Abstract
Objectives
The aim of this study was to determine the prevalence of frailty in a community cohort of patients with heart failure (HF) and to determine whether frailty is associated with healthcare utilization.
Background
Frailty is associated with death in patients with HF, but its prevalence and impact on healthcare utilization in patients with HF are poorly characterized.
Methods
Residents of Olmsted, Dodge, and Fillmore counties in Minnesota with HF between October 2007 and March 2011 were prospectively recruited to undergo frailty assessment. Frailty was defined as 3 or more of the following: unintentional weight loss, exhaustion, weak grip strength, and slowness and low physical activity measured by the SF-12 physical component score. Intermediate frailty was defined as 1 or 2 components. Negative binomial regression was used to examine the association between outpatient visits and frailty; Andersen-Gill models were used to determine if frailty predicted emergency department (ED) visits or hospitalizations.
Results
Among 448 patients (mean age 73 ± 13 years, 57% men), 74% had some degree of frailty (19% frail, 55% intermediate frail). Over a mean follow-up period of 2.0 ± 1.1 years, 20,164 outpatient visits, 1,440 ED visits, and 1,057 hospitalizations occurred. After adjustment for potential confounders, frailty was associated with a 92% increased risk for ED visits and a 65% increased risk for hospitalizations. The population-attributable risk associated with any degree of frailty was 35% for ED visits and 19% for hospitalizations.
Conclusions
Frailty is common among community patients with HF and is a strong and independent predictor of ED visits and hospitalizations. Because frailty is potentially modifiable, it should be incorporated in the clinical evaluation of patients with HF.
Keywords: frailty, healthcare utilization, heart failure, risk
Heart failure (HF), a syndrome associated with substantial morbidity and mortality worldwide, affects approximately 5.7 million Americans (1). Consequently, HF is associated with significant health care utilization and remains the leading cause of hospitalizations among persons 65 years of age or older (1,2). Frailty, a biologic syndrome characterized by a decline in overall function and loss of resistance to stressors (3), is also associated with increased morbidity, mortality, and healthcare utilization among elderly persons (3–10). Although HF primarily affects older persons (1), and some studies suggest that patients with HF have a higher prevalence of frailty than the general elderly population (11–13), the topic of frailty is only recently gaining attention in the cardiology community. Thus, few studies have investigated the prognostic role of frailty in patients with HF (13–15). Although these studies indicate that frailty predicts death among patients with HF, data on the association between frailty and health care utilization in patients with HF are sparse, with only 1 study examining the risk for HF hospitalizations (15). Furthermore, to our knowledge, no other study has investigated the association between frailty and both inpatient and outpatient healthcare utilization in a community population of optimal clinical relevance. Thus, the relationship between frailty and all-cause hospitalizations, emergency department (ED) visits, and outpatient visits among community patients with HF is unknown, and demonstrating a robust association between frailty and outcomes could lead to changes in the clinical evaluation of patients with HF.
To address these gaps in knowledge, our aim was to determine the prevalence of frailty among community patients with HF and to examine whether frailty is associated with hospitalizations, ED visits, and outpatient visits, independently of comorbidities. Furthermore, we aimed to estimate the population attributable risk (PAR) of healthcare utilization that is associated with frailty.
Methods
Study setting
This study was conducted in southeastern Minnesota. As previously described, population-based research is feasible in this area because only a few providers (Mayo Clinic, Olmsted Medical Center, and a few private providers) deliver nearly all health care to the local residents (16). The records from each institution are indexed through the Rochester Epidemiology Project, resulting in the linkage of medical records from all sources of care (16).
Identification of patients
Our methods for identifying patients with HF have been previously described (17–19). In brief, potential patients with HF residing in Olmsted, Dodge, and Fillmore counties were prospectively identified using natural language processing of the electronic health records. The complete records of potential patients were manually reviewed to collect clinical data and to verify the diagnoses of HF, using the Framingham criteria (20). Patients were then contacted about study participation. After consent was obtained, patients completed questionnaires and a hand-grip test, administered by a registered nurse. If a clinical echocardiogram was not available within 6 months before to 2 months after the HF index date, echocardiography was performed as part of the study. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Board.
