Abstract
Intracellular Ca2+ release in many types of cells is mediated by ryanodine receptor Ca2+ release channels (RyRCs) that are assembled into two-dimensional paracrystalline arrays in the endoplasmic/sarcoplasmic reticulum. However, the in situ operating mechanism of the RyRC array is unknown. Here, we found that the elementary Ca2+ release events, Ca2+ sparks from individual RyRC arrays in rat ventricular myocytes, exhibit quantized Ca2+ release flux. Analysis of the quantal property of Ca2+ sparks provided a view of unitary Ca2+ current and gating kinetics of the RyRC in intact cells and revealed that spark activation involves dynamic recruitment of small, variable cohorts of RyRCs. Intriguingly, interplay of RyRCs in multichannel sparks renders an unusual, thermodynamically irreversible mode of channel gating that is unshared by an RyRC acting solo, nor by RyRCs in vitro. Furthermore, an array-based inhibitory feedback, overriding the regenerative Ca2+-induced Ca2+ release of RyRCs, provides a supramolecular mechanism for the microscopic stability of intracellular Ca2+ signaling.
The ryanodine receptor Ca2+ release channel (RyRC) is a prototypical member of the Ca2+ release channel superfamily located in the endoplasmic reticulum/sarcoplasmic reticulum (SR) of eukaryotic cells and plays a pivotal role in intracellular Ca2+ signaling (1-5). Instances of local Ca2+ release, in the form of “Ca2+ sparks” or their equivalents, constitute the elementary Ca2+ signaling events in heart, brain, and muscle cells (6-12). Intriguingly, RyRCs in intact cells are almost exclusively found at discrete spark-generating sites, where ≈100 channels are assembled into two-dimensional paracrystalline arrays (13-15). This pattern of RyRC organization seems to be highly conserved from crustaceans to vertebrates (13, 15, 16), suggesting that array formation is critical to RyRC-mediated Ca2+ signaling in vivo. Thus, understanding array-based RyRC behavior is of fundamental importance for elucidation of intracellular Ca2+ signaling mechanism.
Despite a wealth of information on behavior of RyRCs in planar lipid bilayers and other cell-free systems (5, 17-20), little is known on how RyRCs operate in situ; many fundamental issues regarding the genesis and termination of Ca2+ sparks remain unanswered (5, 21, 22). Based on in vitro properties of RyRCs, the classic Ca2+-induced Ca2+ release (CICR) mechanism (23) would predict an all-or-none activation of the RyRC array and an everlasting local Ca2+ release, which cannot explain the prompt termination of Ca2+ sparks and the microstability of intracellular signaling (24, 25). The relatively constant Ca2+ spark rise time (≈10 ms in the heart) indicates a stereotypical open time of RyRCs (25) whereas RyRCs in vitro always follow exponential open-time distributions (5, 17, 20). The brief spark duration also contrasts sharply with the coupled gating (19) kinetics of RyRCs, in which the open time of physically linked channels acting in unison is prolonged by orders of magnitude (up to 2,500 ms) (20). These paradoxical observations underscore that in vivo operation of RyRCs may involve gating mechanisms that are apparently absent in cell-free systems. Alternatively, the formation of RyRC array may endow the channels with new regulatory mechanisms that are unshared by a corresponding set of solitary RyRCs.
In the present study, we sought to investigate RyRC operation in their native arrays. Because intracellular location of RyRC arrays makes them inaccessible to electrophysiological means, we devised an optical approach to analyze the Ca2+ release flux underlying the spark (Ispark) in intact cardiac myocytes. By splitting Ispark from individual RyRC arrays into single-channel components, we provided a view of the in situ gating of a single RyRC and demonstrated that array-based interaction between RyRCs renders an unusual, irreversible channel behavior and contributes to the microscopic stability of intracellular Ca2+ signaling.
Methods
Confocal Ca2+ Imaging. Enzymatically isolated adult rat cardiac myocytes were loaded with the Ca2+ indicator, fluo-4-AM, as described (26). Confocal line-scan imaging was performed by using a Zeiss LSM 410 or LSM510 confocal microscope equipped with an argon laser (488 nm) and ×40, 1.3 N.A. oil immersion objective. Line-scan images were acquired at sampling rates of 0.38-1.4 ms/line and 0.07 μm/pixel, with radial and axial resolutions of 0.4 and 1.0 μm, respectively. Assuming that the indicator responds to [Ca2+] with little kinetic delay, local Ca2+ concentrations were determined by using the formula [Ca2+] = kdR/(kd/[Ca2+]rest ± 1 - R) (6), where R = F/F0, the resting Ca2+ concentration [Ca2+]rest = 100 nM, and the dissociation constant kd = 1.1 μM. To resolve the quantal property of Ispark, cautions were given to ensure that the focal plane was placed precisely at the tip of the patch pipette throughout the experiments. Environmental vibration was carefully isolated from the recording system so as not to introduce low frequency noise into spark time courses.
