Abstract
Hepatic glucose metabolism is strongly influenced by oxidative stress and pro-inflammatory stimuli. PON2 (paraoxonase 2), an enzyme with undefined antioxidant properties, protects against atherosclerosis. PON2-deficient (PON2-def) mice have elevated hepatic oxidative stress coupled with an exacerbated inflammatory response from PON2-deficient macrophages. In the present paper, we demonstrate that PON2 deficiency is associated with inhibitory insulin-mediated phosphorylation of hepatic IRS-1 (insulin receptor substrate-1). Unexpectedly, we observed a marked improvement in the hepatic IRS-1 phosphorylation state in PON2-def/apoE (apolipoprotein E)−/− mice, relative to apoE−/− mice. Factors secreted from activated macrophage cultures derived from PON2-def and PON2-def/apoE−/− mice are sufficient to modulate insulin signalling in cultured hepatocytes in a manner similar to that observed in vivo. We show that the protective effect on insulin signalling in PON2-def/apoE−/− mice is directly associated with altered production of macrophage proinflammatory mediators, but not elevated intracellular oxidative stress levels. We further present evidence that modulation of the macrophage inflammatory response in PON2-def/apoE−/− mice is mediated by a shift in the balance of NO and ONOO− (peroxynitrite) formation. Our results demonstrate that PON2 plays an important role in hepatic insulin signalling and underscores the influence of macrophage-mediated inflammatory response on hepatic insulin sensitivity.
Keywords: apolipoprotein E (apoE), insulin receptor substrate-1 (IRS-1), liver, macrophage, paraoxonase, paraoxonase 2 (PON2)
INTRODUCTION
Atherosclerosis and diabetes are tightly intertwined disorders linked by extensive epidemiological and clinical data [1]. Accordingly, cardiovascular incidents as a consequence of atherosclerosis are significantly increased in diabetic patients. Oxidative modification of LDL (low-density lipoprotein) is considered to play a major role in the initiation and progression of atherosclerotic plaques [2] and accumulating evidence suggests that increased plasma oxLDL (oxidized LDL) concentrations are likewise associated with the pathogenesis of the metabolic syndrome [3,4]. oxLDL increases the generation of oxidative stress in various insulin-sensitive tissues by increasing the production of intracellular ROS (reactive oxygen species) and lipid peroxidation products, resulting in the activation of numerous signalling kinases and enhancement of DNA-binding activities of transcription factors such as NF-κB (nuclear factor κB) [5–7].
The binding of insulin to the IR (insulin receptor) induces the phosphorylation of several interacting proteins such as IRS-1 (IR substrate-1). IRS-1 contains a number of potential phosphorylation sites that are targeted in response to different signals that modulate its activity. In response to insulin, IRS-1 is typically phosphorylated on specific phosphotyrosine-binding domains, which can couple IRS-1 to activated IR, and recruit signal transducers. Conversely, serine phosphorylation of IRS-1, in particular at Ser307 in mice, has been shown to play a major role in the desensitization of insulin action and is postulated to represent a unifying mechanistic link among numerous factors involved in the establishment of insulin resistance, including inflammatory and ROS-mediated processes [8,9].
PON2 (paraoxonase 2) is an enzyme with undefined antioxidant properties and PON2 deficiency in C57BL/6J and hyperlipidaemic apoE (apolipoprotein E)−/− mice significantly increases susceptibility towards development of atherosclerosis [10,11]. Although its mechanism of action is unclear, PON2 has recently been shown to localize to the inner mitochondrial membrane where it influences electron transport chain function and protects against oxidative stress [11,12]. PON2 is predominantly expressed in vascular cells, and increased levels of ROS and altered functional properties are associated with macrophages obtained from PON2-def (PON2-deficient) mice [10,13]. Ex vivo, these macrophages directly oxidize LDL to a greater extent than control macrophages. Accordingly, LDL obtained from PON2-def and PON2-def/apoE−/−mice is associated with significantly increased lipid peroxidation content and inflammatory properties [10,11].
The liver is the primary site of lipid metabolism and a major site for insulin-mediated glucose uptake, production and storage [14,15]. Despite impaired lipoprotein function, administration of an atherogenic diet to PON2-def mice elicits a significant improvement in the serum cholesterol profile. Although the mechanism is unclear, decreased secretion of apoB (apolipoprotein B) was associated with significantly elevated oxidative stress levels in the livers of PON2-def mice. Presently, it remains to be elucidated whether hepatic insulin signalling is correspondingly altered in PON2-def mice.
In the present study, we demonstrate decreased insulinmediated Tyr895 phosphorylation associated with significant inhibitory Ser307 phosphorylation of IRS-1 in the livers of PON2-def mice. In contrast, we observed a striking improvement in the liver IRS-1 phosphorylation state in PON2-def/apoE−/− mice, despite a comparable increase in oxidative stress levels and susceptibility towards atherosclerosis. We exploited this disparity in order to gain insight into the physiological role contributed by PON2 in atherosclerosis and insulin signalling, and observed that factors secreted from activated macrophages from PON2-def mice were sufficient to modulate insulin signalling in cultured hepatocytes, consistent with that observed in vivo and associated with distinct changes in the pro-inflammatory response. The direct inhibitory role contributed by macrophages from PON2-def mice on hepatic insulin signalling was demonstrated further by restoring macrophage PON2 expression in PON2-def/apoE−/− mice. In summary, results from the present study: (i) implicate a role for PON2 in liver insulin signalling; (ii) underscore the physiological significance of PON2 on macrophage activation in the context of both atherosclerosis and hepatic insulin signalling; and (iii) provide evidence for a novel physiological association between PON2 and apoE.
MATERIALS AND METHODS
Mice and diets
The Animal Research Committee at UCLA approved all procedures used in the present study. Generation of PON2-def C57BL/6J mice has been described previously [10,11]. Mice were maintained on a 6 % fat chow diet after weaning. At 6 weeks of age, female mice (n = 9–10) were placed on the atherogenic diet consisting of 15.8% fat, 1.25% cholesterol and 0.5% cholate (TD90221; Harlan Teklad), a Western diet consisting of 42% fat and 0.15 % cholesterol (TD88137; Harlan Teklad) or a standard chow diet for an additional 15 weeks. Although development of atherosclerosis in apoE−/− mice is spontaneous and exacerbated further by a high-fat Western diet, a high-fat cholate-containing atherogenic diet is required to provoke atherosclerotic lesion development on the C57BL/6J background. The gene-trap vector described previously for the generation of PON2-def mice was inserted within intron 2 and flanked by loxP sites [10]. LysM (Lysozyme M) Cre apoE−/− mice, generously provided by Dr Yibin Wang (Department of Anesthesiology, UCLA, Los Angeles, CA, U.S.A.), were crossed with PON2-def/apoE−/− mice to obtain PON2-def/LysMCre/apoE−/− mice.
Plasma lipoprotein measurements
Mice were starved for 12 h prior to retro-orbital bleeding under anaesthesia using isofluorane. Total plasma cholesterol, HDL (high-density lipoprotein)-cholesterol and total plasma triacylglycerol (triglyceride) concentrations were determined by enzymatic procedures described previously by Hedrick et al. [16]. Assessment of plasma lipoprotein levels was determined at the time of killing at the end of the diet treatment for all conditions.
Glucose and insulin tolerance tests (GTTs and ITTs)
GTTs and ITTs were performed following overnight starving as described previously [17]. Briefly, mice received an intraperitoneal glucose injection of 1 g/kg of body weight for glucose tolerance testing, and an intraperitoneal injection of human recombinant insulin (Eli Lilly) at a dose of 0.75 unit/kg of body weight for insulin tolerance testing. Tail vein blood (5–10 µ1) was assayed for glucose at the indicated time points.
