Abstract
An organic extract of a filamentous fungus (MSX 58801), identified as a Volutella sp. (Hypocreales, Ascomycota), displayed moderate cytotoxic activity against NCI-H460 human large cell lung carcinoma. Bioactivity-directed fractionation led to the isolation of three γ-lactones having the furo[3,4-b]pyran-5-one bicyclic ring system [waol A (1), trans-dihydrowaol A (2), and cis-dihydrowaol A (3)]. The structures were elucidated using a set of spectroscopic and spectrometric techniques; the absolute configuration of 2 was established via a modified Mosher’s ester method. Compounds 1 and 2 were evaluated for cytotoxicity against a human cancer cell panel.
Keywords: Polyketide, Cytotoxicty, γ-Lactone, Filamentous Fungi, Waol A
In pursuit of structurally diverse anticancer leads from nature,1,2 our group has been investigating filamentous fungi, particularly the Mycosynthetix library, representing over 55,000 accessions.3–9 Fungi represent an exciting reservoir of bioactive natural products, as they are an underexplored and renewable resource.10–12
An organic extract of the filamentous fungus MSX 58801, which was isolated from leaf litter in 1991, displayed moderate cytotoxic activity against NCI-H460 human large cell lung carcinoma (~86% inhibition of cell growth when tested at 20 µg/mL).3 Bioactivity-directed fractionation using flash chromatography followed by preparative RP-HPLC resulted in the isolation of three γ-lactones (1–3) containing a furo[3,4-b]pyran-5-one bicyclic ring system, with >95% purity for compounds 1 and 2 according to UPLC (Figure S1, Supplementary data). Compounds 1 and 2 were evaluated for cytotoxicity against a human cancer cell panel.
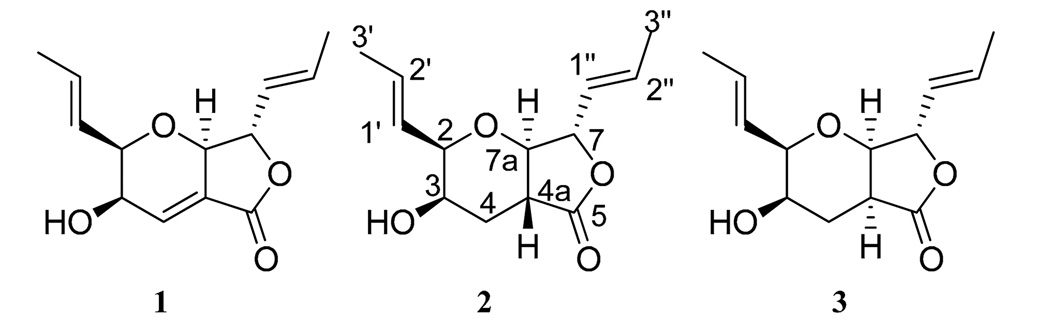
Compound 1 (2.46 mg), which was obtained as a colorless oil, had a molecular formula of C13H16O4 as determined by HRESIMS. The NMR (Figure S2, Supplementary data), HRMS, and optical rotation data identified 1 as the known compound, waol A (FD-211; Figure 1). First isolated in 1995 from a fermentation of Myceliophthora lutea TF-0409,13 the structure of 1 was revised in 2003.14,15
Figure 1.
Structures of compounds 1–3.