Frailty assessment
Frailty was ascertained using a modified version of the Cardiovascular Health Study frailty definition (3). Patients were classified as frail if they met 3 or more of the following criteria: weak grip strength, physical exhaustion, slowness, low physical activity, and unintentional weight loss. Intermediate frailty was defined as meeting 1 or 2 criteria.
Grip strength was measured using a Jamar dynamometer (in kilograms). Grip strength was considered weak if the average of 3 tests was in the lowest 20% of sex-adjusted and body mass index–adjusted community-dwelling older adults (3). Physical exhaustion was assessed according to self-report using a question from the Patient Health Questionnaire (PHQ-9) (21): “Over the past 2 weeks have you been bothered by feeling tired or having little energy?” Patients who answered “more than half the days” or “nearly every day” were classified as experiencing physical exhaustion.
The SF-12, which includes a validated physical component scale, was administered to study participants (22). We used the physical component score as an indicator of slowness and low physical activity, as was done in previous studies (7,23). The SF-12 physical component score ranges from 0 to 100; higher scores indicate better physical health. A physical component score of 25 or less was used as an indicator for both low physical activity and slow walking speed. Unintentional weight loss was assessed by self-report (3,23). The following question was asked: “In the past year, have you lost any weight unintentionally (without trying)?” A response of “10 pounds or more” was classified as unintentional weight loss.
Patient characteristics
Registered nurses, trained in data collection from the medical records, collected characteristics at the time of HF diagnosis from the medical records. Clinicians’ diagnoses were used to define hypertension, hyperlipidemia, chronic obstructive pulmonary disease, myocardial infarction, atrial fibrillation, depression, and smoking status. Diabetes mellitus was defined according to the American Diabetes Association criteria (24), and comorbidity was measured using the Charlson comorbidity index (25).
Estimated glomerular filtration rate was calculated using the creatinine value closest to HF diagnosis (±1 year) with the MDRD (Modification of Diet in Renal Disease) study equation (26). Anemia was defined as hemoglobin <13.0 g/dl in men and <12.0 g/dl in women (27), using the value closest to HF diagnosis (±1 year). Body mass index (in kilograms divided by square meter) was calculated using height at the time of HF diagnosis and weight from the last outpatient visit before HF diagnosis.
Left ventricular ejection fraction (EF) was obtained using the closest value from an echocardiogram within 6 months before to 2 months after HF date. Reduced EF was defined as EF <50% and preserved EF as EF ≥50% (28).
Outcome ascertainment
Participants were followed through September 30, 2011, for healthcare utilization. Outpatient visits, ED visits, and hospitalizations were ascertained via the Olmsted County Healthcare Expenditure and Utilization Database, which contains Olmsted County health care utilization information since 1987. Outpatient visits for tests, imaging, or outpatient procedures were not included. ED visits that resulted in hospitalizations were counted as both ED visits and hospitalizations. For patients enrolled in the study during hospitalization, only subsequent hospitalizations were included in the analysis (2). In-hospital transfers or transfers between Olmsted Medical Center and the Mayo Clinic were counted as 1 hospitalization.
The primary reason for hospitalization was assessed using the primary International Classification of Diseases-Ninth Revision code. This code, which reflects the main reason for hospitalization, is assigned by trained personnel after discharge. The primary reason for hospitalization was grouped by condition.
Statistical analysis
Baseline characteristics are presented as frequency (percent) for categorical variables, as mean ± SD for normally distributed continuous variables, and as median (interquartile range) for continuous variables with skewed distributions. Mantel-Haenszel chi-square tests and linear regression were used to test trends in characteristics across groups. Frailty status was coded as a single 3-level variable (frail, intermediate frail, or not frail).
Negative binomial regression was used to analyze outpatient visits. Outpatient visits during follow-up may cluster; for example, patients may have multiple outpatient visits on a given day or within a span of several days as part of the diagnostic process or for yearly physical examinations. To account for this, the association between frailty and outpatient office visits was evaluated by calculating the number of visits per person-year for each patient. To determine if frailty predicts ED visits or hospitalizations, Andersen-Gill modeling was used to account for repeated events, univariately and while controlling for baseline characteristics. To test whether associations increased with increasing frailty, we tested for a trend using frailty as a single 3-level variable. The proportional hazards assumption was tested using the scaled Schoenfeld residuals and was found to be valid.