Cell-Attached Patch Clamp. Cell-attached patch clamp was established in either GΩ-seal or loose-seal configuration, with glass patch pipettes of 4-5 MΩ. In the loose-seal patches, the membrane potential was determined by proportionally dividing the test voltages between the pipette resistance and the seal resistance (15-30 MΩ) (26). The standard patch pipette filling solution consisted of (in mM) 120 tetraethylammonium chloride, 1 CaCl2, 0.01 tetrodotoxin, and 10 Hepes (pH 7.4). The standard superfusion solution contained (in mM) 135 NaCl, 1 CaCl2, 4 KCl, 1 MgCl2, 10 glucose, and 10 Hepes (pH 7.4). All experiments were performed at room temperature (23-25°C).
Results
Ca2+ Spark Release Flux Exhibited Quantal Substructure. To gain insight into the behavior of RyRCs in situ, we combined confocal microscopy and cell-attached patch clamp technique to activate Ca2+ sparks by single L-type Ca2+ channel (LCC) current (iLCC) (26). In freshly isolated rat ventricular myocytes loaded with the Ca2+-indicator fluo-4, Ca2+ sparks could be triggered by iLCC under the GΩ-seal patch clamp conditions when the pipette contained 20 mM Ca2+ and 10 μM FPL64176, an LCC agonist (Fig. 1A). Likewise, Ca2+ sparks could also be evoked in loose-seal patches during a sequence of depolarization (0.1 Hz) to 0 mV in the presence of 1 mM Ca2+ in the pipette (Fig. 2A). The loose-seal method allows repetitive activation of Ca2+ sparks from in-focus release sites, minimizing the optical blurring associated with out-of-focus events (26).
Fig. 1.
Measurement of Ca2+ release flux of Ca2+ sparks (Ispark). (A) Simultaneous recordings of single LCC currents and triggered sparks in a GΩ-sealed patch. The pipette contained 20 mM Ca2+ and 10 μM FPL64176, an LCC agonist. (B) Representative recordings showing that repolarizations from +60 mV to resting membrane potential (-70 mV) evoked LCC tail current of 0.67 pA. (C) Under the loose-seal patch clamp conditions, the unitary tail current in B produced a tail Ca2+ sparklet when the SR Ca2+ release was disabled by 10 mM caffeine and 10 μM thapsigargin. Nine tail sparklets with >50 ms duration were averaged to improve the signal-to-noise ratio. (D) The time course (open circles) of the averaged sparklet in C was fitted to the equation ΔC = C∞i (1 - exp(-t/τ) with i fixed at 0.67 pA, yielding C∞ = 62 nM/pA and τ = 9.7 ms.
Fig. 2.
Ispark exhibits quantal substructure. (A)Ca2+ sparks triggered in loose-seal patch clamp condition when the SR function was intact. The pipette contained 1 mM Ca2+ without FPL64176. (B) Calibration of Ispark by fitting the spark rising phase to the equation in Fig. 1D using the determined C∞ and τ. The traces shown are for the sparks in A (Ispark = 4.43, 3.47, and 2.26 pA, respectively). (C) Ispark distribution (bars) and its multi-Gaussian components (red curve) for all 384 sparks in 31 patches, and the fitting with Eq. 1 rendered aj = 13.9, 25.5, 19.5, 12.4, 4.5, 3.1, and 1.0 (j = 1 to 7), b = 0.058, and q = 1.24 pA. (D) Evoked Ca2+ sparks in the presence of 100 μM tetracaine. (E) Estimation of Ispark for events in D (Ispark = 1.17 and 1.12 pA, respectively). (F) Histogram of Ispark in the presence of tetracaine (n = 11 cells). Multi-Gaussian fitting (red curve) as in C yielded a1 = 24.5, a2 = 7.0, and aj < 1 for j = 3 to 7, b = 0.077, and q = 1.22 pA.