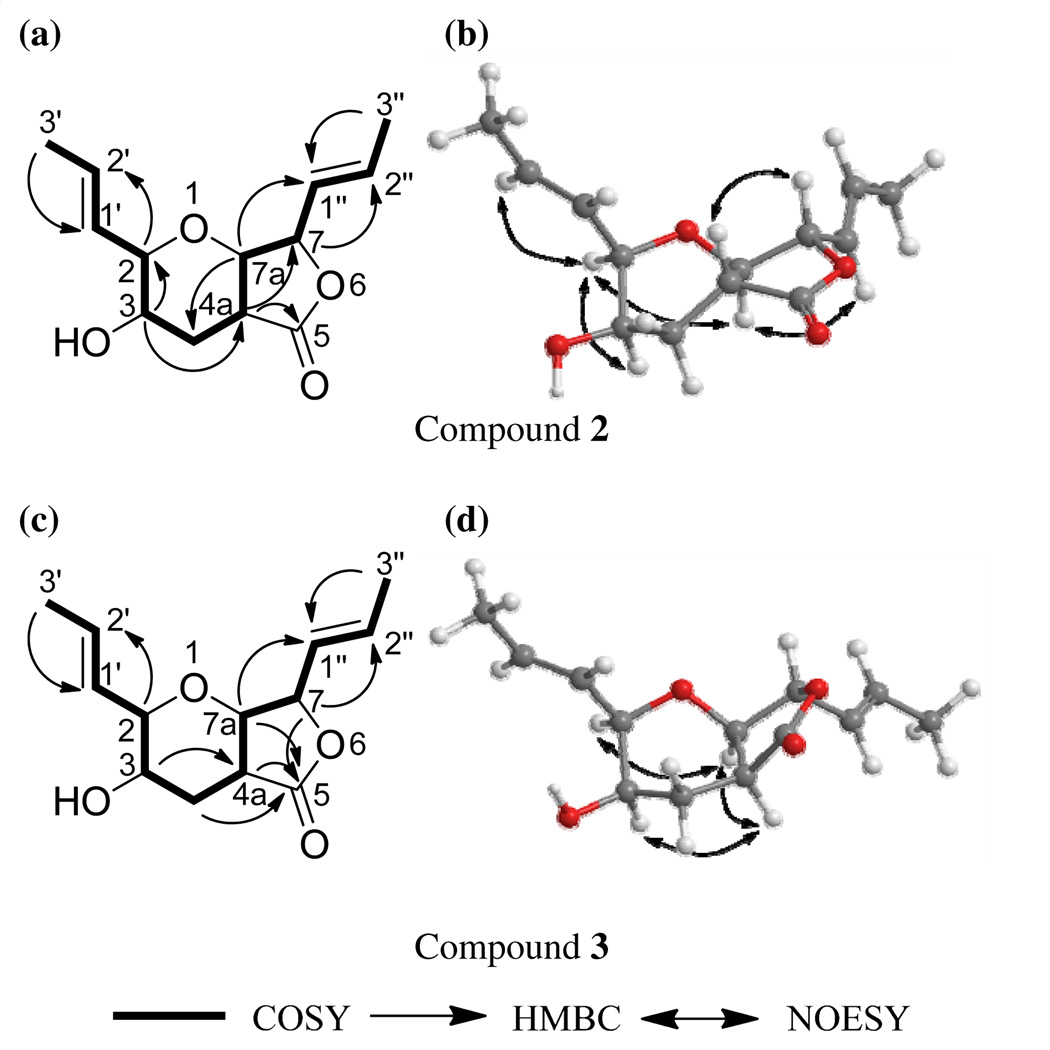
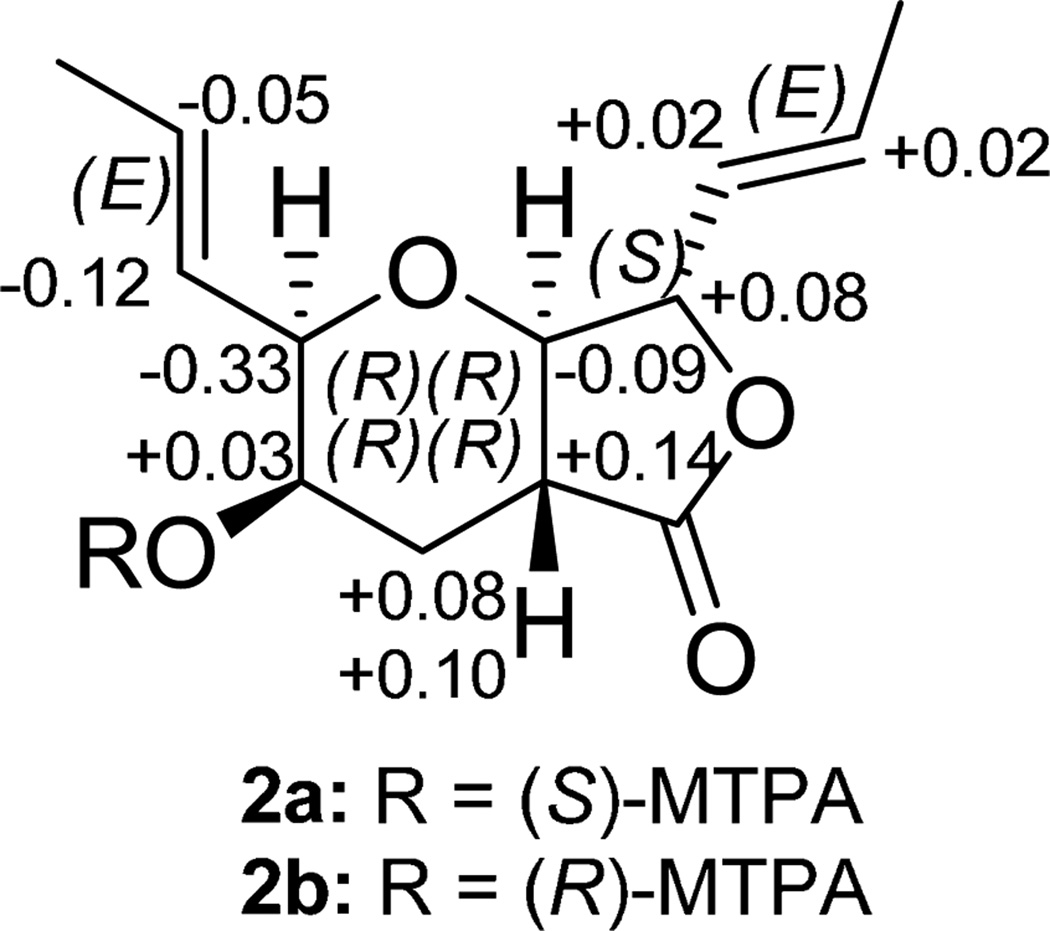
Compound 2 (9.67 mg) was also obtained as a colorless oil.16 The molecular formula was determined as C13H18O4 via HRESIMS, establishing an index of hydrogen deficiency of 5. The NMR data suggested structural similarity with compound 1. However, compound 2 lacked the olefinic proton at δH 6.90, which was replaced by three aliphatic protons (δH 1.79, 2.43, and 2.91). These data suggested a difference between 1 and 2 of a double bond, as supported by a 2 amu difference in the HRMS data. The 1H NMR data of 2 revealed the presence of four olefinic protons, corresponding to two trans-disubstituted olefins (δH 5.52, ddq, J = 15.5, 8.0, 1.7; 5.55, ddq, J = 15.5, 5.2, 1.7; 5.91, dqd, J = 15.5, 6.9, 1.7; and 5.99, dq, J = 15.5, 6.9, for H-1″, H-1′, H-2′, and H-2″, respectively), four oxymethines (δH 3.48, dd, J = 12.0, 8.6; 3.84, bq, J = 2.9; 4.03, ddd, J = 5.2, 2.9, 1.7; and 4.67, dd, J = 8.6, 8.0, for H-7a, H-3, H-2, and H-7, respectively), one methine (δH 2.91, ddd, J = 12.6, 12.0, 3.4, for H-4a), one methylene (δH 1.79, ddd, J = 13.2, 12.6, 2.9; and 2.43, ddd, J = 13.2, 3.4, 2.9, for H-4α and H-4β, respectively), two equivalent methyls (δH 1.77, dd, J = 6.9, 1.7, for H-3′ and H-3″), and one exchangeable proton (δH 1.84, for 3-OH). The 13C NMR data revealed 13 carbons, consistent with the HRMS data and indicative of one carbonyl (δC 173.5 for C-5), four olefinic carbons (δC 125.7, 126.4, 130.6, and 134.3, for C-1″, C-1′, C-2′, and C-2″, respectively), five methines (δC 39.0, 66.3, 81.2, 82.1, and 82.4 for C-4a, C-3, C-2, C-7a, and C-7, respectively), one methylene (δC 