph KS, et al. Increasing prevalence of cerebral palsy among very preterm infants: a population-based study. Pediatrics. 2006;118:e1621–1626. doi: 10.1542/peds.2006-1522. [DOI] [PubMed] [Google Scholar]
- 4.Wilson-Costello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000-2002. Pediatrics. 2007;119:37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- 5.Baier RJ. Genetics of perinatal brain injury in the preterm infant. Front Biosci. 2006;11:1371–1387. doi: 10.2741/1890. [DOI] [PubMed] [Google Scholar]
- 6.Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury: does sex matter? Stroke. 2005;36:193–195. doi: 10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- 7.Deng W, Pleasure J, Pleasure D. Progress in periventricular leukomalacia. Arch Neurol. 2008;65:1291–1295. doi: 10.1001/archneur.65.10.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- 9.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 11.Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 12.Meng SZ, Arai Y, Deguchi K, Takashima S. Early detection of axonal and neuronal lesions in prenatal-onset periventricular leukomalacia. Brain Dev. 1997;19:480–484. doi: 10.1016/s0387-7604(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 13.Deguchi K, Oguchi K, Matsuura N, et al. Periventricular leukomalacia: relation to gestational age and axonal injury. Pediatr Neurol. 1999;20:370–374. doi: 10.1016/s0887-8994(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka F, Ozawa Y, Inage Y, et al. Association of osteopontin with ischemic axonal death in periventricular leukomalacia. Acta Neuropathol. 2000;100:69–74. doi: 10.1007/s004010051194. [DOI] [PubMed] [Google Scholar]
- 15.Leviton A, Gilles FH. Morphologic abnormalities in human infant cerebral white matter related to gestational and postnatal age. Pediatr Res. 1974;8:718–720. doi: 10.1203/00006450-197407000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Schneider H, Schachinger H, Dicht R. Telencephalic leucoencephalopathy in premature infants dying after prolonged artificial respiration. Report on 6 cases. Neuropadiatrie. 1975;6:347–362. doi: 10.1055/s-0028-1091676. [DOI] [PubMed] [Google Scholar]
- 17.Gilles FH, Murphy SF. Perinatal telencephalic leucoencephalopathy. J Neurol Neurosurg Psychiatry. 1969;32:404–413. doi: 10.1136/jnnp.32.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leviton A, Gilles F. Ventriculomegaly, delayed myelination, white matter hypoplasia, and “periventricular” leukomalacia: how are they related? Pediatr Neurol. 1996;15:127–136. doi: 10.1016/0887-8994(96)00157-9. [DOI] [PubMed] [Google Scholar]
- 19.Golden JA, Gilles FH, Rudelli R, Leviton A. Frequency of neuropathological abnormalities in very low birth weight infants. J Neuropathol Exp Neurol. 1997;56:472–478. doi: 10.1097/00005072-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Back SA, Luo NL, Borenstein NS, et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barres BA, Hart IK, Coles HS, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 22.Andrews T, Zhang P, Bhat NR. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Casaccia-Bonnefil P. Cell death in the oligodendrocyte lineage: a molecular perspective of life/death decisions in development and disease. Glia. 2000;29:124–135. doi: 10.1002/(sici)1098-1136(20000115)29:2<124::aid-glia5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Back SA, Gan X, Li Y, et al. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci U S A. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen FE. The role of glutamate receptor maturation in perinatal seizures and brain injury. Int J Dev Neurosci. 2002;20:339–347. doi: 10.1016/s0736-5748(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 27.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 28.Rezaie P, Male D. Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc Res Tech. 1999;45:359–382. doi: 10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Smith ME, van der Maesen K, Somera FP. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J Neurosci Res. 1998;54:68–78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Merrill JE, Ignarro LJ, Sherman MP, et al. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 31.Dommergues MA, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience. 2003;121:619–628. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- 32.Tahraoui SL, Marret S, Bodenant C, et al. Central role of microglia in neonatal excitotoxic lesions of the murine periventricular white matter. Brain Pathol. 2001;11:56–71. doi: 10.1111/j.1750-3639.2001.tb00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadhim H, Tabarki B, Verellen G, et al. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 34.Hirayama A, Okoshi Y, Hachiya Y, et al. Early immunohistochemical detection of axonal damage and glial activation in extremely immature brains with periventricular leukomalacia. Clin Neuropathol. 2001;20:87–91. [PubMed] [Google Scholar]
- 35.Iai M, Takashima S. Thalamocortical development of parvalbumin neurons in normal and periventricular leukomalacia brains. Neuropediatrics. 1999;30:14–18. doi: 10.1055/s-2007-973450. [DOI] [PubMed] [Google Scholar]
- 36.Volpe JJ. Subplate neurons--missing link in brain injury of the premature infant? Pediatrics. 1996;97:112–113. [PubMed] [Google Scholar]
- 37.McQuillen PS, Ferriero DM. Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol. 2005;15:250–260. doi: 10.1111/j.1750-3639.2005.tb00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson S, Li Q, Dechant A, Cohen ML. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huppi PS, Murphy B, Maier SE, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 41.Staudt M, Grodd W, Niemann G, et al. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–125. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- 42.Staudt M, Niemann G, Grodd W, Krageloh-Mann I. The pyramidal tract in congenital hemiparesis: relationship between morphology and function in periventricular lesions. Neuropediatrics. 2000;31:257–264. doi: 10.1055/s-2000-9239. [DOI] [PubMed] [Google Scholar]