We estimated the PAR of outpatient visits, ED visits, and hospitalizations associated with frailty using a standard method (29). The PAR, which assumes a causal relationship, is an estimate of the proportion of outcome events in this population that could have been prevented if all participants were free of frailty.
Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina) and R version 2.14.0 (R Foundation for Statistical Computing, Vienna, Austria). A p value of <0.05 was used as the level of statistical significance.
Results
Patient characteristics
Between October 2007 and March 2011, 985 patients were approached for enrollment, and 560 (57%) consented to the study. We did not have complete frailty assessments on 102 patients and could not obtain health care utilization data on 10 patients, resulting in the final study cohort of 448 (mean age 73 ± 13 years, 57% men) (Table 1). The comorbidity burden was high in this cohort; 299 (67%) of the subjects had Charlson comorbidity indexes of 3 or greater. A total of 173 patients (39%) had incident HF, and the remaining 275 (61%) had prevalent HF.
Table 1.
Baseline Characteristics by Frailty Status
| Variable | Number Missing |
Total (n = 448) |
Not Frail (n = 116) |
Intermediate Frail (n = 248) |
Frail (n = 84) | p Value for Trend |
|---|---|---|---|---|---|---|
| Age (yrs) | 0 | 73.2±13.3 | 69.0±14.0 | 74.4±13.1 | 75.5±11.9 | <0.001 |
| Men | 0 | 257 (57.4%) | 68 (58.6%) | 144 (58.1%) | 45 (53.6%) | 0.505 |
| Cardiovascular risk factors | ||||||
| Hypertension | 0 | 405 (90.4%) | 102 (87.9%) | 223 (89.9%) | 80 (95.2%) | 0.095 |
| Current or former smoker | 0 | 265 (59.2%) | 65 (56.0%) | 141 (56.9%) | 59 (70.2%) | 0.062 |
| Diabetes mellitus | 2 | 175 (39.2%) | 38 (32.8%) | 95 (38.5%) | 42 (50.6%) | 0.013 |
| Hyperlipidemia | 1 | 369 (82.6%) | 89 (76.7%) | 210 (84.7%) | 70 (84.3%) | 0.122 |
| BMI (kg/m2) | 0 | 29.7 (26–35) | 30.3 (27–35) | 29.6 (25–35) | 29.9 (26–37) | 0.873 |
| Comorbidities | ||||||
| Prior MI | 2 | 118 (26.5%) | 24 (20.7%) | 63 (25.6%) | 31 (36.9%) | 0.013 |
| COPD | 0 | 119 (26.6%) | 17 (14.7%) | 70 (28.2%) | 32 (38.1%) | <0.001 |
| Atrial fibrillation/flutter | 0 | 284 (63.4%) | 68 (58.6%) | 155 (62.5%) | 61 (72.6%) | 0.050 |
| Depression | 1 | 179 (40.0%) | 30 (25.9%) | 108 (43.7%) | 41 (48.8%) | 0.001 |
| eGFR (ml/min/1.73 m2) | 49 | 57.1 (42–72) | 61.3 (47–71) | 57.7 (42–72) | 47.7 (39–66) | 0.024 |
| Anemia | 6 | 240 (54.3%) | 36 (31.3%) | 150 (61.7%) | 54 (64.3%) | <0.001 |
| Comorbidity index ≥3 | 2 | 299 (67.0%) | 58 (50.0%) | 175 (71.1%) | 66 (78.6%) | <0.001 |
| HF characteristics and severity indexes | ||||||
| EF (%) | 11 | 46.0±16.3 | 42.4±17.5 | 46.7±15.4 | 48.7±16.4 | 0.005 |
| Prevalent HF | 0 | 272 (60.7%) | 60 (51.7%) | 154 (62.1%) | 58 (69.1%) | 0.011 |
Values are mean ± SD, n (%), or median (interquartile range).
BMI = body mass index; COPD = chronic obstructive pulmonary disease; EF = ejection fraction; eGFR = estimated glomerular filtration rate; HF = heart failure; MI = myocardial infarction.