30.0 for C-4), and two methyls (δC 18.1 and 18.2 for C-3′ and C-3″, respectively) (see Supplementary Figures S3 and S4 for the 1H and 13C NMR spectra and Table S1). The two double bonds and the carbonyl group accounted for three degrees of unsaturations, leaving the remaining two accommodated by the bicyclic ring system. COSY data identified one spin system as H3-3′/H-2′/H-1′/H-2/H-3/H2-4/H-4a/H-7a/H-7/H-1″/H-2″/H3-3″ (Figure 2a). The following key HMBC correlations were observed: H3-3′→C-1′, H3-3″→C-1″, H-2→C-2′, H-7→C-2″, H-3→C-4a, H-7a→C-4, H-4a→C-7, and H-4a→C-5 (Figure 2a). NOESY correlations from H-1″ to H-7a, from H-7a to H-2, and from H-2 to H-3 and H-2′ indicated that H-1″, H-7a, H-2, H-3, and H-2′ were all on the same face. Alternatively, NOESY correlations observed from H-4a to H-7 indicated that these two protons were on the same side of the molecule but opposite to the previous set (Figure 2b). Comparing all of these data with those for 1 yielded the structure of 2 (Figure 1), which was ascribed the trivial name trans-dihydrowaol A. The absolute configuration of 2 was assigned via a modified Mosher’s ester method,17 establishing the configuration as 2R, 3R, 4aR, 7S, and 7aR (Figure 3).18
Figure 2.
Key HMBC, COSY, and NOESY correlations of 2 and 3.
Figure 3.
ΔδH values [Δδ (in ppm) = δS − δR] obtained for (S)- and (R)-MTPA esters (2a and 2b, respectively) of trans-dihydrowaol A (2) in pyridine-d5.