- 43.Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007;30:473–478. doi: 10.1016/j.tins.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Okoshi Y, Mizuguchi M, Itoh M, et al. Altered nestin expression in the cerebrum with periventricular leukomalacia. Pediatr Neurol. 2007;36:170–174. doi: 10.1016/j.pediatrneurol.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Kadhim H, Tabarki B, De Prez C, Sebire G. Cytokine immunoreactivity in cortical and subcortical neurons in periventricular leukomalacia: are cytokines implicated in neuronal dysfunction in cerebral palsy? Acta Neuropathol. 2003;105:209–216. doi: 10.1007/s00401-002-0633-6. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Ari Y. Basic developmental rules and their implications for epilepsy in the immature brain. Epileptic Disord. 2006;8:91–102. [PubMed] [Google Scholar]
- 47.Kesler SR, Vohr B, Schneider KC, et al. Increased temporal lobe gyrification in preterm children. Neuropsychologia. 2006;44:445–453. doi: 10.1016/j.neuropsychologia.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–285. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Ando M, Takashima S, Mito T. Endotoxin, cerebral blood flow, amino acids and brain damage in young rabbits. Brain Dev. 1988;10:365–370. doi: 10.1016/s0387-7604(88)80094-9. [DOI] [PubMed] [Google Scholar]
- 50.Gilles FH, Averill DR, Jr, Kerr CS. Neonatal endotoxin encephalopathy. Ann Neurol. 1977;2:49–56. doi: 10.1002/ana.410020108. [DOI] [PubMed] [Google Scholar]
- 51.Young RS, Hernandez MJ, Yagel SK. Selective reduction of blood flow to white matter during hypotension in newborn dogs: a possible mechanism of periventricular leukomalacia. Ann Neurol. 1982;12:445–448. doi: 10.1002/ana.410120506. [DOI] [PubMed] [Google Scholar]
- 52.Rousset CI, Chalon S, Cantagrel S, et al. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59:428–433. doi: 10.1203/01.pdr.0000199905.08848.55. [DOI] [PubMed] [Google Scholar]
- 53.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- 54.Mallard C, Welin AK, Peebles D, et al. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28:215–223. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- 55.Normann E, Lacaze-Masmonteil T, Eaton F, et al. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e31819984ce. [DOI] [PubMed] [Google Scholar]
- 56.Yoon BH, Kim CJ, Romero R, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- 57.Debillon T, Gras-Leguen C, Verielle V, et al. Effect of maternal antibiotic treatment on fetal periventricular white matter cell death in a rabbit intrauterine infection model. Acta Paediatr. 2003;92:81–86. doi: 10.1111/j.1651-2227.2003.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 58.Debillon T, Gras-Leguen C, Verielle V, et al. Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res. 2000;47:736–742. doi: 10.1203/00006450-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Anderson CA, Higgins RJ, Waldvogel AS, Osburn BI. Tropism of border disease virus for oligodendrocytes in ovine fetal brain cell cultures. Am J Vet Res. 1987;48:822–827. [PubMed] [Google Scholar]
- 60.Acarin L, Gonzalez B, Hidalgo J, et al. Primary cortical glial reaction versus secondary thalamic glial response in the excitotoxically injured young brain: astroglial response and metallothionein expression. Neuroscience. 1999;92:827–839. doi: 10.1016/s0306-4522(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 61.Barks JD, Silverstein FS. Excitatory amino acids contribute to the pathogenesis of perinatal hypoxic-ischemic brain injury. Brain Pathol. 1992;2:235–243. doi: 10.1111/j.1750-3639.1992.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 62.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gressens P, Marret S, Evrard P. Developmental spectrum of the excitotoxic cascade induced by ibotenate: a model of hypoxic insults in fetuses and neonates. Neuropathol Appl Neurobiol. 1996;22:498–502. doi: 10.1111/j.1365-2990.1996.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 64.Innocenti GM, Berbel P. Analysis of an experimental cortical network: I). Architectonics of visual areas 17 and 18 after neonatal injections of ibotenic acid; similarities with human microgyria. J Neural Transplant Plast. 1991;2:1–28. doi: 10.1155/NP.1991.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Innocenti GM, Berbel P. Analysis of an experimental cortical network: II). Connections of visual areas 17 and 18 after neonatal injections of ibotenic acid. J Neural Transplant Plast. 1991;2:29–54. doi: 10.1155/NP.1991.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marret S, Mukendi R, Gadisseux JF, et al. Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol. 1995;54:358–370. doi: 10.1097/00005072-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 67.McDonald JW, Silverstein FS, Johnston MV. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988;459:200–203. doi: 10.1016/0006-8993(88)90306-x. [DOI] [PubMed] [Google Scholar]
- 68.Plaisant F, Clippe A, Vander Stricht D, et al. Recombinant peroxiredoxin 5 protects against excitotoxic brain lesions in newborn mice. Free Radic Biol Med. 2003;34:862–872. doi: 10.1016/s0891-5849(02)01440-5. [DOI] [PubMed] [Google Scholar]
- 69.Dommergues MA, Patkai J, Renauld JC, et al. Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol. 2000;47:54–63. [PubMed] [Google Scholar]
- 70.Patkai J, Mesples B, Dommergues MA, et al. Deleterious effects of IL-9-activated mast cells and neuroprotection by antihistamine drugs in the developing mouse brain. Pediatr Res. 2001;50:222–230. doi: 10.1203/00006450-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 72.Eklind S, Mallard C, Leverin AL, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic--ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 73.Rousset CI, Kassem J, Olivier P, et al. Antenatal bacterial endotoxin sensitizes the immature rat brain to postnatal excitotoxic injury. J Neuropathol Exp Neurol. 2008;67:994–1000. doi: 10.1097/NEN.0b013e31818894a1. [DOI] [PubMed] [Google Scholar]