LPS (lipopolysaccharide)-induced cytokine production
Mice (n = 8) were given an intraperitoneal injection of LPS (5 mg/kg of body weight) derived from Escherichia coli (Sigma). Serum was collected 2 h after injection for determination of TNFα (tumour necrosis factor α) content by commercial ELISA (Invitrogen), and mice were killed at 24 h for tissue collection. Ex vivo studies were performed by injecting thioglycollate solution into the peritoneal cavity in order to elicit the infiltration and accumulation of macrophages, as described previously [10], and subsequently treating cells with 0.1µg/ml LPS for 2 or 24 h in complete medium containing 0.5 % FBS (fetal bovine serum). In some experiments, macrophages were treated overnight with 10 µM uric acid, an ONOO- (peroxynitrite) scavenger that does not inhibit NOS (NO synthase) enzymatic activity prior to LPS treatment.
Cellular ROS and nitrite production
A DCF (2′,7′-dichlorofluorescein) assay was used to quantify intracellular oxidative stress as described previously [10]. Briefly, cells were plated on to 96-well plates (7 × 104 cells/well) and loaded with 100 µM DCF (Invitrogen) for 1 h at 37 °C. Cells were subsequently washed with Krebs-Ringer buffer and treated with 0.1 µg/ml LPS. Fluorescence was measured at the indicated time using a fluorescence microplate reader (Molecular Devices) with an excitation filter of 485 nm and an emission filter at 530 nm. The sum of both nitrite and nitrate in the culture medium was used as an index of total NO production and was determined using a colorimetric assay kit (Cayman).
Immunoblot analyses
For evaluation of insulin signalling ex vivo, peritoneal macrophages were treated with 0.1 µg/ml LPS for 3 h, cells were washed, incubated for an additional 18 h in LPS-free medium and CM (conditioned medium) was collected. The mouse hepatocyte cell line FL83B (A.T.C.C.) was treated with CM for 18 h and subsequently treated with insulin (Sigma) for 10 min. For evaluation of hepatic insulin signalling, starved mice were intravenously injected with either saline or insulin (3.8 units/kg of body weight); 90 s later, mice were killed, and the liver was removed and immediately homogenized. Western blots were performed as described previously [10]. The membranes were incubated with the appropriate dilution of commercially available antibodies (Cell Signaling) to determine the protein expression of IRS-1 [anti-phopho-IRS-1 (Ser307) and anti-phospho-IRS-1 (Tyr895)]. For dot blot analyses, cell protein lysates and appropriately diluted serum were spotted on to a nitrocellulose membrane, blocked in 5 % milk for 2 h, incubated with primary antibody (1:2500 dilution; Abcam) for an additional 2 h and subsequently incubated with the appropriate HRP (horseradish peroxidase)-conjugated secondary antibody (1:10000 dilution; GE Healthcare) for 1 h. Nitrated albumin and treatment of isolated peritoneal macrophages with SIN-1 (3-morpholinosydnonimine), an ONOO- donor, was used to demonstrate the specificity of the anti-nitrotyrosine antibody (Supplementary Figure S1 at http://www.BiochemJ.org/bj/436/bj4360091add.htm).
HSL (homoserine lactone) inactivation bioassay
A portion (4 µg) of crude membrane extracts prepared from isolated peritoneal macrophages was incubated with 10 µM C12 (3-oxo-C12-HSL) in a 50 µl volume of 25 mM Tris/HCl (pH 7.4) and 1 mM CaCl2 at room temperature (25°C). Reactions were stopped with an equal volume of acetonitrile, and 5 µl was used to measure C12 by quantitative bioassay using E. coli MG4 (pKDT17) as described previously [10].
Statistical analysis
Results are means ± S.D. A two-tailed Student’s t test was employed to determine statistically significant differences in group means. A P value of less than 0.05 was considered statistically significant.
RESULTS
PON2 deficiency promotes inhibitory serine phosphorylation of liver IRS-1
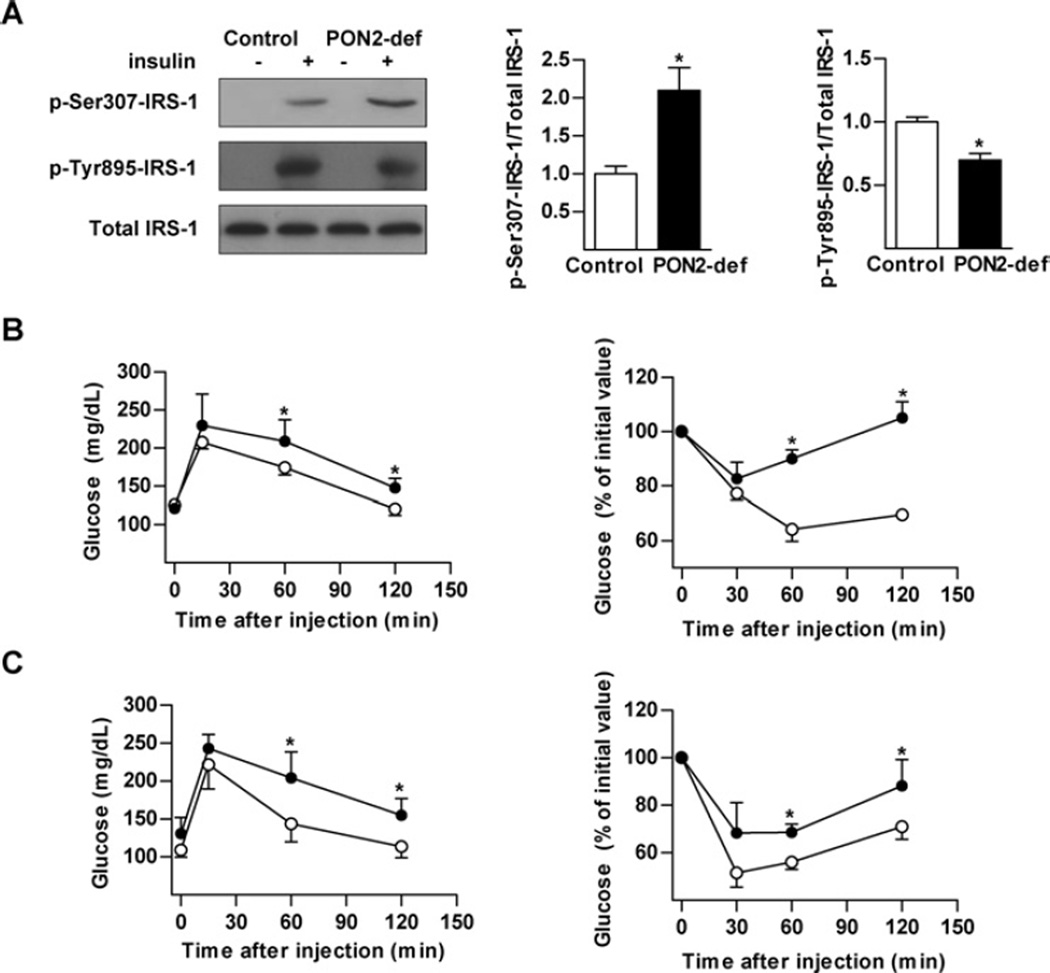
Phosphorylation of IRS-1 at Ser307 is known to inhibit insulin signal transduction in insulin-sensitive tissues in response to a number of stimuli, including oxidative stress. We therefore assessed insulin-stimulated liver IRS-1 phosphorylation in order to evaluate the influence of increased liver oxidative stress characteristic of PON2-def mice. Injection of insulin led to a marked increase in serine phosphorylation of IRS-1 in livers isolated from PON2-def mice, with a corresponding decrease in tyrosine phosphorylation (Figure 1A).
Figure 1. Impaired hepatic insulin signalling in PON2-def mice.
(A) Mice (n = 3) were starved overnight and then administered human insulin at 5 units/kg body of weight intravenously. Liver was collected and insulin signalling was assessed by detection of phosphorylation of IRS-1 at Tyr895 and Ser307. Values are means± S.E.M. (B) GTT (left-hand panel) and ITT (right-hand panel) assessment of mice (n = 8–10) fed on a standard chow diet for 15 weeks. (C) GTT (left-hand panel) and ITT (right-hand panel) assessment of mice (n = 8–10) fed on an atherogenic diet for 15 weeks. ○, control; ●, PON2-def. Values are means ± S.D. *P < 0.05.