To measure Ispark, which relates directly to the unitary Ca2+ flux of RyRCs and the number of channels involved, we created a calibration standard with the aid of “Ca2+ sparklet” (26) produced by iLCC. Specifically, LCC sparklets due to the “tail” iLCC on repolarization from ≈+60 mV (iLCC ≈ 0) to ≈-70 mV (iLCC = -0.67 pA measured separately in GΩ-seal patches, Fig. 1B) were obtained in myocytes whose SR Ca2+ release was abolished by thapsigargin and caffeine. A subset of fully developed “tail” sparklets (longer than 50 ms) was then averaged to improve the signal-to-noise ratio (Fig. 1C). From the resultant high-resolution LCC sparklet, we derived the time course of local Ca2+ concentration as a function of the injected Ca2+ influx (Fig. 1D). The Ispark of any given Ca2+ spark was then deduced by fitting its rising phase to the known relationship (Fig. 2B). Because sparklets and sparks were obtained with identical imaging settings, sharing the same microenvironments with respect to Ca2+ diffusion, buffering, and indicator binding, the use of LCC sparklet as the “yardstick” to gauge Ispark should be essentially model- and parameter-independent.
Our measurement of 384 sparks from 31 patches (Fig. 2C) showed that Ispark varied widely from ≈1 to 10 pA. Notably, the Ispark did not vary continuously but exhibited prominent peaks that were separated at roughly equal intervals (Fig. 2C), suggesting a quantal substructure in the Ispark. By analogy to the classic analysis of quantal release of neurotransmitters (27), the entire histogram of Ispark was fitted to a multicomponent Gaussian function:
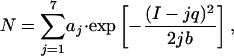 |
1 |
where N is the frequency of observations, aj and b are constants. The fitting yielded a quantal unit of current q = 1.24 pA. By contrast, a similar quantal pattern was not found in the Ispark of spontaneous sparks acquired at random locations (data not shown), indicating that in-focus detection is a prerequisite to revealing the fine Ispark substructure.
Splitting the Elementary Ca2+ Sparks. It is noteworthy that the subpopulation of the smallest Ispark in Fig. 2C consists of only a single q (number of q, nq = 1), suggesting that the q is the smallest building block of Ca2+ release flux underlying the spark. Because ultrastructural studies so far (13-16) do not support any subgrouping of RyRCs within an array in cardiac myocytes, it is most probable that the subpopulation of nq = 1 represent Ca2+ sparks of a single RyRC, and the sparks of higher nq are from multiple RyRCs. To test this hypothesis directly, we devised experiments (Fig. 2D) in which tetracaine was included in the patch pipette at a concentration (100 μM) suitable to block a significant fraction of RyRCs (18). Because inclusion of tetracaine in the patch pipette should reduce the availability of RyRCs only locally, we do not expect a significant change in the “leaky-pump” balance and therefore the SR Ca2+ load. In the presence of tetracaine, Ca2+ sparks evoked beneath the tip of the patch pipette may either be unaffected if they were from a single channel, or be split into smaller sparks if they were multichannel events. We found that 100 μM tetracaine markedly reduced the amplitudes of Ca2+ sparks by decreasing the Ispark (Fig. 2E). Nevertheless, the histogram of decreased Ispark still exhibited quantized variation with the q (1.22 pA) unit unchanged (Fig. 2F). The vast majority of Ispark corresponded to only one (70%) or two (22%) quantal units, with sparks of higher nq essentially eliminated. This result suggests that Ca2+ sparks with large Ispark can be split into subtler events. That tetracaine could neither destroy the quantal structure nor alter the q value indicates that a q arises from the all-or-none Ca2+ release of a single RyRC.
Dynamic Recruitment of RyRCs in an Array. The identification of q as the single RyRC Ca2+ release flux enabled us to analyze the recruitment of RyRCs in individual arrays. As shown in Fig. 3A, Ispark varied widely, even for those from the same patch. Notably, Ispark data points tended to aggregate into regular clusters in the scatter plot. Autocorrelation analysis of Ispark distribution (Fig. 3B) in patches displaying a sufficient number (no less than 15) of events revealed that the Ispark indeed exhibited a periodicity of around 1.2 pA (Fig. 3C, n = 24 patches). These observations indicate that the quantal substructure of Ispark is an inherent property of Ca2+ sparks from individual arrays.
Fig. 3.