Frailty
A total of 332 patients (74%) had some degree of frailty: 84 patients (19%) were classified as frail and 248 (55%) as intermediate frail. A total of 213 patients (48%) had poor grip strength, whereas 174 patients (39%) experienced exhaustion. Among all patients, 54 (12%) experienced 3 components of frailty, 28 (6%) experienced 4, and 2 (0.5%) experienced all 5 components. Baseline characteristics were examined according to degree of frailty (Table 1). Patients who were frail were more likely to be older; to have diabetes; to have had prior myocardial infarctions; and to have chronic obstructive pulmonary disease, atrial fibrillation, depression, anemia, lower estimated glomerular filtration rates, prevalent HF, and a Charlson comorbidity index of 3 or greater. Frail patients were also more likely to have higher EFs (p = 0.005), even after adjustment for age and sex (p = 0.034).
Healthcare utilization
After a mean follow-up period of 2.0 ± 1.1 years, 20,164 outpatient visits, 1,440 ED visits, and 1,057 hospitalizations had occurred. The number of outpatient visits after HF ranged from 1 to 220 (median 35) per person, ED visits ranged from 0 to 44 (median 2) per person, and hospitalizations ranged from 0 to 22 (median 2) per person. Fifty-three percent of ED visits resulted in hospitalizations, whereas 72%of the hospitalizations were preceded by ED visits.
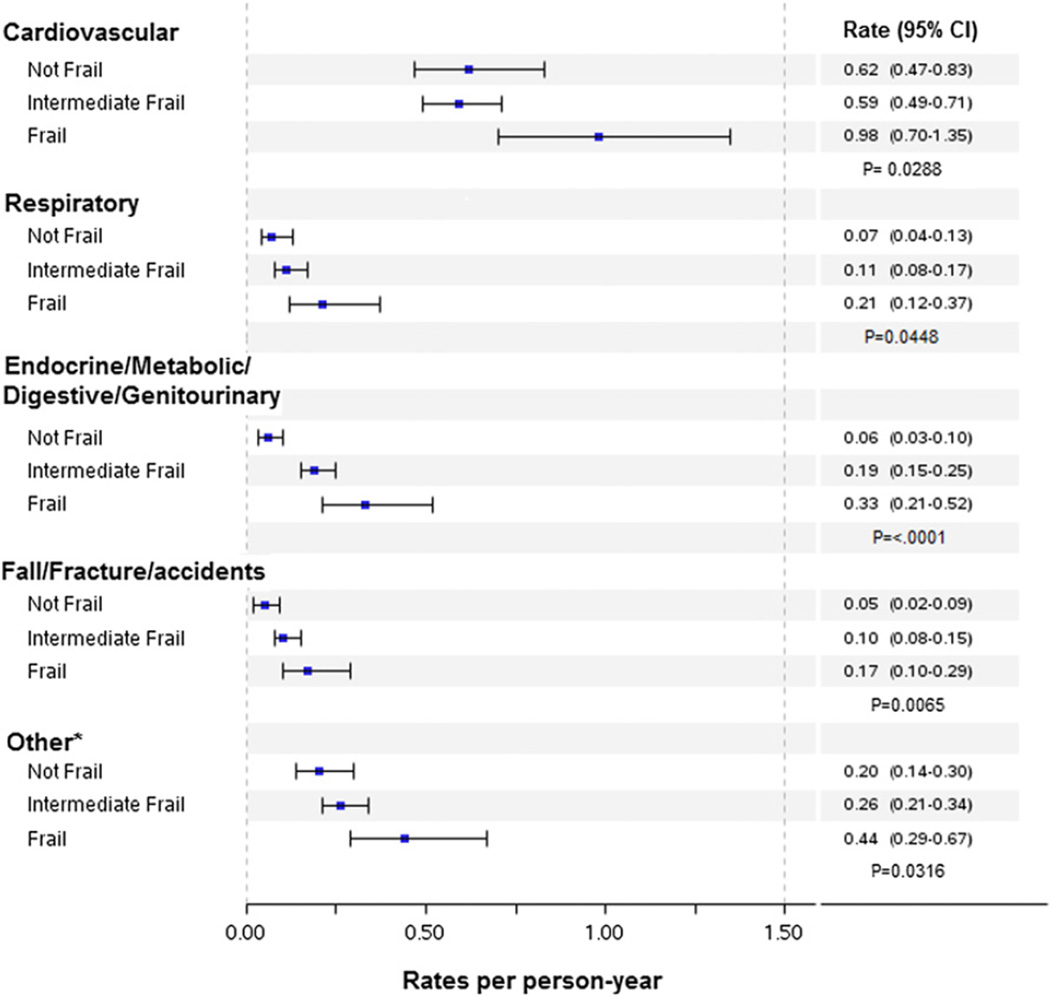
There were strong positive graded associations between frailty and hospitalizations and ED visits, whereas there was a weaker association with outpatient visits (Table 2). The association between frailty and healthcare utilization did not differ significantly according to EF (p > 0.05 for the interaction of frailty and EF for hospitalizations, ED visits, and outpatient visits). After adjustment for age, sex, EF, incident versus prevalent HF, chronic obstructive pulmonary disease, diabetes, anemia, and estimated glomerular filtration rate, the association between frailty and outpatient visits was not statistically significant (intermediate frail risk ratio: 1.08; 95% confidence interval [CI]: 0.92 to 1.27; frail risk ratio: 1.13; 95% CI: 0.92 to 1.39). Frailty was associated with an increased risk for ED visits. After adjustment for covariates, intermediate frail patients had a 60% increased risk for ED visits (hazard ratio [HR]: 1.60; 95% CI: 1.15 to 2.24) compared with those who were not frail, whereas frail patients had 92% increased risk for ED visits (HR: 1.92; 95% CI: 1.30 to 2.83). After adjustment, intermediate frail patients had a 22% increased risk for hospitalizations (HR: 1.22; 95% CI: 0.89 to 1.68), and frail patients had a 65% increased risk for hospitalizations (HR: 1.65; 95% CI: 1.17 to 2.35) compared with those who were not frail. Furthermore, frail patients had higher rates of hospitalizations for all cardiovascular and noncardiovascular conditions (Fig. 1).
Table 2.
Rates and RR (95% CI) or HRs (95% CIs) for Outpatient Visits, ED Visits, and Hospitalizations by Frailty Status
| Variable | Not Frail (n = 116) | Intermediate Frail (n = 248) | Frail (n = 84) | p Value for Trend* |
|---|---|---|---|---|
| Outpatient visits | ||||
| Event rate† | 19.60 | 23.52 | 26.11 | |
| Unadjusted RR | 1.00 | 1.13 (0.98–1.31) | 1.30 (1.08–1.57) | 0.007 |
| Fully adjusted RR‡ | 1.00 | 1.08 (0.92–1.27) | 1.13 (0.92–1.39) | 0.242 |
| ED visits | ||||
| Event rate† | 1.16 | 1.70 | 2.20 | |
| Unadjusted HR | 1.00 | 1.48 (1.12–1.95) | 1.86 (1.32–2.62) | <0.001 |
| Fully adjusted HR‡ | 1.00 | 1.60 (1.15–2.24) | 1.92 (1.30–2.83) | 0.001 |
| Hospitalizations | ||||
| Event rate† | 0.90 | 1.18 | 1.79 | |
| Unadjusted HR | 1.00 | 1.32 (1.01–1.73) | 1.90 (1.38–2.60) | <0.001 |
| Fully adjusted HR‡ | 1.00 | 1.22 (0.89–1.68) | 1.65 (1.17–2.34) | 0.004 |
p value for 1 degree of freedom test.
Unadjusted rate per person-year.
Adjusted for age, sex, ejection fraction, incident versus prevalent heart failure, chronic obstructive pulmonary disease, diabetes, anemia, and estimated glomerular filtration rate.
CI = confidence interval; ED = emergency department; HR = hazard ratio; RR = risk ratio.
Figure 1. Rates of Hospitalizations by Frailty Status and Reason for Admission.
Unadjusted rates (per person-year) of hospitalizations by frailty status and by reason for admission. *Includes infections; diseases of the blood, nervous system, skin, and musculoskeletal system; mental disorders; cancer; pregnancy complications; and ill-defined conditions. CI = confidence interval.
The PAR associated with any degree of frailty (intermediate frail and frail) was 6%, 35%, and 19% for outpatient visits, ED visits, and hospitalizations, respectively.