Compound 3 (1.45 mg) was obtained as a colorless oil.19 The molecular formula was determined as C13H18O4 via HRESIMS, and was the same as compound 2. The NMR data (Table S1 and Figures S5 and S6) suggested structural similarity with 2. Key differences were a coupling constant of 0.6 Hz between H-4a (δH 2.58, ddd, J = 7.5, 2.3, 0.6) and H-7a (δH 4.17, dd, J = 4.6, 0.6) in 3 vs 12 Hz in 2, and a NOESY correlation from H-4a to H-7a in 3 vs H-4a to H-7 in 2 (Figure 2d). These data implied a pseudoaxial/pseudoequatorial cis orientation of H-4a/H-7a. NOESY correlations were also observed from H-2 to H-7a and H-4a, and from H-4a to H-3, indicating that those protons were on the same face (Figure 2d). These data suggested an inversion in the configuration at C-4a in 3 relative to 2, establishing the structure of 3 as an epimer of 2 (Figure 1). The trivial name, cis-dihydrowaol A (3), was ascribed to this compound. The relative configuration of 3 was assigned by comparison with 2 as 2R, 3R, 4aS, 7S, and 7aR. An attempt to establish the absolute configuration via a modified Mosher’s ester method17 was unsuccessful.
Compounds 1 and 2 were tested against two cancer cell lines, MDA-MB-435 (human melanoma) and SW-620 (human colon cancer), using methods described previously;20,3 due to paucity of sample, compound 3 was not tested. While compound 1 showed moderate cytotoxic activity against the SW-620 cancer cell line, compound 2 was inactive against both cancer cell lines (Table 1), suggesting the importance of the double bond for cytotoxicity. Compound 1 was reported by Nozawa et al13 to have broad spectrum activity against cultured tumor cell lines, including adriamycin-resistant HL-60 cells. Several compounds having the furo[3,4-b]pyran-5-one bicyclic ring system have been reported from fungi with diverse biological activities, including antibacterial and cytotoxic activities.21–26
Table 1.