Dynamic recruitment of RyRCs from individual release sites. (A) Scatter plots of Ispark from three representative loose-seal patches. The arrowheads indicate the quantal levels determined by the procedure in B. Small vertical displacements were added to separate overlapping symbols. (B) Autocorrelation function of Ispark data for patches [a] and [b] in A. Ispark histogram was constructed with a bin width of 0.1 pA and, after a minimal (3-kernel) smoothing, its autocorrelation function was then determined with a routine coded in interactive data language (IDL). The cyclic peaks at regular intervals in the autocorrelation function reflect the quantal variation of Ispark. A q is determined for each patch by the first non-zero peak. (C) Distribution of q determined by using autocorrelation analysis (n = 24 patches). Gaussian fitting using the equation N = a·exp[-(I - q)2/2b] yielded a = 11.2, b = 0.030, and q = 1.18 pA. (D) Distribution of nq (Ispark/q, rounded to the nearest integer) in sparks from two representative patches, showing that variable cohorts of RyRCs were recruited from individual RyRC arrays. (E) All-events distribution of nq in Ca2+ sparks from all tested patches.
By counting the nq involved in each Ca2+ spark, we found that the vast majority (88%) of sparks involve multiple (up to 8) RyRCs whereas still 12% of them are single-channel events (nq = 1) (Fig. 3E). The wide variation of nq for the same-patch events (Fig. 3D) contrasts sharply with the notion that Ca2+ sparks are an all-or-none phenomenon (6, 28) and excludes the possibility that Ca2+ sparks involve the entire array (≈100 RyRCs) (29) or any fixed number of RyRCs. Instead, our data indicate that small, variable cohorts of RyRCs are recruited dynamically from a large population of RyRCs in an array.
In Situ Gating Kinesics of a Single RyRC. The ability to visualize single-channel Ca2+ sparks afforded an unprecedented opportunity to probe the gating behavior of a single RyRC in their native physiological environment. Previous studies indicated that RyRC gating in lipid bilayers exhibits an exponential distribution of open duration (17, 20, 25), which cannot explain the stereotyped Ca2+ release duration of typical Ca2+ sparks (25). To probe whether and how gating of a single RyRC differs in vitro vs. in situ, RyRC open duration in situ was measured by the rise time of single-channel Ca2+ sparks. Fig. 4A shows two examples of single-channel sparks, with brief (4.5 ms) and long (41 ms) rise time, respectively. The ensemble data show that the channel open duration followed approximately an exponential distribution with a time constant of 11.6 ms (Fig. 4B). Hence, the in situ gating kinetics of a single RyRC seem to resemble that in lipid bilayers (with a slow open time constant of 13.6 ms) (20), indicating that the mechanism for the stereotyped spark duration must exist at the supramolecular level.
Fig. 4.
In situ kinetics of a single RyRC. (A) Line-scan images (Upper) and their surface plots (Lower) of two single-RyRC Ca2+ sparks with short (4.5 ms, Left) and long (41 ms, Right) rise times, respectively. The different rise times resulted in different spark amplitudes. (B) Distribution of rise time for single-RyRC Ca2+ sparks. Mono-exponential fitting (red curve) yielded a time constant of 11.6 ms.
Transition to an Irreversible Mode of Gating for Interacting RyRs. To probe whether RyRC behavior is reshaped by array-based interplay of Ca2+ release channels, we next measured the rise time of multichannel sparks and categorized the data according to their corresponding nq. Surprisingly, with the increase of nq, the rise-time histogram was progressively transformed from an exponential distribution for nq = 1 (Fig. 4B) to a leftward skewed distribution for nq = 2 or 3 (Fig. 5A), and finally to a nearly Gaussian distribution for nq ≥ 4 (Fig. 5B).
Fig. 5.
Interaction of RyRCs reshapes local Ca2+ release kinetics. (A) Distribution of rise time for Ca2+ sparks of nq = 2 or 3. Note that the histograms become modal as compared with Fig. 4C. (B) Distribution of spark rise time for nq ≥ 4. The red curve represents the fitting to a Gaussian function. (C) Relationship between the average rise time and the nq of Ca2+ sparks. Data are expressed as mean ± SE.
For Markovian channels or channel groups uncoupled from external free energy, microscopic reversibility requires that the distribution of the unit active open time always be the sum of positively weighted decaying exponentials (30-32). Although single-RyRC gating in situ basically satisfies thermodynamic reversibility, the nq-dependent, nonexponential open time distributions for nq ≥ 2 indicate that, when more than one RyRC is activated in a spark, thermodynamic irreversibility ensues. Our observations illustrate that the gating of an ion channel can transit from a thermodynamically reversible to an irreversible mode due to functional channel-channel interaction.