PON2-def mice were subjected to GTTs and ITTs to determine whether further influence on peripheral insulin sensitivity is consequently impaired. Blood glucose levels were significantly higher in PON2-def mice throughout the GTT when compared with control mice. PON2-def mice also exhibited impaired responses to insulin during the ITT (Figure 1B). Similar results were obtained in PON2-def mice fed on an atherogenic diet (Figure 1C), shown previously to develop increased atherosclerosis [10]. Although these data support the observed decrease in hepatic insulin signalling, the ITT is a relatively crude technique for assessment of insulin-stimulated glucose disposal and no significant differences in starving glucose or insulin levels on either the standard or atherogenic diet was observed (Table 1).
Table 1.
Metabolic parameters of PON2-def and C57BL/6J control mice fed on a standard or atherogenic diet for 15 weeks
| Standard diet |
Atherogenic diet |
|||
|---|---|---|---|---|
| Parameter | Control | PON2-def | Control | PON2-def |
| Triacylglycerols (mg/dl) | 13.6 ±4 | 22.0 ± 7* | 6.8 ±3 | 5.4 ± 4 |
| Total cholesterol (mg/dl) | 111.4 ± 14 | 135.5 ± 21* | 296.4 ±52 | 221.6 ±45* |
| HDL-cholesterol (mg/dl) | 103.0 ± 6 | 117.7 ± 18 | 63.0 ±15 | 56.1 ±30 |
| VLDL/LDL-cholesterol (mg/dl) | 14.6 ±5 | 25.0 ± 8* | 162.2 ±21 | 119.8 ± 16* |
| NEFA (mg/dl) | 31.1 ±9 | 30.8 ± 7 | 21.6 ±3 | 19.9 ±4 |
| Glucose (mg/dl) | 133.4 ± 13 | 144.0 ± 19 | 128.4 ±15 | 127.8 ± 14 |
| Insulin (ng/ml) | 0.32 ± 0.05 | 0.33 ± 0.05 | 0.41 ± 0.10 | 0.38 ± 0.10 |
Values are means ± S.D. from 8–10 animals from each group.
P < 0.05.
Metabolic parameters in PON2-def mice
Elevated serum NEFA (non-esterified fatty acid) and lipid content are associated with impaired insulin signalling in peripheral insulin-responsive tissues [18,19]. Despite a previously described improvement in the cholesterol profile on the atherogenic diet [10], we report that PON2-def mice maintained on the standard chow diet developed an approximate 2-fold increase in serum triacylglycerol levels with a significant increase in serum VLDL (very-LDL)/LDL-cholesterol levels (Table 1). This worsened lipid profile, a characteristic of the metabolic syndrome, was not associated with any differences in serum NEFA levels on either diet (Table 1).
Improved liver IRS-1 phosphorylation in PON2-def/apoE−/− mice
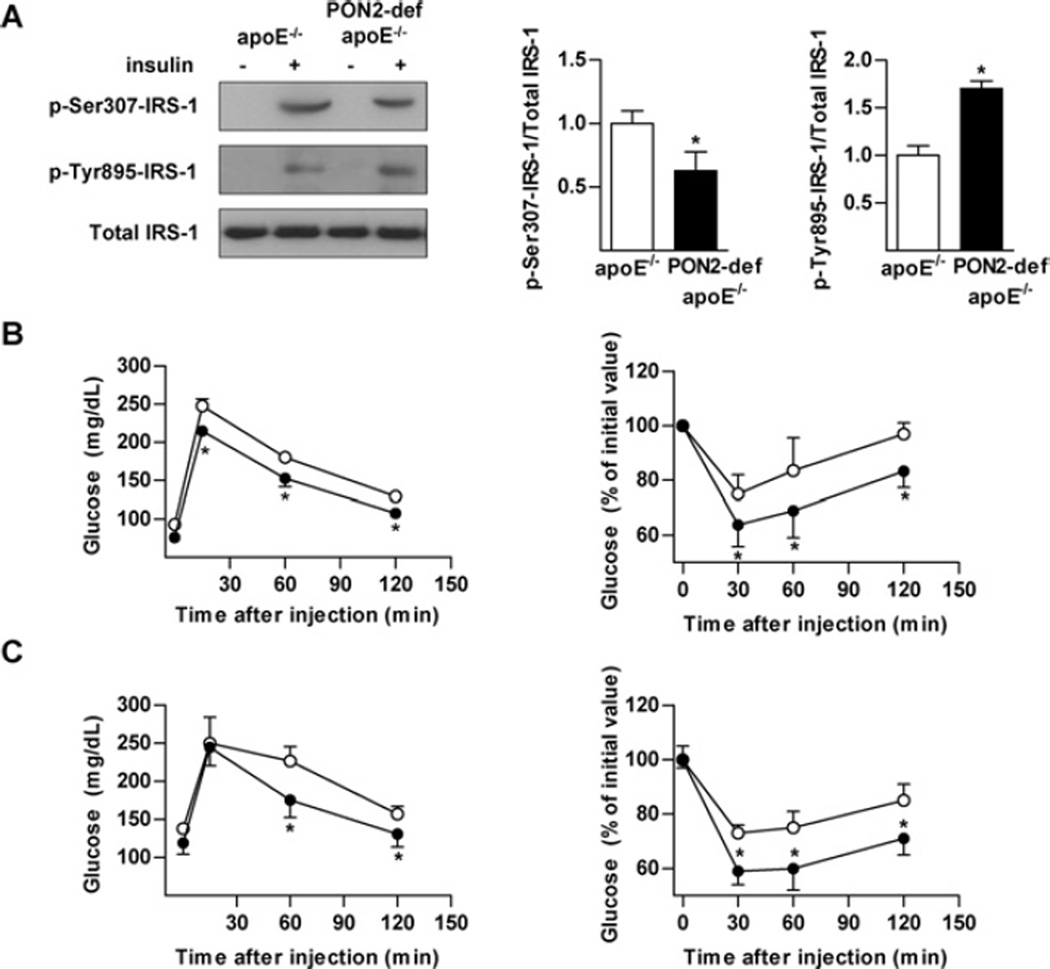
We recently reported an increase in the development of atherosclerosis in PON2-def mice crossed on to the hyperlipidaemic apoE−/− background when compared with apoE−/− mice [10,11]. Consistent with previous observations made inPON2-def mice, the increased atherosclerosis in PON2-def/apoE−/− was correlated with increased oxidative modification and bioactivity of LDL [11]. We assessed insulin stimulated IRS-1 phosphorylation in order to further complement and characterize the association between increased atherosclerosis susceptibility and impaired insulin signalling in PON2-def mice. Unexpectedly, PON2 deficiency did not worsen but markedly reduced serine phosphorylation and increased tyrosine phosphorylation of IRS-1 in livers of PON2-def/apoE−/− mice (Figure 2A). Moreover, this was associated with a concomitant improvement in both GTT and ITT in mice fed on the standard (Figure 2B) and Western (Figure 2C) diets. Similar to results obtained on the C57BL/6J background, no change in starving glucose or insulin levels were observed on either of the administered diets on the apoE−/− background (Table 2).
Figure 2. Improved hepatic insulin signalling in PON2-def/apoE−/− mice.
(A) Mice (n = 3) were starved overnight and then administered human insulin at 5 units/kg of body weight intravenously. Liver was collected and insulin signalling assessed by detection of phosphorylation of IRS-1 at Tyr895 and Ser307. Values are means ± S.E.M. (B) GTT (left-hand panel) and ITT (right-hand panel) assessment of mice (n = 8–10) fed on a standard chow diet for 15 weeks. (C) GTT (left-hand panel) and ITT (right-hand panel) assessment of mice (n = 8–10) fed on a Western diet for 15 weeks. ○, control; ●, PON2-def. Values are means ± S.D. *P < 0.05.