To evaluate the robustness of our results, we further adjusted for hypertension, prior myocardial infarction, atrial fibrillation, cancer, depression, body mass index, and smoking status, which yielded similar results to those found in Table 2. We also conducted a sensitivity analysis, with frailty assessed by only 4 components (an SF-12 physical functioning score ≤25 represented only 1 component). Frailty was defined as having ≥2 components and intermediate frailty as having 1 component. Results were similar to those obtained by assessing frailty with 5 components.
Discussion
Frailty was highly prevalent among community patients with HF and was associated with an increased risk for ED visits and hospitalizations, independently of comorbidities. Frail patients were more likely to be hospitalized for cardiovascular as well as noncardiovascular conditions. The PAR associated with frailty was 35%forEDvisits and 19%for hospitalizations.
Prevalence of frailty in HF
In the present study of community patients with HF, 74% had some degree of frailty (19% frail, 55% intermediate frail), according to a modified version of the definition of frailty used in the Cardiovascular Health Study (3). Furthermore, we found that frail patients were more likely to have higher EFs, which could be explained by the fact that patients with preserved EF are often older than patients with reduced EF and have a higher prevalence of comorbidities (30).
Previously published studies on the prevalence of frailty among patients with HF have used varying definitions, which compromises our ability to make comparisons. However, in general, the data indicate that frailty is prevalent among patients with HF (13–15).
Furthermore, among 4,735 patients enrolled in the Cardiovascular Health Study (11) (mean age 73 years, 43% men), 51% had some degree of frailty (6% were frail and 45% were intermediate frail). Thus, our data indicate that patients with HF experience an excess burden of frailty and could greatly benefit from interventions aimed at preventing or managing frailty.
Frailty and healthcare utilization in HF
The relationship between frailty and outpatient visits among community patients with HF has not been previously studied. Outpatient visits, which tend to denote long-term care, were frequent, but the association between frailty and outpatient visits was not statistically significant after adjustment for comorbidities, suggesting no major link between frailty and scheduled healthcare visits among patients with HF. Because patients with HF have a large burden of comorbidities, typically linked to scheduled outpatient visits, it is conceivable that that frailty does not further increase utilization.
There was a graded association between frailty and hospitalizations. Intermediate frail patients had a 22% increased risk for hospitalizations, and frail patients had a 65% increased risk of being hospitalized compared with those who were not frail. We also found a graded association between frailty and ED visits, with intermediate frail patients having a 60% increased risk for ED visits and frail patients having nearly 2 times the rate of ED visits compared with nonfrail patients. The relationship between frailty and ED visits has not been previously investigated in the community, and only 1 study investigated the association between frailty and hospitalizations among patients with HF (15). This study did not find frailty to predict HF hospitalizations but included only hospitalized patients with HF and aimed to predict only HF-related rehospitalizations, which are now recognized as constituting a minority of hospitalizations among patients with HF (2). Previous studies of the elderly (3,4,6–8) and of patients with coronary disease (31) have reported a significant association between frailty and hospitalizations.
Frail patients are at an increased risk for falls, fractures, and decreased mobility (3,6,7,32). However, frail patients in our study had a higher rate of hospitalizations not only for fractures and other injuries but also for cardiovascular and all other noncardiovascular conditions, suggesting that frail patients may be less capable of managing their care. As recently stated by Joynt and Jha (33), hospitalizations often “result from a complex interplay among patients, hospitals and communities,” and the key drivers of hospitalizations are not always the illness itself but precipitating factors such as poor social support, poverty, or, as suggested in the present study, frailty. Indeed, being frail may mean the difference between being able to function at home and going to the hospital or ED. However, despite growing importance, age-related complexities are not yet integrated in the management of HF (34), underscoring the need for a holistic approach to treating patients with HF that considers all comorbid conditions and frailty.
Limitations, strengths, and clinical implications
On the basis of available data in our cohort, we modified the definition of frailty in the Cardiovascular Health Study (3), because we did not have measurements of walking speed in our patients. However, physical health scores, such as the SF-12 used herein, have been shown to be associated with walking speed and physical activity and have been used as surrogates in previous studies (7,23). Furthermore, the Cardiovascular Health Study frailty definition, on which we based our definition, is a standardized, widely used definition, which has been shown to offer predictive validity for falls, disability, hospitalizations, and death (3). Although southeastern Minnesota is becoming increasingly diverse, the results reported herein need replication in communities of different racial and ethnic composition.