To further delineate the nature of intra-array interaction of RyRCs, we examined the influence of nq on averaged spark rise time. Fig. 5C shows that, with the nq increased from 1 to 7, averaged spark rise time was decreased monotonically from 15.0 ± 1.9 to 8.4 ± 0.8 ms (mean ± SE.). In other words, the more RyRCs recruited in a spark, the faster the spark terminates. It follows that the interaction between RyRCs in their native array is predominantly inhibitory.
Discussion
Ca2+ sparks and equivalent localized Ca2+ release events have been found in all types of muscle cells, neurons, and some nonexcitable cells and are generally accepted as the elementary events of endoplasmic reticulum/SR-mediated Ca2+ signaling (6-12). A major finding reported here is that the elementary Ca2+ sparks actually comprise a finer substructure by virtue of quantized Ispark. The quantal nature of Ca2+ sparks has been established on the basis of the distinct, regular peaks in the Ispark histogram for a large number of in-focus sparks, the periodicity in Ispark data from individual patches, and the splitting of sparks by decreasing RyRC availability using tetracaine. The lack of such quantal substructure for spontaneous events, with their apparent Ispark distorted by out-of-focus blurring, adds to the credence that the quantal substructure of in-focus events is real.
The ability to identify the quantal property of Ispark depends on the variation of data. To minimize detection error, we carefully optimized the recording condition (see Methods) and adopted a new generation of confocal microscope. Nevertheless, the q still exhibited a significant cell-to-cell variation (Fig. 3C, variation factor S = 0.146, defined as  for the equation in Fig. 3C legend). This variation should, in part, reflect the photon shot noise associated with fluorescence measurement and the heterogeneity of release units among cells. The cell-to-cell variation in [Ca2+]rest [S = 0.152 measured with indo-1 ratiometric confocal microscopy (33)], by affecting F/F0, could result in additional variation in q measurement, but its contribution should be partially alleviated because of the positive correlation between [Ca2+]rest and the SR Ca2+ load (and therefore the RyR Ca2+ release flux). Mathematically, neighboring Gaussian peaks can be resolved when S < 0.5. For experimental data with a sufficient number of observations, the quantal peaks would be resolvable with an S considerably smaller than 0.5, as demonstrated in Fig. 2C for nq = 1, 2, and 3. It is noteworthy that the distribution of Ispark becomes smeared at nq higher than 4 (Fig. 2C) because the S value increases at increasing nq.
for the equation in Fig. 3C legend). This variation should, in part, reflect the photon shot noise associated with fluorescence measurement and the heterogeneity of release units among cells. The cell-to-cell variation in [Ca2+]rest [S = 0.152 measured with indo-1 ratiometric confocal microscopy (33)], by affecting F/F0, could result in additional variation in q measurement, but its contribution should be partially alleviated because of the positive correlation between [Ca2+]rest and the SR Ca2+ load (and therefore the RyR Ca2+ release flux). Mathematically, neighboring Gaussian peaks can be resolved when S < 0.5. For experimental data with a sufficient number of observations, the quantal peaks would be resolvable with an S considerably smaller than 0.5, as demonstrated in Fig. 2C for nq = 1, 2, and 3. It is noteworthy that the distribution of Ispark becomes smeared at nq higher than 4 (Fig. 2C) because the S value increases at increasing nq.
On the basis that ultrastructural studies reveal no subgrouping of cardiac RyRCs within array (13-16), a straightforward interpretation is that the q is from a single RyRC. This finding is further supported by several lines of evidence. First, the tiniest Ispark consists of only a single q (Fig. 2C), indicating that the q constitutes the smallest building block of Ispark. Second, local application of tetracaine can diminish Ispark of nq > 1, but not the q per se (Fig. 2F), indicating that the q is pharmacologically indivisible under the present conditions. Third, the rise time constant (11.6 ms) of the Ca2+ sparks with nq = 1 is in good agreement with the major slow component of single cardiac RyRC open duration (13.6 ms) (20) (the fast component of RyR open duration, if it exists, would be difficult to resolve with current optical measurement). In contrast, it is far briefer than that for coupled gating of multiple RyRCs in lipid bilayers (286 ms) (20). In addition, lipid bilayer studies have estimated that a single RyRC in vitro under quasi-physiological conditions carries a Ca2+ flux of 0.5 to 1.4 pA, depending on the exact ionic conditions used (34-36). The q of 1.2 pA falls right within this range and reflects the RyRC Ca2+ release flux in intact functioning cells.