Cytotoxicity of compounds 1 and 2 against two human tumor cell linesa
Positive controls were vinblastine and bortezomib. Vinblastine was tested at concentrations of 3 ng/mL and 1 ng/mL: MDA-MB-435 cells had 37% and 99% viable cells; SW620 cells had 76% and 90% viable cells; respectively. Bortezomib was tested at concentrations of 5 nM and 2.5 nM: MDA-MB-435 cells had 90% and 91% viable cells; SW620 cells had 79% and 71% viable cells; respectively.
melanoma
Supplementary Material
Acknowledgments
This research was supported by program project grant P01 CA125066 from the National Cancer Institute/National Institutes of Health, Bethesda, MD, USA. The high resolution mass spectrometry data were acquired at the Triad Mass Spectrometry Laboratory at the University of North Carolina at Greensboro. Sequence data was generated at the Mycology laboratory of Dr. Andrew N. Miller, Illinois Natural History Survey, University of Illinois at Urbana-Champaign.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Orjala J, Oberlies NH, Pearce CJ, Swanson SM, Kinghorn AD. In: Bioactive Compounds from Natural Sources. Natural Products as Lead Compounds in Drug Discovery. Tringali C, editor. London, UK: Taylor & Francis; 2012. pp. 37–63. [Google Scholar]
- 2.El-Elimat T, Zhang X, Jarjoura D, Moy FJ, Orjala J, Kinghorn AD, Pearce CJ, Oberlies NH. ACS Med. Chem. Lett. 2012;3:645–649. doi: 10.1021/ml300105s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayers S, Graf TN, Adcock AF, Kroll DJ, Matthew S, Carcache de Blanco EJ, Shen Q, Swanson SM, Wani MC, Pearce CJ, Oberlies NH. J. Nat. Prod. 2011;74:1126–1131. doi: 10.1021/np200062x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayers S, Ehrmann BM, Adcock AF, Kroll DJ, Carcache de Blanco EJ, Shen Q, Swanson SM, Falkinham JO, 3rd, Wani MC, Mitchell SM, Pearce CJ, Oberlies NH. J. Pept. Sci. 2012;18:500–510. doi: 10.1002/psc.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayers S, Graf TN, Adcock AF, Kroll DJ, Shen Q, Swanson SM, Matthew S, Carcache de Blanco EJ, Wani MC, Darveaux BA, Pearce CJ, Oberlies NH. J. Antibiot. 2012;65:3–8. doi: 10.1038/ja.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayers S, Graf TN, Adcock AF, Kroll DJ, Shen Q, Swanson SM, Wani MC, Darveaux BA, Pearce CJ, Oberlies NH. Tetrahedron Lett. 2011;52:5128–5230. doi: 10.1016/j.tetlet.2011.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sy-Cordero AA, Graf TN, Adcock AF, Kroll DJ, Shen Q, Swanson SM, Wani MC, Pearce CJ, Oberlies NH. J. Nat. Prod. 2011;74:2137–2142. doi: 10.1021/np2004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa M, Graf TN, Ayers S, Adcock AF, Kroll DJ, Yang J, Swanson SM, Munoz-Acuna U, Carcache de Blanco EJ, Agrawal R, Wani MC, Darveaux BA, Pearce CJ, Oberlies NH. J. Antibiot. 2012;65:559–564. doi: 10.1038/ja.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Elimat T, Figueroa M, Raja HA, Graf TN, Adcock AF, Kroll DJ, Day CS, Wani MC, Pearce CJ, Oberlies NH. J. Nat. Prod. 2013;76:382–387. doi: 10.1021/np300749w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackwell M. Am. J. Bot. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- 11.Hawksworth DL. Mycol. Res. 1991;95:641–655. [Google Scholar]
- 12.Gloer JB. In: Environmental and Microbial Relationships. Kubicek CP, Druzhinina IS, editors. Berlin Heidelberg: Springer; 2007. pp. 257–283. [Google Scholar]
- 13.Nozawa O, Okazaki T, Sakai N, Komurasaki T, Hanada K, Morimoto S, Chen ZX, He BM, Mizoue K. J. Antibiot. 1995;48:113–8. doi: 10.7164/antibiotics.48.113. [DOI] [PubMed] [Google Scholar]
- 14.Gao XL, Nakadai M, Snider B. B. Org. Lett. 2003;5:451–454. doi: 10.1021/ol0273405. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Snider B. B. J. Org. Chem. 2004;69:5517–27. doi: 10.1021/jo0358628. [DOI] [PubMed] [Google Scholar]
- 16.trans-Dihydrowaol A (2): colorless oil; [α]D26 = −56° (c = 0.1, MeOH); 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz) (see Supplementary Data); HRESIMS m/z 239.1278 [M+H]+ (calcd for C13H19O4 239.1278).