Table 2.
Metabolic parameters of PON2-def/apoE−/− and apoE−/− control mice fed on a standard or Western diet for 15 weeks
| Standard diet |
Western diet |
|||
|---|---|---|---|---|
| Parameter | apoE−/− | PON2-def/apoE−/− | apoE−/− | PON2-def/apoE−/− |
| Triacylglycerols (mg/dl) | 40.6 ±8 | 74.0 ± 9* | 41.8 ±7 | 41.0 ±6 |
| Total cholesterol (mg/dl) | 457.4 ±53 | 623.4 ± 38* | 1363.0 ± 15 | 1147.1 ± 155* |
| HDL-cholesterol (mg/dl) | 19.1 ±3 | 21.7 ±4 | 18.1 ±5 | 18.0 ±4 |
| VLDL/LDL-cholesterol (mg/dl) | 414.0 ± 56 | 593.6 ± 40* | 1346.3 ± 15 | 1077.8 ± 178* |
| NEFA (mg/dl) | 27.4 ±8 | 22.7 ±4 | 33.2 ±5 | 31.6 ±6 |
| Glucose (mg/dl) | 97 ±23 | 94 ±27 | 140 ±30 | 157 ±38 |
| Insulin (ng/ml) | 0.43 ± 0.12 | 0.55 ±0.09 | 0.20 ± 0.08 | 0.23 ± 0.12 |
Values are means ± S.D. from 8–10 animals from each group.
P < 0.05.
Metabolic parameters in PON2-def/apoE−/−mice
Despite increased hepatic insulin signalling in PON2-def/apoE−/− mice, the results presented in Table 2 show a diet-dependent influence on serum metabolic parameters analogous to that observed in PON2-def mice. That is, we observed a worsened lipid and cholesterol profile when maintained on the standard chow diet that improved upon switchingontothe high-fat Western diet, in comparison with apoE−/− mice. The mechanism contributing to the diet-influenced affect on lipid metabolism is presently unknown but, taken together, these data suggested that impaired liver insulin signalling is not directly contributed to by serum lipid or NEFA concentrations in the PON2-def mouse model.
Macrophages from PON2-def mice directly influence insulin action in hepatocytes
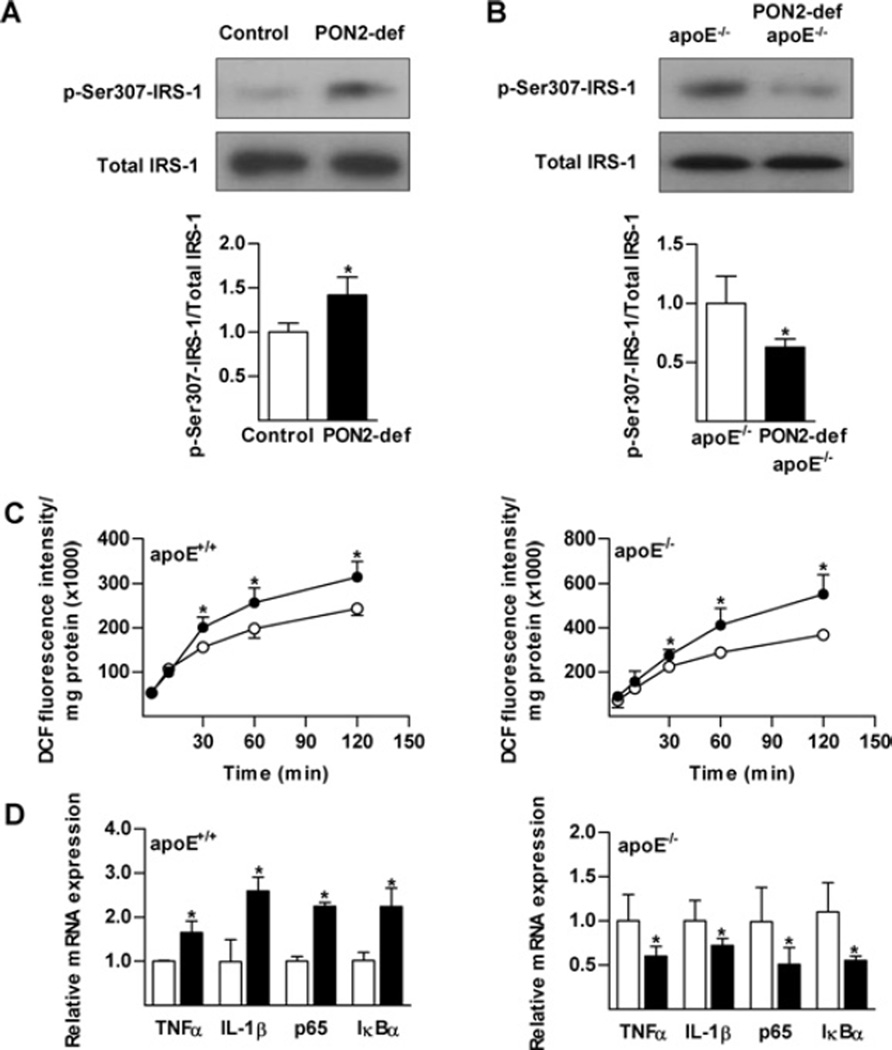
PON2 is highly expressed in macrophages [20] and inflammatory activation of resident macrophages is shown to potentiate insulin signalling in metabolic tissues [21]. We therefore directly tested the effect of macrophage PON2 deficiency on hepatic insulin action in vitro. Insulin sensitivity of the FL83B mouse hepatocyte cell line was assessed following treatment with CM obtained from activated peritoneal macrophages from wild-type control or PON2-def mice. PON2-def CM increased inhibitory serine phosphorylation of IRS-1 to a much greater extent than control CM, in a manner consistent with results observed in vivo (Figure 3A). Conversely, phosphorylation of IRS-1 at Ser307 was correspondingly decreased in hepatocytes treated with CM obtained from activated peritoneal macrophages from PON2-def/apoE−/− mice, relative to control macrophages from apoE−/− mice (Figure 3B). Serine phosphorylation of IRS-1 was not observed in hepatocytes treated with CM obtained from untreated macrophages. These results implicate a fundamental role played by PON2 at the level of macrophage function in the modulation of insulin signalling.
Figure 3. Direct modulation of hepatic insulin signalling by macrophages from PON2-def mice is associated with altered inflammatory response.
Peritoneal macrophages obtained from PON2-def (A) and PON2-def/apoE−/− (B) mice maintained on a standard chow diet were activated (0.1 µg/ml LPS) and allowed to recover for 18 h. FL83B hepatocytes were then treated with CM from macrophages for 18 h and impaired insulin signalling was evaluated by detection of phosphorylation of IRS-1 at Ser307. (C) LPS-elicited production of ROS was measured at the indicated times by quantifying fluorescence emitted by ROS-mediated oxidation of DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate) in macrophages. ○, control; ●, PON2-def. (D) mRNA expression levels of the indicated genes determined using quantitative RT (reverse transcription)–PCR. Values are means ± S.E.M. *P < 0.05.
Altered macrophage pro-inflammatory response
Inhibitory Ser307 phosphorylation of IRS-1 can be mediated by a number of overlapping stimuli, including inflammatory cytokines such as TNFα, NEFAs and oxidative stress. We have shown previously that PON2 deficiency increases the level of oxidative stress in macrophages from both the apoE+/+ and apoE−/− backgrounds [10,11]. To investigate the mechanism by which macrophages from PON2-def influence insulin-stimulated IRS-1 phosphorylation, we treated isolated peritoneal macrophages from PON2-def mice maintained on a standard chow diet with LPS (0.1 µg/ml) for the indicated time. Consistent with previous findings, ROS production was elevated in PON2-def mice on both genetic backgrounds (Figure 3C). Similar results were obtained following treatment with H2O2 or high glucose (results not shown). Using the same cell preparations, we further assessed cytokine gene expression levels. As reported previously [10], LPS elicited an increased level of expression of TNFa and IL-1β (interleukin-1β) in macrophages from PON2-def mice (Figure 3D). However, the increased production of ROS exhibited in macrophages from PON2-def/apoE−/−mice was accompanied by a significantly reduced expression of TNFα and IL-1β (Figure 3D). Both cytokines are products of LPS-mediated activation of the NF-κB pathway and a similar expression profile was observed for p65 and IκBα (inhibitory κBα), an NF-κB subunit and inhibitor respectively. Analogous results were obtained in vivo following intraperitoneal administration of LPS (Supplementary Figure S2 at http://www.BiochemJ.org/bj/436/bj4360091add.htm).