Splitting Ca2+ sparks into quantal units has permitted us to dissect RyRC array operation in the genesis of Ca2+ sparks. It has been controversial whether spark activation involves a single RyRC, a few RyRCs, or the entire RyRC array (6, 10, 28-30, 37, 38). Our results demonstrated that spark origin cannot be any fixed number of channels, nor the entire array. Rather, RyRC recruitment in sparks involves variable cohorts of channels from individual arrays. Although most sparks involve multiple channels, still 12% of sparks are single-channel events. Given the large number (≈100) of RyRCs in a cardiac RyRC array, this result implies that only a small fraction of RyRCs in an array are activated, with the overall activation probability on the order of 0.01 ≈ 0.1 per spark. Such a low activation probability should reserve a wide margin of array activation for various physiological regulations (e.g., during cardiac adrenergic stimulation).
As compared with single-channel sparks, the scarcity of brief or long events at higher nq (comparing Fig. 5 A and B with Fig. 4C) suggests that coupling of RyRCs has the effect of synchronizing Ca2+ release duration, both prolonging shorter openings and curtailing longer ones. The prolongation effect is expected if RyRCs are solely coupled by the regenerative CICR mechanism (23). However, the nq-dependent curtailment of long-duration events suggests that RyRC Ca2+ release must also exert a negative feedback to the release machinery. The inverse relationship between nq and the averaged spark rise time (Fig. 5C) supports this notion and further suggests that the inhibitory component predominates over the coupling among RyRCs.
The inhibitory feedback overriding the regenerative CICR may represent the long-sought mechanism for the termination and stability of intracellular Ca2+ release. We have previously shown that a model RyRC array based on in vitro RyRC properties and local CICR fails to reproduce proper termination of Ca2+ sparks and cannot account for the microscopic stability of intracellular Ca2+ release (24, 25). In light of the present finding, we incorporated an inhibitory coupling by introducing a strong Ca2+-dependent inactivation of RyRCs (Fig. 6A). Monte Carlo simulations of the new model recapitulated salient features of Ca2+ sparks, including the prompt termination of sparks, the inverse relationship between nq and release time (Fig. 6B), and the exponential-to-modal transition of Ca2+ release duration (Fig. 6C). As a proof of principle, the model showed that the dual role of permeating Ca2+ as both agonist and antagonist (3-5, 23, 39, 40) of RyRCs could afford the link by which the free energy in the trans-SR Ca2+ gradients drives the irreversible gating of RyRCs. It is also noteworthy that the inhibitory mechanism inactivates some naive RyRCs before they could ever be fired in a spark (Fig. 6A), resulting in a limited availability of RyRCs in the array.
Fig. 6.
Monte Carlo simulations of a model RyRC array. (A) A snapshot (Right, 1 ms after ignition of a simulated “spark”) of the simulation of RyRC model array with excitatory and inhibitory interactions (Left). The stochastic “cardiac couplon” model of Stern et al. (24) was used, modified by increasing the rate constant for Ca2+-dependent inactivation of the resting-state RyRC 200-fold. (B) Average release duration of simulated sparks as a function of nq. The nq was defined as the peak number of simultaneously open RyRCs, and the release duration was determined as the time at which the number of open RyRCs first fell below half of its peak value. (C) Release-duration statistics of model-generated “sparks.” Note that the model recapitulated salient features of cardiac RyRC arrays in the genesis and termination of Ca2+ sparks.
It should be noted that a strong local Ca2+-dependent inactivation is only one of the candidate mechanisms for spark termination and does not exclude other possibilities, such as regulations from luminal side of SR. In the latter case, there is substantial evidence for luminal Ca2+ activation of RyR (40), but local depletion of SR Ca2+ during a spark remains an open question. It has been recently shown that no detectable Ca2+ gradients exist between the junctional SR (presumably the release sites) and the longitudinal SR (presumably the uptake site) during an action potential-elicited release (41). This finding indicates that the SR network is well-connected, allowing for rapid internal diffusion. Thus, experimental measurements leave it uncertain whether a sufficient diffusion resistance exists within the SR to sustain such local depletion during a spark.