Our study had several notable strengths, including the rigorous validation of each HF diagnosis. This was a community-based study including both inpatients and outpatients, those with preserved and reduced EF and those with incident and prevalent HF, thus capturing the complete spectrum of HF.
We provide new data on the association between frailty and outpatient and ED utilization among patients with HF. We estimated that the PAR associated with frailty was 35% for ED visits and 19% for hospitalizations. This suggests that interventions aimed at reducing frailty could help decrease or control the already overwhelmingly high healthcare utilization and costs associated with HF (2,35). Hence, these data further support the need to assess frailty in the clinical setting, given that interventions, mostly exercise based, appear effective among frail patients (36,37) and have been shown to be safe and efficacious in patients with HF (38).
Conclusions
In the community, frailty is prevalent and is a strong and independent predictor of hospitalizations and ED visits among patients with HF. Because it is independent from coexisting comorbidities, frailty defines new strategies for intervention, and its assessment should be incorporated in the clinical evaluation of patients with HF.
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01 HL72435) and the Rochester Epidemiology Project from the National Institute on Aging (R01 AG034676).
The authors thank Annette McNallan, RN, Kay Traverse, RN, Ellen Koepsell, RN, Ruoxiang Jiang, and Deborah S. Russell for their study support.
Abbreviations and Acronyms
- CI
confidence interval
- ED
emergency department
- EF
ejection fraction
- HF
heart failure
- MDRD
Modification of Diet in Renal Disease
- HR
hazard ratio
- PAR
population-attributable risk
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Fried TR, Mor V. Frailty and hospitalization of long-term stay nursing home residents. J Am Geriatr Soc. 1997;45:265–269. doi: 10.1111/j.1532-5415.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 6.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 7.Fugate Woods N, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 8.Rochat S, Cumming RG, Blyth F, et al. Frailty and use of health and community services by community-dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. 2010;39:228–233. doi: 10.1093/ageing/afp257. [DOI] [PubMed] [Google Scholar]
- 9.Hoeck S, Francois G, Geerts J, Van der Heyden J, Vandewoude M, Van Hal G. Health-care and home-care utilization among frail elderly persons in Belgium. Eur J Public Health. 2012;22:671–677. doi: 10.1093/eurpub/ckr133. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Alexander K, Roger VL, et al. Frailty and its potential relevance to cardiovascular care. Mayo Clin Proc. 2008;83:1146–1153. doi: 10.4065/83.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 12.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 13.Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–316. doi: 10.1016/j.jacc.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lupon J, Gonzalez B, Santaeugenia S, et al. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Esp Cardiol. 2008;61:835–842. [PubMed] [Google Scholar]
- 16.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Pakhomov SV, Buntrock J, Chute CG. Prospective recruitment of patients with congestive heart failure using an ad-hoc binary classifier. J Biomed Inform. 2005;38:145–153. doi: 10.1016/j.jbi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 19.Dunlay SM, Eveleth JM, Shah ND, McNallan SM, Roger VL. Medication adherence among community-dwelling patients with heart failure. Mayo Clin Proc. 2011;86:273–281. doi: 10.4065/mcp.2010.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 21.Martin A, Rief W, Klaiberg A, Braehler E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71–77. doi: 10.1016/j.genhosppsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Clinical practice recommendations 1997. Diabetes Care. 1997;20(Suppl):S1, S70. [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 27.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 28.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 29.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 32.Bilotta C, Nicolini P, Case A, Pina G, Rossi S, Vergani C. Frailty syndrome diagnosed according to the Study of Osteoporotic Fractures (SOF) criteria and adverse health outcomes among community-dwelling older outpatients in Italy. A one-year prospective cohort study. Arch Gerontol Geriatr. 2012;54:e23–e28. doi: 10.1016/j.archger.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366:1366–1369. doi: 10.1056/NEJMp1201598. [DOI] [PubMed] [Google Scholar]
- 34.Forman DE, Rich MW, Alexander KP, et al. Cardiac care for older adults, Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–1810. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta-analysis. Arch Phys Med Rehabil. 2012;93:237–244. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. doi: 10.4061/2011/569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]