Restoring macrophage PON2 expression in vivo is sufficient to modulate hepatic insulin signalling
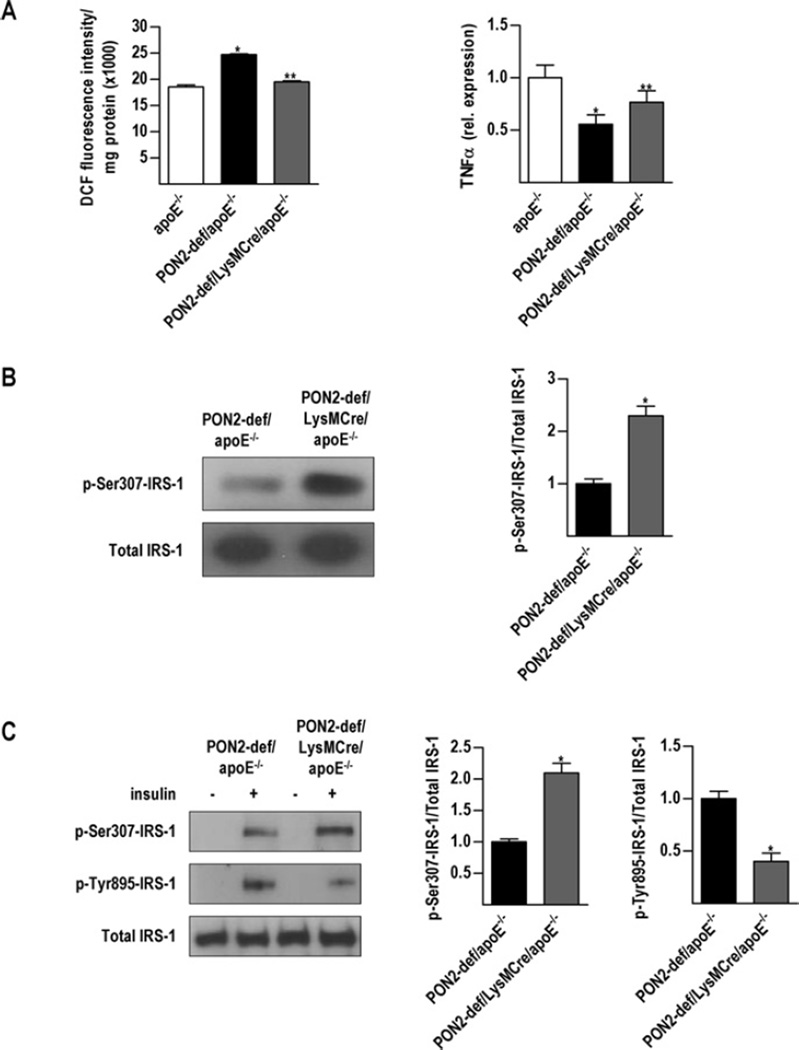
To confirm the assertion that macrophages are directly modulating hepatic insulin signalling in vivo, we generated PON2-def/LysMCre/apoE−/− mice with partially restored macrophage PON2 expression. Briefly, we used the loxP-Cre system to excise the loxP-flanked gene-trap vector inserted within PON2 by expressing Cre recombinase under the control of the well-characterized mouse LysM gene regulatory region. Assessment of isolated peritoneal macrophages showed expression levels of approximately 60 % that of controls (mRNA and protein) and almost 80 % restored lactonase activity (Supplementary Figure S3 at http://www.BiochemJ.org/bj/436/bj4360091add.htm). Figure 4 (A) shows that restoring macrophage PON2 expression reduced intracellular ROS production but significantly increased TNFa expression levels. Despite improved oxidative stress levels, CM obtained from PON2-def/LysMCre/apoE−/−derived macrophages elicited significant phosphorylation of IRS-1 at Ser307, relative to macrophages obtained fromPON2-def/apoE−/− mice (Figure 4B). Administration of insulin in vivo resulted in a dramatic increase in the phosphorylation of hepatic IRS-1 at Ser307 with decreased tyrosine phosphorylation (Figure 4C).
Figure 4. Partial recovery of macrophage PON2 expression is sufficient to modulate hepatic insulin signalling.
Peritoneal macrophages were obtained from PON2-def/LysMCre/apoE−/−mice fed on a standard chow diet. (A) Peritoneal macrophages were treated with 0.1 µg/ml LPS for 90 min and ROS was measured by quantifying the fluorescence emitted by DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate). TNFα mRNA expression levels were determined by quantitative RT (reverse transcription)–PCR. (B) FL83B hepatocytes were treated for 18 h with CM obtained from peritoneal macrophages from PON2-def/apoE−/− and PON2-def/LysMCre/apoE−/− mice and insulin signalling was evaluated by detection of phosphorylation of IRS-1 at Ser307. (C) Mice (n = 3) were starved overnight and then treated with human insulin at 5 units/kg of body weight intravenously. Liver was collected and insulin signalling was assessed by detection of phosphorylation of IRS-1 at Tyr895 and Ser307. Values are means± S.E.M. *P < 0.05 relative to apoE−/−; **P < 0.05 relative to PON2-def/apoE −/−.
Assessment of NO and ONOO~ levels
Oxidative stress and inflammation are co-ordinately regulated and play a fundamental role in the pathogenesis of atherosclerosis and insulin resistance. Therefore we sought to examine the mechanism accounting for the uncoupling of pro-inflammatory cytokine production from elevated cellular oxidative stress in PON2-def/apoE−/− mice. Evidence suggests that a balance subsists between the production of NO and the formation of the nitrating agent ONOO−, which have a profound influence on resolving or prolonging the inflammatory response respectively [22].