Clustering of signaling proteins is an intriguing phenomenon of cell biology (42, 43). Our present study revealed that clustering of RyRCs into arrays endows interacting channels with unique properties. The nq-dependent transition from a thermodynamically reversible to an irreversible mode of gating, which results in the stereotyped duration of Ca2+ sparks, has been heretofore unseen in any ion channels acting solo. The curtailment of active duration in multi-RyRC sparks is also in contrast to the much prolonged open duration in coupled gating of RyRCs in vitro (20), suggesting that not all array-based RyRC properties can be reproduced in a cell-free system.
In summary, we have investigated unitary properties and functional interaction of RyRCs in their native array in intact cardiac myocytes. We found that the number of RyRCs involved in a spark varies dynamically from one to many, which endows Ca2+ sparks with quantal substructure. The interplay of RyRCs in multiquantum sparks results in a thermodynamically irreversible operation of the Ca2+ release channel and stereotyped Ca2+ spark durations. The predominant inhibitory nature of RyRC interaction confines and abbreviates the excitation in the channel array, thus contributing to the microscopic stability of intracellular Ca2+ signaling.
Acknowledgments
We thank Drs. M. B. Cannell, W. J. Lederer, E. G. Lakatta, R. P. Xiao, I. R. Josephson, and S. J. Sollott for critical comments; and Dr. H. A. Spurgeon, Z. Bruce, and S. Wang for technical support. This work was supported by National Institutes of Health intramural and extramural grants (to M.D.S., E.R., and H.C.), a National Institute on Aging Student Training in Academic Research Award (to S.Q.W.), the Major State Basic Research Development Program of China (to H.C.), and the China Ministry of Education Trans-Century Award Program for Talents and Teaching and Research Award Program for Outstanding Young Teachers (to S.Q.W.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RyRC, ryanodine receptor Ca2+ release channel; Ispark, Ca2+ release flux underlying the spark; LCC, L-type Ca2+ channel; q, quantal unit; nq, number of quantal unit; CICR, Ca2+-induced Ca2+ release; SR, sarcoplasmic reticulum.
References
- 1.Clapham, D. E. (1995) Cell 80, 259-268. [DOI] [PubMed] [Google Scholar]
- 2.Berridge, M. J., Lipp, P. & Bootman, M. D. (2000) Nat. Rev. Mol. Cell. Biol. 1, 11-21. [DOI] [PubMed] [Google Scholar]
- 3.Meissner, G. (1994) Annu. Rev. Physiol. 56, 485-508. [DOI] [PubMed] [Google Scholar]
- 4.Bers, D. (2001) Excitation-Contraction Coupling and Cardiac Contractile Force. (Kluwer, Dordrecht, The Netherlands), 2nd Ed.
- 5.Fill, M. & Copello, J. A. (2002) Physiol. Rev. 82, 893-922. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, H., Lederer, W. J. & Cannell, M. B. (1993) Science 262, 740-744. [DOI] [PubMed] [Google Scholar]
- 7.López-López, J. R., Shacklock, P. S., Balke, C. W. & Wier, W. G. (1995) Science 268, 1042-1045. [DOI] [PubMed] [Google Scholar]
- 8.Nelson, M. T., Cheng, H., Rubart, M., Santana, L. F., Bonev, A. D., Knot, H. J. & Lederer, W. J. (1995) Science 270, 633-637. [DOI] [PubMed] [Google Scholar]
- 9.Tsugorka, A., Ríos, E. & Blatter, L. A. (1995) Science 269, 1723-1726. [DOI] [PubMed] [Google Scholar]
- 10.Lipp, P. & Niggli, E. (1998) J. Physiol. (London) 508, 801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollingworth, S., Peet, J., Chandler, W. K. & Baylor, S. M. (2001) J. Gen. Physiol. 118, 653-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haak, L. L., Song, L. S., Molinski, T. F., Pessah, I. N., Cheng, H. & Russell, J. T. (2001) J. Neurosci. 21, 3860-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzini-Armstrong, C., Protasi, F. & Ramesh, V. (1999) Biophys. J. 77, 1528-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin, C. C. & Lai, F. A. (2000) Nat. Cell Biol. 2, 669-671. [DOI] [PubMed] [Google Scholar]