- 17.Hoye TR, Jeffrey CS, Shao F. Nat. Protoc. 2007;2:2451–2458. doi: 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- 18.Preparation of the (R)- and (S)-MTPA ester derivatives of trans-dihydrowaol A (2): To 0.75 mg of compound 2 was added 400 µL of pyridine-d5 and transferred into an NMR tube. To initiate the reaction, 10 µL of S-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl (MTPA) chloride was added into the NMR tube with careful shaking and then monitored immediately by 1H NMR at the following time points 0, 15, 30, and 60 min. The reaction was found to be complete within 30 min, yielding the mono (R)-MTPA ester derivative (2b) of 2. 1H NMR data of 2b (500 MHz, pyridine-d5): 5.93 (1H, m, H-2′), 5.89 (1H, m, H-1′), 5.69 (1H, m, H-2″), 5.60 (1H, m, H-1″), 5.53 (1H, bq, J = 2.3, H-3), 4.81 (1H, dd, J = 8.6, 8.0, H-7), 4.48 (1H, d, J = 5.7, H-2), 3.94 (1H, dd, J = 9.2, 8.6, H-7a), 2.69 (1H, m, H-4a), 2.67 (1H, m, H-4β), 2.29 (1H, m, H-4α), 1.63 (3H, d, J = 6.9, H3-3′), and 1.55 (3H, d, J = 6.3, H3-3″). In an analogues manner, 0.75 mg of compound 2 dissolved in 400 µL pyridine-d5 was reacted in a second NMR tube with 10 µL (R)-(−)-α-MTPA chloride for 30 min, to afford the mono (S)-MTPA ester (2a). 1H NMR data of 2a (500 MHz, pyridine-d5): δH 5.88 (1H, m, H-2′), 5.77 (1H, m, H-1′), 5.70 (1H, m, H-2″), 5.60 (1H, m, H-1″), 5.56 (1H, bq, J = 3.4, H-3), 4.89 (1H, dd, J = 8.6, 8.0, H-7), 4.15 (1H, d, J = 6.9, H-2), 3.85 (1H, m, H-7a), 2.84 (1H, m, H-4a), 2.77 (1H, m, H-4β), 2.37 (1H, m, H-4α), 1.53 (3H, d, J = 6.3, H3-3′), and 1.50 (3H, d, J = 6.3, H3-3″).
- 19.cis-Dihydrowaol A (3): colorless oil; [α]D26 = −32° (c = 0.1, MeOH); 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz) (see Supplementary Data); HRESIMS m/z 239.1280 [M+H]+ (calcd for C13H19O4 239.1278).
- 20.Kim H, Zinkus J, Swanson S, Orjala J. Planta Med. 2012;78:PI83. [Google Scholar]
- 21.Oh H, Swenson DC, Gloer JB, Shearer CA. Tetrahedron Lett. 2001;42:975–977. [Google Scholar]
- 22.Hayashi K, Takizawa M, Noguchi K. 10,287,679, 1998. Chem. Abstr. 1999;130:3122e. Japanese Patent.
- 23.Krohn K, Hussain H, Florke U, Schulz B, Draeger S, Pescitelli G, Salvadori P, Antus S, Kurtan T. Chirality. 2007;19:464–470. doi: 10.1002/chir.20402. [DOI] [PubMed] [Google Scholar]
- 24.Kock I, Krohn K, Egold H, Draeger S, Schulz B, Rheinheimer J. Eur. J. Org. Chem. 2007;2007:2186–2190. [Google Scholar]
- 25.Qin S, Krohn K, Flörke U, Schulz B, Draeger S, Pescitelli G, Salvadori P, Antus S, Kurtán T. Eur. J. Org. Chem. 2009;2009:3279–3284. [Google Scholar]
- 26.Krohn K, Biele C, Drogies K-H, Steingröver K, Aust H-J, Draeger S, Schulz B. Eur. J. Org. Chem. 2002;2002:2331–2336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.