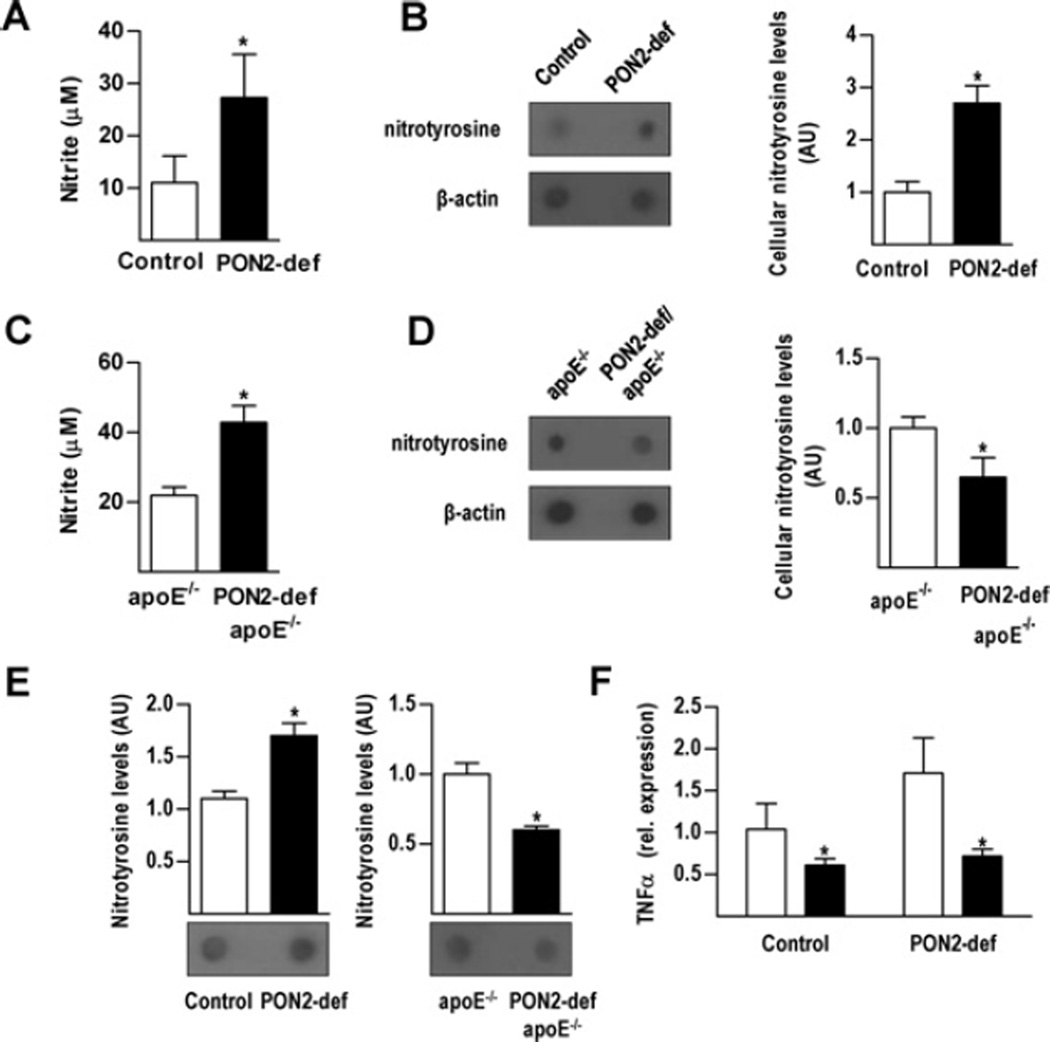
Alongside a 2.5-fold increase in the expression of iNOS (inducible NOS), a target of NF-κB pathway activation, in peritoneal macrophages from PON2-def mice treated with LPS (0.1µg/ml), we observed a relative increase in nitrite accumulation (an indicator of NO synthesis) and a corresponding increase in macrophage protein nitrotyrosylation (a marker of ONOO− formation) (Figures 5A and 5B). Interestingly, activated macrophages from PON2-def/apoE−/− mice exhibited a similar increase in nitrite concentrations, relative to apoE−/− controls, but with reduced expression of iNOS (1.7-fold) and decreased protein nitrotyrosylation (Figures 5C and 5D). Similar results were obtained using a direct ELISA approach (Supplementary Figure S4 at http://www.BiochemJ.org/bj/436/bj4360091add.htm). Serum obtained frommice fed on a standard chow diet exhibited a similar pattern of protein nitrotyrosylation (Figure 5E). To evaluate the influence of ONOO− on NF-κB pathway activation we treated macrophages with uric acid, an ONOO− scavenger that does not influence NO production [23], prior to assessing LPS-mediated TNFα expression levels. Indeed, the results in Figure 5 (F) demonstrate that the expression of TNFα in macrophages from PON2-def mice was sensitive to modulation of cellular ONOO− concentrations.
Figure 5. Macrophage-mediated NO generation and ONOO−formation.
Peritoneal macrophages from PON2-def mice and littermate control were treated overnight with LPS (0.1 µg/ml) for determination of supernatant nitrite concentrations (A) and cellular nitrotyrosine content (B). Peritoneal macrophages from PON2-def/apoE−/− mice and littermate controls were treated overnight with LPS (0.1 µg/ml) for determination of supernatant nitrite concentrations (C) and cellular nitrotyrosine content (D). Protein nitrotyrosine levels were determined in vivo using serum from the indicated standard-diet-fed mice (E). Peritoneal macrophages obtained from PON2-def and littermate control mice were treated overnight with uric acid (10µM) and TNFα expression levels were determined following a subsequent 2 h LPS (0.1 µg/ml) treatment (F). Values are means ± S.D. *P < 0.05. AU, arbitrary units.
DISCUSSION
PON2 is an enzyme with undefined antioxidant properties that has been shown recently to localize within the inner mitochondrial membrane and associate with complex III in the electron transport chain [11]. PON2 deficiency in mice increases susceptibility to atherosclerosis and this has primarily been associated with significantly elevated levels of lipoprotein lipid peroxidation and pro-inflammatory properties [10,11]. The significance and emphasis on lipoprotein function in PON2-def mice is underscored by an anti-atherogenic serum cholesterol profile in mice fed on an atherogenic diet. Although the mechanism remains unclear, increased oxidative stress levels in livers obtained from PON2-def mice have been associated with impaired secretion of apoB [10].
The liver is not only the primary site of lipid metabolism, but is a major site for glucose uptake, production and storage. Its role in glucose metabolism is strongly influenced by systemic as well as local oxidative and inflammatory stimuli [24,25], which in turn influences whole-body insulin responsiveness [15]. Given the elevated oxidative stress levels and abnormal lipid metabolism reported previously in PON2-def and PON2-def/apoE−/− mice [10,11], we hypothesized that atherosclerosis may be accompanied by impaired hepatic insulin signalling. In the present study, we have demonstrated a marked reduction in insulin-mediated tyrosine phosphorylation of IRS-1 in the livers of PON2-def mice. Furthermore, this was associated with a marked increase in inhibitory phosphorylation of IRS-1 at Ser307. Overproduction of VLDL is a hallmark of dyslipidaemia associated with the metabolic syndrome, and lipid metabolism is shown to be directly influenced by hepatic insulin signalling [19]. We observed that impaired hepatic insulin signalling was directly associated with a severely worsened serum lipid and cholesterol profile in PON2-def mice maintained on a standard chow diet. This reveals a role for PON2 in lipid metabolism involving a previously unrecognized diet-dependent physiological mechanism.
Atherosclerosis and insulin resistance are multifactorial diseases that are commonly associated with dyslipidaemia, oxidative stress and chronic inflammation. We show that, despite a corresponding increase in atheroma formation, mitochondrial dysfunction, systemic oxidative stress and diet-influenced metabolic dyslipidaemia, hepatic insulin signalling is effectively improved in PON2-def/apoE−/− mice, relative to apoE−/− littermate controls. These results reveal a functional association between PON2 and apoE, and suggest that loss of hepatic insulin sensitivity contributed to by PON2 deficiency may share common elements with the development of atherosclerosis but manifest independently. Given the striking similarities shared by the two mouse models used in the present study, we sought to identify the basis for this divergence in order to improve our insight regarding the physiological role of PON2 in these highly related diseases.
We subsequently assessed macrophage function given their crucial influence on atherosclerosis and insulin signalling, and the expression and protective influence of both PON2 and apoE in this immune effector cell. In addition, macrophage-elicited inflammatory mediators are shown to modulate insulin signalling in the liver, where they account for over 5% of the total cell population, in a manner analogous to their role in adipose tissue [25]. Primary macrophages isolated from PON2-def mice have been shown previously to be hyper-responsive to numerous agonists, producing comparatively increased levels of ROS and pro-inflammatory cytokines [10]. We demonstrate that factors secreted by activated macrophages from PON2-def mice are sufficient to independently modulate insulin signalling in cultured hepatocytes. Despite a corresponding increase in intracellular ROS production in PON2-def/apoE−/− -derived macrophages, we observed a significant decrease in pro-inflammatory cytokine expression, relative to apoE−/− . The liver is a vital insulin-responsive organ whose function is strongly affected by resident macrophages and inflammatory mediators, and we show that systemic administration of LPS influences the expression of pro-inflammatory cytokines in the livers of PON2-def mice consistent with observed changes in insulin signalling.