- 15.Takekura, H. & Franzini-Armstrong, C. (2002) Biophys. J. 83, 2742-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loesser, K. E., Castellani, L. & Franzini-Armstrong, C. (1992) J. Muscle Res. Cell Motil. 13, 161-173. [DOI] [PubMed] [Google Scholar]
- 17.Xu, L. & Meissner, G. (1998) Biophys. J. 75, 2302-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu, L., Jones, R. & Meissner, G. (1993) J. Gen. Physiol. 101, 207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx, S. O., Ondrias, K. & Marks, A. R. (1998) Science 281, 818-821. [DOI] [PubMed] [Google Scholar]
- 20.Marx, S. O., Gaburjakova, J., Gaburjakova, M., Henrikson, C., Ondrias, K. & Marks, A. R. (2001) Circ. Res. 88, 1151-1158. [DOI] [PubMed] [Google Scholar]
- 21.Cheng, H. & Wang, S. Q. (2002) Front. Biosci. 7, d1867-d1878. [DOI] [PubMed] [Google Scholar]
- 22.Bers, D. M. (2002) Nature 415, 198-205. [DOI] [PubMed] [Google Scholar]
- 23.Fabiato, A. & Fabiato, F. (1975) J. Physiol. (London) 249, 469-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stern, M. D., Song, L. S., Cheng, H., Sham, J. S., Yang, H. T., Boheler, K. R. & Ríos, E. (1999) J. Gen. Physiol. 113, 469-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, S. Q., Song, L. S., Xu, L., Meissner, G., Lakatta, E. G., Ríos, E., Stern, M. D. & Cheng, H. (2002) Biophys. J. 83, 242-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, S. Q., Song, L. S., Lakatta, E. G. & Cheng, H. (2001) Nature 410, 592-595. [DOI] [PubMed] [Google Scholar]
- 27.Katz, B. (1966) Nerve, Muscle and Synapse (McGraw-Hill, New York).
- 28.Bridge, J. H., Ershler, P. R. & Cannell, M. B. (1999) J. Physiol. 518, 469-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobie, E. A., Dilly, K. W., dos Santos Cruz, J., Lederer, W. J. & Jafri, M. S. (2002) Biophys. J. 83, 59-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez, A., Kirsch, W. G., Shirokova, N., Pizarro, G., Brum, G., Pessah, I. N., Stern, M. D., Cheng, H. & Ríos, E. (2000) Proc. Natl. Acad. Sci. USA 97, 4380-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colquhoun, D. & Hawkes. A. G. (1995) in Single Channel Recording, eds. Sakmann, B. & Neher, E. (Plenum, New York), 2nd Ed., pp. 397-482.
- 32.Rengifo, J., Rosales, R., Gonzalez, A., Cheng, H., Stern, M. D. & Ríos, E. (2002) Biophys. J. 83, 2511-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, S. Q. & Zhou, Z. Q. (1999) Life Sci. 65, 871-875. [DOI] [PubMed] [Google Scholar]
- 34.Mejia-Alvarez, R., Kettlun, C., Ríos, E., Stern, M. D. & Fill, M. (1999) J. Gen. Physiol. 113, 177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tinker, A., Lindsay, A. R. G. & Williams, A. J. (1993) Cardiovasc. Res. 27, 1820-1825. [DOI] [PubMed] [Google Scholar]
- 36.Kettlun, C., Gonzalez, A., Ríos, E. & Fill, M. (2003) J. Gen. Physiol. 122, 407-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izu, L. T., Mauban, J. R., Balke, C. W. & Wier, W. G. (2001) Biophys. J. 80, 88-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider, M. F. (1999) J. Gen. Physiol. 113, 365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fill, M., Zahradnikova, A., Villalba-Galea, C. A., Zahradnik, I., Escobar, A. L. & Gyorke, S. (2000) J. Gen. Physiol. 116, 873-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Györke, S., Gyorke, I., Lukyanenko, V., Terentyev, D., Viatchenko-Karpinski, S. & Wiesner, T. F. (2002) Front Biosci. 7, d1454-d1463. [DOI] [PubMed] [Google Scholar]
- 41.Shannon, T. R., Guo, T. & Bers, D. M. (2003) Circ. Res. 93, 40-45. [DOI] [PubMed] [Google Scholar]
- 42.Shuai, J. W. & Jung, P. (2003) Proc. Natl. Acad. Sci. USA 100, 506-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrido, J. J., Giraud, P., Carlier, E., Fernandes, F., Moussif, A., Fache, M. P., Debanne, D. & Dargent, B. (2003) Science 300, 2091-2094. [DOI] [PubMed] [Google Scholar]