In order to directly address the role of PON2 on the macrophage inflammatory response and its influence on hepatic insulin signalling, we selectively re-introduced PON2 expression in macrophages in vivo. As an enzyme with antioxidant properties, this resulted in a significant decrease in the generation of ROS. However, CM obtained from cultures from PON2-def/LysMCre/apoE−/− mice induced a significant increase in the phosphorylation of IRS-1 at Ser307 and this was likewise associated with a correspondingly elevated induction of TNFα. Administration of insulin to PON2-def/LysMCre/apoE−/− mice resulted in a similar inhibitory phosphorylation of hepatic IRS-1, strongly supporting the primary influence of pro-inflammatory mediators on hepatic insulin signalling in PON2-def mice.
NF-κB, a prototypic transcription factor in eukaryotic cells, plays a pivotal role in the transactivation of gene promoters involved in inflammation, immune responses and anti-apoptotic processes. Consequently, NF-κB activation is tightly regulated with aberrant activation highlighted as a key mediator of insulin resistance. ApoE can influence cell function by modulating cellular cholesterol metabolism, but also possesses a direct antioxidant role in macrophages which extends beyond the regulation of lipid transport and has been implicated in the production of NO [26,27]. NO, an important endogenous signalling molecule, plays a dual role in redox regulation of NF-κB [22]: an anti-inflammatory action is attributed to its inhibition of NF-κB-mediated gene expression, whereas its pro-inflammatory action is attributed to its conversion into ONOO− , conversely prolonging NF-κB signalling. Results shown in the present study altogether demonstrate that NF-κB pathway activation in macrophages from PON2-def mice is co-ordinately regulated and sensitive to cellular concentrations of ONOO−. That is, although production of ROS is largely increased in macrophages from PON2-def/apoE−/− mice, we provide evidence that the uncoupling of cytokine gene expression from elevated cellular oxidative stress may be mediated, in part, by reduced ONOO− levels, relative to the production of NO (Figure 6). The significantly reduced content of ONOO− in macrophages from PON2-def/apoE−/− mice, despite elevated NO and O2− (superoxide anion) concentrations that normally react in a near-diffusion rate-limited manner to form ONOO− , further suggests that combined deficiency of both PON2 and apoE may elicit selective neutralization of ONOO− . The extent to which ONOO− promotes NF-κB pathway activation and the mechanism by which ONOO− levels are reduced remains unclear.
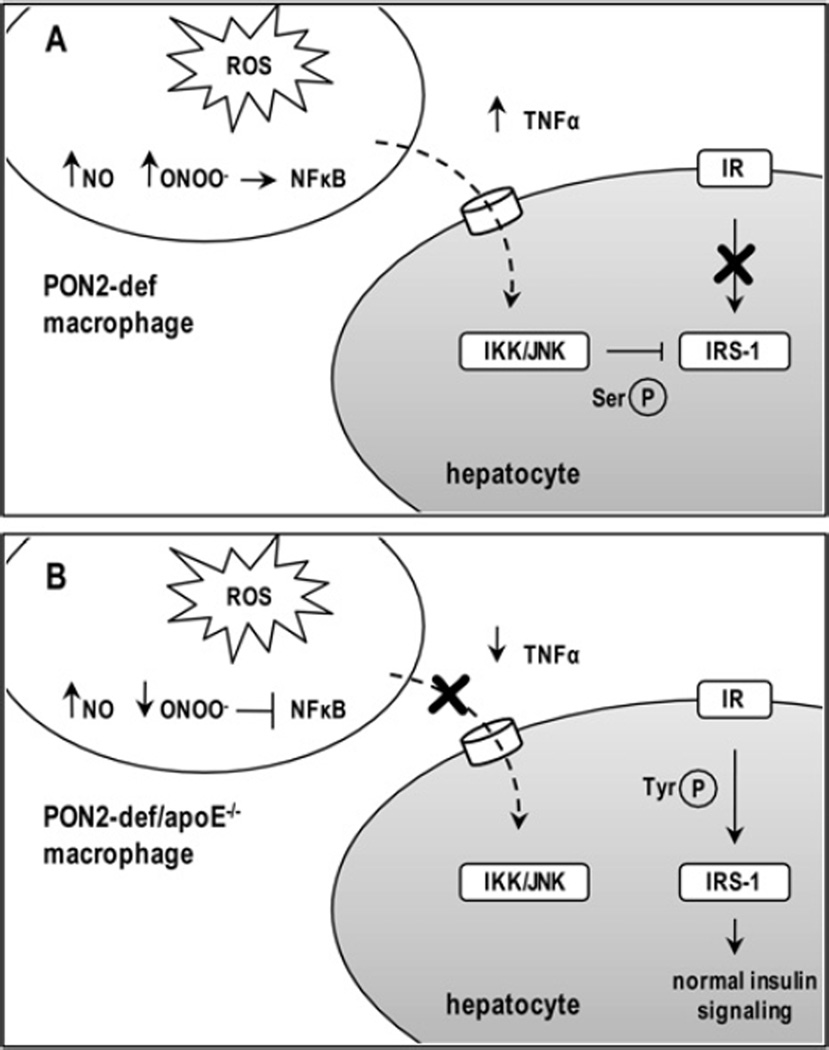
Figure 6. Proposed scheme to explain the influence of macrophage PON2 on hepatic insulin sensitivity.
We show that factors secreted from activated macrophages from PON2-def mice are sufficient to modulate insulin signalling in hepatocytes by eliciting inhibitory serine phosphorylation (Ser-P) of IRS-1. Impaired insulin signalling in macrophages from PON2-def mice was associated with an increased production of ROS, NO, ONOO− and expression of pro-inflammatory cytokines (A). Despite a corresponding increase in intracellular ROS production in macrophages from PON2-def/apoE−/− mice, we observed a significant decrease in the expression of pro-inflammatory cytokines associated with reduced serine phosphorylation and improved tyrosine phosphorylation (Tyr-P) of hepatic IRS-1, relative to apoE −/− littermate controls. Evidence suggests the influence of decreased cellular ONOO− content on NF-κB pathway activation in macrophages from combined PON2-def/apoE−/− mice (B). IKK, inhibitor of NF-κB kinase; JNK, c-Jun N-terminal kinase.
Impaired intracellular insulin signal transduction is considered a major component of insulin resistance and pro-inflammatory cytokines are shown to have a detrimental impact on insulin signalling in peripheral tissues. Despite the evident relationship between pro-inflammatory cytokine production and impaired insulin signalling observed in the present study, the key factor(s) secreted by activated macrophages from PON2-def mice that affect this process have yet to be identified. Therefore the contribution and extent to which the altered pro-inflammatory cytokine response is influencing insulin signalling in PON2-def mice is unclear. For instance, insulin resistance directly associated with iNOS induction is mediated by protein S-nitrosylation, rather than a phosphorylation-dependent inactivation pathway [28,29]. Although we have not assessed S-nitrosylation of insulin signalling intermediates, we have observed differential Ser307 phosphorylation of liver IRS-1, a major mechanism of insulin resistance, which is predominantly activated by exposure to proinflammatory cytokines such as TNFα [9]. Ongoing work in our laboratory is focused on exploring the physiological association of PON2 and apoE, and the direct implication of the modulation of NO on the inflammatory response in activated macrophages from PON2-def mice and on insulin signalling in PON2-def mice.
The divergence in hepatic insulin signalling exhibited in the present study is intriguing given that numerous pathogenic traits shared by PON2-def mice on both genetic backgrounds are closely associated with atherosclerosis and insulin resistance. The present study strengthens the antioxidant capacity ascribed previously to PON2 and supports a model in which the increased atherosclerosis in PON2-def mice can be attributed to oxidative damage. However the present work further suggests that NF-κB-mediated pro-inflammatory cytokine production does not play an essential role in atherogenesis, but rather in modulating insulin sensitivity in PON2-def mice. That is, increased production of ROS is a common denominator in the development of both insulin resistance and atherosclerosis in PON2-def mice, but the influence that PON2 has on the macrophage pro-inflammatory response only appears to have an impact on insulin signalling. Although improved hepatic insulin signalling may be unique to a physiological interaction involving PON2 and apoE, the present study serves to highlight the prominent role of PON2 in modulating the macrophage activation state.
Targeted disruption of hepatic IRS-1 function and abnormal ITT results imply a role for PON2 in systemic insulin sensitivity; however, hyperinsulinaemic clamp studies are required to effectively evaluate the prospective influence of PON2 deficiency on peripheral glucose tolerance. The lack of effect on starving serum insulin and glucose levels demonstrate that the atherosclerosis-susceptible PON2-def mouse models used in the present study do not develop overt insulin resistance. PON2 has been described previously as a stress-activated enzyme [30], and the impact of PON2 deficiency on the inflammatory response and hepatic insulin signalling observed in the present study are all in response to stress stimuli (e.g. bolus insulin injection). Assessment of PON2 deficiency in a mouse model of insulin resistance is a current focus of research in our laboratory.
In summary, we present evidence that increased atherosclerosis susceptibility of PON2-def mice is further associated with impaired hepatic insulin signalling, supporting a number of epidemiological studies previously associating PON2 polymorphisms with diabetic complications. We show that despite numerous attributes shared with PON2-def mice, including increased atherosclerotic lesion development, systemic oxidative stress, diet-influenced dyslipidaemia and mitochondrial dysfunction, PON2-def/apoE−/− mice exhibit improved hepatic insulin signalling, relative to apoE−/− control littermates. Dysregulated inflammation is a well-characterized complication associated with impaired glucose tolerance, and we provide evidence that factors secreted by activated macrophages from PON2-def are capable of altering insulin signalling consistent with that observed in vivo. Overall, the present study provides insight into the mechanistic aetiology of insulin resistance, as it relates to atherosclerosis, and extends our understanding of the vital physiological function that is played by PON2 in both of these metabolic diseases.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Dr Yun Dai, Dr Sherin Devaskar, Dr Diana Shih and Dr Asokan Devarajan for their valuable discussions. We thank Dr Lawrence Castellani and Sarada Charugundla for their expert technical assistance.
FUNDING
This work was supported by the National Heart, Lung and Blood Institute (NHLBI) [grant number 1RO1HL71776 (to S.T.R.)].
Abbreviations used
- apoB
apolipoprotein B
- apoE
apolipoprotein E
- C12
3-oxo-C12-HSL
- CM
conditioned medium
- DCF
2′,7′-dichlorofluorescein
- GTT
glucose tolerance test
- HDL
high-density lipoprotein
- HSL
homoserine lactone
- IL-1β
interleukin-1β
- IR
insulin receptor
- IRS-1
IR substrate-1
- ITT
insulin tolerance test
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide
- LysM
lysozyme M
- NEFA
non-esterified fatty acid
- NF-κB
nuclear factor κB; NOS, NO synthase
- iNOS
inducible NOS
- oxLDL
oxidized LDL
- PON2
paraoxonase 2
- PON2-def
PON2-deficient
- ROS
reactive oxygen species
- TNFα
tumour necrosis factor α
- VLDL
very-LDL
Footnotes
AUTHOR CONTRIBUTION
Noam Bourquard and Carey Ng performed the research. Noam Bourquard wrote the paper. Srinivasa Reddy supervised, reviewed and edited the paper prior to submission.
REFERENCES
- 1.Bonora E, Kiechl S, Oberhollenzer F, Egger G, Bonadonna RC, Muggeo M, Willeit J. Impaired glucose tolerance, Type II diabetes mellitus and carotid atherosclerosis: prospective results from the Bruneck Study. Diabetologia. 2000;43:156–164. doi: 10.1007/s001250050024. [DOI] [PubMed] [Google Scholar]
- 2.Navab M, Hama SY, Reddy ST, Ng CJ, Van Lenten BJ, Laks H, Fogelman AM. Oxidized lipids as mediators of coronary heart disease. Curr. Opin. Lipidol. 2002;13:363–372. doi: 10.1097/00041433-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kopprasch S, Pietzsch J, Kuhlisch E, Fuecker K, Temelkova-Kurktschiev T, Hanefeld M, Kuhne H, Julius U, Graessler J. In vivo evidence for increased oxidation of circulating LDL in impaired glucose tolerance. Diabetes. 2002;51:3102–3106. doi: 10.2337/diabetes.51.10.3102. [DOI] [PubMed] [Google Scholar]
- 4.Tsuzura S, Ikeda Y, Suehiro T, Ota K, Osaki F, Arii K, Kumon Y, Hashimoto K. Correlation of plasma oxidized low-density lipoprotein levels to vascular complications and human serum paraoxonase in patients with type 2 diabetes. Metab. Clin. Exp. 2004;53:297–302. doi: 10.1016/j.metabol.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Maziere C, Auclair M, Djavaheri-Mergny M, Packer L, Maziere JC. Oxidized low density lipoprotein induces activation of the transcription factor NFκB in fibroblasts, endothelial and smooth muscle cells. Biochem. Mol. Biol. Int. 1996;39:1201–1207. doi: 10.1080/15216549600201392. [DOI] [PubMed] [Google Scholar]
- 6.Maziere C, Alimardani G, Dantin F, Dubois F, Conte MA, Maziere JC. Oxidized LDL activates STAT1 and STAT3 transcription factors: possible involvement of reactive oxygen species. FEBS Lett. 1999;448:49–52. doi: 10.1016/s0014-5793(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Yang B, Mehta JL. Ox-LDL induces apoptosis in human coronary artery endothelial cells: role of PKC, PTK, bcl-2, and Fas. Am. J. Physiol. 1998;275:H568–H576. doi: 10.1152/ajpheart.1998.275.2.H568. [DOI] [PubMed] [Google Scholar]
- 8.White MF. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 9.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 10.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, Fogelman AM, Lusis AJ, Young S, Reddy ST. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: anti-atherogenic role for paraoxonase-2. J. Biol. Chem. 2006;281:29491–29500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- 11.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva V, Morvardi S, Clarke C, Vergnes L, Reue K, Teiber JF, Reddy ST. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid. Redox Signaling. 2010;14:341–351. doi: 10.1089/ars.2010.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altenhoefer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Foerstermann U, Horke S. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J. Biol. Chem. 2010;32:24398–24403. doi: 10.1074/jbc.M110.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblat M, Coleman R, Reddy ST, Aviram M. Paraoxonase 2 attenuates macrophage triglyceride accumulation via inhibition of diacylglycerol acyltransferase 1. J. Lipid. Res. 2009;50:870–879. doi: 10.1194/jlr.M800550-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 15.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK−/−β and NF-κB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedrick CC, Castellani LW, Warden CH, Puppione DL, Lusis AJ. Influence of mouse apolipoprotein A-II on plasma lipoproteins in transgenic mice. J. Biol. Chem. 1993;268:20676–20682. [PubMed] [Google Scholar]
- 17.Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, Semenkovich CF. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat. Med. 2000;6:1115–1120. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- 18.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Liang CP, Westerterp M, Senokuchi T, Welch CL, Wang Q, Matsumoto M, Accili D, Tall AR. Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J. Clin. Invest. 2009;119:1029–1041. doi: 10.1172/JCI36523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J. Biol. Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 21.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α-and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 22.Hattori Y, Kasai K, Gross SS. NO suppresses while peroxynitrite sustains NF-κB: a paradigm to rationalize cytoprotective and cytotoxic actions attributed to NO. Cardiovasc. Res. 2004;63:31–40. doi: 10.1016/j.cardiores.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 25.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat. Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 27.Colton CA, Czapiga M, Snell-Callanan J, Chernyshev ON, Vitek MP. Apolipoprotein E acts to increase nitric oxide production in macrophages by stimulating arginine transport. Biochim. Biophys. Acta. 2001;1535:134–144. doi: 10.1016/s0925-4439(00)00092-2. [DOI] [PubMed] [Google Scholar]
- 28.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat. Med. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54:959–967. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblat M, Draganov D, Watson CE, Bisgaier CL, La Du BN, Aviram M. Mouse macrophage paraoxonase 2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23:468–474. doi: 10.1161/01.ATV.0000059385.95664.4D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.